Concentration Dependent Single Chain Properties of Poly(sodium 4-styrenesulfonate) Subjected to Aromatic Interactions with Chlorpheniramine Maleate Studied by Diafiltration and Synchrotron-SAXS
Abstract
:1. Introduction
2. Theory
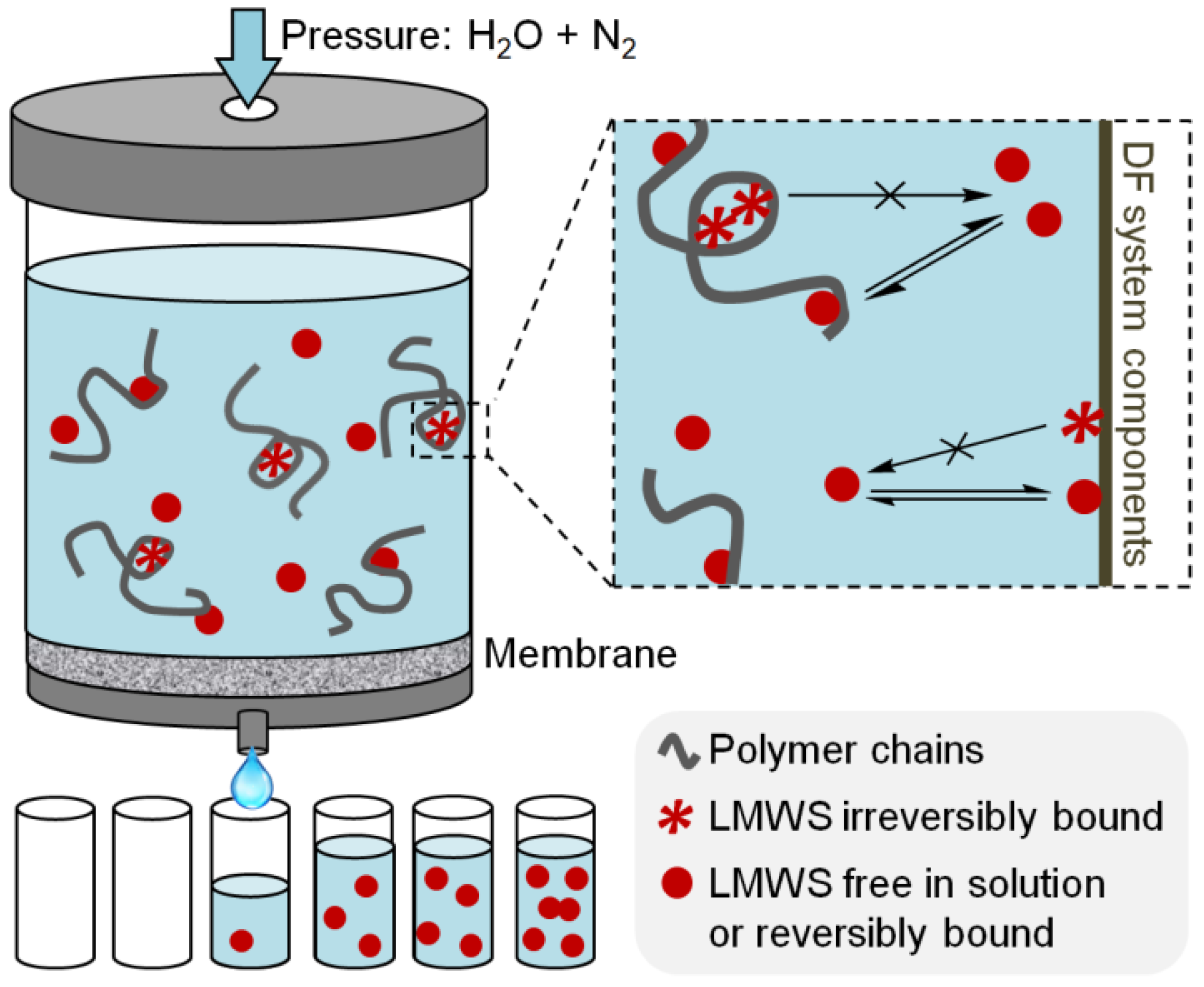
2.1. Diafiltration
2.2. SAXS
3. Experimental Section
3.1. Reagents
3.2. Equipment
3.3. Procedures
3.3.1. Sample Preparation
3.3.2. Diafiltration Measurements
3.3.3. Synchrotron-SAXS Measurements
4. Results and Discussion
4.1. Sample Preparation and DLS Characterization
4.2. Diafiltration Analysis
4.3. SAXS Analysis
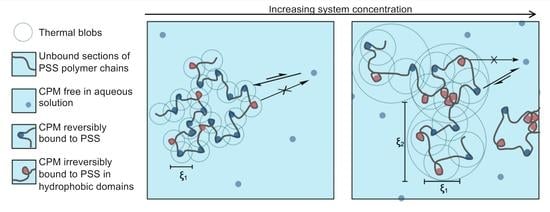
4.4. Aromatic WSP/Aromatic Counterion Complexation and Aggregation Model
4.5. Final Remarks
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Moreno-Villoslada, I.; Flores, M.E.; Marambio, O.G.; Pizarro, G.d.C.; Nishide, H. Polyaromatic-Anion Behavior of Different Polyelectrolytes Containing Benzenecarboxylate Units. J. Phys. Chem. B 2010, 114, 7753–7759. [Google Scholar] [CrossRef]
- Moreno-Villoslada, I.; González, F.; Arias, L.; Villatoro, J.M.; Ugarte, R.; Hess, S.; Nishide, H. Control of CI Basic Violet 10 aggregation in aqueous solution by the use of poly (sodium 4-styrenesulfonate). Dye. Pigment. 2009, 82, 401–408. [Google Scholar] [CrossRef]
- Moreno-Villoslada, I.; González, R.; Hess, S.; Rivas, B.L.; Shibue, T.; Nishide, H. Complex formation between rhodamine B and poly (sodium 4-styrenesulfonate) studied by 1H-NMR. J. Phys. Chem. B 2006, 110, 21576–21581. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Villoslada, I.; Jofré, M.; Miranda, V.; Chandía, P.; González, R.; Hess, S.; Rivas, B.L.; Elvira, C.; San Román, J.; Shibue, T. π-Stacking of rhodamine B onto water-soluble polymers containing aromatic groups. Polymer 2006, 47, 6496–6500. [Google Scholar] [CrossRef]
- Moreno-Villoslada, I.; Torres, C.; González, F.; Shibue, T.; Nishide, H. Binding of methylene blue to polyelectrolytes containing sulfonate groups. Macromol. Chem. Phys. 2009, 210, 1167–1175. [Google Scholar] [CrossRef]
- Moreno-Villoslada, I.; Torres-Gallegos, C.s.; Araya-Hermosilla, R.; Nishide, H. Influence of the linear aromatic density on methylene blue aggregation around polyanions containing sulfonate groups. J. Phys. Chem. B 2010, 114, 4151–4158. [Google Scholar] [CrossRef] [PubMed]
- Araya-Hermosilla, E.; Muñoz, D.; Orellana, S.; Yáñez, A.; Olea, A.F.; Oyarzun-Ampuero, F.; Moreno-Villoslada, I. Immobilization of rhodamine 6G in calcium alginate microcapsules based on aromatic-aromatic interactions with poly (sodium 4-styrenesulfonate). React. Funct. Polym. 2014, 81, 14–21. [Google Scholar] [CrossRef]
- Moreno-Villoslada, I.; González, F.; Rivera, L.; Hess, S.; Rivas, B.L.; Shibue, T.; Nishide, H. Aromatic—Aromatic Interaction between 2, 3, 5-Triphenyl-2 H-tetrazolium Chloride and Poly (sodium 4-styrenesulfonate). J. Phys. Chem. B 2007, 111, 6146–6150. [Google Scholar] [CrossRef]
- Moreno-Villoslada, I.; Soto, M.; González, F.; Montero-Silva, F.; Hess, S.; Takemura, I.; Oyaizu, K.; Nishide, H. Reduction of 2, 3, 5-Triphenyl-2 H-tetrazolium Chloride in the Presence of Polyelectrolytes Containing 4-Styrenesulfonate Moieties. J. Phys. Chem. B 2008, 112, 5350–5354. [Google Scholar] [CrossRef]
- Moreno-Villoslada, I.; Torres, C.; González, F.; Soto, M.; Nishide, H. Stacking of 2, 3, 5-Triphenyl-2 H-tetrazolium Chloride onto Polyelectrolytes Containing 4-Styrenesulfonate Groups. J. Phys. Chem. B 2008, 112, 11244–11249. [Google Scholar] [CrossRef]
- Flores, M.E.; Garcés-Jerez, P.; Fernández, D.; Aros-Perez, G.; González-Cabrera, D.; Álvarez, E.; Cañas, I.; Oyarzun-Ampuero, F.; Moreno-Villoslada, I. Facile Formation of Redox-Active Totally Organic Nanoparticles in Water by In Situ Reduction of Organic Precursors Stabilized through Aromatic—Aromatic Interactions by Aromatic Polyelectrolytes. Macromol. Rapid Commun. 2016, 37, 1729–1734. [Google Scholar] [CrossRef]
- Moreno-Villoslada, I.; Oyarzún, F.; Miranda, V.; Hess, S.; Rivas, B.L. Comparison between the binding of chlorpheniramine maleate to poly (sodium 4-styrenesulfonate) and the binding to other polyelectrolytes. Polymer 2005, 46, 7240–7245. [Google Scholar] [CrossRef]
- Moreno-Villoslada, I.; González, F.; Rivas, B.L.; Shibue, T.; Nishide, H. Tuning the pK a of the antihistaminic drug chlorpheniramine maleate by supramolecular interactions with water-soluble polymers. Polymer 2007, 48, 799–804. [Google Scholar] [CrossRef]
- Orozco, F.; Redondo-Gómez, C.; Vega-Baudrit, J.R.; Moreno-Villoslada, I. On the comparison between diafiltration and isothermal titration calorimetry: Determination of the amount of analytes bound to water-soluble polymers. Polym. Test. 2019, 76, 443–447. [Google Scholar] [CrossRef]
- Villamizar-Sarmiento, M.G.; Molina-Soto, E.F.; Guerrero, J.; Shibue, T.; Nishide, H.; Moreno-Villoslada, I.; Oyarzun-Ampuero, F.A. A New Methodology to Create Polymeric Nanocarriers Containing Hydrophilic Low Molecular-Weight Drugs: A Green Strategy Providing a Very High Drug Loading. Mol. Pharm. 2019, 16, 2892–2901. [Google Scholar] [CrossRef] [PubMed]
- Villamizar-Sarmiento, M.G.; Guerrero, J.; Moreno-Villoslada, I.; Oyarzun-Ampuero, F.A. The key role of the drug self-aggregation ability to obtain optimal nanocarriers based on aromatic-aromatic drug-polymer interactions. Eur. J. Pharm. Biopharm. 2021, 166, 19–29. [Google Scholar] [CrossRef]
- Manning, G.S. The molecular theory of polyelectrolyte solutions with applications to the electrostatic properties of polynucleotides. Q. Rev. Biophys. 1978, 11, 179–246. [Google Scholar] [CrossRef]
- Manning, G.S. Limiting laws and counterion condensation in polyelectrolyte solutions. 8. Mixtures of counterions, species selectivity, and valence selectivity. J. Phys. Chem. 1984, 88, 6654–6661. [Google Scholar] [CrossRef]
- Manning, G.S. Counterion condensation theory constructed from different models. Phys. A Stat. Mech. Its Appl. 1996, 231, 236–253. [Google Scholar] [CrossRef]
- Nordmeier, E.; Dauwe, W. Studies of Polyelectrolyte Solutions I. Counterion Condensation by Poly (styrene sulfonate). Polym. J. 1991, 23, 1297–1305. [Google Scholar] [CrossRef] [Green Version]
- Nordmeier, E. Advances in polyelectrolyte research: Counterion binding phenomena, dynamic processes, and the helix-coil transition of DNA. Macromol. Chem. Phys. 1995, 196, 1321–1374. [Google Scholar] [CrossRef]
- Flores, M.E.; Sano, N.; Araya-Hermosilla, R.; Shibue, T.; Olea, A.F.; Nishide, H.; Moreno-Villoslada, I. Self-association of 5, 10, 15, 20-tetrakis-(4-sulfonatophenyl)-porphyrin tuned by poly (decylviologen) and sulfobutylether-β-cyclodextrin. Dye. Pigment. 2015, 112, 262–273. [Google Scholar] [CrossRef]
- Araya-Hermosilla, E.; Orellana, S.L.; Toncelli, C.; Picchioni, F.; Moreno-Villoslada, I. Novel polyketones with pendant imidazolium groups as nanodispersants of hydrophobic antibiotics. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Araya-Hermosilla, E.; Roscam Abbing, M.; Catalán-Toledo, J.; Oyarzun-Ampuero, F.; Pucci, A.; Raffa, P.; Picchioni, F.; Moreno-Villoslada, I. Synthesis of tuneable amphiphilic-modified polyketone polymers, their complexes with 5,10,15,20-tetrakis-(4-sulfonatophenyl)-porphyrin, and their role in the photooxidation of 1,3,5-triphenylformazan confined in polymeric nanoparticles. Polymer 2019, 167, 215–223. [Google Scholar] [CrossRef]
- Fuenzalida, J.P.; Flores, M.E.; Móniz, I.s.; Feijoo, M.; Goycoolea, F.; Nishide, H.; Moreno-Villoslada, I. Immobilization of hydrophilic low molecular-weight molecules in nanoparticles of chitosan/poly (sodium 4-styrenesulfonate) assisted by aromatic-aromatic interactions. J. Phys. Chem. B 2014, 118, 9782–9791. [Google Scholar] [CrossRef]
- Coronel, A.; Catalán-Toledo, J.; Fernández-Jaramillo, H.; Godoy-Martínez, P.; Flores, M.E.; Moreno-Villoslada, I. Photodynamic action of methylene blue subjected to aromatic-aromatic interactions with poly (sodium 4-styrenesulfonate) in solution and supported in solid, highly porous alginate sponges. Dye. Pigment. 2017, 147, 455–464. [Google Scholar] [CrossRef]
- Pino-Pinto, J.P.; Oyarzun-Ampuero, F.; Orellana, S.L.; Flores, M.E.; Nishide, H.; Moreno-Villoslada, I. Aerogels containing 5, 10, 15, 20-tetrakis-(4-sulfonatophenyl)-porphyrin with controlled state of aggregation. Dye. Pigment. 2017, 139, 193–200. [Google Scholar] [CrossRef]
- Sanhueza, L.; Castro, J.; Urzúa, E.; Barrientos, L.; Oyarzun-Ampuero, F.; Pesenti, H.c.; Shibue, T.; Sugimura, N.; Tomita, W.; Nishide, H. Photochromic Solid Materials Based on Poly (decylviologen) Complexed with Alginate and Poly (sodium 4-styrenesulfonate). J. Phys. Chem. B 2015, 119, 13208–13217. [Google Scholar] [CrossRef]
- Araya-Hermosilla, E.; Catalán-Toledo, J.; Muñoz-Suescun, F.; Oyarzun-Ampuero, F.; Raffa, P.; Polgar, L.M.; Picchioni, F.; Moreno-Villoslada, I. Totally Organic Redox-Active pH-Sensitive Nanoparticles Stabilized by Amphiphilic Aromatic Polyketones. J. Phys. Chem. B 2018, 122, 1747–1755. [Google Scholar] [CrossRef] [PubMed]
- Catalán-Toledo, J.; Nenen, A.; Vallejos, G.A.; Oyarzun-Ampuero, F.; Shibue, T.; Nishide, H.; Moreno-Villoslada, I. A simple and green methodology to assemble poly (4-vinylpyridine) and a sulfonated azo-dye for obtaining stable polymeric nanoparticles. Polymer 2018, 158, 289–296. [Google Scholar] [CrossRef]
- Araya-Hermosilla, R.; Araya-Hermosilla, E.; Torres-Gallegos, C.; Alarcón-Alarcón, C.; Moreno-Villoslada, I. Sensing Cu2+ by controlling the aggregation properties of the fluorescent dye rhodamine 6G with the aid of polyelectrolytes bearing different linear aromatic density. React. Funct. Polym. 2013, 73, 1455–1463. [Google Scholar] [CrossRef]
- Carrillo, J.-M.Y.; Dobrynin, A.V. Detailed Molecular Dynamics Simulations of a Model NaPSS in Water. J. Phys. Chem. B 2010, 114, 9391–9399. [Google Scholar] [CrossRef]
- Mantha, S.; Yethiraj, A. Conformational Properties of Sodium Polystyrenesulfonate in Water: Insights from a Coarse-Grained Model with Explicit Solvent. J. Phys. Chem. B 2015, 119, 11010–11018. [Google Scholar] [CrossRef]
- Zhang, Y.; Douglas, J.F.; Ermi, B.D.; Amis, E.J. Influence of counterion valency on the scattering properties of highly charged polyelectrolyte solutions. J. Chem. Phys. 2001, 114, 3299–3313. [Google Scholar] [CrossRef] [Green Version]
- Matsuoka, H.; Schwahn, D.; Ise, N. Observation of cluster formation in polyelectrolyte solutions by small-angle neutron scattering. 1. A steep upturn of the scattering curves from solutions of sodium poly (styrenesulfonate) at scattering vectors below 0.01.ANG.-1. Macromolecules 1991, 24, 4227–4228. [Google Scholar] [CrossRef]
- Combet, J. Polyelectrolytes and small angle scattering. EPJ Web Conf. 2018, 188, 03001. [Google Scholar] [CrossRef] [Green Version]
- Ise, N.; Okubo, T.; Kunugi, S.; Matsuoka, H.; Yamamoto, K.; Ishii, Y. “Ordered” structure in dilute solutions of sodium polystyrenesulfonates as studied by small-angle x-ray scattering. J. Chem. Phys. 1984, 81, 3294–3306. [Google Scholar] [CrossRef]
- Nishida, K.; Kaji, K.; Kanaya, T.; Shibano, T. Added Salt Effect on the Intermolecular Correlation in Flexible Polyelectrolyte Solutions: Small-Angle Scattering Study. Macromolecules 2002, 35, 4084–4089. [Google Scholar] [CrossRef]
- Nause, R.G.; Hoagland, D.A.; Strey, H.H. Structural Evolution of Complexes of Poly (styrenesulfonate) and Cetyltrimethylammonium Chloride. Macromolecules 2008, 41, 4012–4019. [Google Scholar] [CrossRef]
- Fares, H.M.; Ghoussoub, Y.E.; Delgado, J.D.; Fu, J.; Urban, V.S.; Schlenoff, J.B. Scattering Neutrons along the Polyelectrolyte Complex/Coacervate Continuum. Macromolecules 2018, 51, 4945–4955. [Google Scholar] [CrossRef]
- Essafi, W.; Spiteri, M.-N.; Williams, C.; Boue, F. Hydrophobic Polyelectrolytes in Better Polar Solvent. Structure and Chain Conformation as Seen by SAXS and SANS. Macromolecules 2009, 42, 9568–9580. [Google Scholar] [CrossRef] [Green Version]
- Mertens, H.D.T.; Svergun, D.I. Structural characterization of proteins and complexes using small-angle X-ray solution scattering. J. Struct. Biol. 2010, 172, 128–141. [Google Scholar] [CrossRef] [PubMed]
- Agbabiaka, A.; Wiltfong, M.; Park, C. Small Angle X-Ray Scattering Technique for the Particle Size Distribution of Nonporous Nanoparticles. J. Nanopart. 2013, 2013, 11. [Google Scholar] [CrossRef]
- Dobrynin, A.V.; Colby, R.H.; Rubinstein, M. Scaling Theory of Polyelectrolyte Solutions. Macromolecules 1995, 28, 1859–1871. [Google Scholar] [CrossRef]
- Terao, K.; Morihana, N.; Ichikawa, H. Solution SAXS measurements over a wide temperature range to determine the unperturbed chain dimensions of polystyrene and a cyclic amylose derivative. Polym. J. 2014, 46, 155–159. [Google Scholar] [CrossRef] [Green Version]
- Jouault, N.; Dalmas, F.; Said, S.; Di Cola, E.; Schweins, R.; Jestin, J.; Boué, F. Direct Measurement of Polymer Chain Conformation in Well-Controlled Model Nanocomposites by Combining SANS and SAXS. Macromolecules 2010, 43, 9881–9891. [Google Scholar] [CrossRef]
- De, R.; Das, B. Concentration, medium and salinity-induced shrinkage/expansion of Poly (sodium styrenesulfonate) in 2-ethoxyethanol-Water mixed solvent media as probed by viscosimetry. J. Mol. Struct. 2020, 1199, 126992. [Google Scholar] [CrossRef]
- Han, A.; Colby, R.H. Rheology of Entangled Polyelectrolyte Solutions. Macromolecules 2021, 54, 1375–1387. [Google Scholar] [CrossRef]
- De, R.; Das, B. Coiling/uncoiling behaviour of sodium polystyrenesulfonate in 2-ethoxyethanol-water mixed solvent media as probed using viscometry. Polym. Int. 2014, 63, 1959–1964. [Google Scholar] [CrossRef]
- De, R.; Ray, D.; Das, B. Influence of temperature, added electrolyte, and polymer molecular weight on the counterion-condensation phenomenon in aqueous solution of sodium polystyrenesulfonate: A scaling theory approach. RSC Adv. 2015, 5, 54890–54898. [Google Scholar] [CrossRef]
- Bagchi, D.; Menon, R. Conformational modification of conducting polymer chains by solvents: Small-angle X-ray scattering study. Chem. Phys. Lett. 2006, 425, 114–117. [Google Scholar] [CrossRef]
- Takano, T.; Masunaga, H.; Fujiwara, A.; Okuzaki, H.; Sasaki, T. PEDOT Nanocrystal in Highly Conductive PEDOT:PSS Polymer Films. Macromolecules 2012, 45, 3859–3865. [Google Scholar] [CrossRef]
- Choudhury, P.K.; Bagchi, D.; Sangeeth, C.S.S.; Menon, R. Modified conformation and physical properties in conducting polymers due to varying conjugation and solvent interactions. J. Mater. Chem. 2011, 21, 1607–1614. [Google Scholar] [CrossRef] [Green Version]
- Kaji, K.; Urakawa, H.; Kanaya, T.; Kitamaru, R. Phase diagram of polyelectrolyte solutions. J. Phys. 1988, 49, 993–1000. [Google Scholar] [CrossRef]
- Nishida, K.; Kaji, K.; Kanaya, T. Improved phase diagram of polyelectrolyte solutions. J. Chem. Phys. 2001, 115, 8217–8220. [Google Scholar] [CrossRef] [Green Version]
- Geckeler, K.E.; Bayer, E.; Spivakov, B.Y.; Shkinev, V.M.; Vorob’eva, G.A. Liquid-phase polymer-based retention, a new method for separation and preconcentration of elements. Anal. Chim. Acta 1986, 189, 285–292. [Google Scholar] [CrossRef]
- Geckeler, K.E.; Volchek, K. Removal of Hazardous Substances from Water Using Ultrafiltration in Conjunction with Soluble Polymers. Environ. Sci. Technol. 1996, 30, 725–734. [Google Scholar] [CrossRef]
- Palacio, D.A.; Rivas, B.L.; Urbano, B.F. Ultrafiltration membranes with three water-soluble polyelectrolyte copolymers to remove ciprofloxacin from aqueous systems. Chem. Eng. J. 2018, 351, 85–93. [Google Scholar] [CrossRef]
- Stoner, M.R.; Fischer, N.; Nixon, L.; Buckel, S.; Benke, M.; Austin, F.; Randolph, T.W.; Kendrick, B.S. Protein–solute interactions affect the outcome of ultrafiltration/diafiltration operations. J. Pharm. Sci. 2004, 93, 2332–2342. [Google Scholar] [CrossRef]
- Rivas, B.L.; Pereira, E.D.; Moreno-Villoslada, I. Water-soluble polymer-metal ion interactions. Prog. Polym. Sci. 2003, 28, 173–208. [Google Scholar] [CrossRef]
- Moreno-Villoslada, I.; Miranda, V.; Gutiérrez, R.; Hess, S.; Muñoz, C.; Rivas, B.L. Interactions of 2, 3, 5-triphenyl-2H-tetrazolium chloride with poly (sodium 4-styrenesulfonate) studied by diafiltration and UV-vis spectroscopy. J. Membr. Sci. 2004, 244, 205–213. [Google Scholar] [CrossRef]
- Moreno-Villoslada, I.; Miranda, V.; Jofré, M.; Chandía, P.; Villatoro, J.M.; Bulnes, J.L.; Cortés, M.; Hess, S.; Rivas, B.L. Simultaneous interactions between a low molecular-weight species and two high molecular-weight species studied by diafiltration. J. Membr. Sci. 2006, 272, 137–142. [Google Scholar] [CrossRef]
- Orellana, S.L.; Torres-Gallegos, C.; Araya-Hermosilla, R.; Oyarzun-Ampuero, F.; Moreno-Villoslada, I. Association Efficiency of Three Ionic Forms of Oxytetracycline to Cationic and Anionic Oil-In-Water Nanoemulsions Analyzed by Diafiltration. J. Pharm. Sci. 2015, 104, 1141–1152. [Google Scholar] [CrossRef]
- Barrat, J.-L.; Joanny, F. Theory of Polyelectrolyte Solutions. In Advances in Chemical Physics; Prigogine, I., Rice, S.A., Eds.; Wiley: Hoboken, NJ, USA, 1996; pp. 1–66. [Google Scholar]
- Chalal, M.; Ehrburger-Dolle, F.; Morfin, I.; Bley, F.; Aguilar de Armas, M.-R.; López Donaire, M.-L.; San Roman, J.; Bölgen, N.; Pişkin, E.; Ziane, O.; et al. SAXS Investigation of the Effect of Temperature on the Multiscale Structure of a Macroporous Poly (N-isopropylacrylamide) Gel. Macromolecules 2010, 43, 2009–2017. [Google Scholar] [CrossRef] [Green Version]
- Rubinstein, M.; Colby, R.H. Polymer Physics; Oxford University Press: Oxford, UK, 2003. [Google Scholar]
- Hayashi, H.; Morita, T.; Nishikawa, K. Interpretation of correlation length by small-angle X-ray scattering experiments on fluids near critical point. Chem. Phys. Lett. 2009, 471, 249–252. [Google Scholar] [CrossRef]
- Horkay, F.; Basser, P.J.; Hecht, A.-M.; Geissler, E. Ionic effects in semi-dilute biopolymer solutions: A small angle scattering study. J. Chem. Phys. 2018, 149, 163312. [Google Scholar] [CrossRef] [PubMed]
- Levin, J.; Fox, J.A.; Forde, D.R. Elementary Statistics in Social Research; Pearson Allyn & Bacon: Boston, MA, USA, 2010. [Google Scholar]
- Nishida, K.; Urakawa, H.; Kaji, K.; Gabrys, B.; Higgins, J.S. Electrostatic persistence length of NaPSS polyelectrolytes determined by a zero average contrast SANS technique. Polymer 1997, 38, 6083–6085. [Google Scholar] [CrossRef]
- Combet, J.; Rawiso, M.; Rochas, C.; Hoffmann, S.; Boué, F. Structure of Polyelectrolytes with Mixed Monovalent and Divalent Counterions: SAXS Measurements and Poisson-Boltzmann Analysis. Macromolecules 2011, 44, 3039–3052. [Google Scholar] [CrossRef] [Green Version]
- Borsali, R.; Nguyen, H.; Pecora, R. Small-Angle Neutron Scattering and Dynamic Light Scattering from a Polyelectrolyte Solution: DNA. Macromolecules 1998, 31, 1548–1555. [Google Scholar] [CrossRef]
- Ise, N. Ordering of Ionic Solutes in Dilute Solutions through Attraction of Similarly Charged Solutes—A Change of Paradigm in Colloid and Polymer Chemistry. Angew. Chem. Int. Ed. Engl. 1986, 25, 323–334. [Google Scholar] [CrossRef]
- Limbach, H.J.; Holm, C. Single-Chain Properties of Polyelectrolytes in Poor Solvent. J. Phys. Chem. B 2003, 107, 8041–8055. [Google Scholar] [CrossRef] [Green Version]
- Wu, D.Q.; Chu, B.; Lundberg, R.D.; MacKnight, W.J. Small-angle x-ray scattering (SAXS) studies of sulfonated polystyrene ionomers. 2. Correlation function analysis. Macromolecules 1993, 26, 1000–1007. [Google Scholar] [CrossRef]
- Balu, R.; Mata, J.P.; Knott, R.; Elvin, C.M.; Hill, A.J.; Choudhury, N.R.; Dutta, N.K. Effects of Crowding and Environment on the Evolution of Conformational Ensembles of the Multi-Stimuli-Responsive Intrinsically Disordered Protein, Rec1-Resilin: A Small-Angle Scattering Investigation. J. Phys. Chem. B 2016, 120, 6490–6503. [Google Scholar] [CrossRef]
- Leisner, D.; Imae, T. Interpolyelectrolyte Complex and Coacervate Formation of Poly (glutamic acid) with a Dendrimer Studied by Light Scattering and SAXS. J. Phys. Chem. B 2003, 107, 8078–8087. [Google Scholar] [CrossRef]
- Shrestha, L.K.; Sharma, S.C.; Sato, T.; Glatter, O.; Aramaki, K. Small-angle X-ray scattering (SAXS) study on nonionic fluorinated micelles in aqueous system. J. Colloid Interface Sci. 2007, 316, 815–824. [Google Scholar] [CrossRef] [Green Version]
- Brilliantov, N.V.; Kuznetsov, D.V.; Klein, R. Chain Collapse and Counterion Condensation in Dilute Polyelectrolyte Solutions. Phys. Rev. Lett. 1998, 81, 1433–1436. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, A.; Qiao, W.; Ismail, K. Physicochemical Interactions of Chlorpheniramine Maleate with Sodium Deoxycholate in Aqueous Solution. J. Surfactants Deterg. 2018, 21, 879–887. [Google Scholar] [CrossRef]
- Skerjanc, J.; Kogej, K.; Vesnaver, G. Polyelectrolyte-surfactant interactions: Enthalpy of binding of dodecyl- and cetylpyridinium cations to poly (styrenesulfonate) anion. J. Phys. Chem. 1988, 92, 6382–6385. [Google Scholar] [CrossRef]
- Popov, A.; Zakharova, J.; Wasserman, A.; Motyakin, M.; Kasaikin, V. Macromolecular and Morphological Evolution of Poly (styrene sulfonate) Complexes with Tetradecyltrimethylammonium Bromide. J. Phys. Chem. B 2012, 116, 12332–12340. [Google Scholar] [CrossRef]
- Sitar, S.; Goderis, B.; Hansson, P.; Kogej, K. Phase Diagram and Structures in Mixtures of Poly (styrenesulfonate anion) and Alkyltrimethylammonium Cations in Water: Significance of Specific Hydrophobic Interaction. J. Phys. Chem. B 2012, 116, 4634–4645. [Google Scholar] [CrossRef]








| cCPMtotal mM | cWSPtotal mM | v | u | j and (km) | KdissLMWS/WSP and (KdissLMWS/DS) | Linear Adjustment | R2 | |
|---|---|---|---|---|---|---|---|---|
| Blank | 0.25 | - | 1.03 | −0.03 | (0.83) | (4.9) | y = −0.83x − 7.6 | 0.99 |
| 0.50 | - | 0.97 | 0.03 | (0.81) | (4.3) | y = −0.81x − 7.6 | 0.99 | |
| 0.75 | - | 0.94 | 0.06 | (0.79) | (3.8) | y = −0.79x − 7.1 | 1.00 | |
| 1.00 | - | 0.94 | 0.06 | (0.86) | (6.1) | y = −0.86x − 6.9 | 1.00 | |
| 1.25 | - | 1.03 | 0.03 | (0.84) | (5.3) | y = −0.84x − 6.6 | 1.00 | |
| PAA | 0.25 | 0.5 | 0.95 ± 0.02 | 0.05 ± 0.02 | 0.59 ± 0.13 | 2.8 ± 2.0 | (−0.59 ± 0.13)x + (−8.7 ± 0.2) | 1.00 ± 0.00 |
| 0.50 | 1.0 | 0.89 ± 0.05 | 0.11 ± 0.05 | 0.63 ± 0.08 | 3.5 ± 1.9 | (−0.63 ± 0.08)x + (−8.0 ± 0.2) | 0.99 ± 0.01 | |
| 0.75 | 1.5 | 0.92 ± 0.02 | 0.08 ± 0.02 | 0.63 ± 0.04 | 3.7 ± 1.1 | (−0.63 ± 0.04)x + (−7.6 ± 0.1) | 0.98 ± 0.01 | |
| 1.00 | 2.0 | 0.88 ± 0.01 | 0.12 ± 0.01 | 0.62 ± 0.04 | 2.5 ± 0.6 | (−0.62 ± 0.04)x + (−7.4 ± 0.1) | 0.99 ± 0.00 | |
| 1.25 | 2.5 | 0.86 ± 0.01 | 0.14 ± 0.01 | 0.69 ± 0.04 | 4.5 ± 1.5 | (−0.69 ± 0.04)x + (−7.0 ± 0.1) | 0.99 ± 0.00 | |
| PSS | 0.25 | 0.5 | 0.41 ± 0.00 | 0.59 ± 0.00 | 0.28 ± 0.03 | 0.48 ± 0.07 | (−0.28 ± 0.03)x + (−10.4 ± 0.1) | 0.98 ± 0.02 |
| 0.50 | 1.0 | 0.39 ± 0.08 | 0.61 ± 0.08 | 0.30 ± 0.04 | 0.54 ± 0.11 | (−0.30 ± 0.04)x + (−9.7 ± 0.1) | 0.98 ± 0.01 | |
| 0.75 | 1.5 | 0.27 ± 0.01 | 0.73 ± 0.01 | 0.35 ± 0.02 | 0.70 ± 0.09 | (−0.35 ± 0.02)x + (−9.5 ± 0.0) | 0.99 ± 0.00 | |
| 1.00 | 2.0 | 0.27 ± 0.01 | 0.73 ± 0.01 | 0.37 ± 0.01 | 0.69 ± 0.03 | (−0.37 ± 0.10)x + (−9.2 ± 0.1) | 0.98 ± 0.00 | |
| 1.25 | 2.5 | 0.24 ± 0.02 | 0.76 ± 0.02 | 0.38 ± 0.00 | 0.78 ± 0.01 | (−0.38 ± 0.00)x + (−9.0 ± 0.1) | 0.99 ± 0.01 |
| PSSn/CPMn/2 (mM/mM) | q Range for Equation (10) (nm−1) | R2 | ξ1 ± δξ1 (nm) | q Range for Equation (11) (nm−1) | R2 | ξ2 ± δξ2 (nm) |
|---|---|---|---|---|---|---|
| 0.5/0.25 | 0.49–1.23 | 0.78 | 0.60 ± 0.01 | - | - | - |
| 2.0/1.0 | 0.53–1.27 | 0.90 | 0.70 ± 0.01 | - | - | - |
| 10/5.0 | 0.61–1.22 | 0.90 | 0.80 ± 0.01 | 0.20–0.52 | 0.924 | 1.70 ± 0.02 |
| 20/10 | 0.67–1.19 | 0.94 | 1.00 ± 0.01 | 0.22–0.53 | 0.978 | 2.30 ± 0.03 |
| 30/15 | 0.74–1.21 | 0.85 | 1.10 ± 0.02 | 0.20–0.54 | 0.976 | 2.70 ± 0.05 |
| 35/18 | 0.74–1.21 | 0.95 | 1.20 ± 0.02 | 0.20–0.55 | 0.989 | 2.90 ± 0.03 |
| 40/20 | 0.73–1.20 | 0.94 | 1.20 ± 0.02 | 0.20–0.55 | 0.992 | 3.00 ± 0.04 |
| 50/25 | 0.73–1.20 | 0.97 | 1.40 ± 0.02 | 0.20–0.55 | 0.993 | 3.40 ± 0.03 |
| 60/30 | 0.67–1.17 | 0.98 | 1.50 ± 0.02 | 0.18–0.53 | 0.995 | 4.0 ± 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orozco, F.; Hoffmann, T.; Flores, M.E.; Lisoni, J.G.; Vega-Baudrit, J.R.; Moreno-Villoslada, I. Concentration Dependent Single Chain Properties of Poly(sodium 4-styrenesulfonate) Subjected to Aromatic Interactions with Chlorpheniramine Maleate Studied by Diafiltration and Synchrotron-SAXS. Polymers 2021, 13, 3563. https://doi.org/10.3390/polym13203563
Orozco F, Hoffmann T, Flores ME, Lisoni JG, Vega-Baudrit JR, Moreno-Villoslada I. Concentration Dependent Single Chain Properties of Poly(sodium 4-styrenesulfonate) Subjected to Aromatic Interactions with Chlorpheniramine Maleate Studied by Diafiltration and Synchrotron-SAXS. Polymers. 2021; 13(20):3563. https://doi.org/10.3390/polym13203563
Chicago/Turabian StyleOrozco, Felipe, Thomas Hoffmann, Mario E. Flores, Judit G. Lisoni, José Roberto Vega-Baudrit, and Ignacio Moreno-Villoslada. 2021. "Concentration Dependent Single Chain Properties of Poly(sodium 4-styrenesulfonate) Subjected to Aromatic Interactions with Chlorpheniramine Maleate Studied by Diafiltration and Synchrotron-SAXS" Polymers 13, no. 20: 3563. https://doi.org/10.3390/polym13203563