Effects of Lipase and Xylanase Pretreatment on the Structure and Pulping Properties of Wheat Straw
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Analysis of Enzyme
2.3. Bio-Enzyme Pretreatment of Wheat Straw
2.4. Alkali-Oxygen Pulping Process
2.5. Properties of Wheat Straw
2.6. Properties of Pulp
2.7. Characterization of Fiber
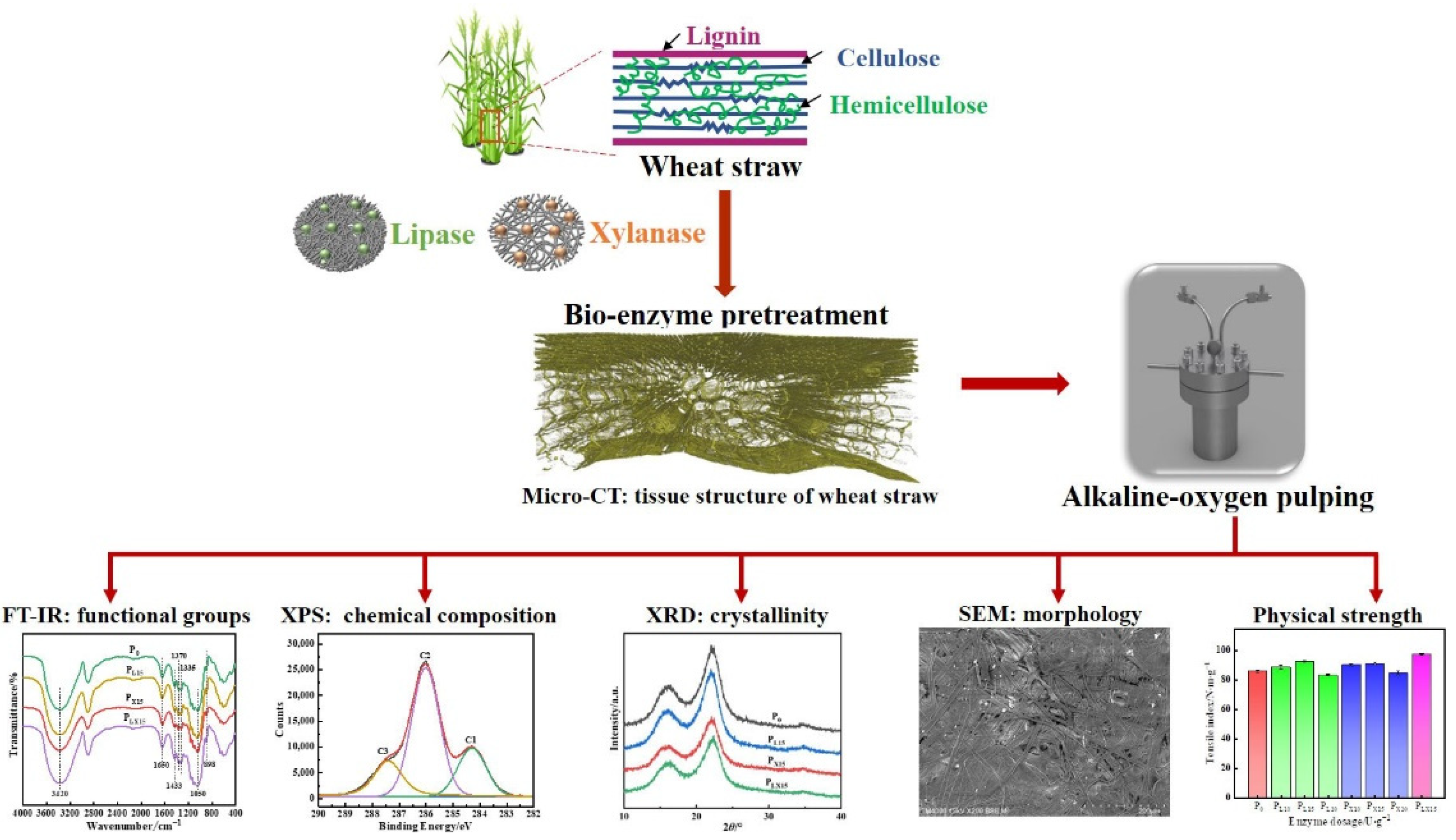
3. Results and Discussion
3.1. Pulp Properties
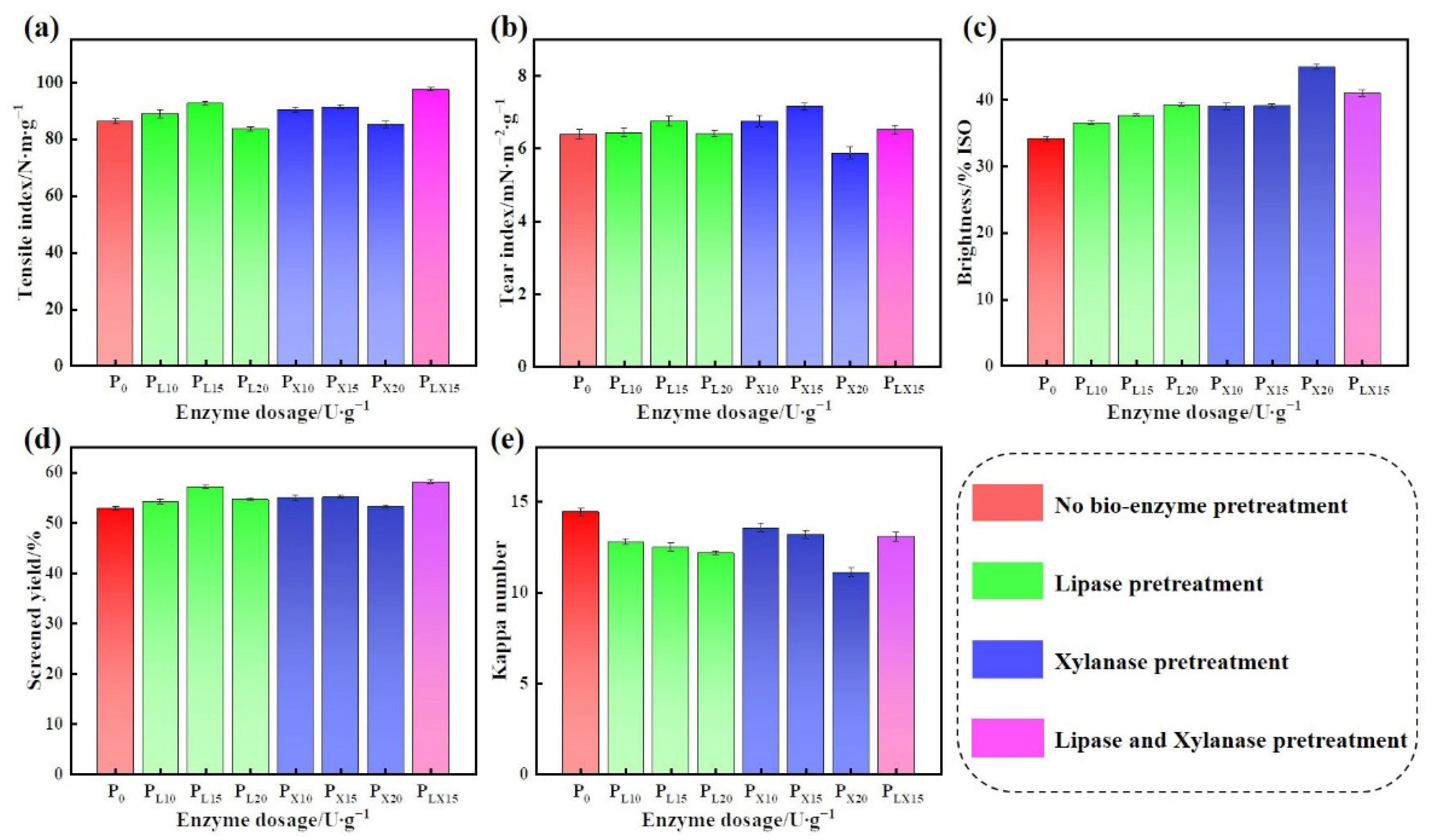
3.1.1. Effect of Bio-Enzyme Dosage on the Properties of Pulp
3.1.2. Effect of Bio-Enzyme Pretreatment on Surface Morphology of Pulp Fibers
3.1.3. Effect of Bio-Enzyme Pretreatment on Pulp Fiber Quality
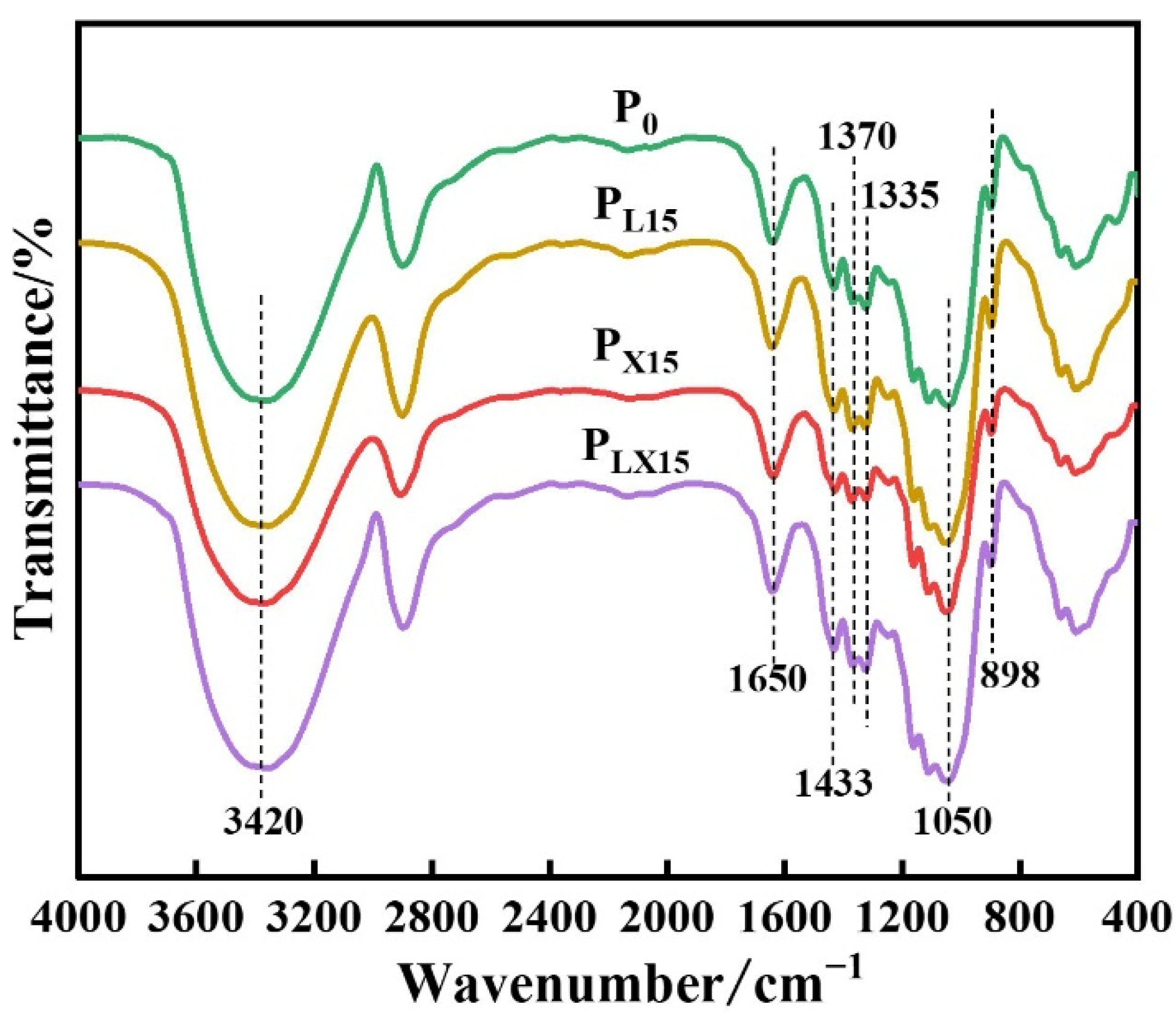
3.1.4. FTIR Analysis of the Bio-Enzyme Pretreated Pulp
3.1.5. Crystallinity Analysis of the Bio-Enzyme Pretreated Pulp
3.1.6. XPS Analysis of the Chemical Composition of the Bio-Enzyme Pretreated Pulp Fiber Surface
3.2. Effect of Bio-Enzyme Pretreatment on Wheat Straw Microstructure
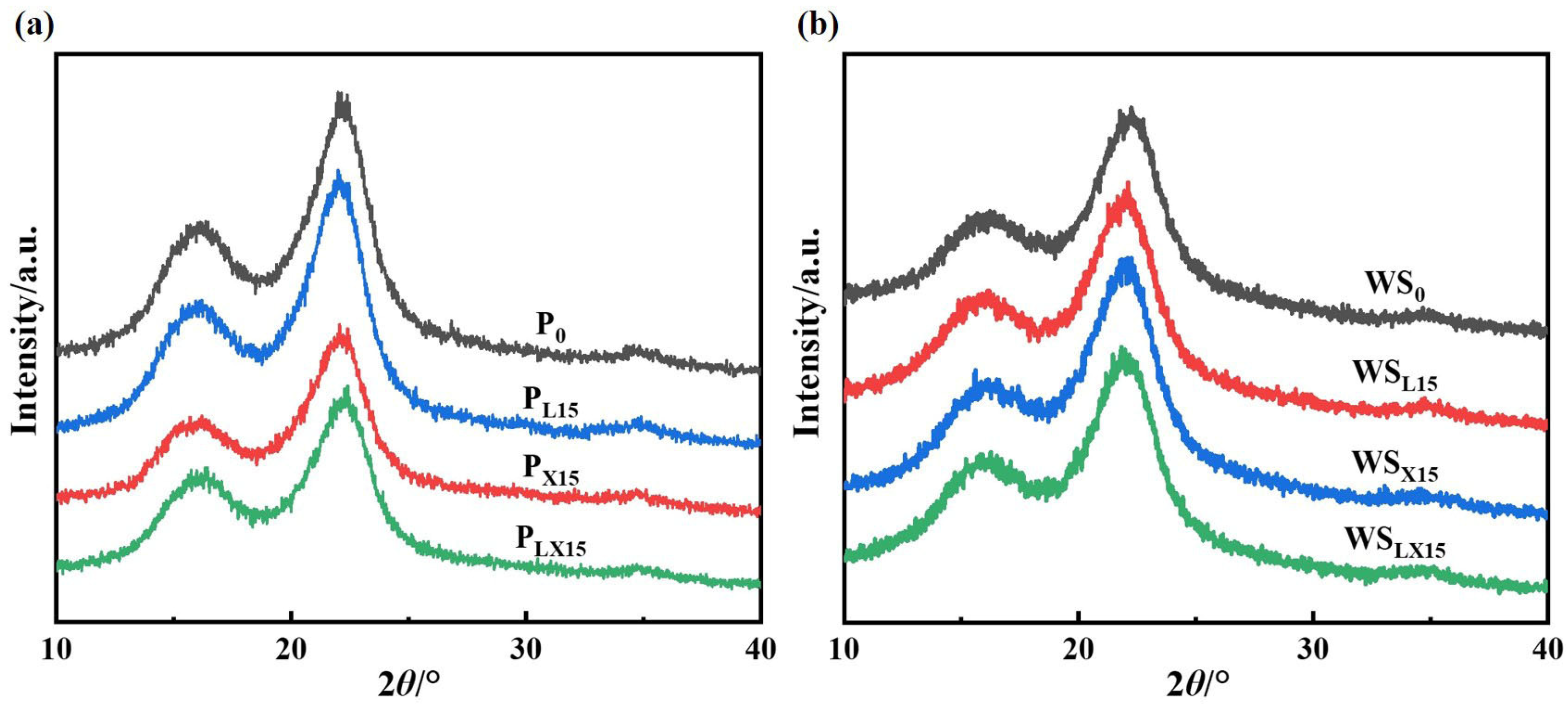
3.2.1. Crystallinity of Wheat Straw
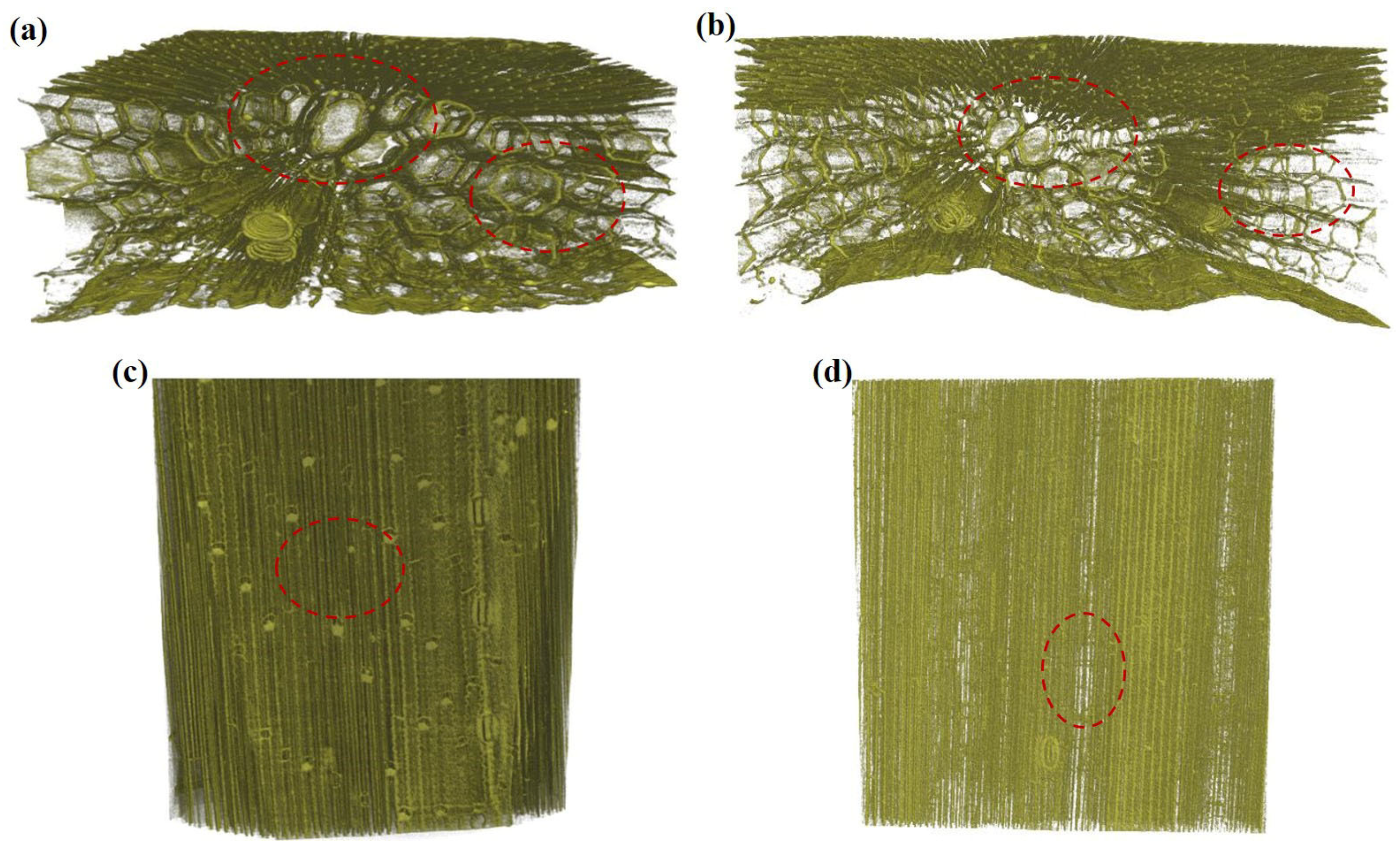
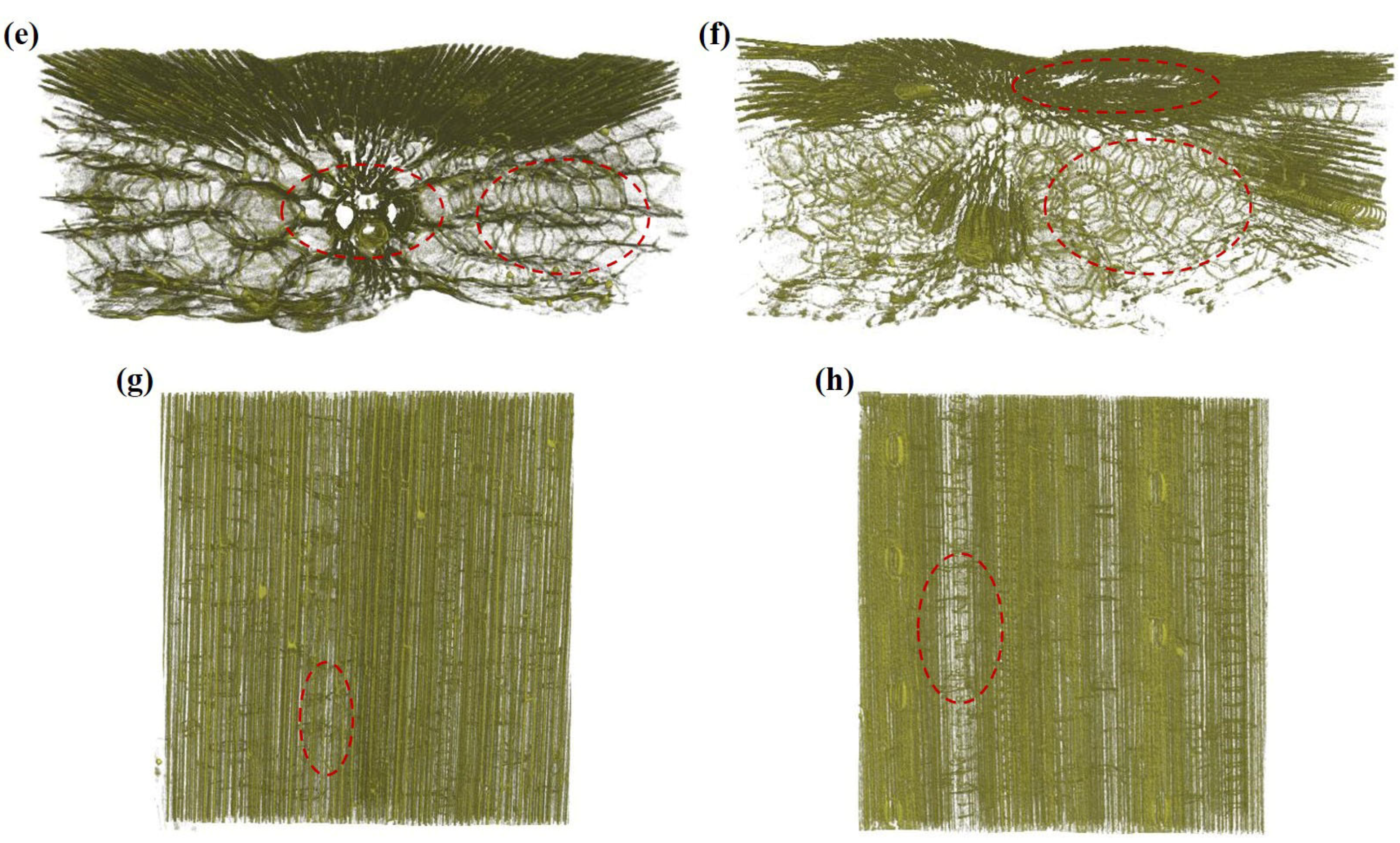
3.2.2. Three-Dimensional Tissue Structure of Wheat Straw
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, J.; Pan, X. Woody biomass pretreatment for cellulosic ethanol production: Technology and energy consumption evaluation. Bioresour. Technol. 2010, 101, 4992–5002. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Xu, X.; Wang, C.; Bai, Y.; Fu, C.; Zhang, L.; Fu, R.; Wang, Y. Environmental burdens of the comprehensive utilization of straw: Wheat straw utilization from a life-cycle perspective. J. Clean. Prod. 2020, 259, 120702. [Google Scholar] [CrossRef]
- Gonzalo, A.; Bimbela, F.; Sánchez, J.; Labidi, J.; Marín, F.; Arauzo, J. Evaluation of different agricultural residues as raw materials for pulp and paper production using a semichemical process. J. Clean. Prod. 2017, 156, 184–193. [Google Scholar] [CrossRef]
- El-Sayed, E.S.A.; El-Sakhawy, M. Non-wood fibers as raw material for pulp and paper industry. Nord. Pulp Pap. Res. J. 2020, 35, 215–230. [Google Scholar] [CrossRef]
- Man, Y.; Li, J.; Hong, M.; Han, Y. Energy transition for the low-carbon pulp and paper industry in China. Renew. Sustain. Energy Rev. 2020, 131, 109998. [Google Scholar] [CrossRef]
- Mandeep; Gupta, G.K.; Shukla, P. Insights into the resources generation from pulp and paper industry wastes: Challenges, perspectives and innovations. Bioresour. Technol. 2019, 297, 122496. [Google Scholar] [CrossRef]
- Yue, F.; Chen, K.-L.; Lu, F. Low Temperature Soda-Oxygen Pulping of Bagasse. Molecules 2016, 21, 85. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.-J.; Hong, S.-B.; Eom, T.-J. Preparation of Eucalyptus pulp by mild condition of low-temperature, atmospheric pressure, and short-reaction-time with high-boiling-point solvent and pulp properties. Cellulose 2017, 25, 753–761. [Google Scholar] [CrossRef]
- Mboowa, D. A review of the traditional pulping methods and the recent improvements in the pulping processes. Biomass-Convers. Biorefinery 2021, 1–12. [Google Scholar] [CrossRef]
- Ding, N.; Liu, H.; Sun, Y.; Tang, X.; Lei, T.; Xu, F.; Zeng, X.; Lin, L. Lignin degradation in cooking with active oxygen and solid Alkali process: A mechanism study. J. Clean. Prod. 2021, 278, 123984. [Google Scholar] [CrossRef]
- Demirbas, A.; Pehlivan, E.; Altun, T. Potential evolution of Turkish agricultural residues as bio-gas, bio-char and bio-oil sources. Int. J. Hydrogen Energy 2006, 31, 613–620. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, K. Pyrolysis Behavior of the Black Liquor Derived from Soda–Anthraquinone and Soda–Oxygen Pulping of Rice Straw at Different Reaction End Points. Energy Fuels 2017, 31, 514–522. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, K. Effects of pH and Suspended Matter on the Physico-Chemical Properties of Black Liquor from Alkali-Oxygen Pulping of Rice Straw. BioResources 2016, 11, 4252–4267. [Google Scholar] [CrossRef] [Green Version]
- Toquero, C.; Bolado, S. Effect of four pretreatments on enzymatic hydrolysis and ethanol fermentation of wheat straw. Influence of inhibitors and washing. Bioresour. Technol. 2014, 157, 68–76. [Google Scholar] [CrossRef]
- Ma, C.-Y.; Xu, L.-H.; Sun, Q.; Shen, X.-J.; Wen, J.-L.; Yuan, T.-Q. Tailored one-pot lignocellulose fractionation to maximize biorefinery toward controllable producing lignin nanoparticles and facilitating enzymatic hydrolysis. Chem. Eng. J. 2022, 450, 138315. [Google Scholar] [CrossRef]
- Sulzenbacher, D.; Atzmüller, D.; Hawe, F.; Richter, M.; Cristobal-Sarramian, A.; Zwirzitz, A. Optimization of steam explosion parameters for improved biotechnological use of wheat straw. Biomass-Convers. Biorefinery 2021, 1–12. [Google Scholar] [CrossRef]
- Li, X.; Dilokpimol, A.; Kabel, M.A.; de Vries, R.P. Fungal xylanolytic enzymes: Diversity and applications. Bioresour. Technol. 2021, 344, 126290. [Google Scholar] [CrossRef]
- Selvam, K.; Priya, M.S.; Arungandhi, K. Pretreatment of wood chips and pulps with Thelephora sp. to reduce chemical consumption in paper industries. Int. J. ChemTech Res. 2011, 3, 471–476. [Google Scholar]
- Lin, X.; Wu, Z.; Zhang, C.; Liu, S.; Nie, S. Enzymatic pulping of lignocellulosic biomass. Ind. Crop. Prod. 2018, 120, 16–24. [Google Scholar] [CrossRef]
- Bornscheuer, U.T.; Bessler, C.; Srinivas, R.; Krishna, S.H. Optimizing lipases and related enzymes for efficient application. Trends Biotechnol. 2002, 20, 433–437. [Google Scholar] [CrossRef]
- Yang, Q.; Zhan, H.; Wang, S.; Fu, S.; Li, K. Modification of eucalyptus CTMP fibres with white-rot fungus Trametes hirsute—Effects on fibre morphology and paper physical strengths. Bioresour. Technol. 2008, 99, 8118–8124. [Google Scholar] [CrossRef]
- Nagpal, R.; Bhardwaj, N.K.; Mishra, O.P.; Mahajan, R. Cleaner bio-pulping approach for the production of better strength rice straw paper. J. Clean. Prod. 2021, 318, 128539. [Google Scholar] [CrossRef]
- Wei, S.; Liu, K.; Ji, X.; Wang, T.; Wang, R. Application of enzyme technology in biopulping and biobleaching. Cellulose 2021, 28, 10099–10116. [Google Scholar] [CrossRef]
- Walia, A.; Guleria, S.; Mehta, P.; Chauhan, A.; Parkash, J. Microbial xylanases and their industrial application in pulp and paper biobleaching: A review. 3 Biotech 2017, 7, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Dedhia, B.S.; Vetal, M.D.; Rathod, V.K.; Levente, C. Xylanase and laccase aided bio-bleaching of wheat straw pulp. Can. J. Chem. Eng. 2013, 92, 131–138. [Google Scholar] [CrossRef]
- Sharma, A.; Balda, S.; Gupta, N.; Capalash, N.; Sharma, P. Enzyme cocktail: An opportunity for greener agro-pulp bio-bleaching in paper industry. J. Clean. Prod. 2020, 271, 122573. [Google Scholar] [CrossRef]
- Pereira, E.B.; Castro, H.F.D.; Moraes, F.F.D.; Zanin, G.M. Kinetic studies of lipase from Candida rugosa. Appl. Biochem. Biotechnol. 2001, 91–93, 739–752. [Google Scholar] [CrossRef]
- Gusakov, A.V.; Kondratyeva, E.G.; Sinitsyn, A.P. Comparison of two methods for assaying reducing sugars in the deter-mination of carbohydrase activities. Int. J. Anal. Chem. 2011, 2011, 283658. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X. Effect of Pretreatment on Wheat Straw Biomechanical Pulping Performance. Master Thesis, Qilu University of Technology, Jinan, China, 2020. [Google Scholar]
- Wang, Y.; Ji, X.; Liu, S.; Tian, Z.; Si, C.; Wang, R.; Yang, G.; Wang, D. Effects of two different enzyme treatments on the mi-crostructure of outer surface of wheat straw. Adv. Compos. Hybrid Mater. 2022, 5, 934–947. [Google Scholar] [CrossRef]
- TAPPI T236 om-99; Kappa Number of Pulp. TAPPI Press: Atlanta, GA, USA, 2004.
- TAPPI T494 om-01; Tensile Breaking Properties of Paper and Paperboard. TAPPI Press: Atlanta, GA, USA, 2001.
- TAPPI T414 om-04; Internal Tearing Resistance of Paper (Elmendorf-Type Method). TAPPI Press: Atlanta, GA, USA, 2004.
- TAPPI T217 wd-77; Brightness of Pulp. TAPPI Press: Atlanta, GA, USA, 2004.
- Esteves, C.V.; Sevastyanova, O.; Östlund, S.; Brännvall, E. Differences and similarities between kraft and oxygen delignifica-tion of softwood fibers: Effects on mechanical properties. Cellulose 2021, 28, 3775–3788. [Google Scholar] [CrossRef]
- Gao, Y.; Xu, J.; Zhang, Y.; Yu, Q.; Yuan, Z.; Liu, Y. Effects of different pretreatment methods on chemical composition of sug-arcane bagasse and enzymatic hydrolysis. Bioresour. Technol. 2013, 144, 396–400. [Google Scholar] [CrossRef]
- Wong, K.K.; Kibblewhite, R.P.; Signal, F.A. Effect of Xylanase and Dosage on the Refining Properties of Unbleached Softwood Kraft Pulp. J. Wood Chem. Technol. 1999, 19, 203–212. [Google Scholar] [CrossRef]
- Nishimura, H.; Kamiya, A.; Nagata, T.; Katahira, M.; Watanabe, T. Direct evidence for α ether linkage between lignin and carbohydrates in wood cell walls. Sci. Rep. 2018, 8, 6538. [Google Scholar] [CrossRef] [Green Version]
- Dedhia, B.S.; Csoka, L.; Rathod, V.K. Xylanase and Ultrasound Assisted Pulping of Wheat Straw. Appl. Biochem. Biotechnol. 2012, 168, 731–741. [Google Scholar] [CrossRef]
- Cebreiros, F.; Seiler, S.; Dalli, S.S.; Lareo, C.; Saddler, J. Enhancing cellulose nanofibrillation of eucalyptus Kraft pulp by combining enzymatic and mechanical pretreatments. Cellulose 2020, 28, 189–206. [Google Scholar] [CrossRef]
- Xu, H.; Chen, K.; Zhang, L.; Wu, Y. Synchronous silicon removal and viscosity reduction in the soda-oxygen pulping of wheat straw. Cellulose 2021, 28, 9081–9089. [Google Scholar] [CrossRef]
- Rebola, S.M.; Azevedo, C.A.; Evtuguin, D.V. Effect of cooking and bleaching conditions on the properties of eucalyptus kraft fluff pulps. Cellulose 2021, 28, 4411–4426. [Google Scholar] [CrossRef]
- Martins, C.C.N.; Dias, M.C.; Mendonça, M.C.; Durães, A.F.S.; Silva, L.E.; Félix, J.R.; Damásio, R.A.P.; Tonoli, G.H.D. Optimizing cellulose microfibrillation with NaOH pretreatments for unbleached Eucalyptus pulp. Cellulose 2021, 28, 11519–11531. [Google Scholar] [CrossRef]
- Akbarpour, I.; Ghasemian, A.; Resalati, H.; Saraeian, A. Biodeinking of mixed ONP and OMG waste papers with cellulase. Cellulose 2018, 25, 1265–1280. [Google Scholar] [CrossRef]
- Siqueira, G.; Tapin-Lingua, S.; Bras, J.; Perez, D.D.S.; Dufresne, A. Morphological investigation of nanoparticles obtained from combined mechanical shearing, and enzymatic and acid hydrolysis of sisal fibers. Cellulose 2010, 17, 1147–1158. [Google Scholar] [CrossRef]
- Chandra, R.P.; Wu, J.; Saddler, J.N. The Application of Fiber Quality Analysis (FQA) and Cellulose Accessibility Measurements To Better Elucidate the Impact of Fiber Curls and Kinks on the Enzymatic Hydrolysis of Fibers. ACS Sustain. Chem. Eng. 2019, 7, 8827–8833. [Google Scholar] [CrossRef]
- Chen, G.; Wan, J.; Ma, Y.; Wang, Y. Characterization of fibers after xylanase and modified laccase-glutamate system bio-bleaching of old newsprint pulp. Nord. Pulp Pap. Res. J. 2021, 36, 21–32. [Google Scholar] [CrossRef]
- Kesari, K.; Leppänen, M.; Ceccherini, S.; Seitsonen, J.; Väisänen, S.; Altgen, M.; Johansson, L.-S.; Maloney, T.; Ruokolainen, J.; Vuorinen, T. Chemical characterization and ultrastructure study of pulp fibers. Mater. Today Chem. 2020, 17, 100324. [Google Scholar] [CrossRef]
- Huang, C.; He, J.; Li, X.; Min, D.; Yong, Q. Facilitating the enzymatic saccharification of pulped bamboo residues by degrading the remained xylan and lignin–carbohydrates complexes. Bioresour. Technol. 2015, 192, 471–477. [Google Scholar] [CrossRef]
- Foster, E.J.; Moon, R.J.; Agarwal, U.P.; Bortner, M.J.; Bras, J.; Espinosa, S.C.; Chan, K.J.; Clift, M.J.D.; Cranston, E.D.; Eichhorn, S.J.; et al. Current characterization methods for cellulose nano-materials. Chem. Soc. Rev. 2018, 47, 2609–2679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clarke, K.; Li, X.; Li, K. The mechanism of fiber cutting during enzymatic hydrolysis of wood biomass. Biomass-Bioenergy 2011, 35, 3943–3950. [Google Scholar] [CrossRef]
- Wang, J.; Xu, H.; Zhang, Y.; Dai, F.; Wang, Z.; Wang, H.; Zhong, T. Morphology, structure and property of high consistency mechano-enzymatic fibrillated cellulose: Effect of treatment consistency of bamboo pulp fibers. Ind. Crop. Prod. 2022, 188, 115731. [Google Scholar] [CrossRef]
- Král, P.; Ráhel’, J.; Stupavská, M.; Šrajer, J.; Klímek, P.; Mishra, P.K.; Wimmer, R. XPS depth profile of plasma-activated surface of beech wood (Fagus sylvatica) and its impact on polyvinyl acetate tensile shear bond strength. Wood Sci. Technol. 2014, 49, 319–330. [Google Scholar] [CrossRef]
- Inari, G.N.; Pétrissans, M.; Dumarcay, S.; Lambert, J.; Ehrhardt, J.J.; Šernek, M.; Gérardin, P. Limitation of XPS for analysis of wood species containing high amounts of lipophilic extractives. Wood Sci. Technol. 2010, 45, 369–382. [Google Scholar] [CrossRef]
- Luo, L.; Yuan, X.; Zhang, S.; Wang, X.; Li, M.; Wang, S. Effect of Pretreatments on the Enzymatic Hydrolysis of High-Yield Bamboo Chemo-Mechanical Pulp by Changing the Surface Lignin Content. Polymers 2021, 13, 787. [Google Scholar] [CrossRef]
- Kellock, M.; Rahikainen, J.; Marjamaa, K.; Kruus, K. Lignin-derived inhibition of monocomponent cellulases and a xylanase in the hydrolysis of lignocellulosics. Bioresour. Technol. 2017, 232, 183–191. [Google Scholar] [CrossRef]
- Chen, M.; Lu, J.; Cheng, Y.; Li, Q.; Wang, H. Novel process for the coproduction of xylo-oligosaccharide and glucose from reed scraps of reed pulp mill. Carbohydr. Polym. 2019, 215, 82–89. [Google Scholar] [CrossRef]
- Liu, Y.-Y.; Liu, M.-R.; Li, H.-L.; Li, B.-Y.; Zhang, C.-H. Characteristics of high yield pulp fibers by xylanase treatment. Cellulose 2016, 23, 3281–3289. [Google Scholar] [CrossRef]
- Wang, J.; Wan, J.; Ma, Y.; Wang, Y. Macroscopic and microscopic properties of fibers after enzymatic deinking of mixed office waste paper. Cellulose 2019, 26, 9863–9875. [Google Scholar] [CrossRef]
- Sun, E.; Zhang, Y.; Yong, C.; Qu, P.; Huang, H.; Xu, Y. Biological fermentation pretreatment accelerated the depolymerization of straw fiber and its mechanical properties as raw material for mulch film. J. Clean. Prod. 2020, 284, 124688. [Google Scholar] [CrossRef]
- Zhang, L.; Larsson, A.; Moldin, A.; Edlund, U. Comparison of lignin distribution, structure, and morphology in wheat straw and wood. Ind. Crop. Prod. 2022, 187, 115432. [Google Scholar] [CrossRef]
- Yu, H.; Liu, R.; Shen, D.; Wu, Z.; Huang, Y. Arrangement of cellulose microfibrils in the wheat straw cell wall. Carbohydr. Polym. 2008, 72, 122–127. [Google Scholar] [CrossRef]
- Shen, J.-H.; Liu, Z.-M.; Li, J.; Niu, J. Wettability changes of wheat straw treated with chemicals and enzymes. J. For. Res. 2011, 22, 107–110. [Google Scholar] [CrossRef]
- Ko, C.-H.; Yang, C.-Y.; Chang, F.-C.; Lin, L.-D. Effect of Paenibacillus cellulase pretreatment for fiber surface. J. Environ. Manag. 2019, 241, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cui, B.; Xie, H.; Sun, H.; Ji, T.; Li, S.; Jia, X.; Wang, W. Anisotropic, ultrastrong and light-transmission film designed on wheat straw. J. Mater. Chem. A 2022, 10, 12968–12976. [Google Scholar] [CrossRef]
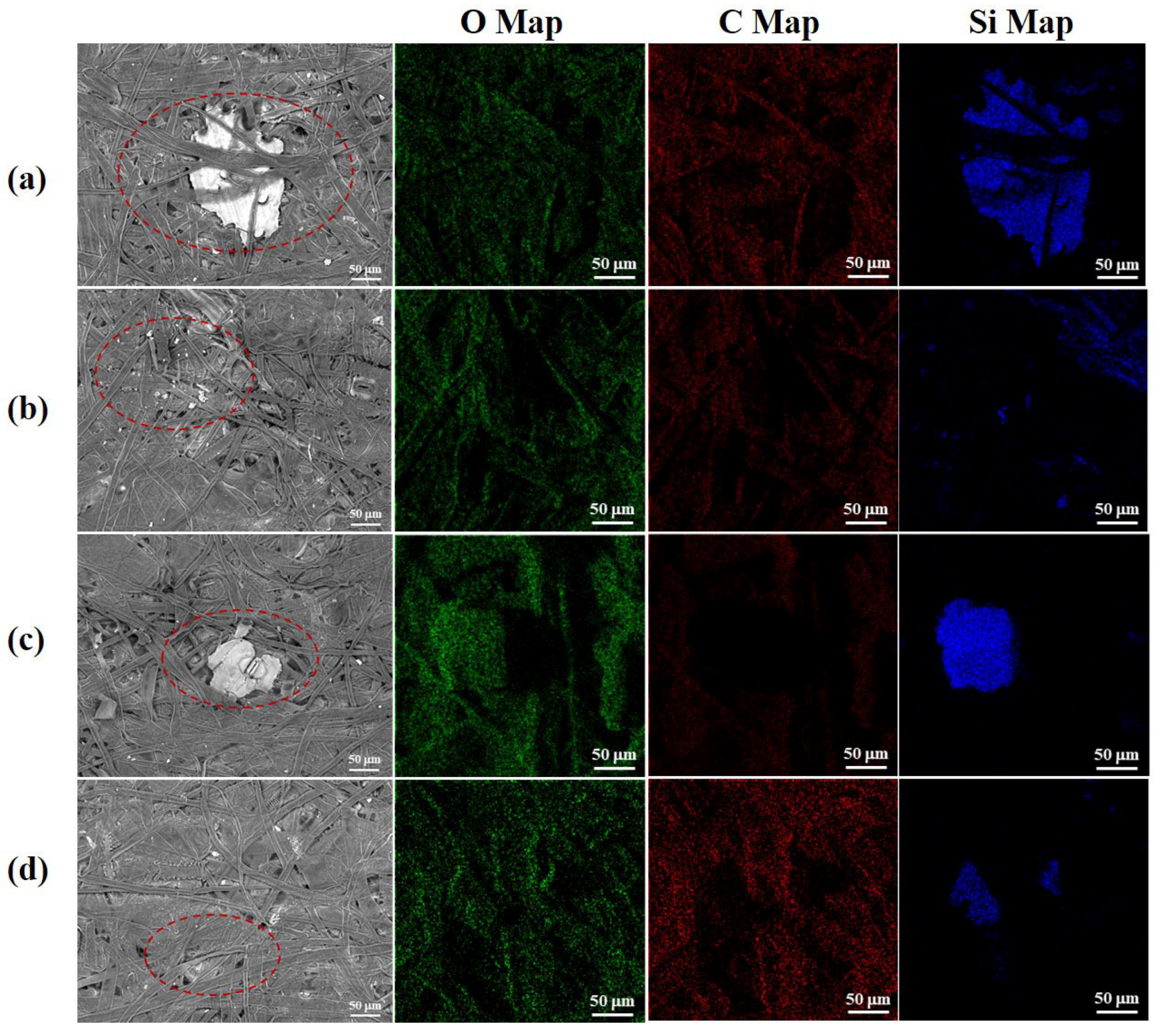

| Pulp | Length /mm | Width /μm | Aspect Ratio | Fines /% | Kink Index | Shape Factor /% | Curl Index |
|---|---|---|---|---|---|---|---|
| P0 | 0.711 | 20.6 | 34.5 | 50.7 | 1.695 | 88.6 | 0.129 |
| PL15 | 0.751 | 19.6 | 38.3 | 50.1 | 1.347 | 90.0 | 0.111 |
| PX15 | 0.746 | 19.8 | 37.7 | 49.1 | 1.252 | 90.2 | 0.109 |
| PLX15 | 0.806 | 19.7 | 40.9 | 49.3 | 1.060 | 90.8 | 0.101 |
| Pulp | O 1s /% | C 1s /% | C1 /% | C2 /% | C3 /% | C1/C2 | O/C |
|---|---|---|---|---|---|---|---|
| P0 | 32.38 | 66.24 | 43.97 | 42.51 | 13.52 | 1.03 | 0.49 |
| PL15 | 38.66 | 60.48 | 21.57 | 58.36 | 20.07 | 0.37 | 0.64 |
| PX15 | 39.36 | 59.86 | 23.70 | 57.75 | 18.55 | 0.41 | 0.66 |
| PLX15 | 37.56 | 61.70 | 29.71 | 52.69 | 17.60 | 0.56 | 0.61 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, Q.; Chen, J.; Yang, G.; Liu, K.; Wang, Y.; Zhang, K. Effects of Lipase and Xylanase Pretreatment on the Structure and Pulping Properties of Wheat Straw. Polymers 2022, 14, 5129. https://doi.org/10.3390/polym14235129
Jia Q, Chen J, Yang G, Liu K, Wang Y, Zhang K. Effects of Lipase and Xylanase Pretreatment on the Structure and Pulping Properties of Wheat Straw. Polymers. 2022; 14(23):5129. https://doi.org/10.3390/polym14235129
Chicago/Turabian StyleJia, Qianqian, Jiachuan Chen, Guihua Yang, Kefeng Liu, Yueying Wang, and Kai Zhang. 2022. "Effects of Lipase and Xylanase Pretreatment on the Structure and Pulping Properties of Wheat Straw" Polymers 14, no. 23: 5129. https://doi.org/10.3390/polym14235129





