Recent Advances in Application of Polyoxometalates in Lignocellulose Pretreatment and Transformation
Abstract
:1. Introduction
2. Advances in the Pretreatment of Lignocellulose with POMs in the GVL/Water System
3. Advances in the Pretreatment of Lignocellulose with POMs in the DES System
4. Advances in the Pretreatment of Lignocellulose with POMs in the ILs System
5. Advances in the Pretreatment of Lignocellulose with POMs in Other Systems
6. Advances in the Complete Conversion of Lignocellulose Catalyzed by POMs
6.1. Reduction Conditions
6.2. Oxidation Conditions
6.3. Other Conditions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Straathof, A.J. Transformation of biomass into commodity chemicals using enzymes or cells. Chem. Rev. 2014, 114, 1871–1908. [Google Scholar] [CrossRef]
- Besson, M.; Gallezot, P.; Pinel, C. Conversion of biomass into chemicals over metal catalysts. Chem. Rev. 2014, 114, 1827–1870. [Google Scholar] [CrossRef]
- He, M.; Sun, Y.; Han, B. Green carbon science: Scientific basis for integrating carbon resource processing, utilization, and recycling. Angew. Chem. Int. Ed. Engl. 2013, 52, 9620–9633. [Google Scholar] [CrossRef]
- Sriariyanun, M.; Kitsubthawee, K. Trends in lignocellulosic biorefinery for production of value-added biochemicals. Appl. Sci. Eng. Prog. 2020, 13, 283–284. [Google Scholar] [CrossRef]
- Gundupalli, M.P.; Sriariyanun, M. Recent trends and updates for chemical pretreatment of lignocellulosic biomass. Appl. Sci. Eng. Prog. 2022, 16, 5842. [Google Scholar] [CrossRef]
- Kee, P.E.; Cheng, Y.-S.; Chang, J.-S.; Yim, H.S.; Tan, J.C.Y.; Lam, S.S.; Lan, J.C.-W.; Ng, H.S.; Khoo, K.S. Insect biorefinery: A circular economy concept for biowaste conversion to value-added products. Environ. Res. 2023, 221, 115284. [Google Scholar] [CrossRef] [PubMed]
- Pulidindi, I.N.; Kimchi, B.B.; Gedanken, A. Can cellulose be a sustainable feedstock for bioethanol production? Renew. Energy 2014, 71, 77–80. [Google Scholar] [CrossRef]
- Hernández, C.A.; Ziarelli, F.; Gaime, I.; Farnet Da Silva, A.M.; García, G.; García-Pérez, J.A.; Gutiérrez-Rivera, B.; Alarcón, E.J.C. Chemical and Biological Pretreatments on Sugarcane Bagasse to Enhance its Enzymatic Hydrolysis. ChemistrySelect 2017, 2, 4213–4218. [Google Scholar] [CrossRef]
- Wettstein, S.G.; Alonso, D.M.; Gürbüz, E.I.; Dumesic, J.A. A roadmap for conversion of lignocellulosic biomass to chemicals and fuels. Curr. Opin. Chem. Eng. 2012, 1, 218–224. [Google Scholar] [CrossRef]
- Dutta, S.; Iris, K.; Tsang, D.C.; Su, Z.; Hu, C.; Wu, K.C.; Yip, A.C.; Ok, Y.S.; Poon, C. Influence of green solvent on levulinic acid production from lignocellulosic paper waste. Bioresour. Technol. 2020, 298, 122544. [Google Scholar] [CrossRef]
- Dutta, S.; Yu, I.K.; Tsang, D.C.; Fan, J.; Clark, J.H.; Jiang, Z.; Su, Z.; Hu, C.; Poon, C.S. Efficient depolymerization of cellulosic paper towel waste using organic carbonate solvents. ACS Sustain. Chem. Eng. 2020, 8, 13100–13110. [Google Scholar] [CrossRef]
- Talekar, S.; Patti, A.F.; Vijayraghavan, R.; Arora, A. Complete utilization of waste pomegranate peels to produce a hydrocolloid, punicalagin rich phenolics, and a hard carbon electrode. ACS Sustain. Chem. Eng. 2018, 6, 16363–16374. [Google Scholar] [CrossRef]
- Albert, J.; Jess, A.; Kern, C.; Pöhlmann, F.; Glowienka, K.; Wasserscheid, P. Engineering, formic acid-based fischer–tropsch synthesis for green fuel production from wet waste biomass and renewable excess energy. ACS Sustain. Chem. Eng. 2016, 4, 5078–5086. [Google Scholar] [CrossRef]
- Tucki, K.; Orynycz, O.; Wasiak, A.; Świć, A.; Wichłacz, J. The Impact of Fuel Type on the Output Parameters of a New Biofuel Burner. Energies 2019, 12, 1383. [Google Scholar] [CrossRef]
- Tucki, K.; Orynycz, O.; Wasiak, A.; Świć, A.; Mruk, R.; Botwińska, K. Estimation of Carbon Dioxide Emissions from a Diesel Engine Powered by Lignocellulose Derived Fuel for Better Management of Fuel Production. Energies 2020, 13, 561. [Google Scholar] [CrossRef]
- Alonso, D.M.; Bond, J.Q.; Dumesic, J.A. Catalytic conversion of biomass to biofuels. Green Chem. 2010, 12, 1493–1513. [Google Scholar] [CrossRef]
- Wang, S.; Luo, Z. Biomass components and characteristics. In Pyrolysis of Biomass; Chemie Ingenieur Technik Berlin; ACS Publications: Washington, DC, USA, 2016; Volume 72, pp. 1–32. [Google Scholar]
- Chen, Z.; Wan, C. Biological valorization strategies for converting lignin into fuels and chemicals. Renew. Sustain. Energy Rev. 2017, 73, 610–621. [Google Scholar] [CrossRef]
- Himmel, M.E.; Ding, S.Y.; Johnson, D.K.; Adney, W.S.; Nimlos, M.R.; Brady, J.W.; Foust, T.D. Biomass recalcitrance: Engineering plants and enzymes for biofuels production. Science 2007, 315, 804–807. [Google Scholar] [CrossRef]
- Cheng, Y.-S.; Mutrakulcharoen, P.; Chuetor, S.; Cheenkachorn, K.; Tantayotai, P.; Panakkal, E.J.; Sriariyanun, M. Recent situation and progress in biorefining process of lignocellulosic biomass: Toward green economy. Appl. Sci. Eng. Prog. 2020, 13, 299–311. [Google Scholar]
- Panakkal, E.J.; Kitiborwornkul, N.; Sriariyanun, M.; Ratanapoompinyo, J.; Yasurin, P.; Asavasanti, S.; Rodiahwati, W.; Tantayotai, P. Production of food flavouring agents by enzymatic reaction and microbial fermentation. Appl. Sci. Eng. Prog. 2021, 14, 297–312. [Google Scholar] [CrossRef]
- Wang, S.; Dai, G.; Yang, H.; Luo, Z. Lignocellulosic biomass pyrolysis mechanism: A state-of-the-art review. Prog. Energy Combust. Sci. 2017, 62, 33–86. [Google Scholar] [CrossRef]
- Meng, X.; Sun, Q.; Kosa, M.; Huang, F.; Pu, Y.; Ragauskas, A.J. Physicochemical Structural Changes of Poplar and Switchgrass during Biomass Pretreatment and Enzymatic Hydrolysis. ACS Sustain. Chem. Eng. 2016, 4, 4563–4572. [Google Scholar] [CrossRef]
- Tian, D.; Shen, F.; Yang, G.; Deng, S.; Long, L.; He, J.; Zhang, J.; Huang, C.; Luo, L. Liquid hot water extraction followed by mechanical extrusion as a chemical-free pretreatment approach for cellulosic ethanol production from rigid hardwood. Fuel 2019, 252, 589–597. [Google Scholar] [CrossRef]
- Yan, M.; Wu, T.; Ma, J.; Lu, H.; Zhou, X. Characteristic comparison of lignocellulose nanofibrils from wheat straw having different mechanical pretreatments. Appl. Polym. 2022, 139, e53054. [Google Scholar] [CrossRef]
- Raj, T.; Kapoor, M.; Gaur, R.; Christopher, J.; Lamba, B.; Tuli, D.K.; Kumar, R. Physical and chemical characterization of various Indian agriculture residues for biofuels production. Energy Fuels 2015, 29, 3111–3118. [Google Scholar] [CrossRef]
- Zhang, L.; Lu, H.; Yu, J.; Wang, Z.; Fan, Y.; Zhou, X. Dissolution of lignocelluloses with a high lignin content in a N-methylmorpholine-N-oxide monohydrate solvent system via simple glycerol-swelling and mechanical pretreatments. Agric. Food Chem. 2017, 65, 9587–9594. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Xiao, X.; Li, C.; Sheng, X.; Zhang, J.; Ping, Q. Boosting VFAs production during the anaerobic acidification of lignocellulose waste pulp and paper mill excess sludge: Ultrasonic pretreatment and inoculating rumen microorganisms. Ind. Crops Prod. 2022, 188, 115613. [Google Scholar] [CrossRef]
- Duque, A.; Manzanares, P.; Ballesteros, I.; Ballesteros, M. Steam explosion as lignocellulosic biomass pretreatment. Biomass Fractionation Technol. A Lignocellul. Feedstock Based Biorefin. 2016, 30, 349–368. [Google Scholar]
- Auxenfans, T.; Crônier, D.; Chabbert, B.; Paës, G. Understanding the structural and chemical changes of plant biomass following steam explosion pretreatment. Biotechnol. Biofuels 2017, 10, 36. [Google Scholar] [CrossRef]
- Sheng, Y.; Xu, Y. Nuclear magnetic resonance analysis of ascorbic acid assisted lignocellulose decomposition in dilute acid pretreatment and its stimulation on enzymatic hydrolysis. Bioresour. Technol. 2022, 343, 126147. [Google Scholar] [CrossRef]
- Jung, Y.H.; Kim, I.J.; Kim, H.K.; Kim, K.H. Dilute acid pretreatment of lignocellulose for whole slurry ethanol fermentation. Bioresour. Technol. 2013, 132, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhu, Y.; Liu, T.; Sun, S.; Ren, J.; Wu, A.; Li, H. A novel wet-mechanochemical pretreatment for the efficient enzymatic saccharification of lignocelluloses: Small dosage dilute alkali assisted ball milling. Energy Convers. Manag. 2019, 194, 46–54. [Google Scholar] [CrossRef]
- Yamaguchi, A.; Hiyoshi, N.; Sato, O.; Shirai, M. Sorbitol dehydration in high temperature liquid water. Green Chem. 2011, 13, 873–881. [Google Scholar] [CrossRef]
- Singh, J.; Suhag, M.; Dhaka, A. Augmented digestion of lignocellulose by steam explosion, acid and alkaline pretreatment methods: A review. Carbohydr. Polym. 2015, 117, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Tromp, M.; Sietsma, J.R.; van Bokhoven, J.A.; van Strijdonck, G.P.; van Haaren, R.J.; van der Eerden, A.M.; van Leeuwen, P.W.; Koningsberger, D.C. Deactivation processes of homogeneous Pd catalysts using in situ time resolved spectroscopic techniques. Chem. Commun. 2003, 1, 128–129. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.M.; Zhang, T.; Kumar, R.; Wyman, C.E. THF co-solvent enhances hydrocarbon fuel precursor yields from lignocellulosic biomass. Green Chem. 2013, 15, 3140–3145. [Google Scholar] [CrossRef]
- Gschwend, F.J.; Chambon, C.L.; Biedka, M.; Brandt-Talbot, A.; Fennell, P.S.; Hallett, J.P. Quantitative glucose release from softwood after pretreatment with low-cost ionic liquids. Green Chem. 2019, 21, 692–703. [Google Scholar] [CrossRef]
- da Costa Sousa, L.; Chundawat, S.P.; Balan, V.; Dale, B.E. Cradle-to-grave’assessment of existing lignocellulose pretreatment technologies. Curr. Opin. Biotechnol. 2009, 20, 339–347. [Google Scholar] [CrossRef]
- Akhtar, N.; Gupta, K.; Goyal, D.; Goyal, A. Recent advances in pretreatment technologies for efficient hydrolysis of lignocellulosic biomass. Environ. Prog. Sustain. Energy 2016, 35, 489–511. [Google Scholar] [CrossRef]
- Zhang, K.; Pei, Z.; Wang, D. Organic solvent pretreatment of lignocellulosic biomass for biofuels and biochemicals: A review. Bioresour. Technol. 2016, 199, 21–33. [Google Scholar] [CrossRef]
- Kapoor, M.; Raj, T.; Vijayaraj, M.; Chopra, A.; Gupta, R.P.; Tuli, D.K.; Kumar, R. Structural features of dilute acid, steam exploded, and alkali pretreated mustard stalk and their impact on enzymatic hydrolysis. Carbohydr. Polym. 2015, 124, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Gaur, R.; Semwal, S.; Raj, T.; Yadav Lamba, B.; Ramu, E.; Gupta, R.P.; Kumar, R.; Puri, S.K. Intensification of steam explosion and structural intricacies impacting sugar recovery. Bioresour. Technol. 2017, 241, 692–700. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Shi, S.; Jia, X.; Xia, F.; Ma, H.; Gao, J.; Xu, J. Advances in catalytic conversion of lignocellulose to chemicals and liquid fuels. J. Energy Chem. 2019, 36, 74–86. [Google Scholar] [CrossRef]
- Damayanti, D.; Supriyadi, D.; Amelia, D.; Saputri, D.R.; Devi, Y.L.L.; Auriyani, W.A.; Wu, H.S. Conversion of Lignocellulose for bioethanol production, applied in bio-polyethylene terephthalate. Polymers 2021, 13, 2886. [Google Scholar] [CrossRef] [PubMed]
- Vedernikovs, N.; Khroustalyova, G.; Muiznieks, I.; Rapoport, A. New concept for conversion of lignocellulose to ethanol and furfural. Appl. Microbiol. Biotechnol. 2023, 107, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, M.G.; Pereira, S.D.P.S.; Fernandes, S.A.; da Silva, M.J. Enhancement of levoglucosan production via fast pyrolysis of sugarcane bagasse by pretreatment with Keggin heteropolyacids. Ind. Crops Prod. 2020, 154, 112680. [Google Scholar] [CrossRef]
- Li, X.X.; Wang, Y.X.; Wang, R.H.; Cui, C.Y.; Tian, C.B.; Yang, G.Y. Designed Assembly of Heterometallic Cluster Organic Frameworks Based on Anderson-Type Polyoxometalate Clusters. Angew. Chem. Int. Ed. Engl. 2016, 55, 6462–6466. [Google Scholar] [CrossRef]
- Li, Z.; Li, Y.; Chen, Y.; Wang, Q.; Jadoon, M.; Yi, X.; Duan, X.; Wang, X. Developing Dawson-Type Polyoxometalates Used as Highly Efficient Catalysts for Lignocellulose Transformation. ACS Catal. 2022, 12, 9213–9225. [Google Scholar] [CrossRef]
- Almohalla, M.; Rodríguez-Ramos, I.; Guerrero-Ruiz, A. Comparative study of three heteropolyacids supported on carbon materials as catalysts for ethylene production from bioethanol. Catal. Sci. Technol. 2017, 7, 1892–1901. [Google Scholar] [CrossRef]
- Guo, H.; Wang, Y.; Tian, L.; Wei, W.; Zhu, T.; Liu, Y. Unveiling the mechanisms of a novel polyoxometalates (POMs)-based pretreatment technology for enhancing methane production from waste activated sludge. Bioresour. Technol. 2021, 342, 125934. [Google Scholar] [CrossRef]
- Luo, X.; Wu, H.; Li, C.; Li, Z.; Li, H.; Zhang, H.; Li, Y.; Su, Y.; Yang, S. Heteropoly Acid-Based Catalysts for Hydrolytic Depolymerization of Cellulosic Biomass. Front. Chem. 2020, 8, 580146. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.S.; Yang, G.Y. Recent advances in polyoxometalate-catalyzed reactions. Chem. Rev. 2015, 115, 4893–4962. [Google Scholar] [CrossRef] [PubMed]
- Zhai, H.; Gong, T.; Xu, B.; Cheng, Q.; Paley, D.; Qie, B.; Jin, T.; Fu, Z.; Tan, L.; Lin, Y.H.; et al. Stabilizing Polyether Electrolyte with a 4 V Metal Oxide Cathode by Nanoscale Interfacial Coating. ACS Appl. Mater. Interfaces 2019, 11, 28774–28780. [Google Scholar] [CrossRef] [PubMed]
- Luterbacher, J.S.; Rand, J.M.; Alonso, D.M.; Han, J.; Youngquist, J.T.; Maravelias, C.T.; Pfleger, B.F.; Dumesic, J.A. Nonenzymatic sugar production from biomass using biomass-derived γ-valerolactone. Science 2014, 343, 277–280. [Google Scholar] [CrossRef]
- Li, S.-X.; Li, M.-F.; Yu, P.; Fan, Y.-M.; Shou, J.-N.; Sun, R.-C. Valorization of bamboo by γ-valerolactone/acid/water to produce digestible cellulose, degraded sugars and lignin. Bioresour. Technol. 2017, 230, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Qin, Y.; Wen, P.; Zhang, T.; Zhang, J. Alkaline post-incubation improves cellulose hydrolysis after γ-valerolactone/water pretreatment. Bioresour. Technol. 2019, 278, 440–443. [Google Scholar] [CrossRef]
- Luterbacher, J.S.; Azarpira, A.; Motagamwala, A.H.; Lu, F.; Ralph, J.; Dumesic, J.A. Lignin monomer production integrated into the γ-valerolactone sugar platform. Energy Environ. Sci. 2015, 8, 2657–2663. [Google Scholar] [CrossRef]
- Lê, H.Q.; Zaitseva, A.; Pokki, J.P.; Ståhl, M.; Alopaeus, V.; Sixta, H. Solubility of organosolv lignin in γ-valerolactone/water binary mixtures. ChemSusChem 2016, 9, 2939–2947. [Google Scholar] [CrossRef]
- Horváth, I.T.; Mehdi, H.; Fábos, V.; Boda, L.; Mika, L.T. γ-Valerolactone—A sustainable liquid for energy and carbon-based chemicals. Green Chem. 2008, 10, 238–242. [Google Scholar] [CrossRef]
- Shuai, L.; Questell-Santiago, Y.M.; Luterbacher, J.S. A mild biomass pretreatment using γ-valerolactone for concentrated sugar production. Green Chem. 2016, 18, 937–943. [Google Scholar] [CrossRef]
- Sener, C.; Motagamwala, A.H.; Alonso, D.M.; Dumesic, J.A. Enhanced Furfural Yields from Xylose Dehydration in the gamma-Valerolactone/Water Solvent System at Elevated Temperatures. ChemSusChem 2018, 11, 2321–2331. [Google Scholar] [CrossRef] [PubMed]
- Trevorah, R.M.; Huynh, T.; Vancov, T.; Othman, M.Z. Bioethanol potential of Eucalyptus obliqua sawdust using gamma-valerolactone fractionation. Bioresour. Technol. 2018, 250, 673–682. [Google Scholar] [CrossRef]
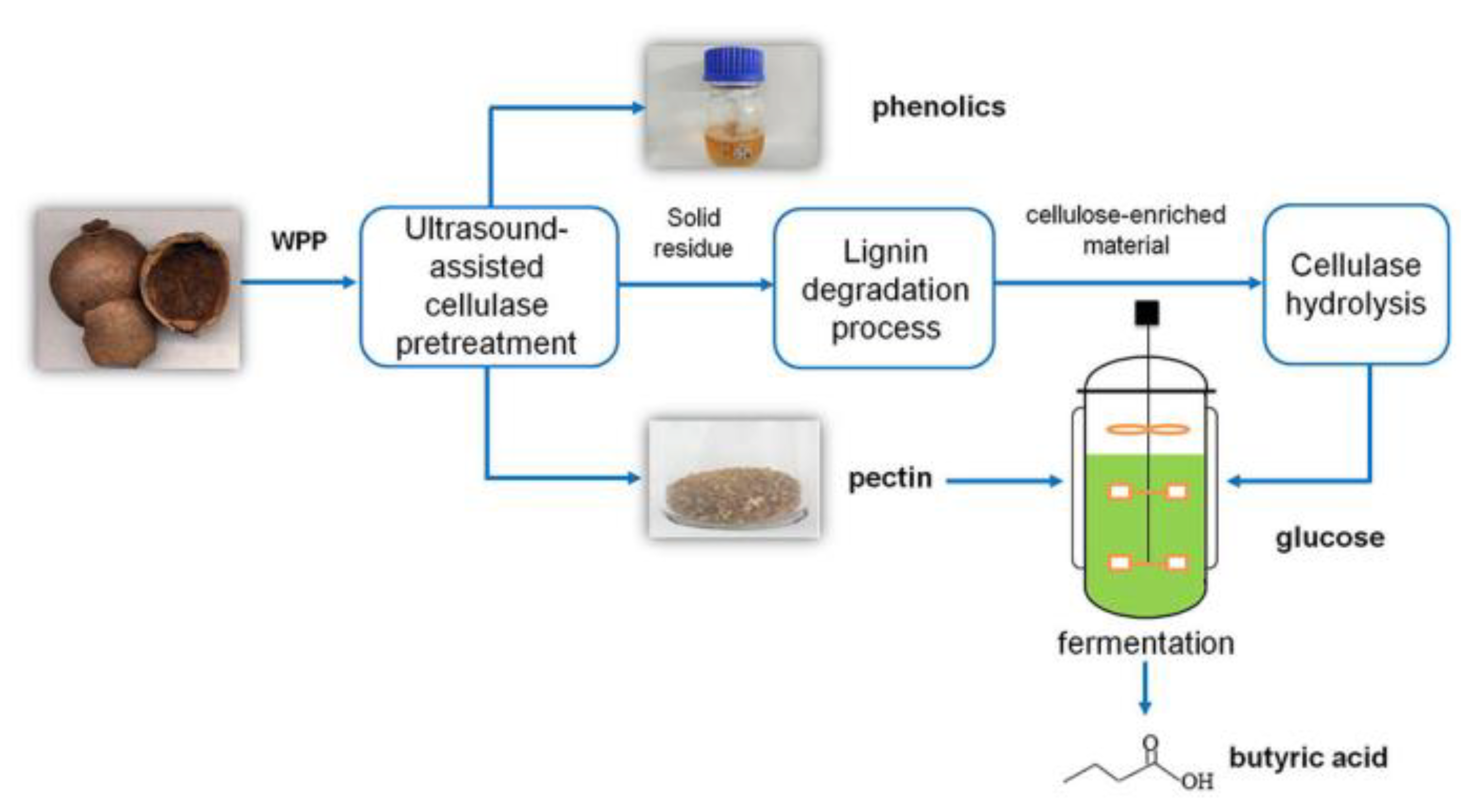
- Wang, T.; Zhao, Q.; Li, C.; He, F.; Jiang, L.; Aisa, H.A. Integrating chemical and biological catalysis for simultaneous production of polyphenolics and butyric acid from waste pomegranate peels. Sci. Total Environ. 2021, 778, 146095. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zheng, W.; Wang, Z.; Ma, Y.; Jiang, L.; Wang, T. Efficient degradation of lignin in raw wood via pretreatment with heteropoly acids in gamma-valerolactone/water. Bioresour. Technol. 2018, 261, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Q.; Hse, C.-Y.; Long, F.; Wang, F.; Shupe, T.F.; Xu, J. Sequential Fractionation of Lignocellulosic Biomass Using CO2-Assisted Hydrolysis Combined with γ-Valerolactone Treatment. Energy Technol. 2020, 8, 1900949. [Google Scholar] [CrossRef]
- Procentese, A.; Raganati, F.; Olivieri, G.; Russo, M.E.; Rehmann, L.; Marzocchella, A. Low-energy biomass pretreatment with deep eutectic solvents for bio-butanol production. Bioresour. Technol. 2017, 243, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Procentese, A.; Raganati, F.; Olivieri, G.; Russo, M.E.; Rehmann, L.; Marzocchella, A. Deep eutectic solvents pretreatment of agro-industrial food waste. Biotechnol. Biofuels 2018, 11, 37. [Google Scholar] [CrossRef]
- Sun, X.; Zhou, Z.; Tian, D.; Zhao, J.; Zhang, J.; Deng, P.; Zou, H.; Lu, C. Acidic deep eutectic solvent assisted mechanochemical delignification of lignocellulosic biomass at room temperature. Int. J. Biol. Macromol. 2023, 234, 123593. [Google Scholar] [CrossRef]
- Guo, Z.; Zhang, Q.; You, T.; Ji, Z.; Zhang, X.; Qin, Y.; Xu, F. Heteropoly acids enhanced neutral deep eutectic solvent pretreatment for enzymatic hydrolysis and ethanol fermentation of Miscanthus x giganteus under mild conditions. Bioresour. Technol. 2019, 293, 122036. [Google Scholar] [CrossRef]
- Guo, Z.; Li, D.; You, T.; Zhang, X.; Xu, F.; Zhang, X.; Yang, Y. New Lignin Streams Derived from Heteropoly Acids Enhanced Neutral Deep Eutectic Solvent Fractionation: Toward Structural Elucidation and Antioxidant Performance. ACS Sustain. Chem. Eng. 2020, 8, 12110–12119. [Google Scholar] [CrossRef]
- Xie, J.; Xu, J.; Cheng, Z.; Zhu, S.; Wang, B. Phosphotungstic acid assisted with neutral deep eutectic solvent boost corn straw pretreatment for enzymatic saccharification and lignin extraction. Ind. Crops Prod. 2021, 172, 114058. [Google Scholar] [CrossRef]
- Plechkova, N.V.; Seddon, K.R. Applications of ionic liquids in the chemical industry. Chem. Soc. Rev. 2008, 37, 123–150. [Google Scholar] [CrossRef] [PubMed]
- Seddon, W.L. Mechanisms of temperature acclimation in the channel catfish Ictalurus punctatus: Isozymes and quantitative changes. Comp. Biochem. Physiol. 1997, 68, 351–356. [Google Scholar] [CrossRef]
- Raj, T.; Gaur, R.; Lamba, B.Y.; Singh, N.; Gupta, R.P.; Kumar, R.; Puri, S.K.; Ramakumar, S.S.V. Characterization of ionic liquid pretreated plant cell wall for improved enzymatic digestibility. Bioresour. Technol. 2018, 249, 139–145. [Google Scholar] [CrossRef]
- Raj, T.; Gaur, R.; Dixit, P.; Gupta, R.P.; Kagdiyal, V.; Kumar, R.; Tuli, D.K. Ionic liquid pretreatment of biomass for sugars production: Driving factors with a plausible mechanism for higher enzymatic digestibility. Carbohydr. Polym. 2016, 149, 369–381. [Google Scholar] [CrossRef] [PubMed]
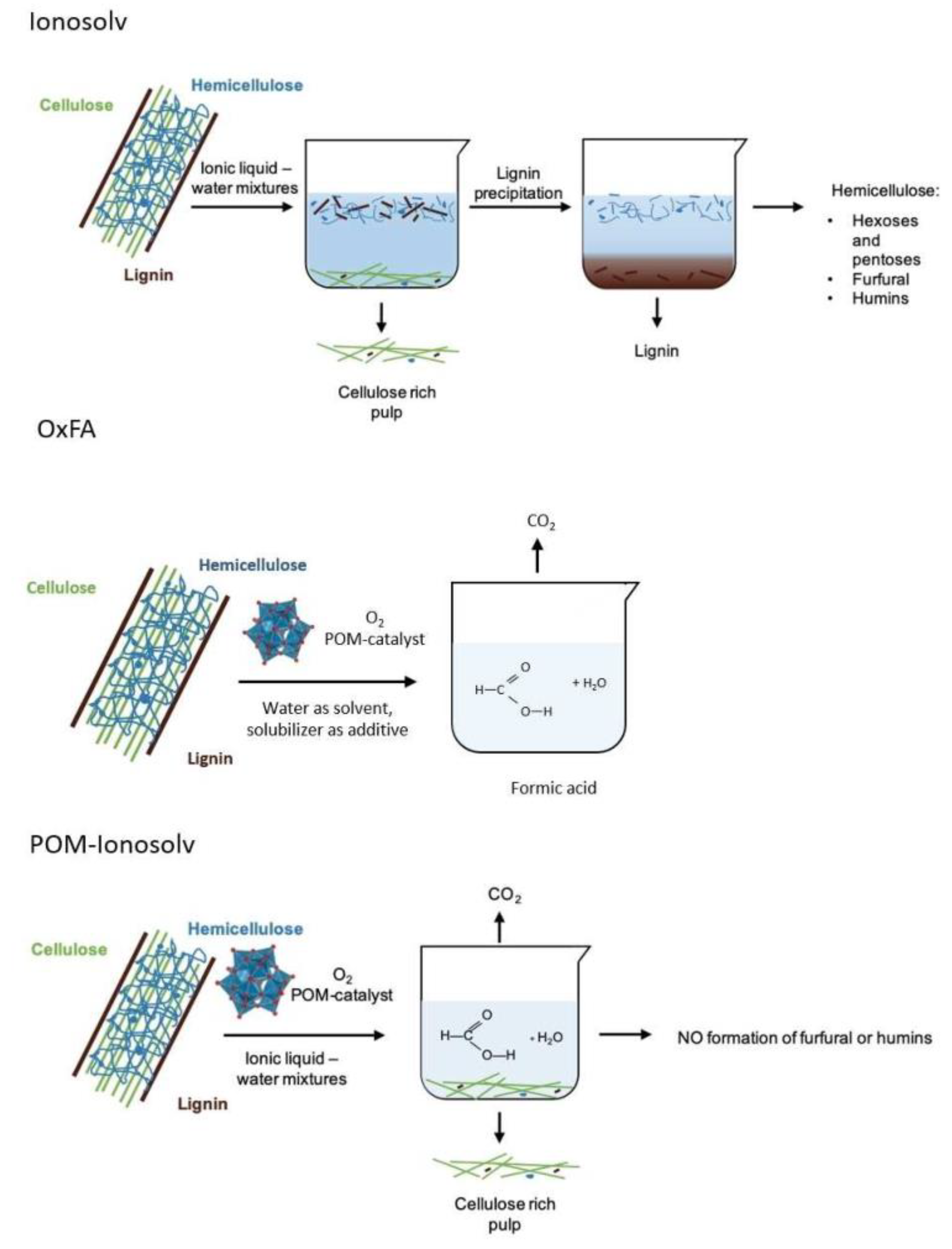
- Bukowski, A.; Esau, D.; Rafat Said, A.A.; Brandt-Talbot, A.; Albert, J. Combining Cost-Efficient Cellulose and Short-Chain Carboxylic Acid Production: The Polyoxometalate (POM)-Ionosolv Concept. Chempluschem 2020, 85, 373–386. [Google Scholar] [CrossRef]
- Shi, N.; Liu, D.; Huang, Q.; Guo, Z.; Jiang, R.; Wang, F.; Chen, Q.; Li, M.; Shen, G.; Wen, F. Product-oriented decomposition of lignocellulose catalyzed by novel polyoxometalates-ionic liquid mixture. Bioresour. Technol. 2019, 283, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, X.; Li, Z.; Song, J.; Wang, X. Full Utilization of Lignocellulose with Ionic Liquid Polyoxometalates in a One-Pot Three-Step Conversion. ChemSusChem 2019, 12, 4936–4945. [Google Scholar] [CrossRef]
- Hronec, M.; Fulajtárová, K.; Klempová, T.; Čertík, M. Fractionation of Wheat Straw Catalyzed by Recyclable Terephthalic Acid. ChemistrySelect 2019, 4, 6060–6065. [Google Scholar] [CrossRef]
- Yang, W.; Du, X.; Liu, W.; Wang, Z.; Dai, H.; Deng, Y. Direct Valorization of Lignocellulosic Biomass into Value-Added Chemicals by Polyoxometalate Catalyzed Oxidation under Mild Conditions. Ind. Eng. Chem. Res. 2019, 58, 22996–23004. [Google Scholar] [CrossRef]
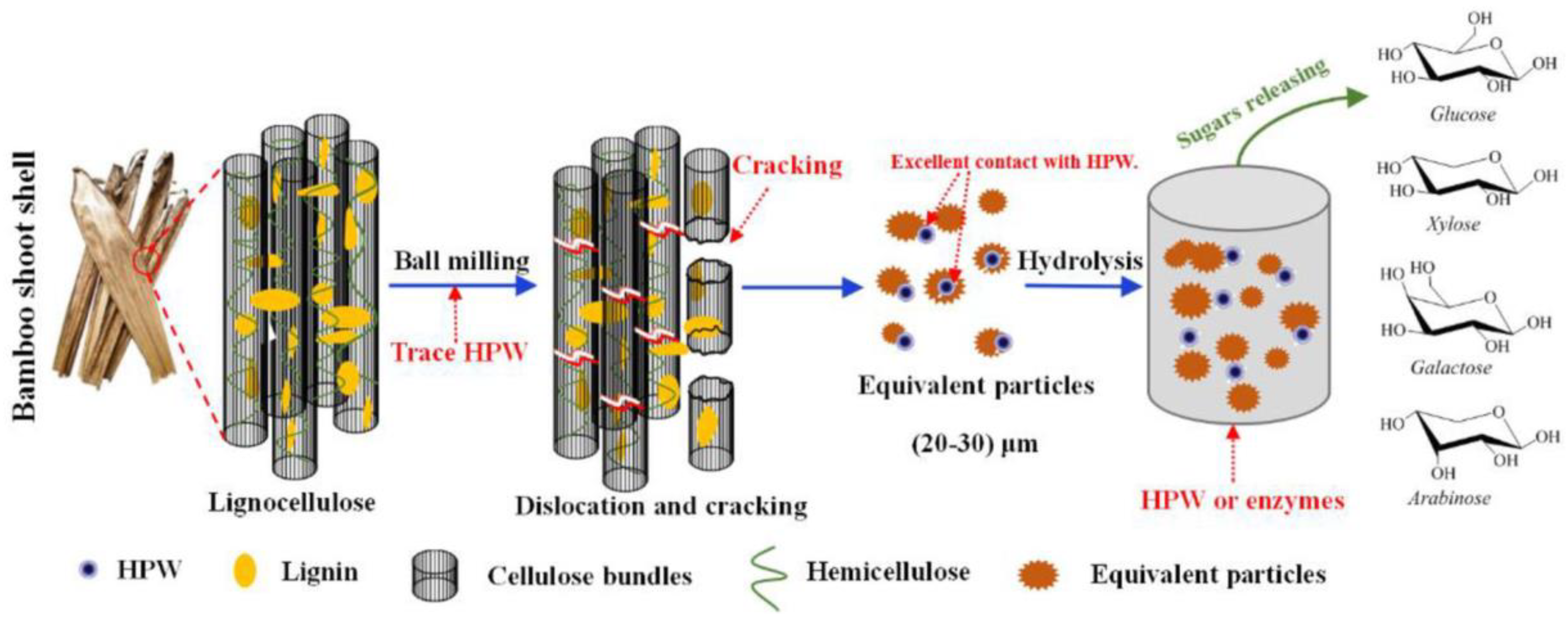
- Xiao, Z.; Yang, Q.; Wu, X.; He, Y. Ball milling promotes saccharification of agricultural biomass by heteropolyacid and enzyme: Unlock the lignin cage for sugars recovery. Biomass Convers. Biorefinery 2020, 12, 4105–4115. [Google Scholar] [CrossRef]
- Liu, Y.; Zhong, L.; Wang, C.; Yang, G.; Chen, J.; Yoo, C.G.; Lyu, G. Synergistic effects of heteropoly acids and sulfolane on fractionation and utilization of willow. Ind. Crops Prod. 2022, 188, 115712. [Google Scholar] [CrossRef]
- Wang, X.; Duan, C.; Zhao, C.; Meng, J.; Qin, X.; Xu, Y.; Ni, Y. Heteropoly acid catalytic treatment for reactivity enhancement and viscosity control of dissolving pulp. Bioresour. Technol. 2018, 253, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Garron, A.; Maksoud, W.A.; Larabi, C.; Arquillière, P.; Szeto, K.C.; Walter, J.-J.; Santini, C.C. Direct thermo-catalytic transformation of pine wood into low oxygenated fuel: Influence of the support. Catal. Today 2015, 255, 75–79. [Google Scholar] [CrossRef]
- Al Maksoud, W.; Larabi, C.; Garron, A.; Szeto, K.C.; Walter, J.J.; Santini, C.C. Direct thermocatalytic transformation of pine wood into low oxygenated biofuel. Green Chem. 2014, 16, 3031–3038. [Google Scholar] [CrossRef]
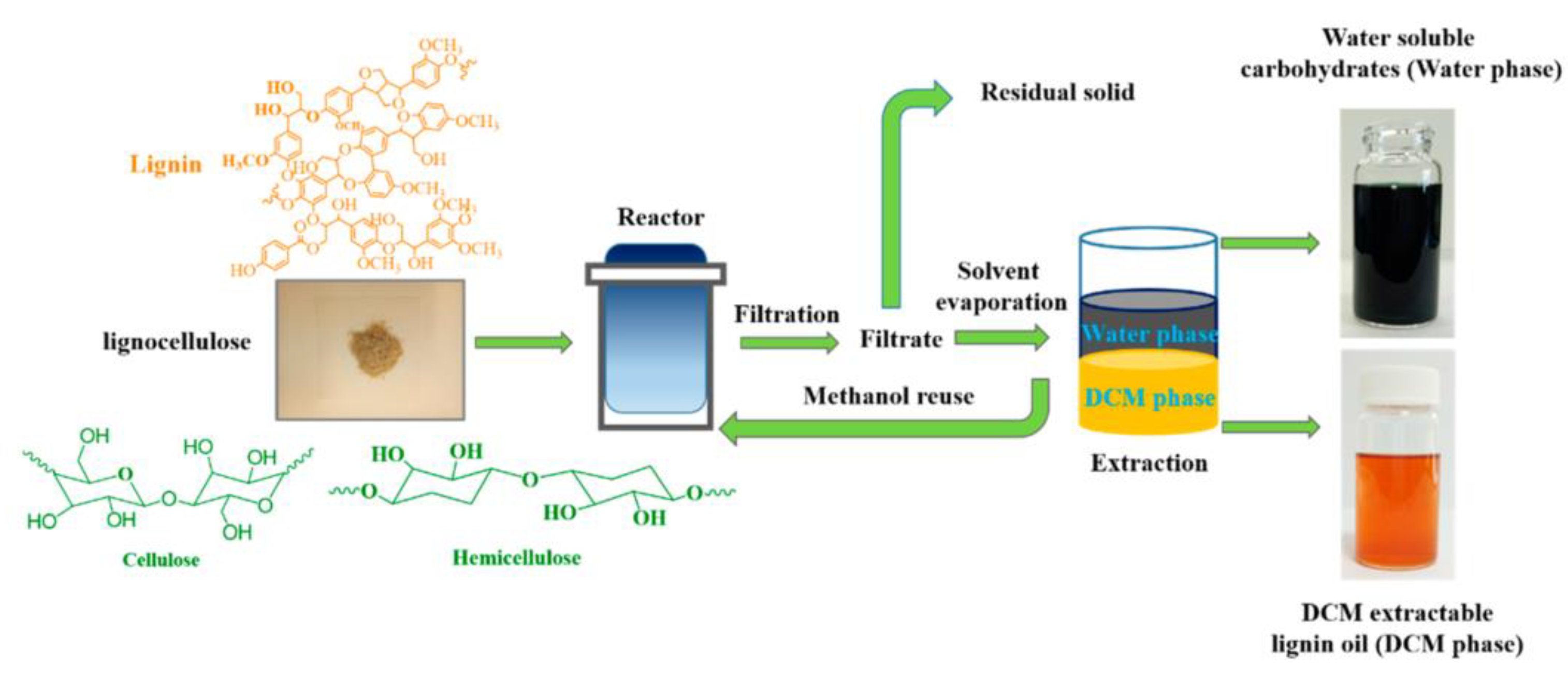
- Du, X.; Tricker, A.W.; Yang, W.; Katahira, R.; Liu, W.; Kwok, T.T.; Gogoi, P.; Deng, Y. Oxidative Catalytic Fractionation and Depolymerization of Lignin in a One-Pot Single-Catalyst System. ACS Sustain. Chem. Eng. 2021, 9, 7719–7727. [Google Scholar] [CrossRef]
- Nayak, A.; Pulidindi, I.N.; Rao, C.S. Novel strategies for glucose production from biomass using heteropoly acid catalyst. Renew. Energy 2020, 159, 215–220. [Google Scholar] [CrossRef]
- Tao, C.; Peng, L.; Zhang, J.; He, L. Al-modified heteropolyacid facilitates alkyl levulinate production from cellulose and lignocellulosic biomass: Kinetics and mechanism studies. Fuel Process. Technol. 2021, 213. [Google Scholar] [CrossRef]
- Yang, H.; Bai, Y.; Ouyang, D.; Wang, F.; Liu, D.; Zhao, X. Coupling biomass pretreatment for enzymatic hydrolysis and direct biomass-to-electricity conversion with molybdovanadophosphoric heteropolyacids as anode electron transfer carriers. J. Energy Chem. 2021, 58, 133–146. [Google Scholar] [CrossRef]
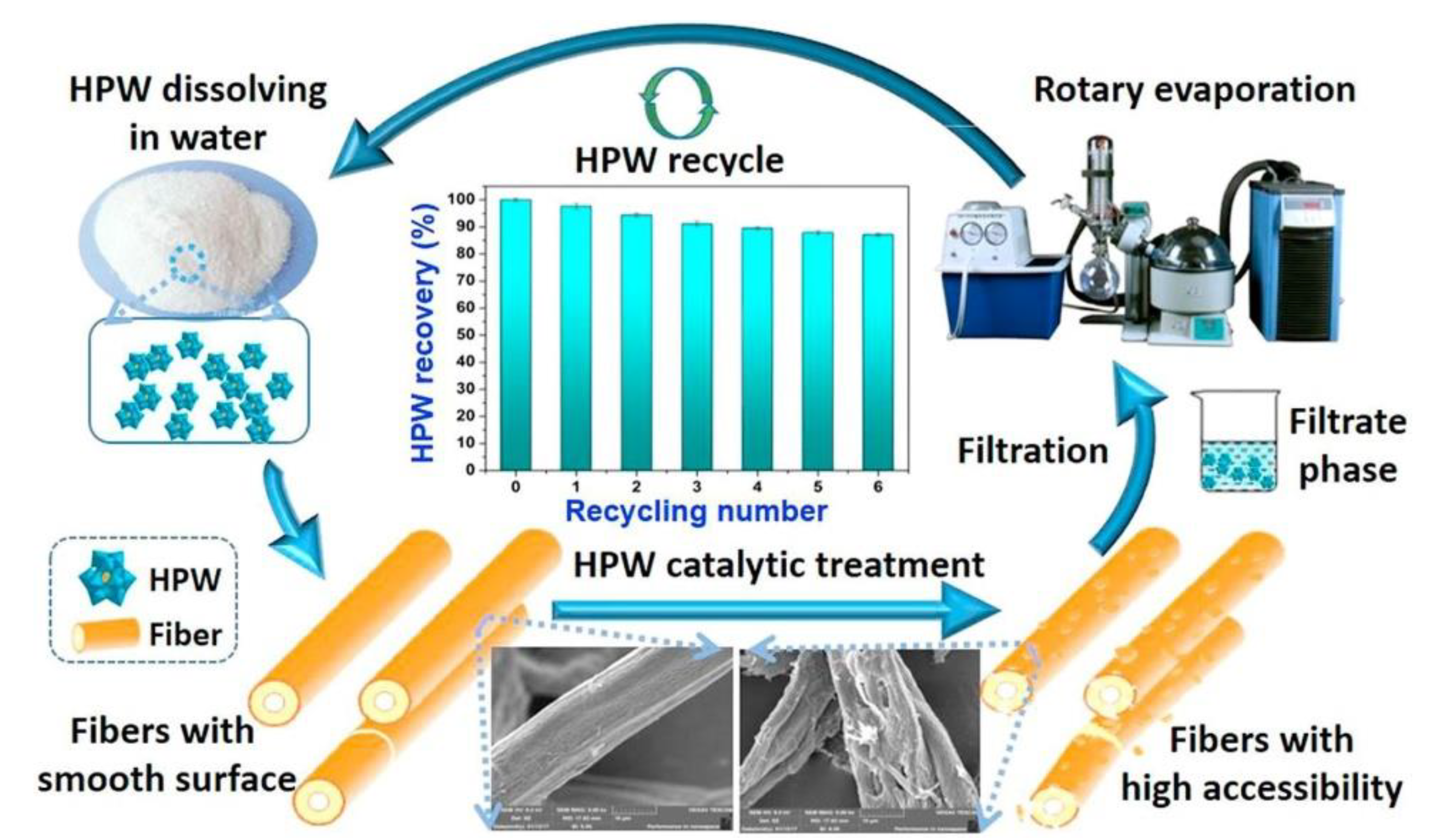
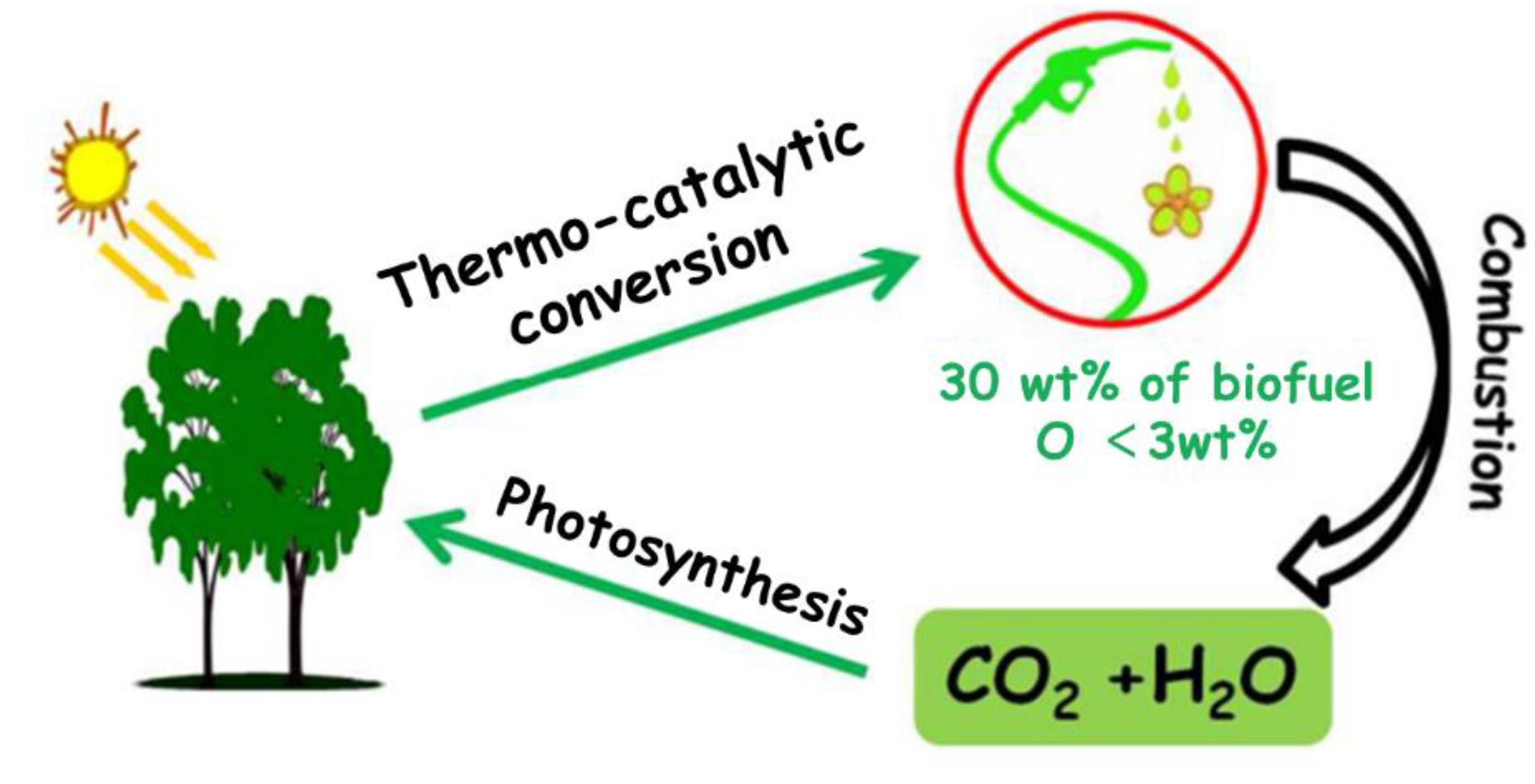
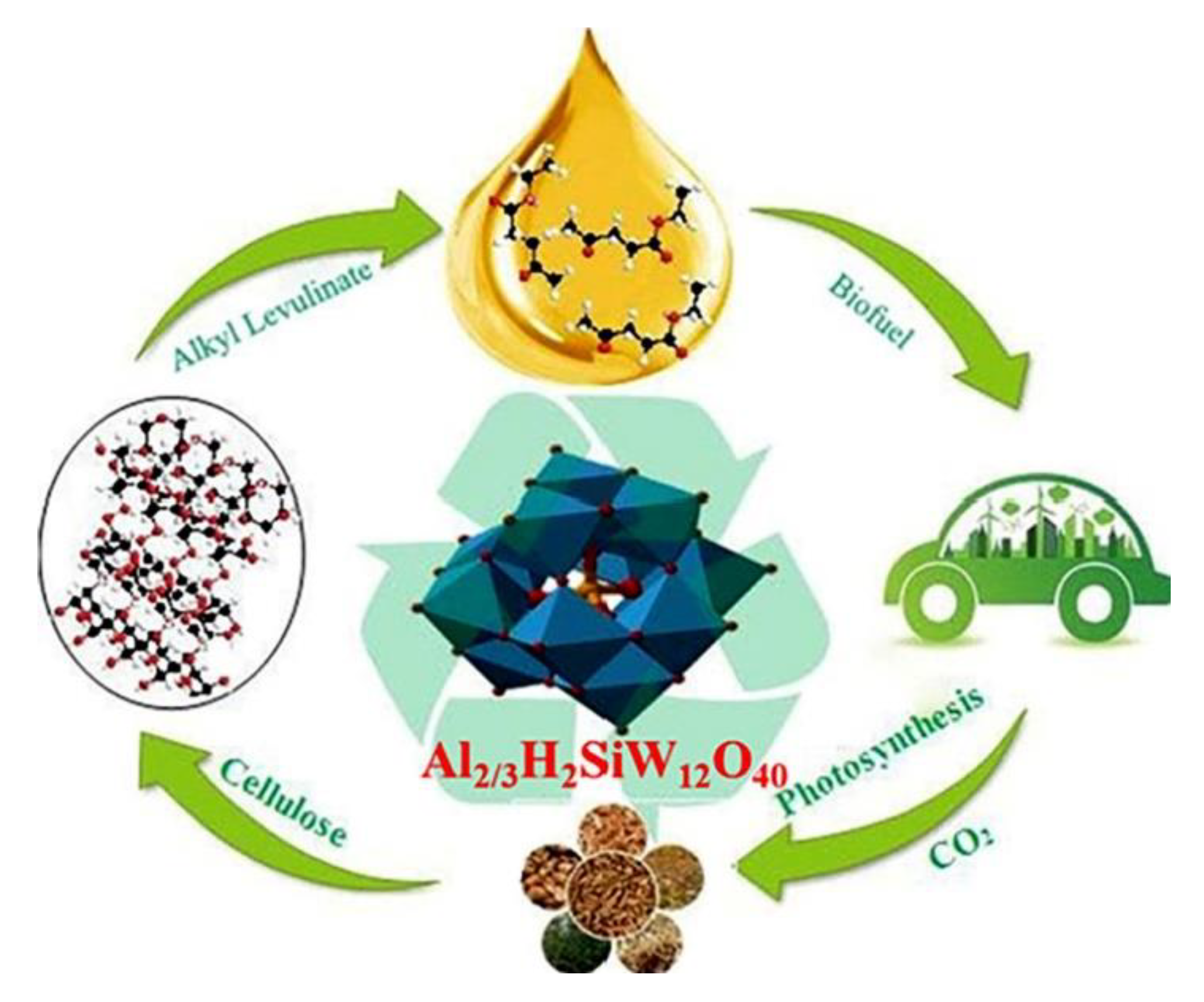

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, H.; Xu, W.; Zhang, D.; Li, X.; Shi, J. Recent Advances in Application of Polyoxometalates in Lignocellulose Pretreatment and Transformation. Polymers 2023, 15, 2401. https://doi.org/10.3390/polym15102401
Deng H, Xu W, Zhang D, Li X, Shi J. Recent Advances in Application of Polyoxometalates in Lignocellulose Pretreatment and Transformation. Polymers. 2023; 15(10):2401. https://doi.org/10.3390/polym15102401
Chicago/Turabian StyleDeng, Haoyu, Wenbiao Xu, Dan Zhang, Xiangyu Li, and Junyou Shi. 2023. "Recent Advances in Application of Polyoxometalates in Lignocellulose Pretreatment and Transformation" Polymers 15, no. 10: 2401. https://doi.org/10.3390/polym15102401
APA StyleDeng, H., Xu, W., Zhang, D., Li, X., & Shi, J. (2023). Recent Advances in Application of Polyoxometalates in Lignocellulose Pretreatment and Transformation. Polymers, 15(10), 2401. https://doi.org/10.3390/polym15102401





