Advances in Polysaccharide Production Based on the Co-Culture of Microbes
Abstract
1. Introduction
2. Classification and Application of Polysaccharides
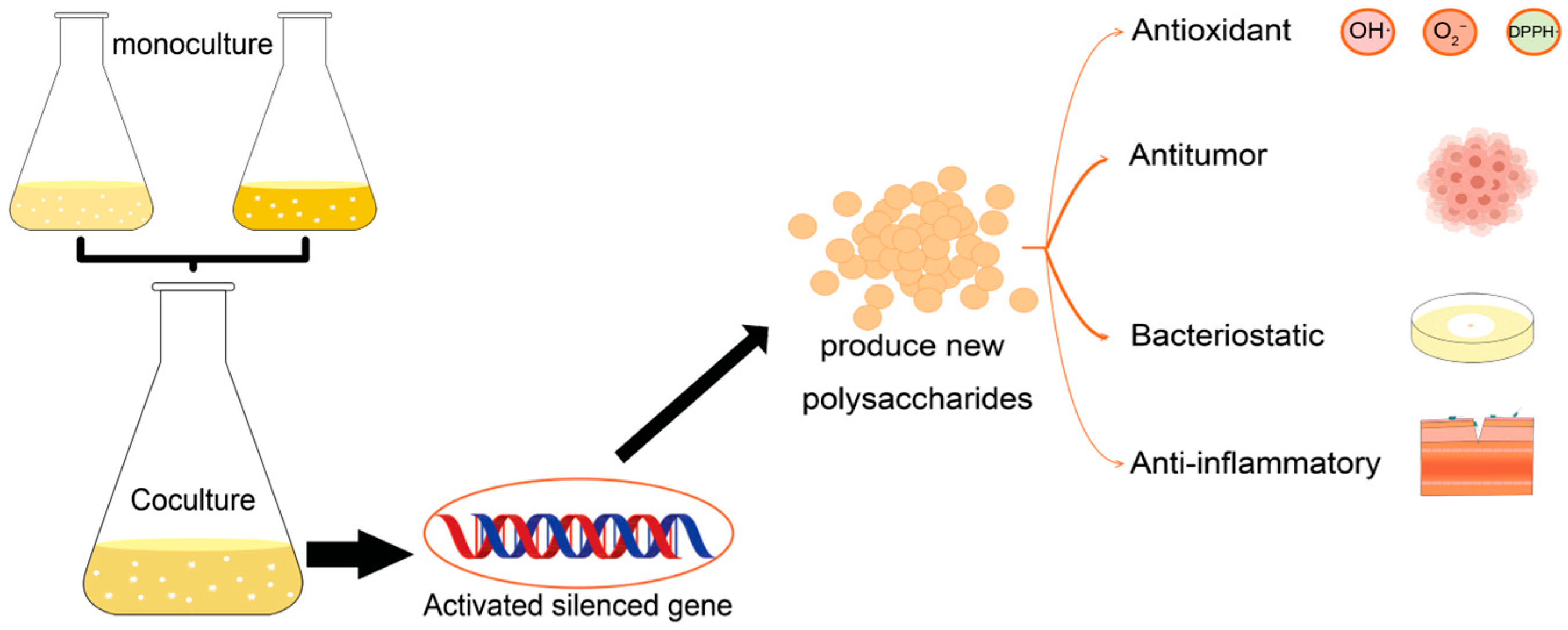
3. Microbial Co-Culture
3.1. Advantages and Applications of Co-Culture
3.2. Metabolites
4. Comparison of Co- and Pure Culture-Produced Polysaccharides
5. Co-Culture-Derived Microbial Polysaccharides
5.1. Polysaccharide Yields
5.2. Antioxidant Activity
5.2.1. DPPH Radical Scavenging Activity
5.2.2. OH∙ Radical Scavenging Activity
5.2.3. O2− Radical Scavenging Activity
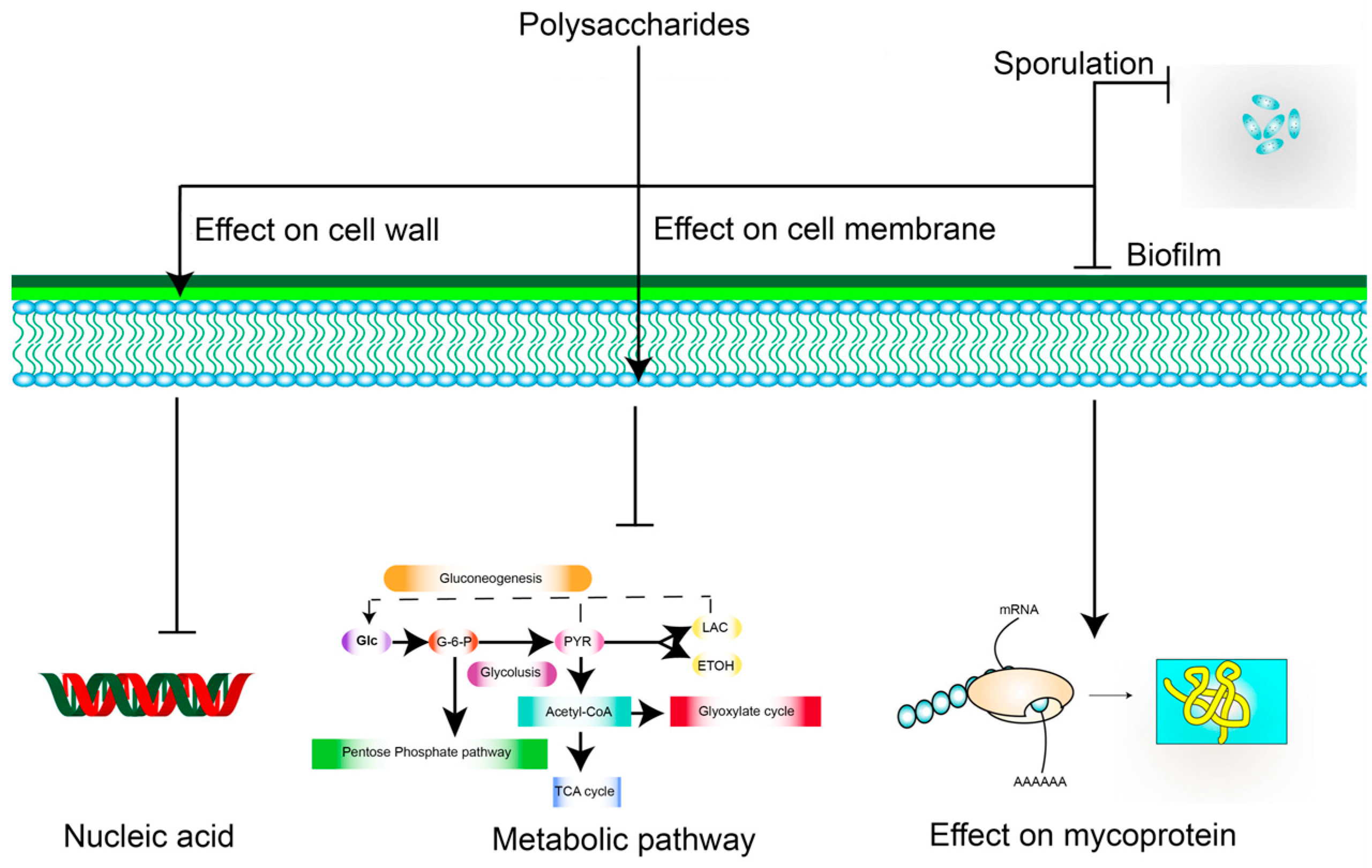
5.3. Antibacterial Activity
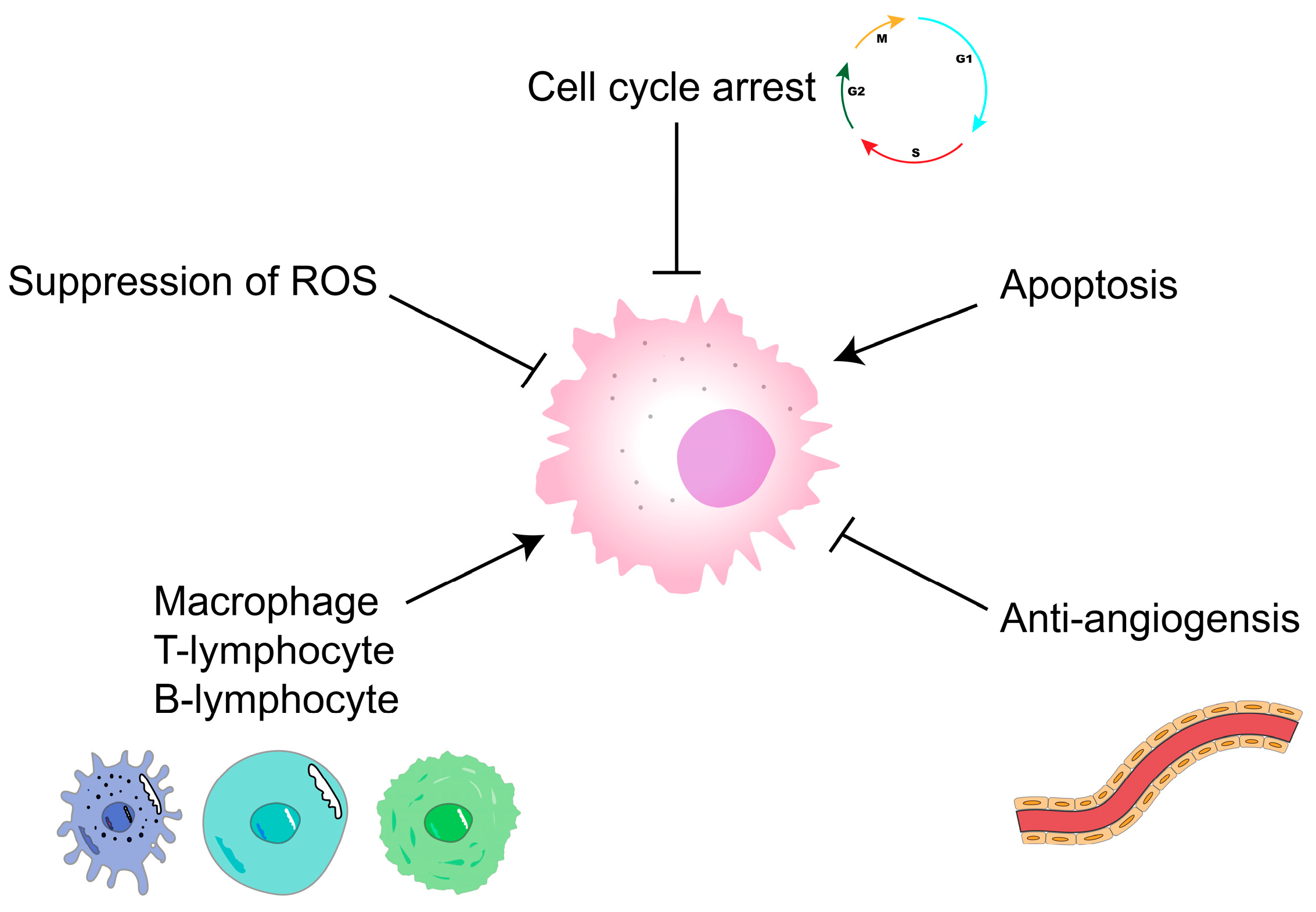
5.4. Antitumor Activity
5.5. Anti-Inflammatory Activity
6. Summary and Prospect
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, X.X.; Yang, J.; Shen, M.Y.; Chen, Y.; Yu, Q.; Xie, J.H. Structure, function and advance application of microwave-treated polysaccharide: A review. Trends Food Sci. Technol. 2022, 123, 198–209. [Google Scholar] [CrossRef]
- Ji, X.L.; Hou, C.Y.; Shi, M.M.; Yan, Y.Z.; Liu, Y.Q. An insight into the research concerning Panax ginseng C. A. Meyer polysaccharides: A review. Food Rev. Int. 2020, 38, 1149–1165. [Google Scholar] [CrossRef]
- Bertsch, A.; Roy, D.; LaPointe, G. Enhanced exopolysaccharide production by Lactobacillus rhamnosus in co-culture with Saccharomyces cerevisiae. Appl. Sci. 2019, 9, 4026. [Google Scholar] [CrossRef]
- Liu, C.; Kakeya, H. Cryptic chemical communication: Secondary metabolic responses revealed by microbial co-culture. Chem.-Asian J. 2020, 15, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Nicault, M.; Zaiter, A.; Dumarcay, S.; Chaimbault, P.; Gelhaye, E.; Leblond, P.; Bontemps, C. Elicitation of antimicrobial active compounds by Streptomyces-fungus co-cultures. Microorganisms 2021, 9, 178. [Google Scholar] [CrossRef]
- Murakami, S.; Hayashi, N.; Inomata, T.; Kato, H.; Hitora, Y.; Tsukamoto, S. Induction of secondary metabolite production by fungal co-culture of Talaromyces pinophilus and Paraphaeosphaeria sp. J. Nat. Med. 2020, 74, 545–549. [Google Scholar] [CrossRef]
- Hoshino, S.; Onaka, H.; Abe, I. Activation of silent biosynthetic pathways and discovery of novel secondary metabolites in actinomycetes by co-culture with mycolic acid-containing bacteria. J. Ind. Microbiol. Biotech. 2019, 46, 363–374. [Google Scholar] [CrossRef]
- Angelis, S.; Novak, A.C.; Sydney, E.B.; Soccol, V.T.; Carvalho, J.C.; Pandey, A.; Noseda, M.D.; Tholozan, J.L.; Lorquin, J.; Soccol, C.R. Co-culture of microalgae, cyanobacteria, and macromycetes for exopolysaccharides production: Process preliminary optimization and partial characterization. Appl. Biochem. Biotech. 2012, 167, 1092–1106. [Google Scholar] [CrossRef]
- Gong, H.X.; Li, W.A.; Sun, J.L.; Jia, L.; Guan, Q.X.; Guo, Y.Y.; Wang, Y.H. A review on plant polysaccharide based on drug delivery system for construction and application, with emphasis on traditional Chinese medicine polysaccharide. Int. J. Biol. Macromol. 2022, 211, 711–728. [Google Scholar] [CrossRef]
- Zhao, W.; Liu, W.; Li, J.; Lin, X.; Wang, Y. Preparation of animal polysaccharides nanofibers by electrospinning and their potential biomedical applications. J. Biomed. Mater. Res. A 2015, 103, 807–818. [Google Scholar] [CrossRef]
- Yildiz, H.; Karatas, N. Microbial exopolysaccharides: Resources and bioactive properties. Process Biochem. 2018, 72, 41–46. [Google Scholar] [CrossRef]
- Sasikumar, K.; Vaikkath, D.K.; Devendra, L.; Nampoothiri, K.M. An exopolysaccharide (EPS) from a Lactobacillus plantarum BR2 with potential benefits for making functional foods. Bioresour. Technol. 2017, 241, 1152–1156. [Google Scholar] [CrossRef] [PubMed]
- Camacho-Chab, J.C.; Castaneda-Chavez, M.D.; Chan-Bacab, M.J.; Aguila-Ramirez, R.N.; Galaviz-Villa, I.; Bartolo-Perez, P.; Lango-Reynoso, F.; Tabasco-Novelo, C.; Gaylarde, C.; Ortega-Morales, B.O. Biosorption of cadmium by non-toxic extracellular polymeric substances (EPS) synthesized by bacteria from marine intertidal biofilms. Int. J. Environ. Res. Public Health 2018, 15, 314. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Rodriguez, A.; Vasto-Anzaldo, X.G.; Perez, D.B.; Vazquez-Garza, E.; Chapoy-Villanueva, H.; Garcia-Rivas, G.; Garza-Cervantes, J.A.; Gomez-Lugo, J.J.; Gomez-Loredo, A.E.; Gonzalez, M.T.G.; et al. Microbial competition of Rhodotorula mucilaginosa UANL-001L and E. coli increase biosynthesis of non-toxic exopolysaccharide with applications as a wide-spectrum antimicrobial. Sci. Rep. 2018, 8, 14. [Google Scholar] [CrossRef]
- Liang, J.J.; Zhang, M.N.; Wang, X.N.; Ren, Y.C.; Yue, T.L.; Wang, Z.L.; Gao, Z.P. Edible fungal polysaccharides, the gut microbiota, and host health. Carbohyd. Polym. 2021, 273, 12. [Google Scholar] [CrossRef]
- Wang, X.Y.; Wang, W.J.; Wang, L.M.; Yu, C.; Zhang, G.L.; Zhu, H.L.; Wang, C.W.; Zhao, S.J.; Hu, C.A.A.; Liu, Y.L. Lentinan modulates intestinal microbiota and enhances barrier integrity in a piglet model challenged with lipopolysaccharide. Food Funct. 2019, 10, 479–489. [Google Scholar] [CrossRef]
- Chen, M.Y.; Xiao, D.; Liu, W.; Song, Y.F.; Zou, B.R.; Li, L.; Li, P.; Cai, Y.; Liu, D.L.; Liao, Q.F.; et al. Intake of Ganoderma lucidum polysaccharides reverses the disturbed gut microbiota and metabolism in type 2 diabetic rats. Int. J. Biol. Macromol. 2020, 155, 890–902. [Google Scholar] [CrossRef]
- Knowles, S.L.; Raja, H.A.; Roberts, C.D.; Oberlies, N.H. Fungal-fungal co-culture: A primer for generating chemical diversity. Nat. Prod. Rep. 2022, 39, 1557–1573. [Google Scholar] [CrossRef]
- Rosero-Chasoy, G.; Rodriguez-Jasso, R.M.; Aguilar, C.N.; Buitron, G.; Chairez, I.; Ruiz, H.A. Microbial co-culturing strategies for the production high value compounds, a reliable framework towards sustainable biorefinery implementation—An overview. Bioresour. Technol. 2021, 321, 11. [Google Scholar] [CrossRef]
- Zhuang, L.; Zhang, H.R. Utilizing cross-species co-cultures for discovery of novel natural products. Curr. Opin. Biotech. 2021, 69, 252–262. [Google Scholar] [CrossRef]
- Kapoore, R.V.; Padmaperuma, G.; Maneein, S.; Vaidyanathan, S. Co-culturing microbial consortia: Approaches for applications in biomanufacturing and bioprocessing. Crit. Rev. Biotech. 2022, 42, 46–72. [Google Scholar] [CrossRef] [PubMed]
- Diender, M.; Olm, I.P.; Sousa, D.Z. Synthetic co-cultures: Novel avenues for bio-based processes. Curr. Opin. Biotech. 2021, 67, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.R.; Wang, X.N. Modular co-culture engineering, a new approach for metabolic engineering. Metab. Eng. 2016, 37, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Akdemir, H.; Liu, Y.X.; Zhuang, L.; Zhang, H.R.; Koffas, M.A.G. Utilization of microbial cocultures for converting mixed substrates to valuable bioproducts. Curr. Opin. Microbiol. 2022, 68, 9. [Google Scholar] [CrossRef]
- Li, Z.H.; Wang, X.N.; Zhang, H.R. Balancing the non-linear rosmarinic acid biosynthetic pathway by modular co-culture engineering. Metab. Eng. 2019, 54, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Lou, H.X. Strategies to diversify natural products for drug discovery. Med. Res. Rev. 2018, 38, 1255–1294. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef]
- Onaka, H.; Mori, Y.; Igarashi, Y.; Furumai, T. Mycolic acid-containing bacteria induce natural-product biosynthesis in Streptomyces species. Appl. Environ. Microbiol. 2011, 77, 400–406. [Google Scholar] [CrossRef]
- Yu, M.L.; Li, Y.X.; Banakar, S.P.; Liu, L.; Shao, C.L.; Li, Z.Y.; Wang, C.Y. New metabolites from the co-culture of marine-derived actinomycete Streptomyces rochei MB037 and fungus Rhinocladiella similis 35. Front. Microbiol. 2019, 10, 11. [Google Scholar] [CrossRef]
- Li, F.L.; Yan, S.; Huang, Z.Y.; Gao, W.X.; Zhang, S.T.; Mo, S.Y.; Lin, S.; Wang, J.P.; Hu, Z.X.; Zhang, Y.H. Inducing new bioactive metabolites production from coculture of Pestalotiopsis sp. and Penicillium bialowiezense. Bioorg. Chem. 2021, 110, 9. [Google Scholar] [CrossRef]
- Kurata, S.; Yamada, K.; Takatsu, K.; Hanada, S.; Koyama, O.; Yokomaku, T.; Kamagata, Y.; Kanagawa, T.; Kurane, R. Extracellular acidic polysaccharide production by a two-membered bacterial coculture. Biosci. Biotech. Biochem. 2003, 67, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, Z.; Wang, Y.P.; Anjum, N.; Ahmad, H.; Ahmad, A.; Raza, M. Characterization of new exopolysaccharides produced by coculturing of L. kefiranofaciens with yoghurt strains. Int. J. Biol. Macromol. 2013, 59, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Zhu, L.P.; Xu, X.Y.; Tan, L.L.; Sadilek, M.; Fan, H.; Hu, B.; Shen, X.T.; Yang, J.; Qiao, B.; et al. Discovery of novel xylosides in co-culture of basidiomycetes Trametes versicolor and Ganoderma applanatum by integrated metabolomics and bioinformatics. Sci. Rep. 2015, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Liu, S. Research on Co-Fermentation of Fungal Polysaccharides by Sanghuangporus lonicericola and Cordyceps militaris; Central South University of Forestry and Technology: Changsha, China, 2019. [Google Scholar]
- Yin, H. Study on Extraction, Determination of Content and Antioxygenic Activity of Mixed Fermentation Amylase of Ganoderma lucidum; Tricholoma matsutake Fruitbodies and Aweto; Jilin University: Changchun, China, 2008. [Google Scholar]
- Suo, M.Y. Study on the Co-Fermentation of Ganoderma lucidum and Saccharomyces boulardii and Its Effect on Polysaccharides Yield and Activity; Tianjin University of Science and Technology: Tianjin, China, 2020. [Google Scholar]
- Wang, Y.Z. The Comparison of Fermentation, Co-Fermentation and Polysaccharides Activities of Marasmius androsaceus and Cordyceps militaris; Jilin University: Changchun, China, 2007. [Google Scholar]
- Wu, J.Y.; Kaewnarin, K.; Nie, X.M.; Li, Q.B.; He, N.; Huang, J.L.; Geng, A.L. Biological activities of a polysaccharide from the coculture of Ganoderma lucidum and Flammulina velutipes mycelia in submerged fermentation. Process Biochem. 2021, 109, 10–18. [Google Scholar] [CrossRef]
- Bertrand, S.; Bohni, N.; Schnee, S.; Schumpp, O.; Gindro, K.; Wolfender, J.L. Metabolite induction via microorganism co-culture: A potential way to enhance chemical diversity for drug discovery. Biotechnol. Adv. 2014, 32, 1180–1204. [Google Scholar] [CrossRef]
- Ma, X.; Yang, M.; He, Y.; Zhai, C.T.; Li, C.L. A review on the production, structure, bioactivities and applications of Tremella polysaccharides. Int. J. Immunopath. Pharm. 2021, 35, 14. [Google Scholar] [CrossRef]
- Sun, L.; Yang, Y.B.; Wang, R.; Li, S.; Qiu, Y.B.; Lei, P.; Gao, J.; Xu, H.; Zhang, F.L.; Lv, Y.F. Effects of exopolysaccharide derived from Pantoea alhagi NX-11 on drought resistance of rice and its efficient fermentation preparation. Int. J. Biol. Macromol. 2020, 162, 946–955. [Google Scholar] [CrossRef]
- Xue, C.Z.; Wang, L.B.; Wu, T.; Zhang, S.P.; Tang, T.; Wang, L.; Zhao, Q.Y.; Sun, Y.H. Characterization of co-cultivation of Cyanobacteria on growth, productions of polysaccharides and extracellular proteins, nitrogenase activity, and photosynthetic activity. Appl. Biochem. Biotech. 2017, 181, 340–349. [Google Scholar] [CrossRef]
- Wang, M.Y.; Zhao, Z.Z.; Zhou, X.; Hu, J.R.; Xue, J.; Liu, X.; Zhang, J.S.; Liu, P.; Tong, S.S. Simultaneous use of stimulatory agents to enhance the production and hypoglycaemic activity of polysaccharides from Inonotus obliquus by submerged fermentation. Molecules 2019, 24, 4400. [Google Scholar] [CrossRef]
- Wang, X.Y.Z.; Dong, J.J.; Xu, G.C.; Han, R.Z.; Ni, Y. Enhanced curdlan production with nitrogen feeding during polysaccharide synthesis by Rhizobium radiobacter. Carbohydr. Polym. 2016, 150, 385–391. [Google Scholar] [CrossRef]
- Peng, R.; Fu, Y.Z.; Zou, J.; Qiu, H.; Gan, L.T.; Yi, H.L.; Yao, R.Y.; Luo, X.Y. Improvement of polysaccharide and triterpenoid production of Ganoderma lucidum through mutagenesis of protoplasts. Biotechnol. Biotechnol. Equip. 2016, 30, 381–387. [Google Scholar] [CrossRef]
- Wang, Y.F.; Yang, X.; Chen, P.; Yang, S.L.; Zhang, H. Homologous overexpression of genes in Cordyceps militaris improves the production of polysaccharides. Food Res. Int. 2021, 147, 8. [Google Scholar] [CrossRef] [PubMed]
- Han, P.P.; Shen, S.G.; Wang, H.Y.; Yao, S.Y.; Tan, Z.L.; Zhong, C.; Jia, S.R. Applying the strategy of light environment control to improve the biomass and polysaccharide production of Nostoc flagelliforme. J. Appl. Phycol. 2017, 29, 55–65. [Google Scholar] [CrossRef]
- Tong, L.L.; Wang, Y.; Yuan, L.; Liu, M.Z.; Du, Y.H.; Mu, X.Y.; Yang, Q.H.; Wei, S.X.; Li, J.Y.; Wang, M.A.; et al. Enhancement of polysaccharides production using microparticle enhanced technology by Paraisaria dubia. Microb. Cell. Fact. 2022, 21, 14. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Wu, J.Y.; Chang, C.Y.; Yu, S.T.; Liu, Y.C. Enhanced exopolysaccharide production by Cordyceps militaris using repeated batch cultivation. J. Biosci. Bioeng. 2019, 127, 499–505. [Google Scholar] [CrossRef]
- Lin, S.; Liu, Z.Q.; Baker, P.J.; Yi, M.; Wu, H.; Xu, F.; Teng, Y.; Zheng, Y.G. Enhancement of cordyceps polysaccharide production via biosynthetic pathway analysis in Hirsutella sinensis. Int. J. Biol. Macromol. 2016, 92, 872–880. [Google Scholar] [CrossRef]
- Fan, L.P.; Li, J.W.; Deng, K.Q.; Ai, L.Z. Effects of drying methods on the antioxidant activities of polysaccharides extracted from Ganoderma lucidum. Carbohydr. Polym. 2012, 87, 1849–1854. [Google Scholar] [CrossRef]
- Chang, S.H.; Wu, C.H.; Tsai, G.J. Effects of chitosan molecular weight on its antioxidant and antimutagenic properties. Carbohydr. Polym. 2018, 181, 1026–1032. [Google Scholar] [CrossRef]
- Redouan, E.; Emmanuel, P.; Michelle, P.; Bernard, C.; Josiane, C.; Cedric, D. Evaluation of antioxidant capacity of ulvan-like polymer obtained by regioselective oxidation of gellan exopolysaccharide. Food Chem. 2011, 127, 976–983. [Google Scholar] [CrossRef]
- Lo, T.C.T.; Chang, C.A.; Chiu, K.H.; Tsay, P.K.; Jen, J.F. Correlation evaluation of antioxidant properties on the monosaccharide components and glycosyl linkages of polysaccharide with different measuring methods. Carbohydr. Polym. 2011, 86, 320–327. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, G.L. The antioxidant activities of carboxymethylated cushaw polysaccharide. Int. J. Biol. Macromol. 2019, 121, 666–670. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Huang, G.L. Synthesis and antioxidant activities of garlic polysaccharide-Fe(III) complex. Int. J. Biol. Macromol. 2020, 145, 813–818. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.Y.; Sun, J.S.; Xu, Z.Y.; Li, H.C.; Hu, J.; Ning, H.J.; Qin, Z.F.; Pei, H.S.; Sun, T.; Zhang, X.Q. Effect of heat stress on production and in-vitro antioxidant activity of polysaccharides in Ganoderma lucidum. Bioprocess. Biosyst. Eng. 2018, 41, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.S.; Chen, J.R.; Ren, P.F.; Zhang, Y.W.; Onayango, S.O. Ultrasound irradiation alters the spatial structure and improves the antioxidant activity of the yellow tea polysaccharide. Ultrason. Sonochem. 2021, 70, 11. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Xu, L.L.; Zhou, Q.W.; Hao, S.X.; Zhou, T.; Xie, H.J. Enhanced in vitro antioxidant activity of polysaccharides from Enteromorpha Prolifera by enzymatic degradation. J. Food Biochem. 2016, 40, 275–283. [Google Scholar] [CrossRef]
- Li, S.Q.; Dai, S.H.; Shah, N.P. Sulfonation and antioxidative evaluation of polysaccharides from Pleurotus mushroom and Streptococcus thermophilus bacteria: A review. Compr. Rev. Food. Sci. Food Saf. 2017, 16, 282–294. [Google Scholar] [CrossRef]
- Mirzadeh, M.; Arianejad, M.R.; Khedmat, L. Antioxidant, antiradical, and antimicrobial activities of polysaccharides obtained by microwave-assisted extraction method: A review. Carbohydr. Polym. 2020, 229, 12. [Google Scholar] [CrossRef]
- Amiri, S.; Mokarram, R.R.; Khiabani, M.S.; Bari, M.R.; Khaledabad, M.A. Exopolysaccharides production by Lactobacillus acidophilus LA5 and Bifidobacterium animalis subsp. lactis BB12: Optimization of fermentation variables and characterization of structure and bioactivities. Int. J. Biol. Macromol. 2019, 123, 752–765. [Google Scholar] [CrossRef]
- Halliwell, B. Commentary for “oxygen free radicals and iron in relation to biology and medicine: Some problems and concepts”. Arch. Biochem. Biophys. 2022, 718, 2. [Google Scholar] [CrossRef]
- Mu, S.; Yang, W.J.; Huang, G.L. Antioxidant activities and mechanisms of polysaccharides. Chem. Biol. Drug Des. 2021, 97, 628–632. [Google Scholar] [CrossRef]
- Lyu, Y.; Wang, M.; Zhang, Y.; Zhang, X.; Liu, X.; Li, F.; Wang, D.; Wei, M.; Yu, X. Antioxidant properties of water-soluble polysaccharides prepared by co-culture fermentation of straw and shrimp shell. Front. Nutr. 2022, 9, 1047932. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.G.; Dang, J.; Wang, Q.L.; Yu, M.F.; Jiang, L.; Mei, L.J.; Shao, Y.; Tao, Y.D. Optimization of polysaccharides from Lycium ruthenicum fruit using RSM and its anti-oxidant activity. Int. J. Biol. Macromol. 2013, 61, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Jiang, J.; Li, W. Co-cultured Lepista sordida and Pholiota nameko polysaccharide-iron(iii) chelates exhibit good antioxidant activity. RSC Adv. 2020, 10, 27259–27265. [Google Scholar] [CrossRef]
- Mousavi, S.A.; Ghotaslou, R.; Kordi, S.; Khoramdel, A.; Aeenfar, A.; Kahjough, S.T.; Akbarzadeh, A. Antibacterial and antifungal effects of chitosan nanoparticles on tissue conditioners of complete dentures. Int. J. Biol. Macromol. 2018, 118, 881–885. [Google Scholar] [CrossRef]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef]
- Zhang, X.F.; Guo, Y.J.; Guo, L.Y.; Jiang, H.; Ji, Q.H. In vitro evaluation of antioxidant and antimicrobial activities of Melaleuca alternifolia essential oil. BioMed Res. Int. 2018, 2018, 8. [Google Scholar] [CrossRef]
- Mazarei, F.; Jooyandeh, H.; Noshad, M.; Hojjati, M. Polysaccharide of caper (Capparis spinosa L.) leaf: Extraction optimization, antioxidant potential and antimicrobial activity. Int. J. Biol. Macromol. 2017, 95, 224–231. [Google Scholar] [CrossRef]
- Ardean, C.; Davidescu, C.M.; Nemes, N.S.; Negrea, A.; Ciopec, M.; Duteanu, N.; Negrea, P.; Duda-Seiman, D.; Musta, V. Factors influencing the antibacterial activity of chitosan and chitosan modified by functionalization. Int. J. Mol. Sci. 2021, 22, 7449. [Google Scholar] [CrossRef]
- He, F.; Yang, Y.; Yang, G.; Yu, L. Studies on antibacterial activity and antibacterial mechanism of a novel polysaccharide from Streptomyces virginia H03. Food Control. 2010, 21, 1257–1262. [Google Scholar] [CrossRef]
- Sonenberg, N.; Hinnebusch, A.G. Regulation of translation initiation in eukaryotes: Mechanisms and biological targets. Cell 2009, 136, 731–745. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, Y.T.; Zheng, W.; Han, X.X.; Jiang, Y.H.; Hu, P.L.; Tang, Z.X.; Shi, L.E. The antibacterial activity and antibacterial mechanism of a polysaccharide from Cordyceps cicadae. J. Funct. Food. 2017, 38, 273–279. [Google Scholar] [CrossRef]
- Liang, R.; Li, X.Y.; Yuan, W.L.; Jin, S.Y.; Hou, S.T.; Wang, M.; Wang, H.Z. Antifungal activity of nanochitin whisker against crown rot diseases of wheat. J. Agric. Food Chem. 2018, 66, 9907–9913. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.C.; Sun, Q.; Zhang, H.R.; Wang, J.Q.; Fu, Q.Z.; Qiao, H.Z.; Wang, Q. Insight into antibacterial mechanism of polysaccharides: A review. LWT-Food Sci. Technol. 2021, 150, 6. [Google Scholar] [CrossRef]
- Chen, J.Y.; Ma, X.T.; Edgar, K.J. A versatile method for preparing polysaccharide conjugates via thiol-michael addition. Polymers 2021, 13, 1905. [Google Scholar] [CrossRef] [PubMed]
- Li, X.L.; Thakur, K.; Zhang, Y.Y.; Tu, X.F.; Zhang, Y.S.; Zhu, D.Y.; Zhang, J.G.; Wei, Z.J. Effects of different chemical modifications on the antibacterial activities of polysaccharides sequentially extracted from peony seed dreg. Int. J. Biol. Macromol. 2018, 116, 664–675. [Google Scholar] [CrossRef] [PubMed]
- Wang, L. Biological Activity of Co-Culture Polysaccharides from Lepista sordida and Pholiota nameko and Their Applications; Qingdao Agricultural University: Qingdao, China, 2018. [Google Scholar]
- Cor, D.; Knez, Z.; Hrncic, M.K. Antitumour, antimicrobial, antioxidant and antiacetylcholinesterase effect of Ganoderma lucidum terpenoids and polysaccharides: A review. Molecules 2018, 23, 649. [Google Scholar] [CrossRef]
- Chen, L.; Huang, G.L. Antitumor activity of polysaccharides: An overview. Curr. Drug Targets 2018, 19, 89–96. [Google Scholar] [CrossRef]
- Habtemariam, S. Trametes versicolor (synn. Coriolus versicolor) polysaccharides in cancer therapy: Targets and efficacy. Biomedicines 2020, 8, 135. [Google Scholar] [CrossRef]
- Hsu, H.Y.; Hwang, P.A. Clinical applications of fucoidan in translational medicine for adjuvant cancer therapy. Clin. Transl. Med. 2019, 8, 18. [Google Scholar] [CrossRef]
- Li, F.P.; Liu, H.Z. A potential adjuvant agent of chemotherapy: Sepia ink polysaccharides. Mar. Drugs 2018, 16, 106. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Chen, M.; Ding, Y.Y.; Yang, P.Y.; Wang, M.J.; Zhang, H.H.; He, Y.Q.; Ma, H.L. Polysaccharides as potential anti-tumor biomacromolecules -A review. Front. Nutr. 2022, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, P.; Jeya, M.; Moon, H.J.; Lee, K.M.; Kim, I.W.; Kim, J.H.; Lee, J.K. Antitumor activity of methylan polysaccharide derivatives. Biotechnol. Lett. 2010, 32, 891–895. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.L.; Huang, H.L. The derivatization and antitumor mechanisms of polysaccharides. Future Med. Chem. 2017, 9, 1931–1938. [Google Scholar] [CrossRef]
- Zhu, J.; Xu, J.; Jiang, L.L.; Huang, J.Q.; Yan, J.Y.; Chen, Y.W.; Yang, Q. Improved antitumor activity of cisplatin combined with Ganoderma lucidum polysaccharides in U14 cervical carcinoma-bearing mice. Kaohsiung J. Med. Sci. 2019, 35, 222–229. [Google Scholar] [CrossRef]
- Gui, Y.; Cheng, J.; Chen, Z.G. Oridonin improves the therapeutic effect of lentinan on lung cancer. Exp. Ther. Med. 2021, 22, 9. [Google Scholar] [CrossRef]
- Bai, C.; Wang, S.Z.; Yang, J.F.; Li, Y. The comparison of the effect of anti-tumor with G-lucidum and T-mastutake co-fermentation exopolysaccharide. Microbiol. China 2004, 31, 76–80. [Google Scholar]
- Li, T.T. The Investigation of Tricholoma matsutake and Cordyceps militaris Co-Cultivating and the Activities of Polysaccharides; Jilin University: Changchun, China, 2008. [Google Scholar]
- Wen, Z.S.; Xiang, X.W.; Jin, H.X.; Guo, X.Y.; Liu, L.J.; Huang, Y.N.; OuYang, X.K.; Qu, Y.L. Composition and anti-inflammatory effect of polysaccharides from Sargassum horneri in RAW264.7 macrophages. Int. J. Biol. Macromol. 2016, 88, 403–413. [Google Scholar] [CrossRef]
- Bai, R.R.; Yao, C.S.; Zhong, Z.C.; Ge, J.M.; Bai, Z.Q.; Ye, X.Y.; Xie, T.; Xie, Y.Y. Discovery of natural anti-inflammatory alkaloids: Potential leads for the drug discovery for the treatment of inflammation. Eur. J. Med. Chem. 2021, 213, 22. [Google Scholar] [CrossRef]
- Yuan, D.; Li, C.; Huang, Q.; Fu, X.; Dong, H. Current advances in the anti-inflammatory effects and mechanisms of natural polysaccharides. Crit. Rev. Food Sci. Nutr. 2022, 21. [Google Scholar] [CrossRef]
- Cui, C.; Chen, S.; Wang, X.Y.; Yuan, G.W.; Jiang, F.; Chen, X.Y.; Wang, L. Characterization of Moringa oleifera roots polysaccharide MRP-1 with anti-inflammatory effect. Int. J. Biol. Macromol. 2019, 132, 844–851. [Google Scholar] [CrossRef] [PubMed]
| No. | Types of Polysaccharides | Source | Method | Reference |
|---|---|---|---|---|
| 1 | Curdlan | Rhizobium radiobacter | Add nitrogen source | [44] |
| 2 | Ganoderma lucidum polysaccharides | Ganoderma lucidum | Protoplast mutation | [45] |
| 3 | Cordyceps polysaccharides | Cordyceps militaris | Co-overexpression | [46] |
| 4 | Cyanobacterium Nostoc flagelliforme EPSs | Cyanobacterium Nostoc flagelliforme | Light environment control | [47] |
| 5 | Inonotus obliquus EPSs | Inonotus obliquus | Stimulatory agents | [43] |
| 6 | Paraisaria dubia polysaccharides | Paraisaria dubia | Morphological induction | [48] |
| 7 | Cordyceps militaris EPSs | Cordyceps militaris | Repeated batch approach | [49] |
| 8 | Cordyceps polysaccharide | Hirsutella sinensis | Biosynthetic pathway | [50] |
| No. | Source | Method | Action Targets | Reference |
|---|---|---|---|---|
| 1 | Momordica charantia | Carboxymethylated | Scavenging O2− and OH∙ radicals | [55] |
| 2 | Allium sativum | Garlic polysaccharide-Fe (III) complex | Scavenging O2− | [56] |
| 3 | Ganoderma lucidum | Heat stress | Scavenging OH∙ radicals, DPPH, and ferric reducing antioxidant power | [57] |
| 4 | Phellinus igniarius | Ultrasound | Scavenging OH∙ radicals ABTS radicals, and ferric reducing ability | [58] |
| 5 | Enteromorpha prolifera | Enzymatic degradation | Scavenging OH∙, DPPH, and O2− radicals | [59] |
| 6 | Pleurotus and Streptococcus thermophilus | Sulfonation | Scavenging OH∙ radicals, ABTS, DPPH, and O2− | [60] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, W.; Guo, X.; Xu, X.; Zou, D.; Zou, H.; Yang, X. Advances in Polysaccharide Production Based on the Co-Culture of Microbes. Polymers 2023, 15, 2847. https://doi.org/10.3390/polym15132847
Peng W, Guo X, Xu X, Zou D, Zou H, Yang X. Advances in Polysaccharide Production Based on the Co-Culture of Microbes. Polymers. 2023; 15(13):2847. https://doi.org/10.3390/polym15132847
Chicago/Turabian StylePeng, Wanrong, Xueying Guo, Xinyi Xu, Dan Zou, Hang Zou, and Xingyong Yang. 2023. "Advances in Polysaccharide Production Based on the Co-Culture of Microbes" Polymers 15, no. 13: 2847. https://doi.org/10.3390/polym15132847
APA StylePeng, W., Guo, X., Xu, X., Zou, D., Zou, H., & Yang, X. (2023). Advances in Polysaccharide Production Based on the Co-Culture of Microbes. Polymers, 15(13), 2847. https://doi.org/10.3390/polym15132847







