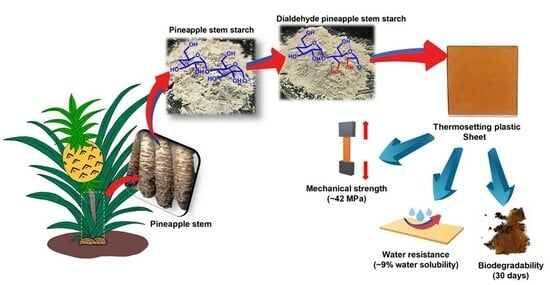
Development of Biodegradable Thermosetting Plastic Using Dialdehyde Pineapple Stem Starch
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Dialdehyde Pineapple Stem Starch
2.3. Determination of Aldehyde Content of DPSS
2.4. Preparation of Starch-Based Thermosetting Plastic
2.5. Characterization of Modified Starch and Plastic
2.5.1. Fourier Transform Infrared (FTIR) Spectroscopy
2.5.2. Proton Nuclear Magnetic Resonance (1H-NMR) Spectroscopy
2.5.3. Determination of Intrinsic Viscosity
2.5.4. X-ray Diffractometry (XRD)
2.5.5. Mechanical Properties
2.5.6. Morphology
2.5.7. Water Absorption and Solubility
2.5.8. Soil Burial Degradation Test
2.5.9. Statistical Analysis
3. Results
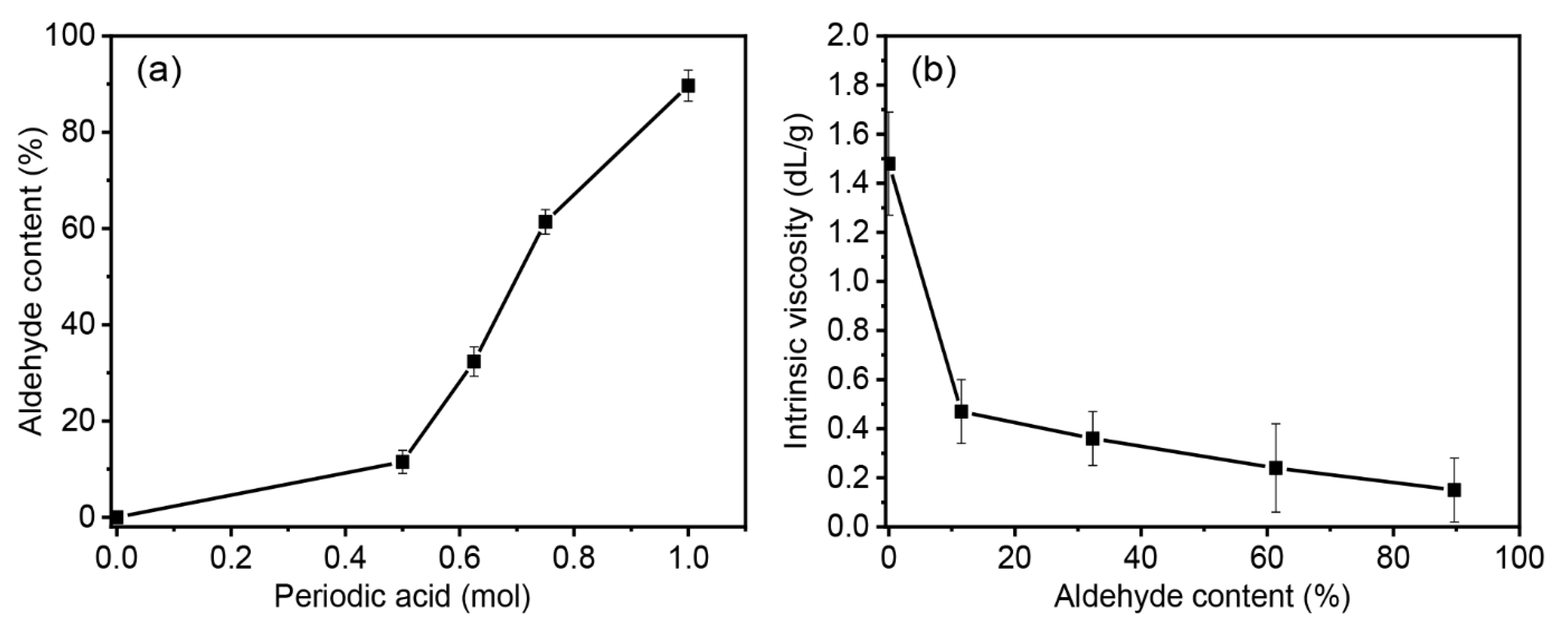
3.1. Aldehyde Contents and Intrinsic Viscosity of DPSS
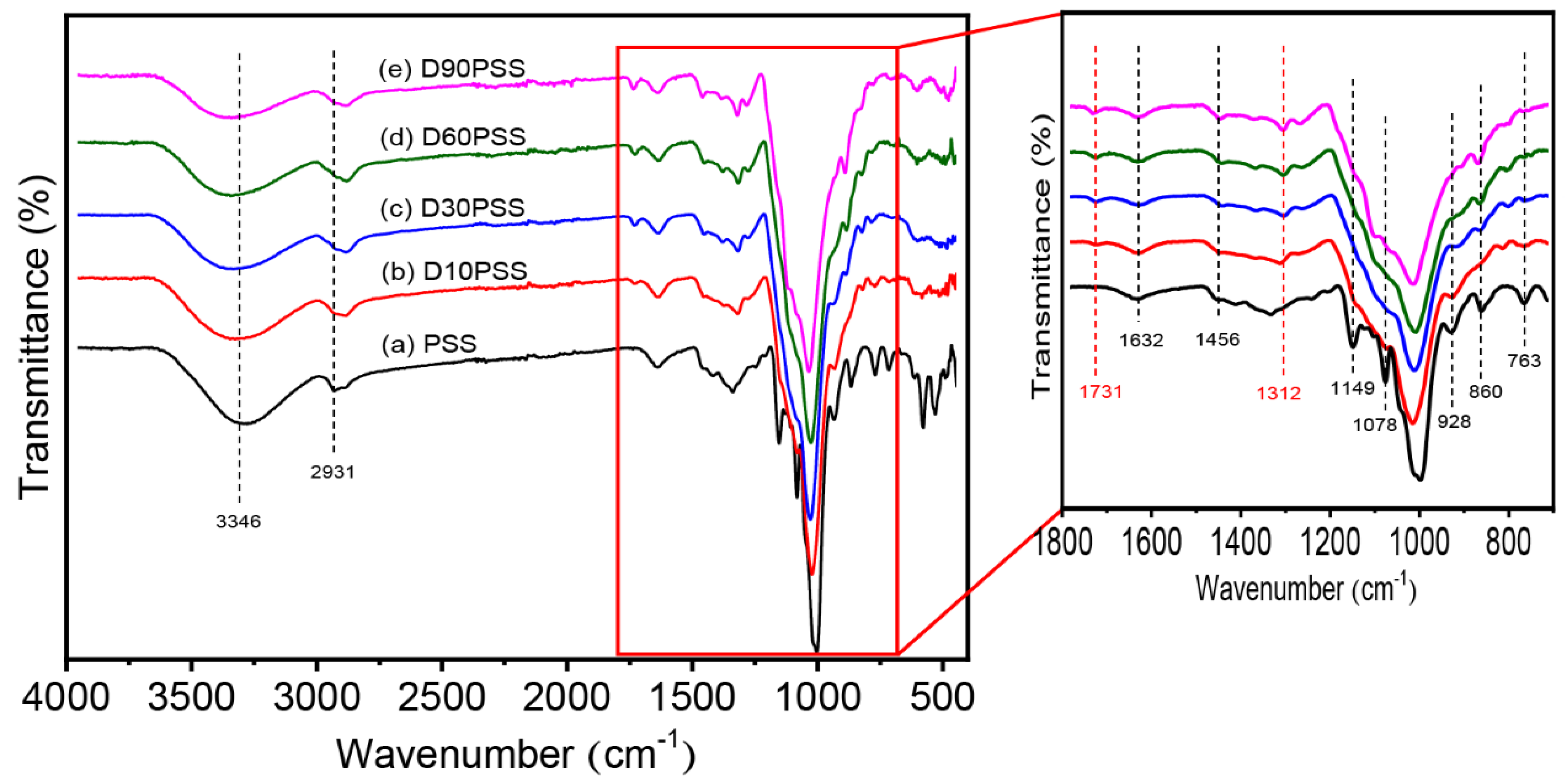
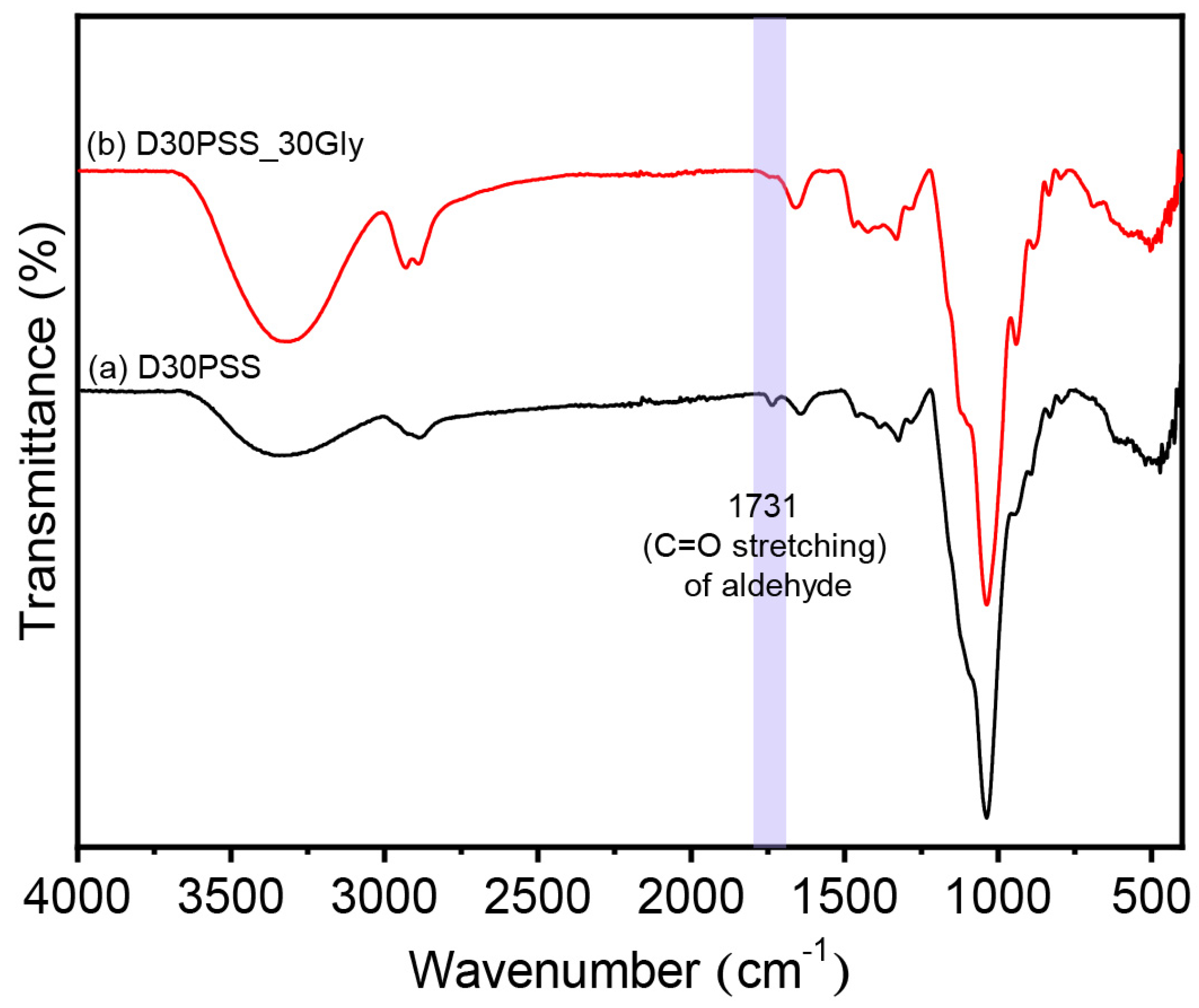
3.2. Chemical Structure of DPSS
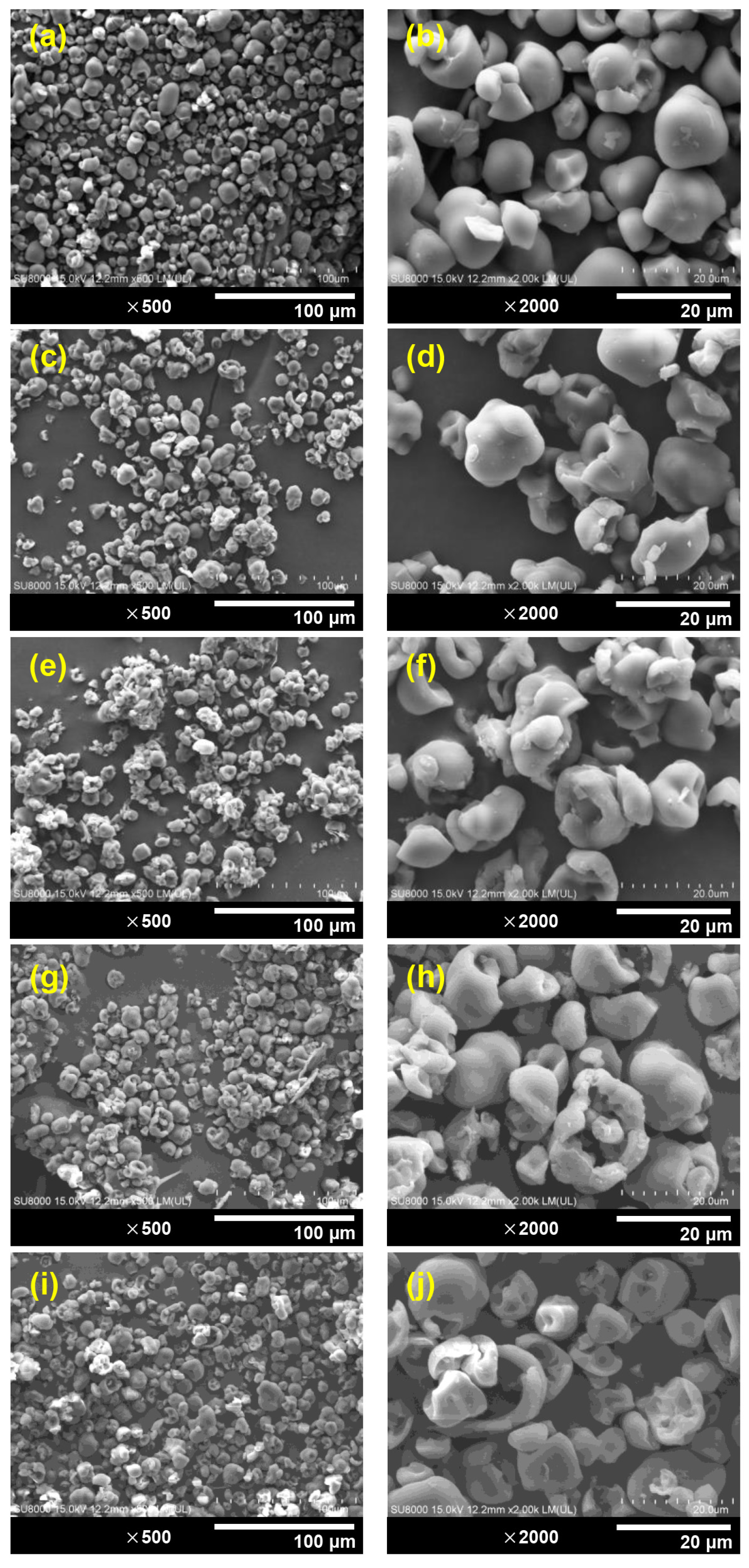
3.3. Morphological Aspects
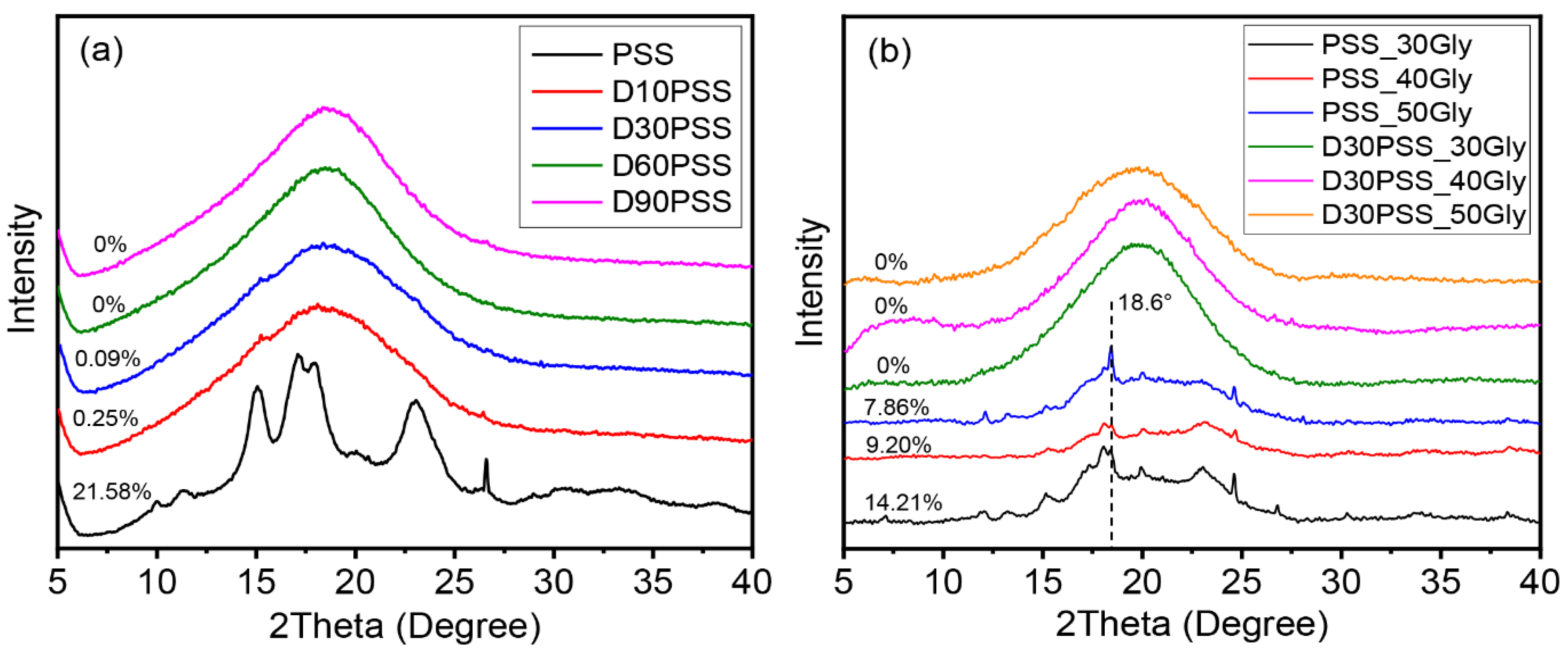
3.4. X-ray Diffraction (XRD)
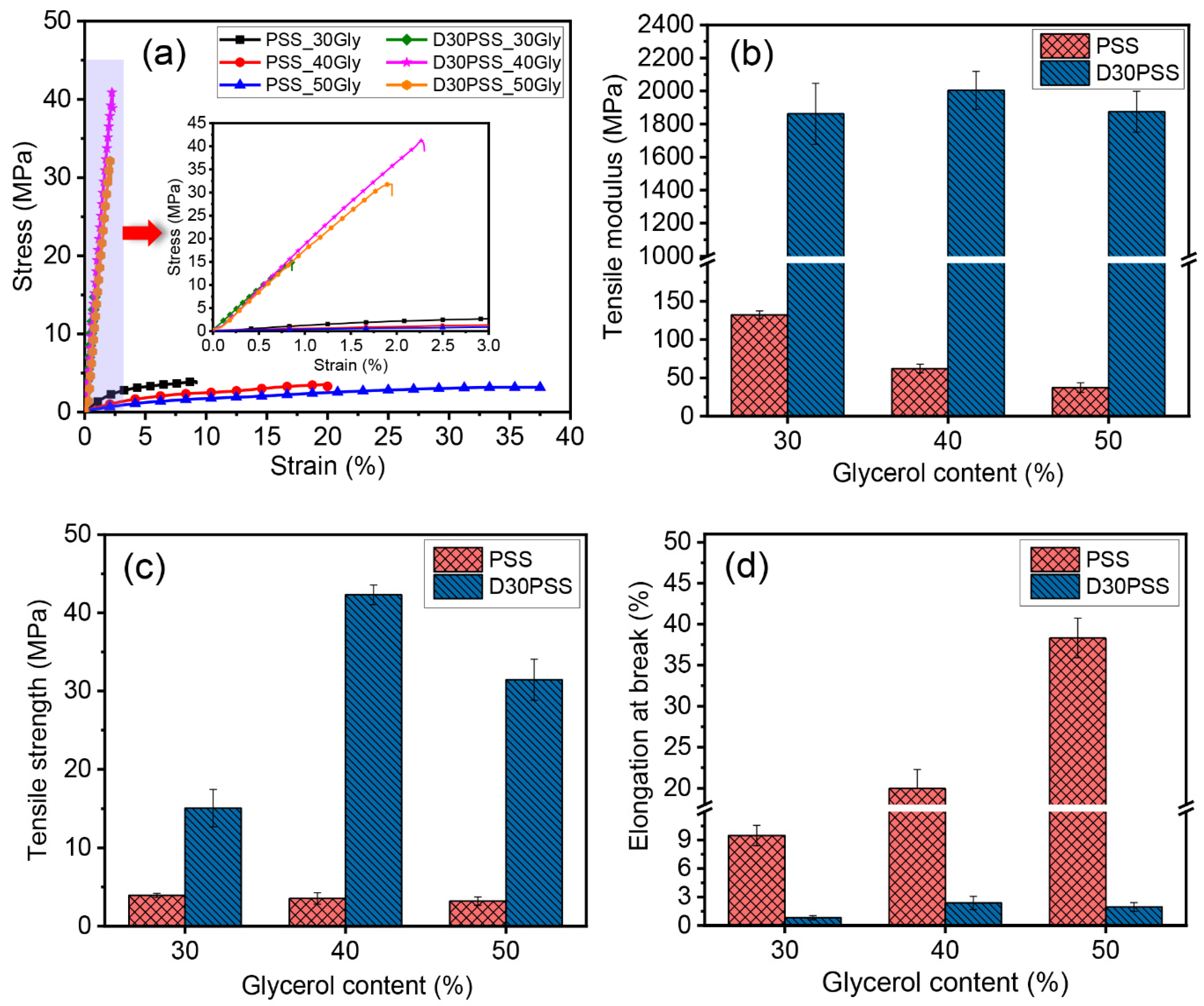
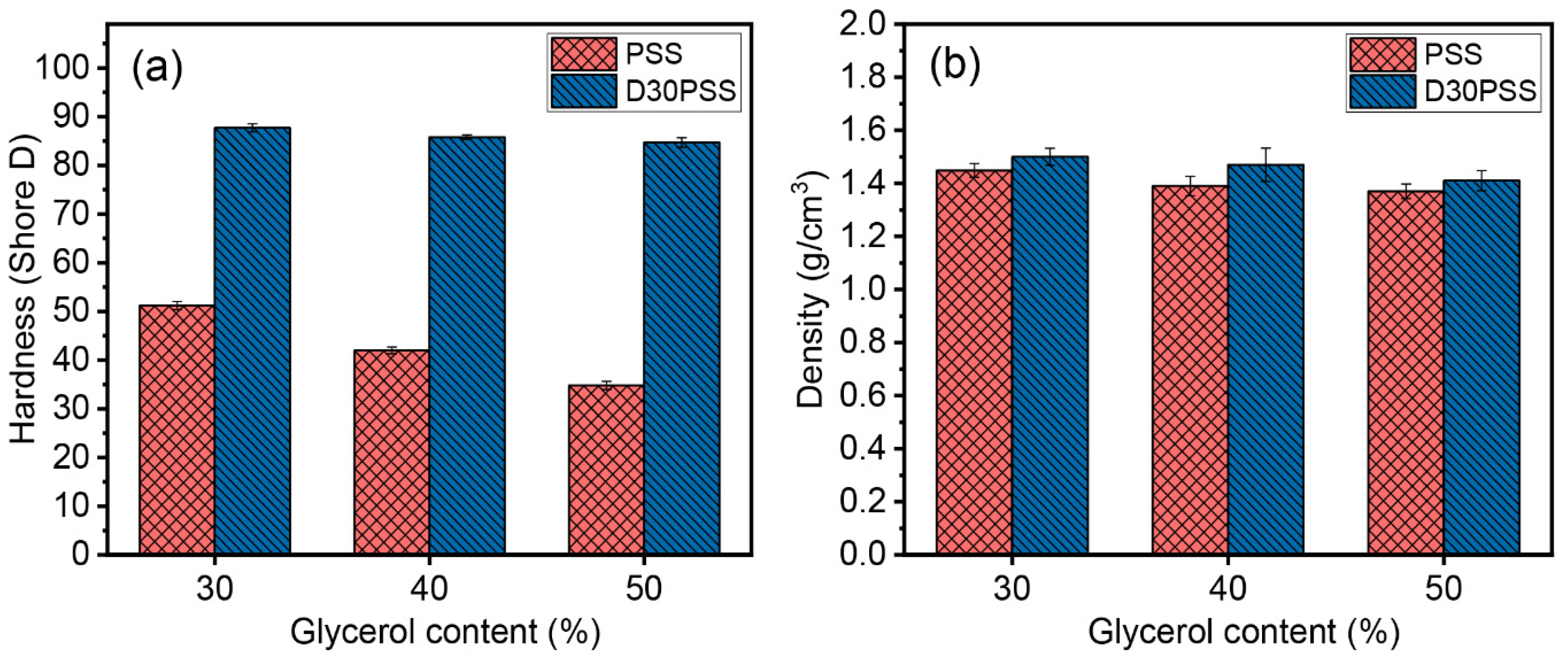
3.5. Mechanical Properties
3.6. Water Absorption and Solubility
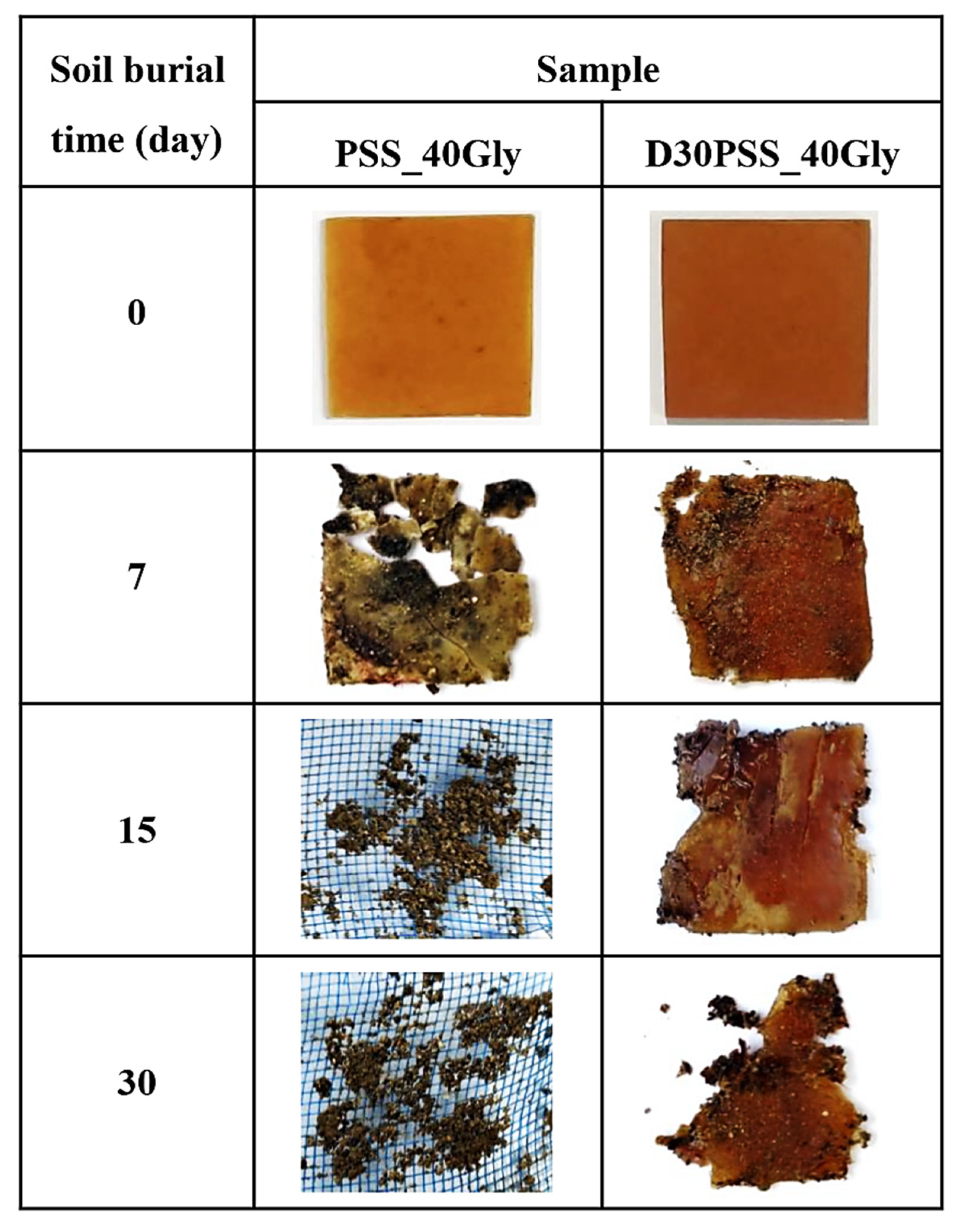
3.7. Soil Burial Degradability
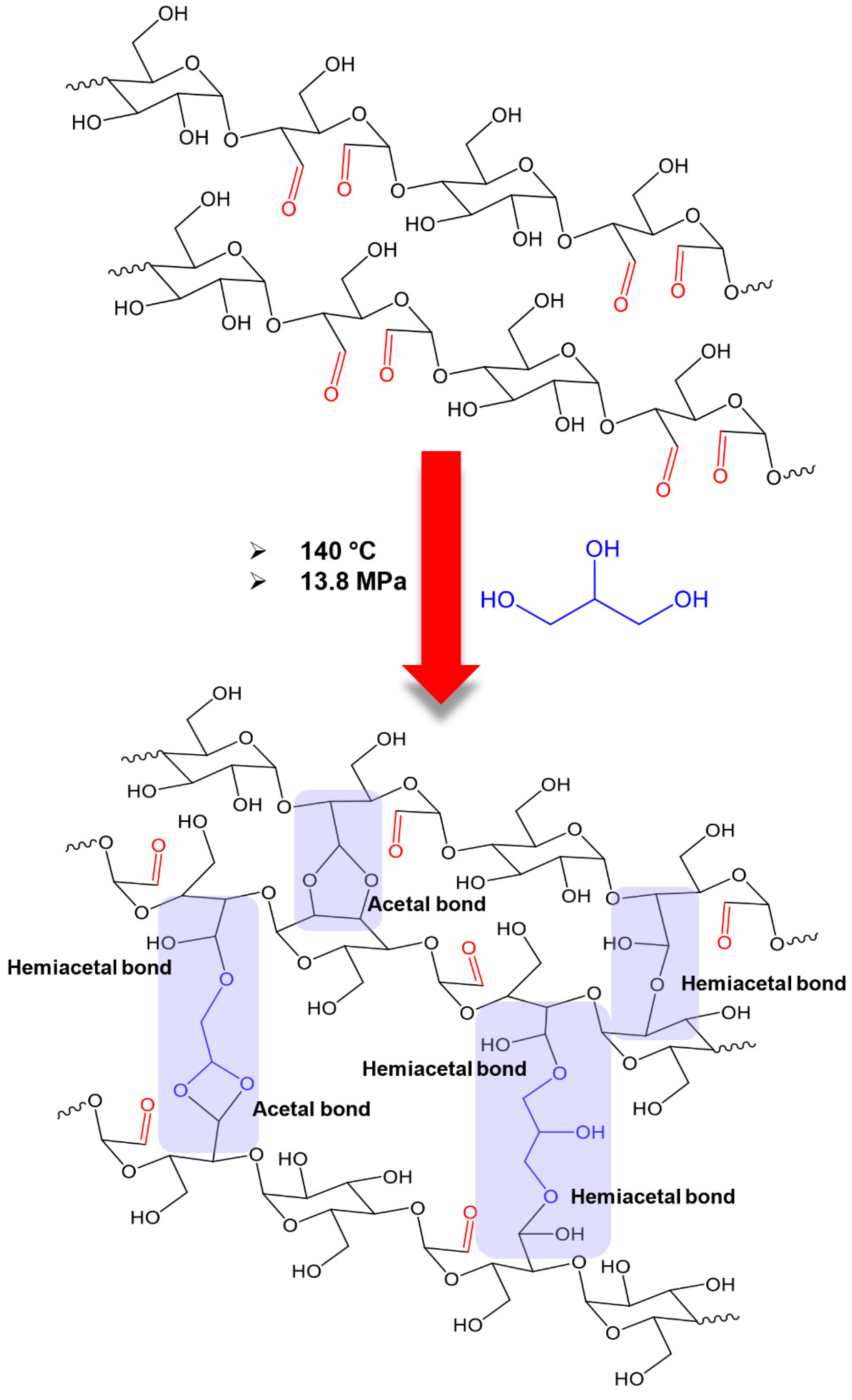
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- D’Amato, D.; Korhonen, J.; Toppinen, A. Circular, green, and bio economy: How do companies in land-use intensive sectors align with sustainability concepts? Ecol. Econ. 2019, 158, 116–133. [Google Scholar] [CrossRef]
- The Effects of the COVID-19 Pandemic on Plastics Use and Waste, Global Plastics Outlook: Economic Drivers, Environmental Impacts and Policy Options. Available online: https://www.oecd-ilibrary.org/sites/9e4fd47f-en/index.html?itemId=/content/component/9e4fd47f-en# (accessed on 14 August 2023).
- Wongprapinkul, B.; Vassanadumrongdee, S. A systems thinking approach towards single-use plastics reduction in food delivery business in Thailand. Sustainability 2022, 14, 9173. [Google Scholar] [CrossRef]
- Wang, G.-X.; Huang, D.; Ji, J.-H.; Völker, C.; Wurm, F.R. Seawater-degradable polymers—Fighting the marine plastic pollution. Adv. Sci. 2021, 8, 2001121. [Google Scholar] [CrossRef] [PubMed]
- Annemette, K.; Marcus, P.; Charlotte, L.; Ewa, G. A Review of Standards for Biodegradable Plastics. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/817684/review-standards-for-biodegradable-plastics-IBioIC.pdf (accessed on 13 August 2023).
- Jia, M.Z. Biodegradable Plastics: Breaking down the Facts, Greenpeace East Asia 2020. Available online: https://www.greenpeace.org/static/planet4-eastasia-stateless/84075f56-biodegradable-plastics-report.pdf (accessed on 13 August 2023).
- Muniyasamy, S.; Ofosu, O.; John, M.-J.; Anandjiwala, R.-D. Mineralization of poly(lactic acid) (PLA), poly(3-hydroxybutyrate-co-valerate) (PHBV) and PLA/PHBV blend in compost and soil environments. J. Renew. Mater. 2016, 4, 133–145. [Google Scholar] [CrossRef]
- Surendren, A.; Mohanty, A.K.; Liu, Q.; Misra, M. A review of biodegradable thermoplastic starches, their blends and composites: Recent developments and opportunities for single-use plastic packaging alternatives. Green Chem. 2022, 24, 8606–8636. [Google Scholar] [CrossRef]
- Lauer, M.K.; Smith, R.C. Recent advances in starch-based films toward food packaging applications: Physicochemical, mechanical, and functional properties. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3031–3083. [Google Scholar] [CrossRef]
- Cheng, H.; Chen, L.; McClements, D.J.; Yang, T.; Zhang, Z.; Ren, F.; Miao, M.; Tian, Y.; Jin, Z. Starch-based biodegradable packaging materials: A review of their preparation, characterization and diverse applications in the food industry. Trends Food Sci. Technol. 2021, 114, 70–82. [Google Scholar] [CrossRef]
- Lai, W.-F.; Wong, W.-T. Edible clusteroluminogenic films obtained from starch of different botanical origins for food packaging and quality management of frozen foods. Membranes 2022, 12, 437. [Google Scholar] [CrossRef]
- Liu, D.; Zhao, P.; Chen, J.; Yan, Y.; Wu, Z. Recent advances and applications in starch for intelligent active food packaging: A review. Foods 2022, 11, 2879. [Google Scholar] [CrossRef]
- Onyeaka, H.; Obileke, K.; Makaka, G.; Nwokolo, N. Current research and applications of starch-based biodegradable films for food packaging. Polymers 2022, 14, 1126. [Google Scholar] [CrossRef]
- Zuo, Y.; He, X.; Li, P.; Li, W.; Wu, Y. Preparation and characterization of hydrophobically grafted starches by in situ solid phase polymerization. Polymers 2019, 11, 72. [Google Scholar] [CrossRef]
- Sandhu, K.S.; Siroha, A.K.; Punia, S.; Sangwan, L.; Nehra, M.; Purewal, S.S. Effect of degree of cross linking on physicochemical, rheological and morphological properties of sorghum starch. Carbohydr. Polym. Technol. Appl. 2021, 2, 100073. [Google Scholar] [CrossRef]
- Bajer, D.; Burkowska-But, A. Innovative and environmentally safe composites based on starch modified with dialdehyde starch, caffeine, or ascorbic acid for applications in the food packaging industry. Food Chem. 2022, 374, 131639. [Google Scholar] [CrossRef]
- Tiozon, R.J.N.; Bonto, A.P.; Sreenivasulu, N. Enhancing the functional properties of rice starch through biopolymer blending for industrial applications: A review. Int. J. Biol. Macromol. 2021, 192, 100–117. [Google Scholar] [CrossRef] [PubMed]
- Kochkina, N.E.; Butikova, O.A.; Lukin, N.D. Ecofriendly films based on low-substituted starch acetate enhanced by polyvinyl alcohol additions. Iran. Polym. J. 2022, 31, 1361–1371. [Google Scholar] [CrossRef]
- Martinez Villadiego, K.; Arias Tapia, M.J.; Useche, J.; Escobar Macías, D. Thermoplastic starch (TPS)/polylactic acid (PLA) blending methodologies: A review. J. Polym. Environ. 2022, 30, 75–91. [Google Scholar] [CrossRef]
- Vorwerg, W.; Dijksterhuis, J.; Borghuis, J.; Radosta, S.; Kröger, A. Film properties of hydroxypropyl starch. Starch-Stärke 2004, 56, 297–306. [Google Scholar] [CrossRef]
- Reddy, N.; Yang, Y. Citric acid cross-linking of starch films. Food Chem. 2010, 118, 702–711. [Google Scholar] [CrossRef]
- da Rosa Zavareze, E.; Pinto, V.Z.; Klein, B.; El Halal, S.L.M.; Elias, M.C.; Prentice-Hernández, C.; Dias, A.R.G. Development of oxidised and heat–moisture treated potato starch film. Food Chem. 2012, 132, 344–350. [Google Scholar] [CrossRef]
- Colussi, R.; Pinto, V.Z.; El Halal, S.L.M.; Biduski, B.; Prietto, L.; Castilhos, D.D.; da Rosa Zavareze, E.; Dias, A.R.G. Acetylated rice starches films with different levels of amylose: Mechanical, water vapor barrier, thermal, and biodegradability properties. Food Chem. 2017, 221, 1614–1620. [Google Scholar] [CrossRef]
- Tanetrungroj, Y.; Prachayawarakorn, J. Effect of dual modification on properties of biodegradable crosslinked-oxidized starch and oxidized-crosslinked starch films. Int. J. Biol. Macromol. 2018, 120, 1240–1246. [Google Scholar] [CrossRef]
- Veelaert, S.; de Wit, D.; Gotlieb, K.F.; Verhé, R. The gelation of dialdehyde starch. Carbohydr. Polym. 1997, 32, 131–139. [Google Scholar] [CrossRef]
- Veelaert, S.; de Wit, D.; Gotlieb, K.F.; Verhé, R. Chemical and physical transitions of periodate oxidized potato starch in water. Carbohydr. Polym. 1997, 33, 153–162. [Google Scholar] [CrossRef]
- Wongsagon, R.; Shobsngob, S.; Varavinit, S. Preparation and physicochemical properties of dialdehyde tapioca starch. Starch-Stärke 2005, 57, 166–172. [Google Scholar] [CrossRef]
- Rinju, R.; Harikumaran-Thampi, B.-S. Characteristics of starch extracted from the stem of pineapple plant (Ananas comosus)-an agro waste from pineapple farms. Braz. Arch. Biol. Technol. 2021, 64, e21190276. [Google Scholar] [CrossRef]
- Chaosap, C.; Sahatsanon, K.; Sitthigripong, R.; Sawanon, S.; Setakul, J. The effects of using pineapple stem starch as an alternative starch source and ageing period on meat quality, texture profile, ribonucleotide content, and fatty acid composition of longissimus thoracis of fattening dairy steers. Foods 2021, 10, 2319. [Google Scholar] [CrossRef]
- Chu, P.H.; Jenol, M.A.; Phang, L.Y.; Ibrahim, M.F.; Prasongsuk, S.; Bankeeree, W.; Punnapayak, H.; Lotrakul, P.; Abd-Aziz, S. Starch extracted from pineapple (Ananas comosus) plant stem as a source for amino acids production. Chem. Biol. Technol. Agric. 2021, 8, 29. [Google Scholar] [CrossRef]
- Nakthong, N.; Wongsagonsup, R.; Amornsakchai, T. Characteristics and potential utilizations of starch from pineapple stem waste. Ind. Crops Prod. 2017, 105, 74–82. [Google Scholar] [CrossRef]
- Namphonsane, A.; Suwannachat, P.; Chia, C.H.; Wongsagonsup, R.; Smith, S.M.; Amornsakchai, T. Toward a Circular Bioeconomy: Exploring Pineapple Stem Starch Film as a Plastic Substitute in Single Use Applications. Membranes 2023, 13, 458. [Google Scholar] [CrossRef]
- Thongphang, C.; Namphonsane, A.; Thanawan, S.; Chia, C.H.; Wongsagonsup, R.; Smith, S.M.; Amornsakchai, T. Toward a circular bioeconomy: Development of pineapple stem starch composite as a plastic-sheet substitute for single-use applications. Polymers 2023, 15, 2388. [Google Scholar] [CrossRef]
- Bumrungnok, K.; Threepopnatkul, P.; Amornsakchai, T.; Chia, C.H.; Wongsagonsup, R.; Smith, S.M. Toward a circular bioeconomy: Exploring pineapple stem starch film as protective coating for fruits and vegetables. Polymers 2023, 15, 2493. [Google Scholar] [CrossRef] [PubMed]
- Namphonsane, A.; Amornsakchai, T.; Chia, C.H.; Goh, K.L.; Thanawan, S.; Wongsagonsup, R.; Smith, S.M. Development of biodegradable rigid foams from pineapple field waste. Polymers 2023, 15, 2895. [Google Scholar] [CrossRef] [PubMed]
- Ninsuwan, K.; Nimnuan, J.; Watcharakitti, J.; Siriwong, C.; Amornsakchai, T.; Smith, S.M. Antifungal Activity of Water-Based Adhesives Derived from Pineapple Stem Flour with Apple Cider Vinegar as an Additive. Polymers 2023, 15, 1735. [Google Scholar] [CrossRef] [PubMed]
- Tangsrianugul, N.; Hongsanyatham, S.; Kapcum, C.; Sungayuth, N.; Boonsanong, N.; Somprasong, N.; Smith, S.M.; Amornsakchai, T.; Pinyo, J.; Wongsagonsup, R. Physicochemical and sensory properties of corn grits and pineapple stem starch-based extruded snacks enriched with oyster mushroom powder. Int. J. Food Sci. Technol. 2023, 58, 1528–1540. [Google Scholar] [CrossRef]
- Sriprablom, J.; Suphantharika, M.; Smith, S.M.; Amornsakchai, T.; Pinyo, J.; Wongsagonsup, R. Physicochemical, Rheological, In-Vitro Digestibility, and Emulsifying Properties of Starch Extracted from Pineapple Stem Agricultural Waste. Foods 2023, 12, 2028. [Google Scholar] [CrossRef]
- Corzo, C.A.; Waliszewski, K.N.; Welti-Chanes, J. Pineapple fruit bromelain affinity to different protein substrates. Food Chem. 2012, 133, 631–635. [Google Scholar] [CrossRef]
- Manzoor, Z.; Nawaz, A.; Mukhtar, H.; Haq, I. Bromelain: Methods of extraction, purification and therapeutic applications. Braz. Arch. Biol. Technol. 2016, 59, e16150010. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, P.; Wang, Y.; Gao, W. Study on physico-chemical properties of dialdehyde yam starch with different aldehyde group contents. Thermochim. Acta 2011, 512, 196–201. [Google Scholar] [CrossRef]
- Hofreiter, B.T.; Alexander, B.H.; Wolff, I.A. Rapid estimation of dialdehyde content of periodate oxystarch through quantitative alkali consumption. Anal. Chem. 1955, 27, 1930–1931. [Google Scholar] [CrossRef]
- ASTM D882; Standard Test Method for Tensile Properties of Thin Plastic Sheeting. American Society for Testing and Materials (ASTM): West Conshohocken, PA, USA, 2018.
- ASTM D2240; Standard Test Method for Rubber Property—Durometer Hardness. American Society for Testing and Materials (ASTM): West Conshohocken, PA, USA, 2021.
- ASTM D792; Standard Test Methods for Density and Specific Gravity (Relative Density) of Plastics by Displacement. American Society for Testing and Materials (ASTM): West Conshohocken, PA, USA, 2020.
- Seligra, P.G.; Medina Jaramillo, C.; Famá, L.; Goyanes, S. Biodegradable and non-retrogradable eco-films based on starch–glycerol with citric acid as crosslinking agent. Carbohydr. Polym. 2016, 138, 66–74. [Google Scholar] [CrossRef]
- Zhang, S.-D.; Wang, X.-L.; Zhang, Y.-R.; Yang, K.-K.; Wang, Y.-Z. Preparation of a new dialdehyde starch derivative and investigation of its thermoplastic properties. J. Polym. Res. 2010, 17, 439–446. [Google Scholar] [CrossRef]
- Wannous, A.; Milaneh, S.; Said, M.; Atassi, Y. New approach for starch dialdehyde preparation using microwave irradiation for removal of heavy metal ions from water. SN Appl. Sci. 2022, 4, 133. [Google Scholar] [CrossRef]
- Li, J.H.; Vasanthan, T. Hypochlorite oxidation of field pea starch and its suitability for noodle making using an extrusion cooker. Food Res. Int. 2003, 36, 381–386. [Google Scholar] [CrossRef]
- Fiedorowicz, M.; Para, A. Structural and molecular properties of dialdehyde starch. Carbohydr. Polym. 2006, 63, 360–366. [Google Scholar] [CrossRef]
- Zuo, Y.; Gu, J.; Yang, L.; Qiao, Z.; Tan, H.; Zhang, Y. Synthesis and characterization of maleic anhydride esterified corn starch by the dry method. Int. J. Biol. Macromol. 2013, 62, 241–247. [Google Scholar] [CrossRef]
- Abdullah, A.H.D.; Chalimah, S.; Primadona, I.; Hanantyo, M.H.G. Physical and chemical properties of corn, cassava, and potato starchs. IOP Conf. Ser. Earth Environ. Sci. 2018, 160, 012003. [Google Scholar] [CrossRef]
- Yu, J.; Chang, P.R.; Ma, X. The preparation and properties of dialdehyde starch and thermoplastic dialdehyde starch. Carbohydr. Polym. 2010, 79, 296–300. [Google Scholar] [CrossRef]
- Martucci, J.F.; Ruseckaite, R.A. Tensile properties, barrier properties, and biodegradation in soil of compression—Molded gelatin-dialdehyde starch films. J. Appl. Polym. Sci. 2009, 112, 2166–2178. [Google Scholar] [CrossRef]
- Chi, H.; Xu, K.; Wu, X.; Chen, Q.; Xue, D.; Song, C.; Zhang, W.; Wang, P. Effect of acetylation on the properties of corn starch. Food Chem. 2008, 106, 923–928. [Google Scholar] [CrossRef]
- Namazi, H.; Fathi, F.; Dadkhah, A. Hydrophobically modified starch using long-chain fatty acids for preparation of nanosized starch particles. Sci. Iran. 2011, 18, 439–445. [Google Scholar] [CrossRef]
- Zhang, S.-D.; Zhang, Y.-R.; Zhu, J.; Wang, X.-L.; Yang, K.-K.; Wang, Y.-Z. Modified corn starches with improved comprehensive properties for preparing thermoplastics. Starch-Stärke 2007, 59, 258–268. [Google Scholar] [CrossRef]
- Krauss, B.H. Anatomy of the vegetative organs of the pineapple, Ananas comosus (L.) Merr Merr. I. Introduction, organography, the stem, and the lateral branch or axillary buds. Bot. Gaz. 1948, 110, 159–217. [Google Scholar] [CrossRef]
- Zuo, Y.; Liu, W.; Xiao, J.; Zhao, X.; Zhu, Y.; Wu, Y. Preparation and characterization of dialdehyde starch by one-step acid hydrolysis and oxidation. Int. J. Biol. Macromol. 2017, 103, 1257–1264. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Park, S.; Roh, H.-G.; Oh, S.; Kim, S.; Kim, M.; Kim, D.; Park, J. Preparation and characterization of superabsorbent polymers based on starch aldehydes and carboxymethyl cellulose. Polymers 2018, 10, 605. [Google Scholar] [CrossRef]
- Shujun, W.; Jinglin, Y.; Wenyuan, G.; Jiping, P.; Hongyan, L.; Jiugao, Y. Granule structural changes in native Chinese Yam (Dioscorea opposita Thunb var. Anguo) starch during acid hydrolysis. Carbohydr. Polym. 2007, 69, 286–292. [Google Scholar] [CrossRef]
- Yin, Q.-F.; Ju, B.-Z.; Zhang, S.-F.; Wang, X.-B.; Yang, J.-Z. Preparation and characteristics of novel dialdehyde aminothiazole starch and its adsorption properties for Cu (II) ions from aqueous solution. Carbohydr. Polym. 2008, 72, 326–333. [Google Scholar] [CrossRef]
- Ma, X.; Jian, R.; Chang, P.R.; Yu, J. Fabrication and characterization of citric acid-modified starch nanoparticles/plasticized-starch composites. Biomacromolecules 2008, 9, 3314–3320. [Google Scholar] [CrossRef]
- Sarko, A.; Wu, H.-C.H. The crystal structures of A-, B- and C-polymorphs of amylose and starch. Starch-Stärke 1978, 30, 73–78. [Google Scholar] [CrossRef]
- Barroso, A.G.; Garcia, R.H.; Del Mastro, N.L. X-ray diffraction pattern and relative crystallinity of irradiated arrowroot starch. Braz. J. Radiat. Sci. 2019, 7, 1–7. [Google Scholar] [CrossRef]
- Yong, H.; Bai, R.; Bi, F.; Liu, J.; Qin, Y.; Liu, J. Synthesis, characterization, antioxidant and antimicrobial activities of starch aldehyde-quercetin conjugate. Int. J. Biol. Macromol. 2020, 156, 462–470. [Google Scholar] [CrossRef]
- Bodîrlău, R.; Teacă, C.-A.; Spiridon, I.; Tudorachi, N. Effects of chemical modification on the structure and mechanical properties of starch-based biofilms. Monatsh. Chem. 2012, 143, 335–343. [Google Scholar] [CrossRef]
- Xu, H.; Canisag, H.; Mu, B.; Yang, Y. Robust and flexible films from 100% starch cross-linked by biobased disaccharide derivative. ACS Sustain. Chem. Eng. 2015, 3, 2631–2639. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, F.; Peang, H.; Wang, Y. An investigation of the effect of semi-acetal formation on the properties of dialdehyde starch and its thermoplastic blend with glycerol. J. Macromol. Sci. Phys. Part B 2015, 54, 836–850. [Google Scholar] [CrossRef]
- Jia, Y.; Asoh, T.-A.; Hsu, Y.-I.; Uyama, H. Wet strength improvement of starch-based blend films by formation of acetal/hemiacetal bonding. Polym. Degrad. Stab. 2020, 177, 109197. [Google Scholar] [CrossRef]
- Sastri, V.R. 3—Materials used in medical devices. In Plastics in Medical Devices, 3rd ed.; Sastri, V.R., Ed.; William Andrew Publishing: Norwich, NY, USA, 2022; pp. 41–64. [Google Scholar]
- Ma, X.; Yu, J.; Kennedy, J.F. Studies on the properties of natural fibers-reinforced thermoplastic starch composites. Carbohydr. Polym. 2005, 62, 19–24. [Google Scholar] [CrossRef]
- Yang, J.-H.; Yu, J.-G.; Ma, X.-F. Preparation and properties of ethylenebisformamide plasticized potato starch (EPTPS). Carbohydr. Polym. 2006, 63, 218–223. [Google Scholar] [CrossRef]
- Sahari, J.; Sapuan, S.M.; Zainudin, E.S.; Maleque, M.A. A new approach to use Arenga Pinnata as sustainable biopolymer: Effects of plasticizers on physical properties. Procedia Chem. 2012, 4, 254–259. [Google Scholar] [CrossRef]
- Tarique, J.; Sapuan, S.M.; Khalina, A. Effect of glycerol plasticizer loading on the physical, mechanical, thermal, and barrier properties of arrowroot (Maranta arundinacea) starch biopolymers. Sci. Rep. 2021, 11, 13900. [Google Scholar] [CrossRef]
- Perez-gago, M.B.; Krochta, J.M. Denaturation time and temperature effects on solubility, tensile properties, and oxygen permeability of whey protein edible films. J. Food Sci. 2001, 66, 705–710. [Google Scholar] [CrossRef]
- Fakhouri, F.M.; Maria Martelli, S.; Canhadas Bertan, L.; Yamashita, F.; Innocentini Mei, L.H.; Collares Queiroz, F.P. Edible films made from blends of manioc starch and gelatin—Influence of different types of plasticizer and different levels of macromolecules on their properties. LWT 2012, 49, 149–154. [Google Scholar] [CrossRef]
- Basiak, E.; Lenart, A.; Debeaufort, F. Effect of starch type on the physico-chemical properties of edible films. Int. J. Biol. Macromol. 2017, 98, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Junlapong, K.; Boonsuk, P.; Chaibundit, C.; Chantarak, S. Highly water resistant cassava starch/poly(vinyl alcohol) films. Int. J. Biol. Macromol. 2019, 137, 521–527. [Google Scholar] [CrossRef] [PubMed]



| Sample Code | Starch (Weight Fraction, %) | Glycerol (Weight Fraction, %) | |
|---|---|---|---|
| PSS | D30PSS | ||
| PSS_30Gly | 100 | - | 30 |
| PSS_40Gly | 100 | - | 40 |
| PSS_50Gly | 100 | - | 50 |
| D30PSS_30Gly | - | 100 | 30 |
| D30PSS_40Gly | - | 100 | 40 |
| D30PSS_50Gly | - | 100 | 50 |
| Sample Code | Aldehyde Content (%) | Intrinsic Viscosity (dL/g) |
|---|---|---|
| PSS | - | 1.48 ± 0.21 a |
| D10PSS | 11.5 ± 2.39 d | 0.47 ± 0.13 b |
| D30PSS | 32.35 ± 3.06 c | 0.36 ± 0.10 b |
| D60PSS | 61.37 ± 2.55 b | 0.24 ± 0.18 b |
| D90PSS | 89.68 ± 3.22 a | 0.15 ± 0.13 b |
| Sample Code | Tensile Properties | Hardness (Shore D) | Density (g/cm3) | ||
|---|---|---|---|---|---|
| E * (MPa) | σ ** (MPa) | ε *** (%) | |||
| PSS_30Gly | 132.42 ± 5.06 b | 3.92 ± 0.26 d | 9.48 ± 1.07 c | 51.20 ± 0.84 d | 1.45 ± 0.03 a |
| PSS_40Gly | 62.19 ± 5.68 c | 3.52 ± 0.74 d | 20.00 ± 2.27 b | 42.00 ± 0.71 e | 1.39 ± 0.04 b |
| PSS_50Gly | 37.24 ± 6.35 d | 3.19 ± 0.53 d | 38.33 ± 2.41 a | 34.80 ± 0.83 f | 1.37 ± 0.03 c |
| D30PSS_30Gly | 1862.03 ± 184.35 a | 15.08 ± 2.38 c | 0.83 ± 0.21 e | 87.70 ± 0.84 a | 1.50 ± 0.03 a |
| D30PSS_40Gly | 2004.08 ± 114.82 a | 42.31 ± 1.26 a | 2.38 ± 0.69 d | 85.80 ± 0.45 b | 1.47 ± 0.06 a |
| D30PSS_50Gly | 1874.93 ± 124.24 a | 31.45 ± 2.63 b | 1.95 ± 0.46 d | 84.70 ± 0.97 c | 1.41 ± 0.04 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tessanan, W.; Phinyocheep, P.; Amornsakchai, T. Development of Biodegradable Thermosetting Plastic Using Dialdehyde Pineapple Stem Starch. Polymers 2023, 15, 3832. https://doi.org/10.3390/polym15183832
Tessanan W, Phinyocheep P, Amornsakchai T. Development of Biodegradable Thermosetting Plastic Using Dialdehyde Pineapple Stem Starch. Polymers. 2023; 15(18):3832. https://doi.org/10.3390/polym15183832
Chicago/Turabian StyleTessanan, Wasan, Pranee Phinyocheep, and Taweechai Amornsakchai. 2023. "Development of Biodegradable Thermosetting Plastic Using Dialdehyde Pineapple Stem Starch" Polymers 15, no. 18: 3832. https://doi.org/10.3390/polym15183832
APA StyleTessanan, W., Phinyocheep, P., & Amornsakchai, T. (2023). Development of Biodegradable Thermosetting Plastic Using Dialdehyde Pineapple Stem Starch. Polymers, 15(18), 3832. https://doi.org/10.3390/polym15183832