Myrica esculenta Leaf Extract—Assisted Green Synthesis of Porous Magnetic Chitosan Composites for Fast Removal of Cd (II) from Water: Kinetics and Thermodynamics of Adsorption
Abstract
:1. Introduction
2. Materials and Method
2.1. Materials
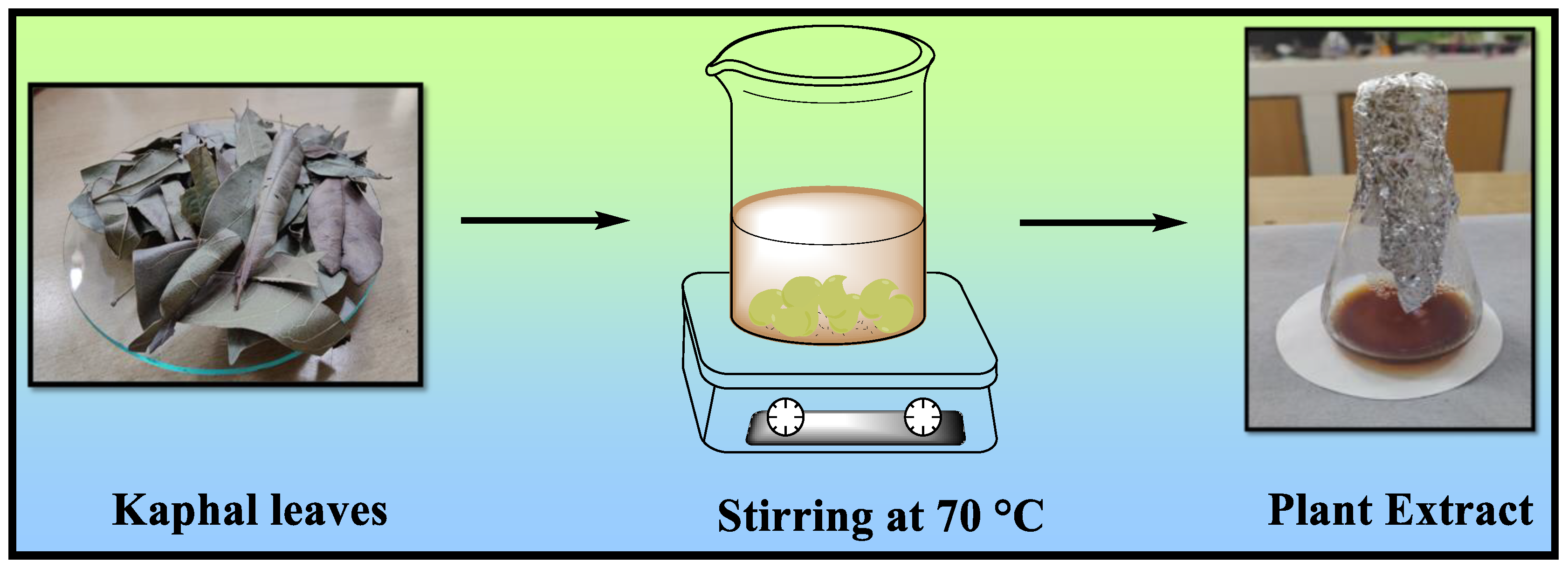
2.2. Preparation of Aqueous Leaf Extract of Myrica esculenta (Kaphal)
2.3. Characterization of the Adsorbents
2.4. Batch Adsorption Studies for the Adsorption of Cd (II) Using MNPs and Chitosan/Fe3O4 Composite as Adsorbents
3. Result and Discussion
3.1. Adsorbent Characterization
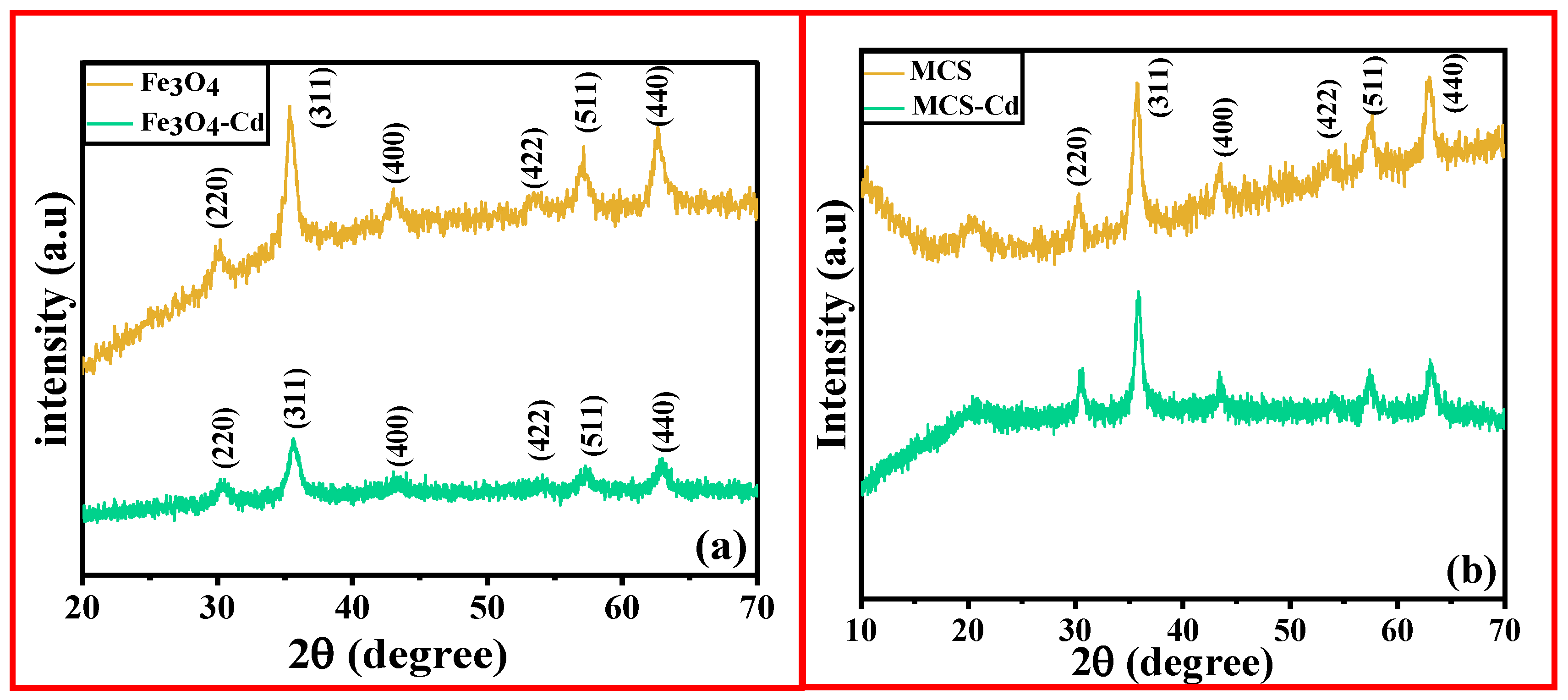
3.1.1. X-ray Diffraction
3.1.2. BET Sorptometry for Evaluating the Textural Properties of Adsorbents
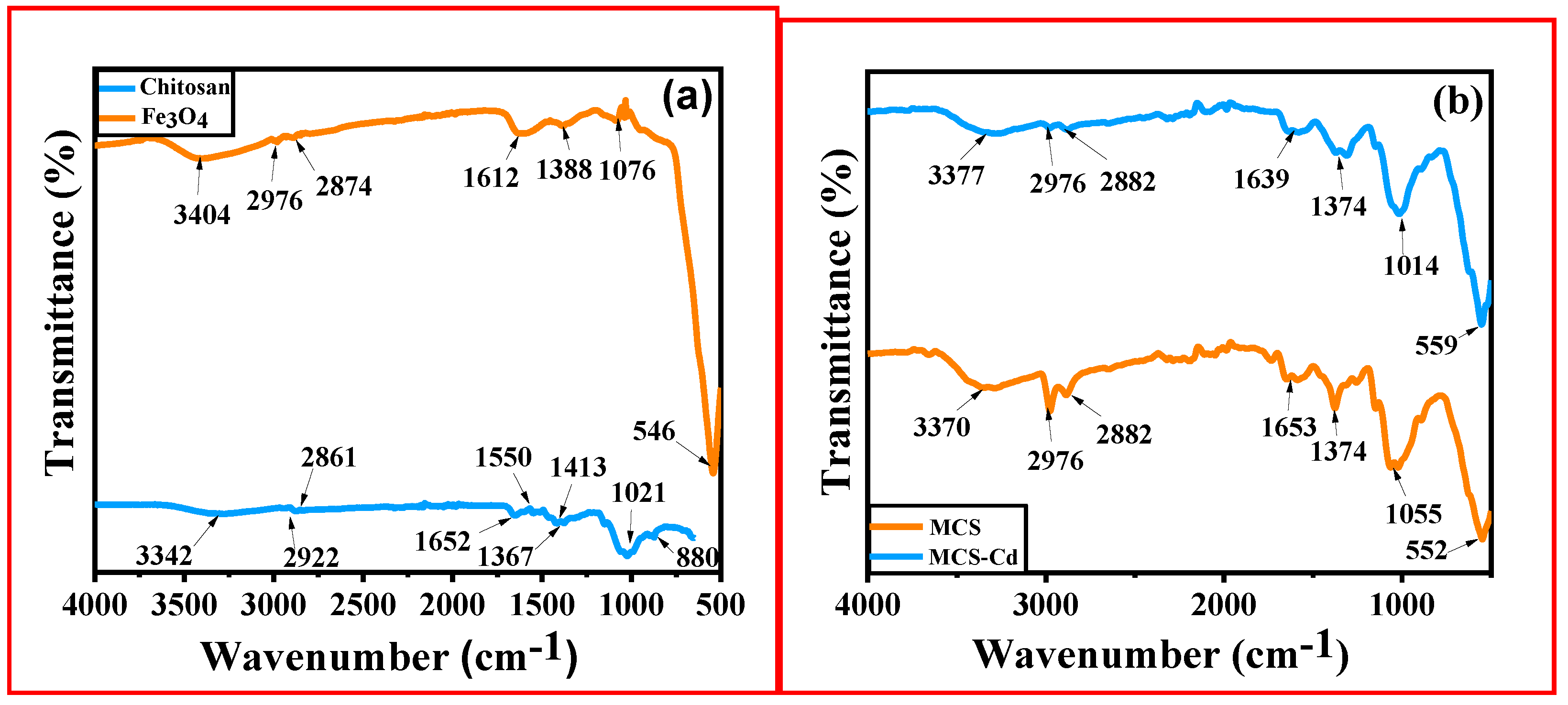
3.1.3. Fourier Transform Infrared (FT-IR) Spectroscopy
3.1.4. FE-SEM-EDX
3.1.5. Vibrating Sample Magnetometer Study for Evaluating the Magnetic Property of the Adsorbents
3.2. Adsorption Experiments
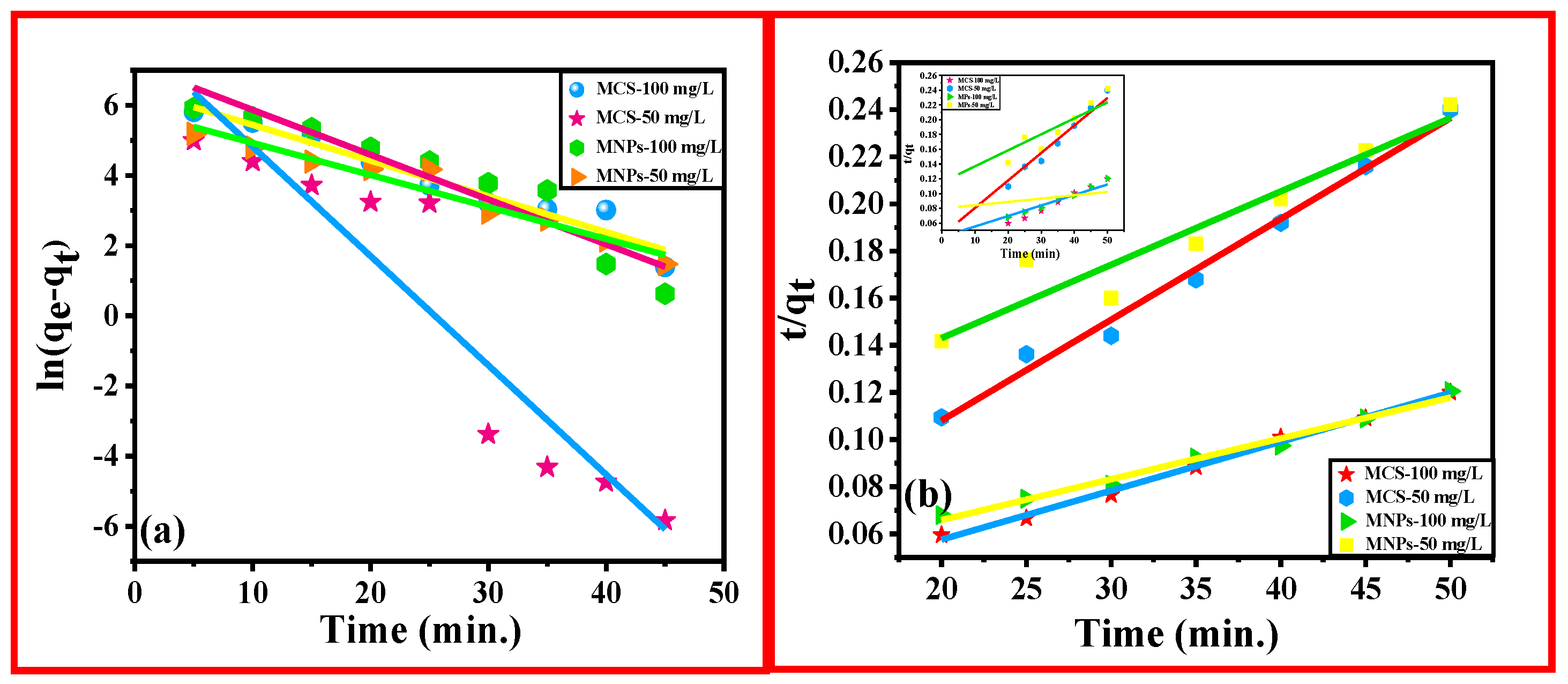
3.2.1. Study of Kinetics of Adsorption of Cd (II) onto the Adsorbents, MNPs, and MCS Composite
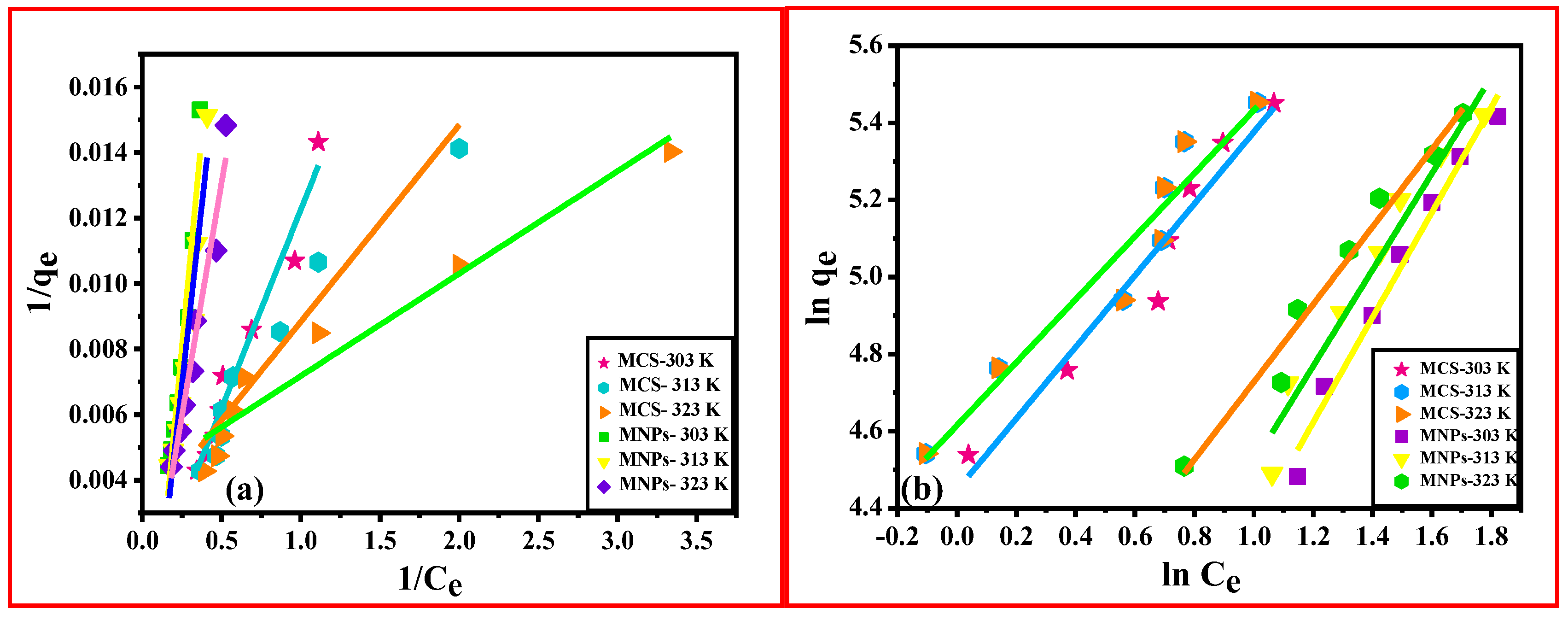
3.2.2. Adsorption Isotherm Models
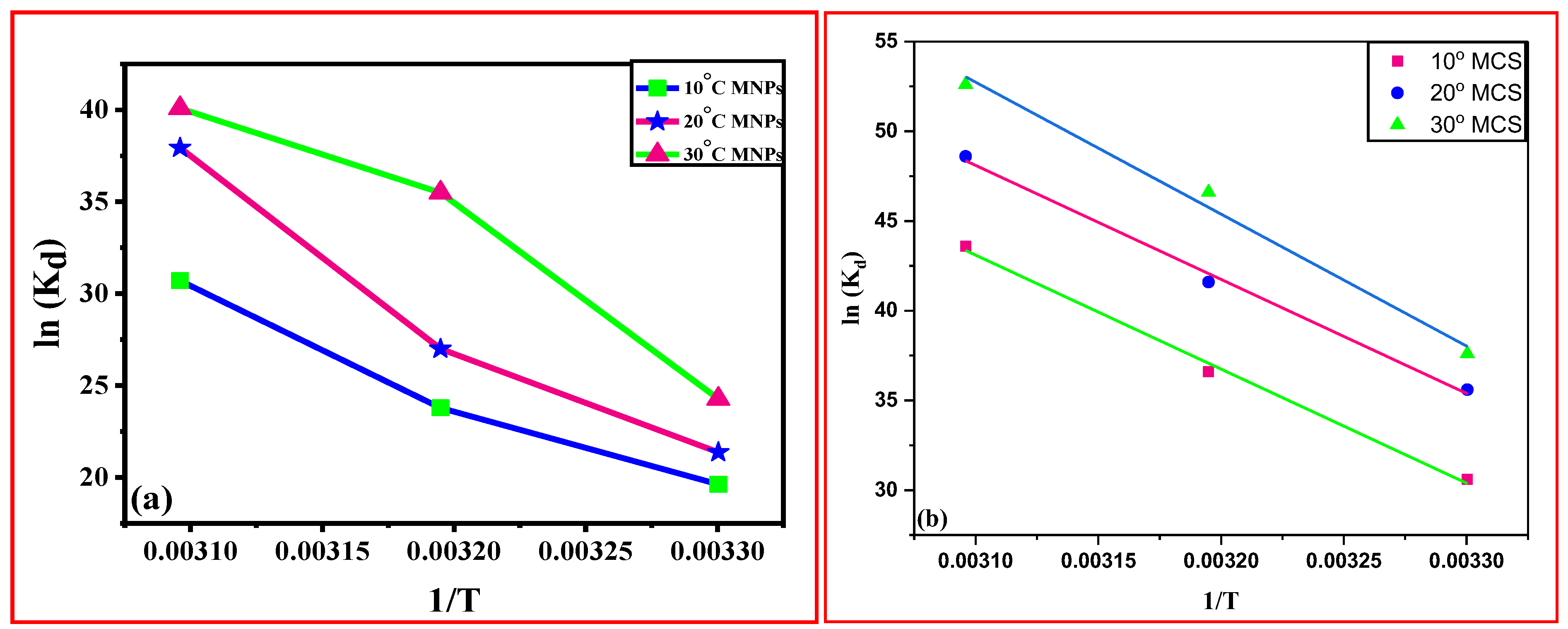
3.3. Thermodynamic Studies
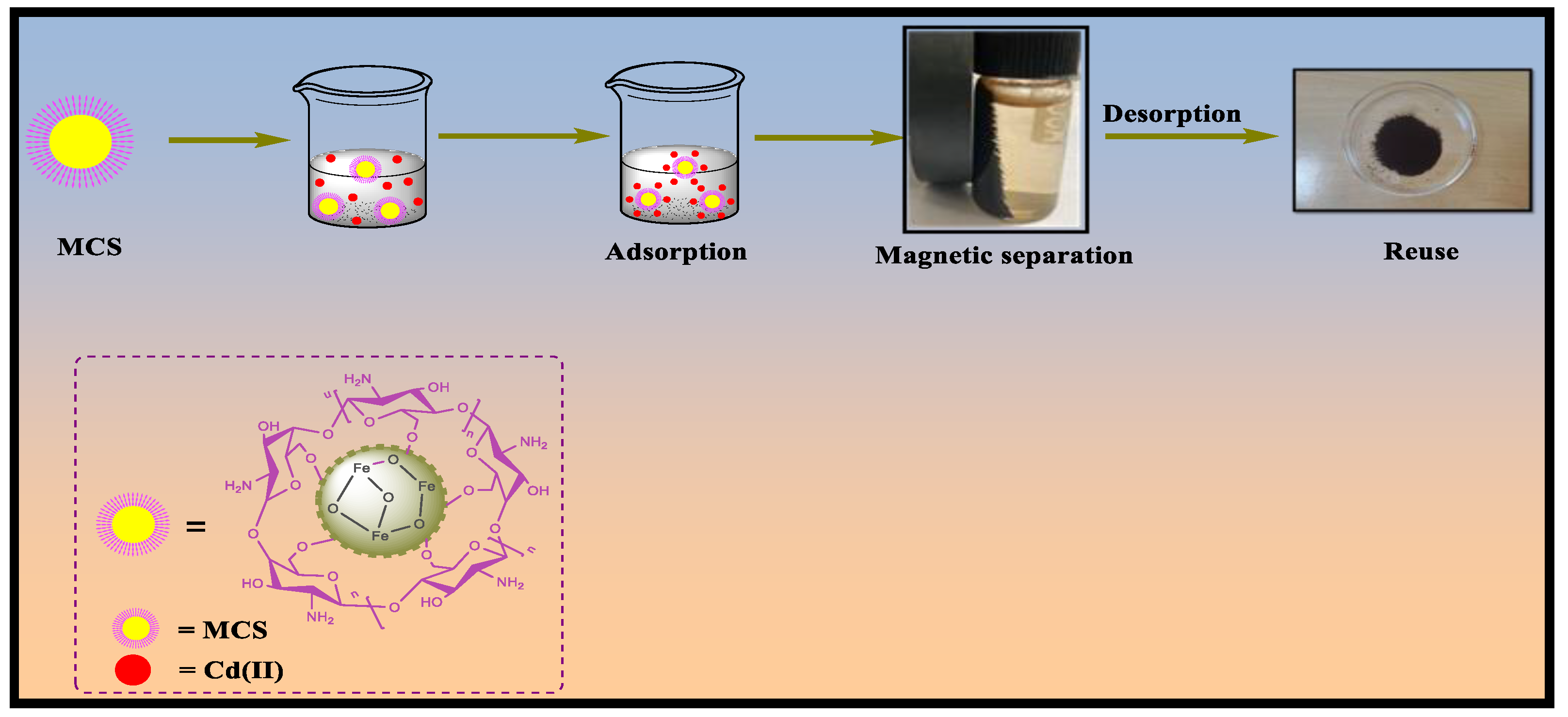
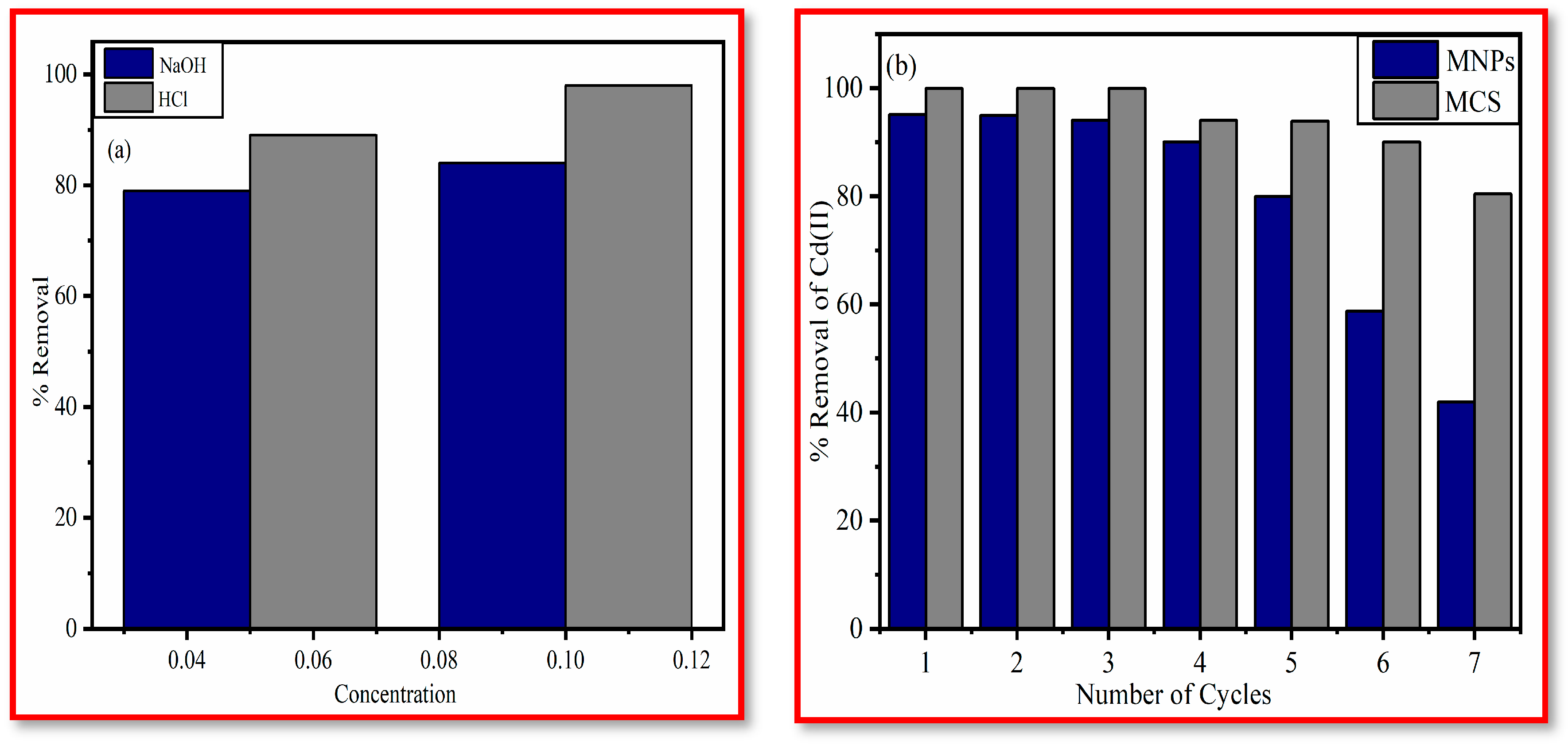
3.4. Reusability of Adsorbent
3.5. Comparative Studies
3.6. Adsorption Studies
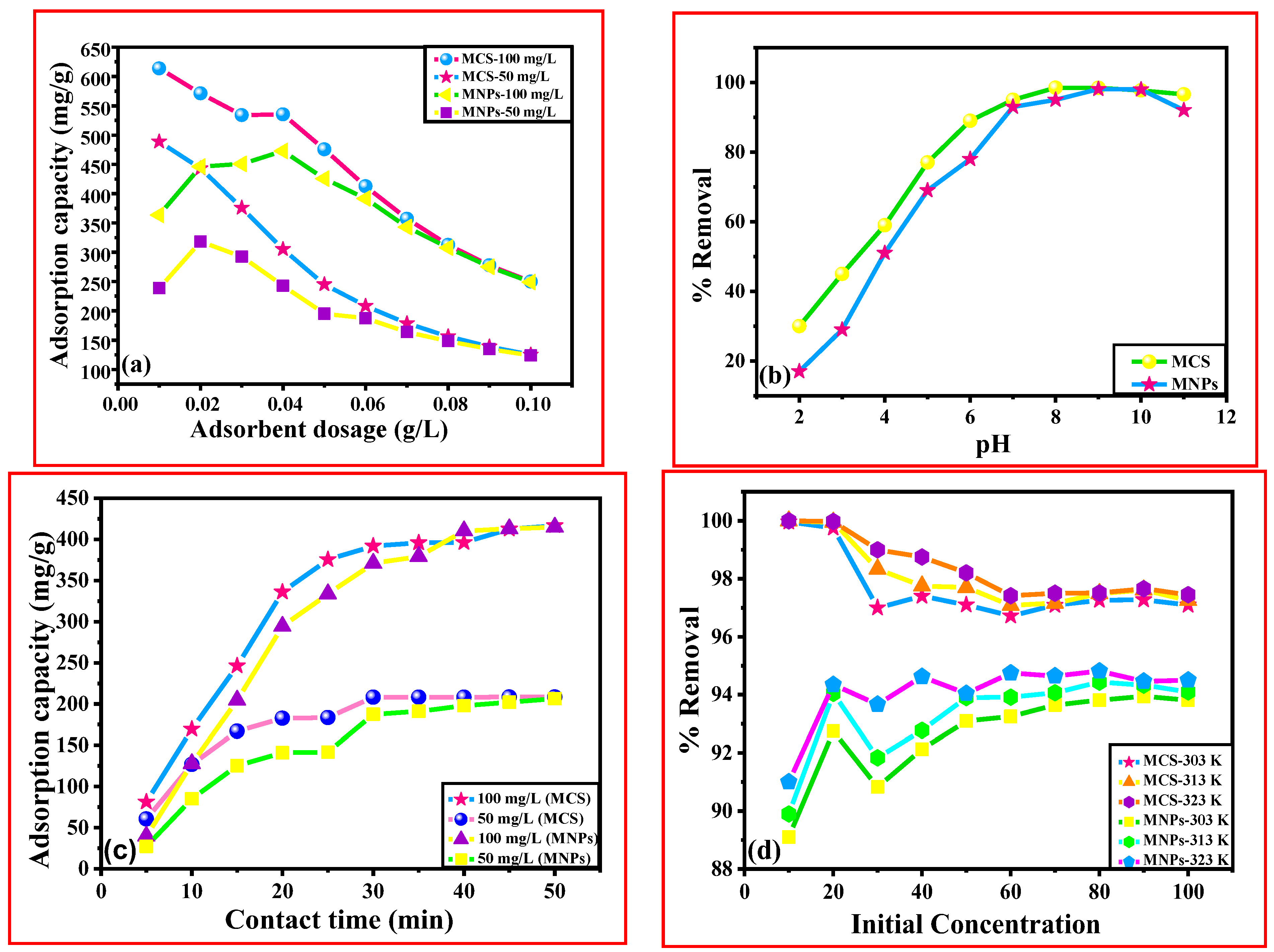
3.6.1. Variation of Adsorbent Dose
3.6.2. Variation in pH
3.6.3. Variation of Contact Time
3.6.4. Variation of Concentration
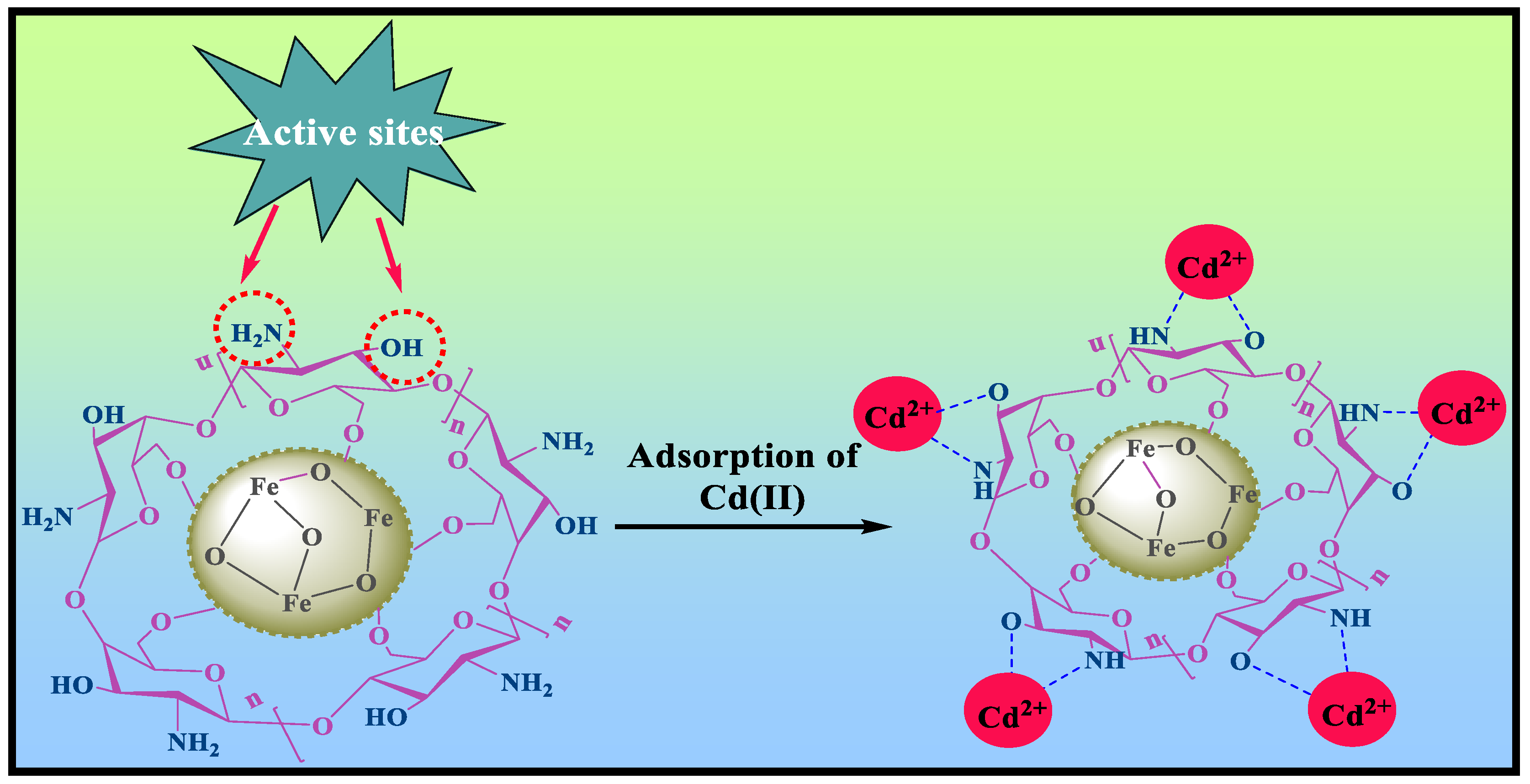
4. Adsorption Mechanism
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mahvi, A.H.; Balarak, D.; Bazrafshan, E. Remarkable Reusability of Magnetic Fe3O4-Graphene Oxide Composite: A Highly Effective Adsorbent for Cr (VI) Ions. Int. J. Environ. Anal. Chem. 2023, 103, 3501–3521. [Google Scholar] [CrossRef]
- Li, L.; Zou, J.; Han, Y.; Liao, Z.; Lu, P.; Nezamzadeh-Ejhieh, A.; Liu, J.; Peng, Y. Recent advances in Al (iii)/In (iii)-based MOFs for the detection of pollutants. New J. Chem. 2022, 46, 19577–19592. [Google Scholar] [CrossRef]
- Adelabu, I.O.; Saleh, T.A.; Garrison, T.F.; Al Hamouz, O.C.S. Synthesis of Polyamine-CNT Composites for the Removal of Toxic Cadmium Metal Ions from Wastewater. J. Mol. Liq. 2020, 297, 111827. [Google Scholar] [CrossRef]
- Begum, S.; Yuhana, N.Y.; Saleh, N.M.; Kamarudin, N.H.N.; Sulong, A.B. Review of Chitosan Composite as a Heavy Metal Adsorbent: Material Preparation and Properties. Carbohydr. Polym. 2021, 259, 117613. [Google Scholar] [CrossRef]
- Gupta, K.; Joshi, P.; Gusain, R.; Khatri, O.P. Recent Advances in Adsorptive Removal of Heavy Metal and Metalloid Ions by Metal Oxide-Based Nanomaterials. Coord. Chem. Rev. 2021, 445, 214100. [Google Scholar] [CrossRef]
- Mahmoud, M.E.; Mohamed, A.K. Novel Derived Pectin Hydrogel from Mandarin Peel Based Metal-Organic Frameworks Composite for Enhanced Cr (VI) and Pb (II) Ions Removal. Int. J. Biol. Macromol. 2020, 164, 920–931. [Google Scholar] [CrossRef] [PubMed]
- Yanrong, C.A.I.; Weili, J.; Chun, C. Research Progress on Removal of Metal Ions from Water by Marine Waste Biomass Based Adsorbent. J. Light Ind. 2022, 37, 100–110. [Google Scholar]
- Zhang, H.; Dang, Q.; Liu, C.; Yu, D.; Wang, Y.; Pu, X.; Liu, Y.; Liang, Y.; Cha, D. Fabrication of Methyl Acrylate and Tetraethylenepentamine Grafted Magnetic Chitosan Microparticles for Capture of Cd (II) from Aqueous Solutions. J. Hazard. Mater. 2019, 366, 346–357. [Google Scholar] [CrossRef]
- Imran, M.; Khan, Z.U.H.; Iqbal, J.; Shah, N.S.; Muzammil, S.; Ali, S.; Muhammad, N.; Aziz, A.; Murtaza, B.; Naeem, M.A. Potential of Siltstone and Its Composites with Biochar and Magnetite Nanoparticles for the Removal of Cadmium from Contaminated Aqueous Solutions: Batch and Column Scale Studies. Environ. Pollut. 2020, 259, 113938. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Ayati, A.; Davoodi, R.; Tanhaei, B.; Karimi, F.; Malekmohammadi, S.; Orooji, Y.; Fu, L.; Sillanpää, M. Recent Advances in Using of Chitosan-Based Adsorbents for Removal of Pharmaceutical Contaminants: A Review. J. Clean. Prod. 2021, 291, 125880. [Google Scholar] [CrossRef]
- Ahamad, T.; Naushad, M.; Alshehri, S.M. Fabrication of Magnetic Polymeric Resin for the Removal of Toxic Metals from Aqueous Medium: Kinetics and Adsorption Mechanisms. J. Water Process Eng. 2020, 36, 101284. [Google Scholar] [CrossRef]
- Zheng, M.; Chen, J.; Zhang, L.; Cheng, Y.; Lu, C.; Liu, Y.; Singh, A.; Trivedi, M.; Kumar, A.; Liu, J. Metal organic frameworks as efficient adsorbents for drugs from wastewater. Mater. Today Commun. 2022, 31, 103514. [Google Scholar] [CrossRef]
- Doshi, B.; Ayati, A.; Tanhaei, B.; Repo, E.; Sillanpää, M. Partially Carboxymethylated and Partially Cross-Linked Surface of Chitosan versus the Adsorptive Removal of Dyes and Divalent Metal Ions. Carbohydr. Polym. 2018, 197, 586–597. [Google Scholar] [CrossRef]
- Li, D.; Tian, X.; Wang, Z.; Guan, Z.; Li, X.; Qiao, H.; Ke, H.; Luo, L.; Wei, Q. Multifunctional Adsorbent Based on Metal-Organic Framework Modified Bacterial Cellulose/Chitosan Composite Aerogel for High Efficient Removal of Heavy Metal Ion and Organic Pollutant. Chem. Eng. J. 2020, 383, 123127. [Google Scholar] [CrossRef]
- Karimi, F.; Ayati, A.; Tanhaei, B.; Sanati, A.L.; Afshar, S.; Kardan, A.; Dabirifar, Z.; Karaman, C. Removal of Metal Ions Using a New Magnetic Chitosan Nano-Bio-Adsorbent; A Powerful Approach in Water Treatment. Environ. Res. 2022, 203, 111753. [Google Scholar] [CrossRef]
- Chen, B.; Long, F.; Chen, S.; Cao, Y.; Pan, X. Magnetic Chitosan Biopolymer as a Versatile Adsorbent for Simultaneous and Synergistic Removal of Different Sorts of Dyestuffs from Simulated Wastewater. Chem. Eng. J. 2020, 385, 123926. [Google Scholar] [CrossRef]
- Liu, J.; Chen, Y.; Han, T.; Cheng, M.; Zhang, W.; Long, J.; Fu, X. A Biomimetic SiO2@Chitosan Composite as Highly-Efficient Adsorbent for Removing Heavy Metal Ions in Drinking Water. Chemosphere 2019, 214, 738–742. [Google Scholar] [CrossRef]
- Chen, B.; Zhao, H.; Chen, S.; Long, F.; Huang, B.; Yang, B.; Pan, X. A Magnetically Recyclable Chitosan Composite Adsorbent Functionalized with EDTA for Simultaneous Capture of Anionic Dye and Heavy Metals in Complex Wastewater. Chem. Eng. J. 2019, 356, 69–80. [Google Scholar] [CrossRef]
- Huang, Y.; Hu, C.; An, Y.; Xiong, Z.; Hu, X.; Zhang, G.; Zheng, H. Magnetic Phosphorylated Chitosan Composite as a Novel Adsorbent for Highly Effective and Selective Capture of Lead from Aqueous Solution. J. Hazard. Mater. 2021, 405, 124195. [Google Scholar] [CrossRef]
- Shaumbwa, V.R.; Liu, D.; Archer, B.; Li, J.; Su, F. Preparation and Application of Magnetic Chitosan in Environmental Remediation and Other Fields: A Review. J. Appl. Polym. Sci. 2021, 138, 51241. [Google Scholar] [CrossRef]
- Mohseni-Bandpi, A.; Kakavandi, B.; Kalantary, R.R.; Azari, A.; Keramati, A. Development of a Novel Magnetite–Chitosan Composite for the Removal of Fluoride from Drinking Water: Adsorption Modeling and Optimization. RSC Adv. 2015, 5, 73279–73289. [Google Scholar] [CrossRef]
- Wu, K.; Su, D.; Liu, J.; Saha, R.; Wang, J.-P. Magnetic Nanoparticles in Nanomedicine: A Review of Recent Advances. Nanotechnology 2019, 30, 502003. [Google Scholar] [CrossRef] [PubMed]
- Kabra, A.; Martins, N.; Sharma, R.; Kabra, R.; Baghel, U.S. Myrica Esculenta Buch.-Ham. Ex D. Don: A Natural Source for Health Promotion and Disease Prevention. Plants 2019, 8, 149. [Google Scholar] [CrossRef] [PubMed]
- de Jesús Ruíz-Baltazar, Á.; Reyes-López, S.Y.; de Lourdes Mondragón-Sánchez, M.; Robles-Cortés, A.I.; Pérez, R. Eco-Friendly Synthesis of Fe3O4 Nanoparticles: Evaluation of Their Catalytic Activity in Methylene Blue Degradation by Kinetic Adsorption Models. Results Phys. 2019, 12, 989–995. [Google Scholar] [CrossRef]
- Appu, M.; Lian, Z.; Zhao, D.; Huang, J. Biosynthesis of Chitosan-Coated Iron Oxide (Fe3O4) Hybrid Nanocomposites from Leaf Extracts of Brassica oleracea L. and Study on Their Antibacterial Potentials. 3 Biotech 2021, 11, 271. [Google Scholar] [CrossRef]
- Rai, P.; Usman, U.L.; Banerjee, S. Sequestration of Cadmium Ions from Aqueous Phase Using Magnetic Chitosan Composite. Mater. Today Proc. 2022, 49, 3375–3383. [Google Scholar] [CrossRef]
- Jaafari, J.; Barzanouni, H.; Mazloomi, S.; Farahani, N.A.A.; Sharafi, K.; Soleimani, P.; Haghighat, G.A. Effective Adsorptive Removal of Reactive Dyes by Magnetic Chitosan Nanoparticles: Kinetic, Isothermal Studies and Response Surface Methodology. Int. J. Biol. Macromol. 2020, 164, 344–355. [Google Scholar] [CrossRef]
- Bouafia, A.; Laouini, S.E.; Khelef, A.; Tedjani, M.L.; Guemari, F. Effect of Ferric Chloride Concentration on the Type of Magnetite (Fe3O4) Nanoparticles Biosynthesized by Aqueous Leaves Extract of Artemisia and Assessment of Their Antioxidant Activities. J. Clust. Sci. 2021, 32, 1033–1041. [Google Scholar] [CrossRef]
- Varma, R.; Vasudevan, S. Extraction, Characterization, and Antimicrobial Activity of Chitosan from Horse Mussel Modiolus Modiolus. ACS Omega 2020, 5, 20224–20230. [Google Scholar] [CrossRef]
- Subedi, N.; Lähde, A.; Abu-Danso, E.; Iqbal, J.; Bhatnagar, A. A Comparative Study of Magnetic Chitosan (Chi@Fe3O4) and Graphene Oxide Modified Magnetic Chitosan (Chi@Fe3O4GO) Nanocomposites for Efficient Removal of Cr (VI) from Water. Int. J. Biol. Macromol. 2019, 137, 948–959. [Google Scholar] [CrossRef]
- Nhiem, N.X.; Van Kiem, P.; Van Minh, C.; Tai, B.H.; Cuong, N.X.; Thu, V.K.; Kim, Y.H. A new monoterpenoid glycoside from Myrica esculenta and the inhibition of angiotensin I-converting enzyme. Chem. Pharm. Bull. 2010, 58, 1408–1410. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Lian, Z.; Xian, H.; Jiang, R.; Wang, N.; Weng, Y.; Peng, X.; Wang, S.; Ouyang, X. Adsorption of Pb (II) from Aqueous Solution by Polyacrylic Acid Grafted Magnetic Chitosan Nanocomposite. Int. J. Biol. Macromol. 2020, 154, 1537–1547. [Google Scholar] [CrossRef]
- Zhu, A.; Yuan, L.; Liao, T. Suspension of Fe3O4 Nanoparticles Stabilized by Chitosan and O-Carboxymethylchitosan. Int. J. Pharm. 2008, 350, 361–368. [Google Scholar] [CrossRef]
- Jiang, Z.; Li, N.; Li, P.-Y.; Liu, B.; Lai, H.-J.; Jin, T. One-Step Preparation of Chitosan-Based Magnetic Adsorbent and Its Application to the Adsorption of Inorganic Arsenic in Water. Molecules 2021, 26, 1785. [Google Scholar] [CrossRef]
- Peralta, M.E.; Nisticò, R.; Franzoso, F.; Magnacca, G.; Fernandez, L.; Parolo, M.E.; León, E.G.; Carlos, L. Highly Efficient Removal of Heavy Metals from Waters by Magnetic Chitosan-Based Composite. Adsorption 2019, 25, 1337–1347. [Google Scholar] [CrossRef]
- Liu, Y.; Shen, L. From Langmuir Kinetics to First-and Second-Order Rate Equations for Adsorption. Langmuir 2008, 24, 11625–11630. [Google Scholar] [CrossRef] [PubMed]
- Sarojini, G.; Venkateshbabu, S.; Rajasimman, M. Facile Synthesis and Characterization of Polypyrrole—Iron Oxide—Seaweed (PPy-Fe3O4-SW) Nanocomposite and Its Exploration for Adsorptive Removal of Pb(II) from Heavy Metal Bearing Water. Chemosphere 2021, 278, 130400. [Google Scholar] [CrossRef] [PubMed]
- Peers, A.M. Elovich Adsorption Kinetics and the Heterogeneous Surface. J. Catal. 1965, 4, 499–503. [Google Scholar] [CrossRef]
- Revellame, E.D.; Fortela, D.L.; Sharp, W.; Hernandez, R.; Zappi, M.E. Adsorption Kinetic Modeling Using Pseudo-First Order and Pseudo-Second Order Rate Laws: A Review. Clean. Eng. Technol. 2020, 1, 100032. [Google Scholar] [CrossRef]
- Sahoo, T.R.; Prelot, B. Chapter 7—Adsorption Processes for the Removal of Contaminants from Wastewater: The Perspective Role of Nanomaterials and Nanotechnology. In Micro and Nano Technologies; Bonelli, B., Freyria, F.S., Rossetti, I., Sethi, R.B.T.-N., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 161–222. ISBN 978-0-12-818489-9. [Google Scholar]
- Ayub, A.; Irfan, A.; Raza, Z.A.; Abbas, M.; Muhammad, A.; Ahmad, K.; Munwar, A. Development of Poly (1-Vinylimidazole)-Chitosan Composite Sorbent under Microwave Irradiation for Enhanced Uptake of Cd (II) Ions from Aqueous Media. Polym. Bull. 2021, 79, 807–827. [Google Scholar] [CrossRef]
- Pavithra, S.; Thandapani, G.; Sugashini, S.; Sudha, P.N.; Alkhamis, H.H.; Alrefaei, A.F.; Almutairi, M.H. Batch Adsorption Studies on Surface Tailored Chitosan/Orange Peel Hydrogel Composite for the Removal of Cr (VI) and Cu (II) Ions from Synthetic Wastewater. Chemosphere 2021, 271, 129415. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, X.; Peng, L.; Han, S.; Hao, C.; Jiang, C.; Wang, H.; Fan, X. Effective Removal of Heavy Metals from Water Using Porous Lignin-Based Adsorbents. Chemosphere 2021, 279, 130504. [Google Scholar] [CrossRef] [PubMed]
- Jadoun, S.; Fuentes, J.P.; Urbano, B.F.; Yáñez, J. Highly Efficient Remediation of Cu (II) from Water by Novel Poly(o-Phenylenediamine)/Zinc Oxide Nanohybrids: Kinetic, Equilibrium, and Thermodynamic Studies. J. Water Process Eng. 2023, 53, 103663. [Google Scholar] [CrossRef]
- Massoudinejad, M.; Rasoulzadeh, H.; Ghaderpoori, M. Magnetic Chitosan Nanocomposite: Fabrication, Properties, and Optimization for Adsorptive Removal of Crystal Violet from Aqueous Solutions. Carbohydr. Polym. 2019, 206, 844–853. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Yadav, V.; Kumari, S.; Ghosh, D.; Rawat, P.; Vij, A.; Srivastava, C.; Saini, S.; Sharma, V.; Hassan, M.I. Deciphering the Potent Application of Nanobentonite and α-Fe2O3/Bentonite Nanocomposite in Dye Removal: Revisiting the Insights of Adsorption Mechanism. Appl. Nanosci. 2023, 13, 883–897. [Google Scholar] [CrossRef]
- Inyinbor, A.A.; Adekola, F.A.; Olatunji, G.A. Kinetics and Isothermal Modeling of Liquid Phase Adsorption of Rhodamine B onto Urea Modified Raphia Hookerie Epicarp. Appl. Water Sci. 2017, 7, 3257–3266. [Google Scholar] [CrossRef]
- Togue Kamga, F. Modeling Adsorption Mechanism of Paraquat onto Ayous (Triplochiton scleroxylon) Wood Sawdust. Appl. Water Sci. 2019, 9, 1. [Google Scholar] [CrossRef]
- Manzoor, K.; Ahmad, M.; Ahmad, S.; Ikram, S. Removal of Pb (Ii) and Cd (Ii) from Wastewater Using Arginine Cross-Linked Chitosan–Carboxymethyl Cellulose Beads as Green Adsorbent. RSC Adv. 2019, 9, 7890–7902. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Zhang, Z.; Cui, W.; Zhang, X.; Wang, S. Removing Copper and Cadmium from Water and Sediment by Magnetic Microspheres-MnFe2O4/Chitosan Prepared by Waste Shrimp Shells. J. Environ. Chem. Eng. 2021, 9, 104647. [Google Scholar] [CrossRef]
- Salehi, N.; Moghimi, A.; Shahbazi, H. Preparation of Cross-Linked Magnetic Chitosan with Methionine-Glutaraldehyde for Removal of Heavy Metals from Aqueous Solutions. Int. J. Environ. Anal. Chem. 2022, 102, 2305–2321. [Google Scholar] [CrossRef]
- Babakhani, A.; Sartaj, M. Removal of Cadmium (II) from Aqueous Solution Using Tripolyphosphate Cross-Linked Chitosan. J. Environ. Chem. Eng. 2020, 8, 103842. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, R.; Wang, C.; Zhou, G.; Hua, C.; Cao, Y.; Song, Z. Novel Environmental-Friendly Nano-Composite Magnetic Attapulgite Functionalized by Chitosan and EDTA for Cadmium (II) Removal. J. Alloys Compd. 2020, 817, 153286. [Google Scholar] [CrossRef]
- Fan, H.-L.; Zhou, S.-F.; Jiao, W.-Z.; Qi, G.-S.; Liu, Y.-Z. Removal of Heavy Metal Ions by Magnetic Chitosan Nanoparticles Prepared Continuously via High-Gravity Reactive Precipitation Method. Carbohydr. Polym. 2017, 174, 1192–1200. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Tao, Y.; Chen, S.; Li, H.; Chen, P.; Wei, M.; Wang, H.; Li, K.; Mazzeo, M.; Duan, Y. A Flexible Plasma-Treated Silver-Nanowire Electrode for Organic Light-Emitting Devices. Sci. Rep. 2017, 7, 16468. [Google Scholar] [CrossRef] [PubMed]
- Ahamad, T.; Naushad, M.; Eldesoky, G.E.; Alqadami, A.A.; Khan, A. Synthesis and Characterization of Egg-Albumen-Formaldehyde Based Magnetic Polymeric Resin (MPR): Highly Efficient Adsorbent for Cd (II) Ion Removal from Aqueous Medium. J. Mol. Liq. 2019, 286, 110951. [Google Scholar] [CrossRef]
- Alosaimi, E.H.; Alsohaimi, I.H.; Dahan, T.E.; Chen, Q.; Melhi, S. Adsorptive Performance of Tetracarboxylic Acid-Modified Magnetic Silica Nanocomposite for Recoverable Efficient Removal of Toxic Cd (II) from Aqueous Environment: Equilibrium, Isotherm, and Reusability Studies. J. Mol. Liq. 2021, 334, 116069. [Google Scholar] [CrossRef]
- Mahmoud, M.E.; Amira, M.F.; Seleim, S.M.; Mohamed, A.K. Amino-Decorated Magnetic Metal-Organic Framework as a Potential Novel Platform for Selective Removal of Chromium (Vl), Cadmium (II) and Lead (II). J. Hazard. Mater. 2020, 381, 120979. [Google Scholar] [CrossRef]
- Wu, Z.; Deng, W.; Zhou, W.; Luo, J. Novel Magnetic Polysaccharide/Graphene Oxide@Fe3O4 Gel Beads for Adsorbing Heavy Metal Ions. Carbohydr. Polym. 2019, 216, 119–128. [Google Scholar] [CrossRef]
- Wang, L.; Hu, D.; Kong, X.; Liu, J.; Li, X.; Zhou, K.; Zhao, H.; Zhou, C. Anionic Polypeptide Poly (γ-Glutamic Acid)-Functionalized Magnetic Fe3O4-GO-(o-MWCNTs) Hybrid Nanocomposite for High-Efficiency Removal of Cd (II), Cu (II) and Ni (II) Heavy Metal Ions. Chem. Eng. J. 2018, 346, 38–49. [Google Scholar] [CrossRef]
- Liu, G.; Liao, L.; Dai, Z.; Qi, Q.; Wu, J.; Ma, L.Q.; Tang, C.; Xu, J. Organic Adsorbents Modified with Citric Acid and Fe3O4 Enhance the Removal of Cd and Pb in Contaminated Solutions. Chem. Eng. J. 2020, 395, 125108. [Google Scholar] [CrossRef]
- Naushad, M.; Ahamad, T.; Al-Ghanim, K.A.; Ala’a, H.; Eldesoky, G.E.; Khan, A.A. A Highly Porous Nanocomposite (Fe3O4@ BFR) for the Removal of Toxic Cd (II) Ions from Aqueous Environment: Adsorption Modelling and Regeneration Study. Compos. Part B Eng. 2019, 172, 179–185. [Google Scholar] [CrossRef]
- Behbahani, E.S.; Dashtian, K.; Ghaedi, M. Fe3O4-FeMoS4: Promise Magnetite LDH-Based Adsorbent for Simultaneous Removal of Pb (II), Cd (II), and Cu (II) Heavy Metal Ions. J. Hazard. Mater. 2021, 410, 124560. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Xie, Y.; Ren, X.; Song, G.; Alsaedi, A.; Hayat, T.; Chen, C. Efficient Removal of Cd (II) by Core-Shell Fe3O4@ Polydopamine Microspheres from Aqueous Solution. J. Mol. Liq. 2019, 295, 111724. [Google Scholar] [CrossRef]
- Liu, C.; Jiang, X.; Wang, X.; Wang, Q.; Li, L.; Zhang, F.; Liang, W. Magnetic Polyphenol Nanocomposite of Fe3O4/SiO2/PP for Cd (II) Adsorption from Aqueous Solution. Environ. Technol. 2022, 43, 935–948. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Dong, X.; Zhao, L. Application of Magnetic Chitosan Nanocomposites Modified by Graphene Oxide and Polyethyleneimine for Removal of Toxic Heavy Metals and Dyes from Water. Int. J. Biol. Macromol. 2021, 192, 118–125. [Google Scholar] [CrossRef]
- Abou El-Reash, Y.G. Magnetic Chitosan Modified with Cysteine-Glutaraldehyde as Adsorbent for Removal of Heavy Metals from Water. J. Environ. Chem. Eng. 2016, 4, 3835–3847. [Google Scholar] [CrossRef]
- Li, B.; Zhou, F.; Huang, K.; Wang, Y.; Mei, S.; Zhou, Y.; Jing, T. Environmentally Friendly Chitosan/PEI-Grafted Magnetic Gelatin for the Highly Effective Removal of Heavy Metals from Drinking Water. Sci. Rep. 2017, 7, 43082. [Google Scholar] [CrossRef]
- Zeng, X.; Zhang, G.; Wen, J.; Li, X.; Zhu, J.; Wu, Z. Simultaneous Removal of Aqueous Same Ionic Type Heavy Metals and Dyes by a Magnetic Chitosan/Polyethyleneimine Embedded Hydrophobic Sodium Alginate Composite: Performance, Interaction and Mechanism. Chemosphere 2023, 318, 137869. [Google Scholar] [CrossRef]
- Le, T.T.N.; Le, V.T.; Dao, M.U.; Nguyen, Q.V.; Vu, T.T.; Nguyen, M.H.; Tran, D.L.; Le, H.S. Preparation of Magnetic Graphene Oxide/Chitosan Composite Beads for Effective Removal of Heavy Metals and Dyes from Aqueous Solutions. Chem. Eng. Commun. 2019, 206, 1337–1352. [Google Scholar] [CrossRef]
- Fan, L.; Luo, C.; Sun, M.; Li, X.; Qiu, H. Highly Selective Adsorption of Lead Ions by Water-Dispersible Magnetic Chitosan/Graphene Oxide Composites. Colloids Surf. B Biointerfaces 2013, 103, 523–529. [Google Scholar] [CrossRef]
- Lv, L.; Chen, N.; Feng, C.; Zhang, J.; Li, M. Heavy Metal Ions Removal from Aqueous Solution by Xanthate-Modified Cross-Linked Magnetic Chitosan/Poly (Vinyl Alcohol) Particles. RSC Adv. 2017, 7, 27992–28000. [Google Scholar] [CrossRef]
- Liu, T.; Han, X.; Wang, Y.; Yan, L.; Du, B.; Wei, Q.; Wei, D. Magnetic Chitosan/Anaerobic Granular Sludge Composite: Synthesis, Characterization and Application in Heavy Metal Ions Removal. J. Colloid Interface Sci. 2017, 508, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Peighambardoust, S.J.; Foroutan, R.; Peighambardoust, S.H.; Khatooni, H.; Ramavandi, B. Decoration of Citrus Limon Wood Carbon with Fe3O4 to Enhanced Cd2+ Removal: A Reclaimable and Magnetic Nanocomposite. Chemosphere 2021, 282, 131088. [Google Scholar] [CrossRef] [PubMed]
- Homayonfard, A.; Miralinaghi, M.; Shirazi, R.H.S.M.; Moniri, E. Efficient Removal of Cadmium (II) Ions from Aqueous Solution by CoFe2O4/Chitosan and NiFe2O4/Chitosan Composites as Adsorbents. Water Sci. Technol. 2018, 78, 2297–2307. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Wang, Y.; Li, Y.; Wei, Q.; Hu, L.; Yan, T.; Feng, R.; Yan, L.; Du, B. Phosphorylated Chitosan/CoFe2O4 Composite for the Efficient Removal of Pb (II) and Cd (II) from Aqueous Solution: Adsorption Performance and Mechanism Studies. J. Mol. Liq. 2019, 277, 181–188. [Google Scholar] [CrossRef]
- Song, X.; Li, L.; Zhou, L.; Chen, P. Magnetic Thiolated/Quaternized-Chitosan Composites Design and Application for Various Heavy Metal Ions Removal, Including Cation and Anion. Chem. Eng. Res. Des. 2018, 136, 581–592. [Google Scholar] [CrossRef]
- Reddy, D.H.K.; Lee, S.M. Application of magnetic chitosan composites for the removal of toxic metal and dyes from aqueous solutions. Adv. Colloid Interface Sci. 2013, 201, 68–93. [Google Scholar] [CrossRef]
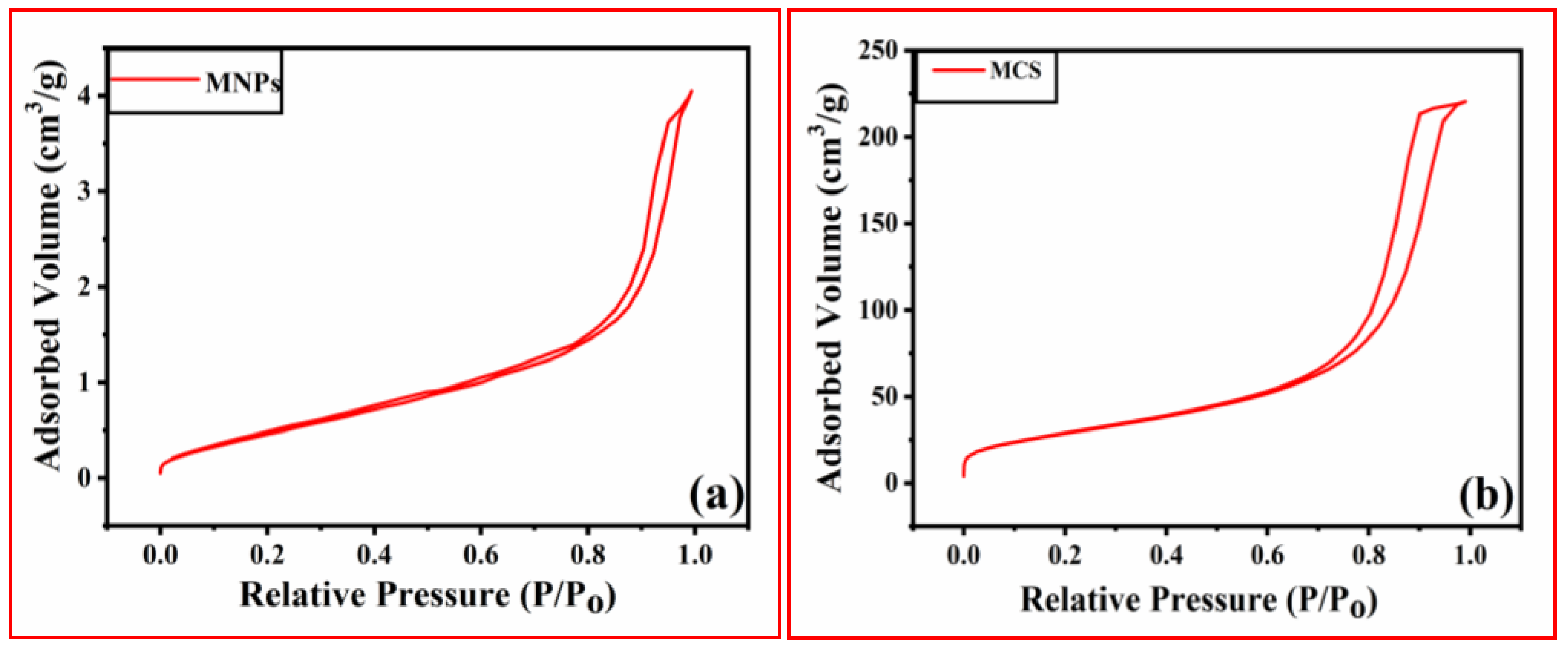


| Parameters | MNPs | MCS |
|---|---|---|
| BET specific surface area (m2/g) | 105 | 173 |
| Langmuir surface area (m2/g) | 201 | 537 |
| Average pore size (Å) | 64.36 | 49.77 |
| Total pore volume (cc/g) | 0.3410 | 0.4305 |
| S. No. | Materials | Qe(mg/g) | Reference |
|---|---|---|---|
| 1 | Egg-albumen-formaldehyde-based magnetic polymeric resin | 149.3 | [56] |
| 2 | Mesoporous magnetic nanocomposite | 158.68 | [57] |
| 3 | Amino-decorated magnetic metal-organic framework | 693.0 | [58] |
| 4 | Carboxymethyl chitosan/sodium alginate/graphene oxide@ Fe3O4 beads | 86.28 | [59] |
| 6 | Poly(γ-glutamic acid) modified magnetic Fe3O4-GO-(o-MWCNTs) hybrid nanocomposite | 625.00 | [60] |
| 7 | Citric acid- and Fe3O4-modified sugarcane bagasse | 33.2 | [61] |
| 8 | Fe3O4@Biuret-formaldehyde pre polymeric resin | 92.6 | [62] |
| 9 | Fe3O4/FeMoS4/MgAl-LDH nanocomposite | 140.50 | [63] |
| 10 | Fe3O4@PDA microspheres | 296.4 | [64] |
| 11 | Fe3O4/SiO2/PP | 30.1 | [65] |
| 12 | Fe3O4 nanoparticles | 290 | This work |
| 13 | Fe3O4/Chitosan composite | 426 | This work |
| S. No. | Materials | Heavy Metals | Qe(mg/g) | Reference |
|---|---|---|---|---|
| 1. | Magnetic chitosan composite | Ni(II) Cu(II) Pb(II) | 108.9 216.8 220.9 | [35] |
| 2. | Magnetic chitosan nanocomposites modified with graphene oxide and polyethyleneimine |
As(V) Hg(II) |
220.26 124.84 | [66] |
| 3. | Chitosan magnetic beads modified with cysteine glutaraldehyde Schiff’s base | Cu(II) Cr(VI) | 156.49 138.53 | [67] |
| 4. | PEI-grafted magnetic gelatin | Pb(II) Cd (II) | 341 321 | [68] |
| 5. | Magnetic chitosan/polyethyleneimine embedded hydrophobic sodium alginate composite | Cr(VI) Cu(II) | 87.53 351.03 | [69] |
| 6. | Magnetic graphene oxide/chitosan composite beads | Ni(II) | 80.48 | [70] |
| 7. | Magnetic Fe3O4/Chitosan nanoparticles | Pb(II) Cd (II) | 79.24 36.42 | [54] |
| 8. | Magnetic chitosan/graphene oxide (MCGO) materials | Pb(II) | 76.94 | [71] |
| 9. | Xanthate-modified cross-linked magnetic chitosan/poly(vinyl alcohol) particles | Pb(II) Cu(II) | 59.855 139.797 | [72] |
| 10. | Magnetic anaerobic granule sludge/chitosan composite | Pb(II) Cu(II) | 97.97 83.65 | [73] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yadav, A.; Raghav, S.; Jangid, N.K.; Srivastava, A.; Jadoun, S.; Srivastava, M.; Dwivedi, J. Myrica esculenta Leaf Extract—Assisted Green Synthesis of Porous Magnetic Chitosan Composites for Fast Removal of Cd (II) from Water: Kinetics and Thermodynamics of Adsorption. Polymers 2023, 15, 4339. https://doi.org/10.3390/polym15214339
Yadav A, Raghav S, Jangid NK, Srivastava A, Jadoun S, Srivastava M, Dwivedi J. Myrica esculenta Leaf Extract—Assisted Green Synthesis of Porous Magnetic Chitosan Composites for Fast Removal of Cd (II) from Water: Kinetics and Thermodynamics of Adsorption. Polymers. 2023; 15(21):4339. https://doi.org/10.3390/polym15214339
Chicago/Turabian StyleYadav, Anjali, Sapna Raghav, Nirmala Kumari Jangid, Anamika Srivastava, Sapana Jadoun, Manish Srivastava, and Jaya Dwivedi. 2023. "Myrica esculenta Leaf Extract—Assisted Green Synthesis of Porous Magnetic Chitosan Composites for Fast Removal of Cd (II) from Water: Kinetics and Thermodynamics of Adsorption" Polymers 15, no. 21: 4339. https://doi.org/10.3390/polym15214339
APA StyleYadav, A., Raghav, S., Jangid, N. K., Srivastava, A., Jadoun, S., Srivastava, M., & Dwivedi, J. (2023). Myrica esculenta Leaf Extract—Assisted Green Synthesis of Porous Magnetic Chitosan Composites for Fast Removal of Cd (II) from Water: Kinetics and Thermodynamics of Adsorption. Polymers, 15(21), 4339. https://doi.org/10.3390/polym15214339










