The Effect of Sub- and Near-Critical Carbon Dioxide Assisted Manufacturing on Medical Thermoplastic Polyurethane
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
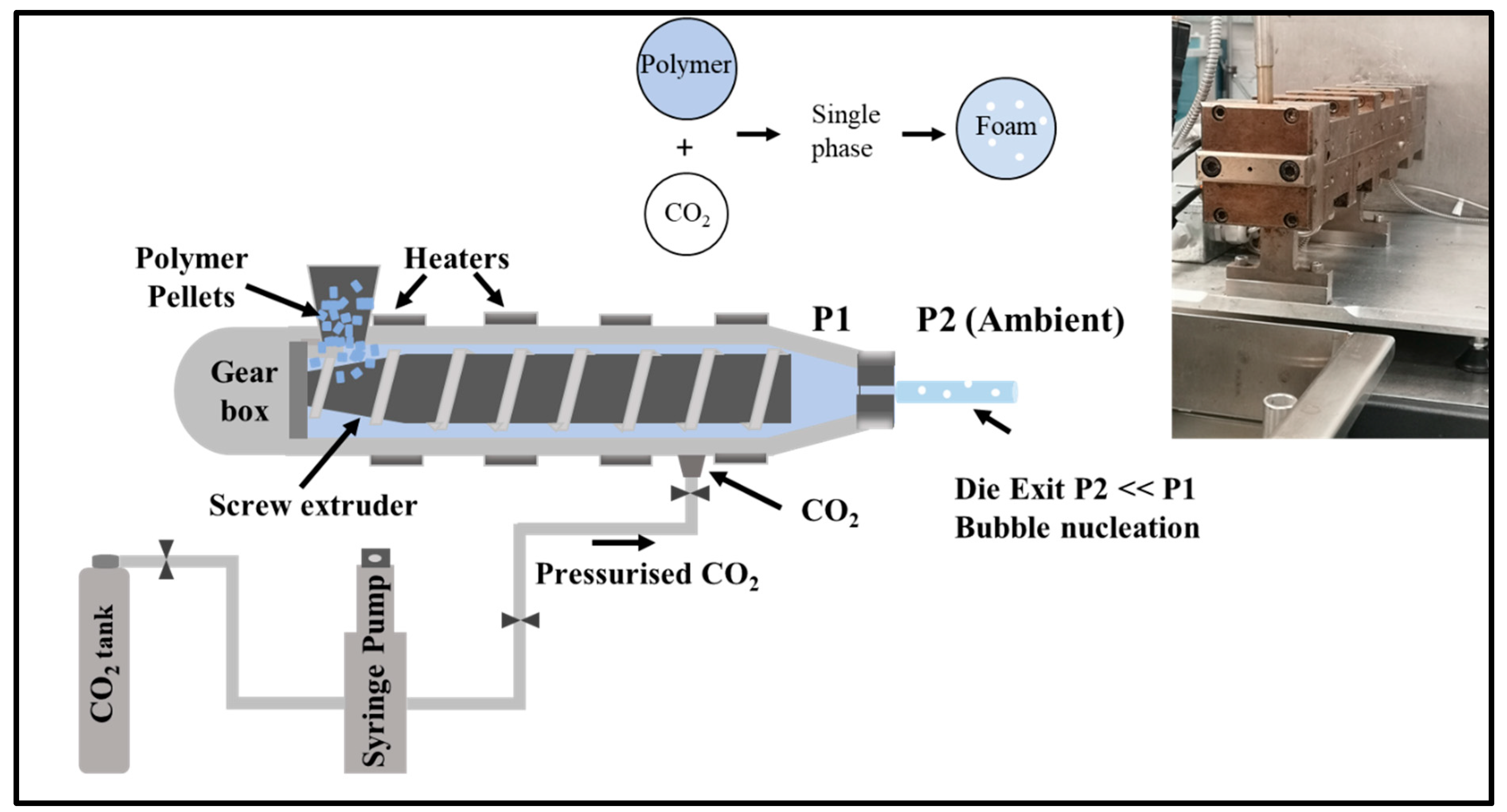
2.2. Experimental
2.3. Characterisation
2.3.1. Scanning Electron Microscopy (SEM)
2.3.2. Throughput Measurement
2.3.3. Differential Scanning Calorimetry (DSC)
2.3.4. Thermogravimetric Analysis (TGA)
2.3.5. Rheological Properties
2.3.6. Tensile Tests
3. Results and Discussion
3.1. Effect of CO2 on Process Parameters
3.2. Throughput of Pellethane Treated with and without CO2
3.3. Morphological Characterisation
3.4. Rheological Properties
3.5. DSC
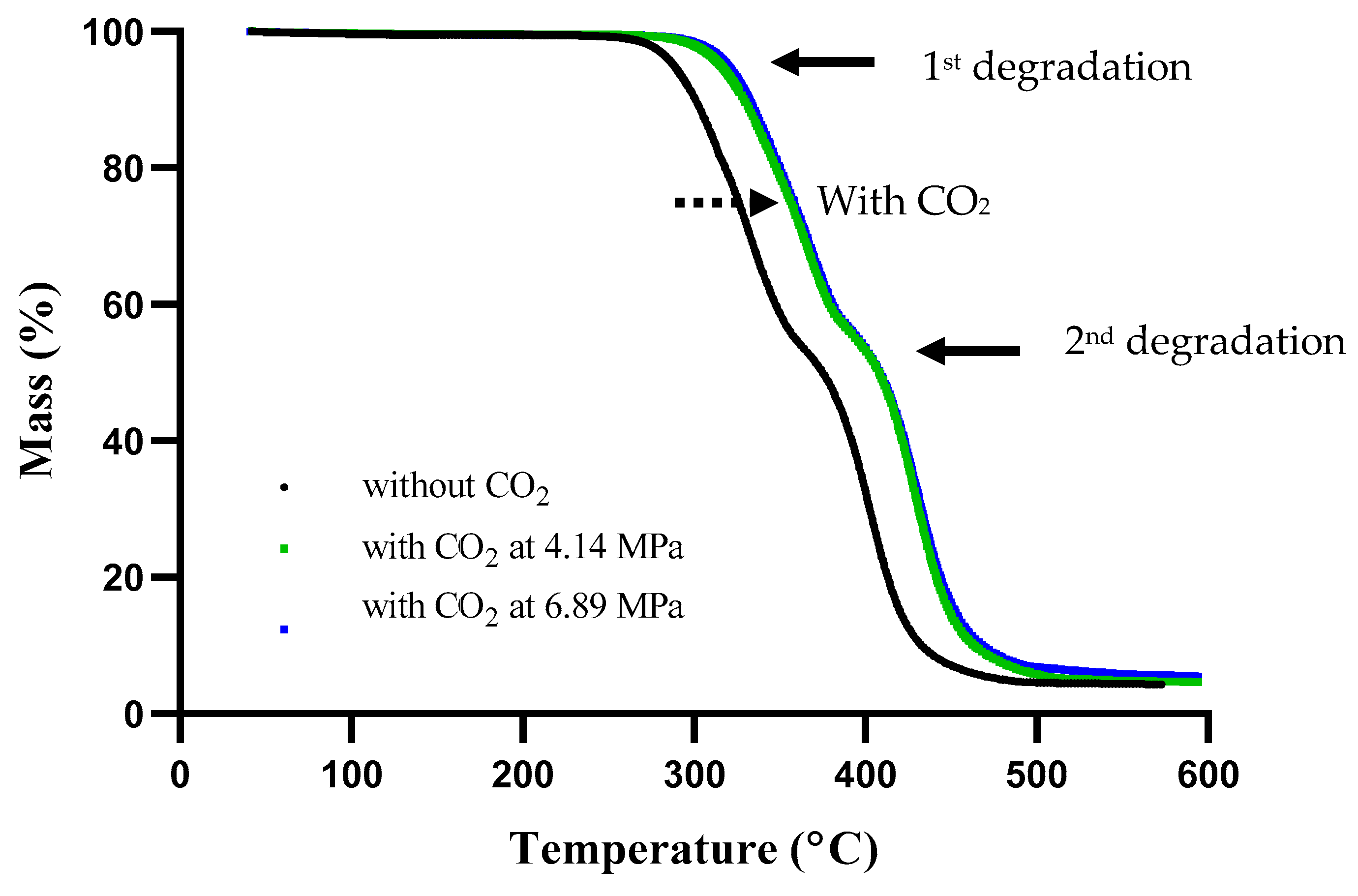
3.6. Thermal Stability
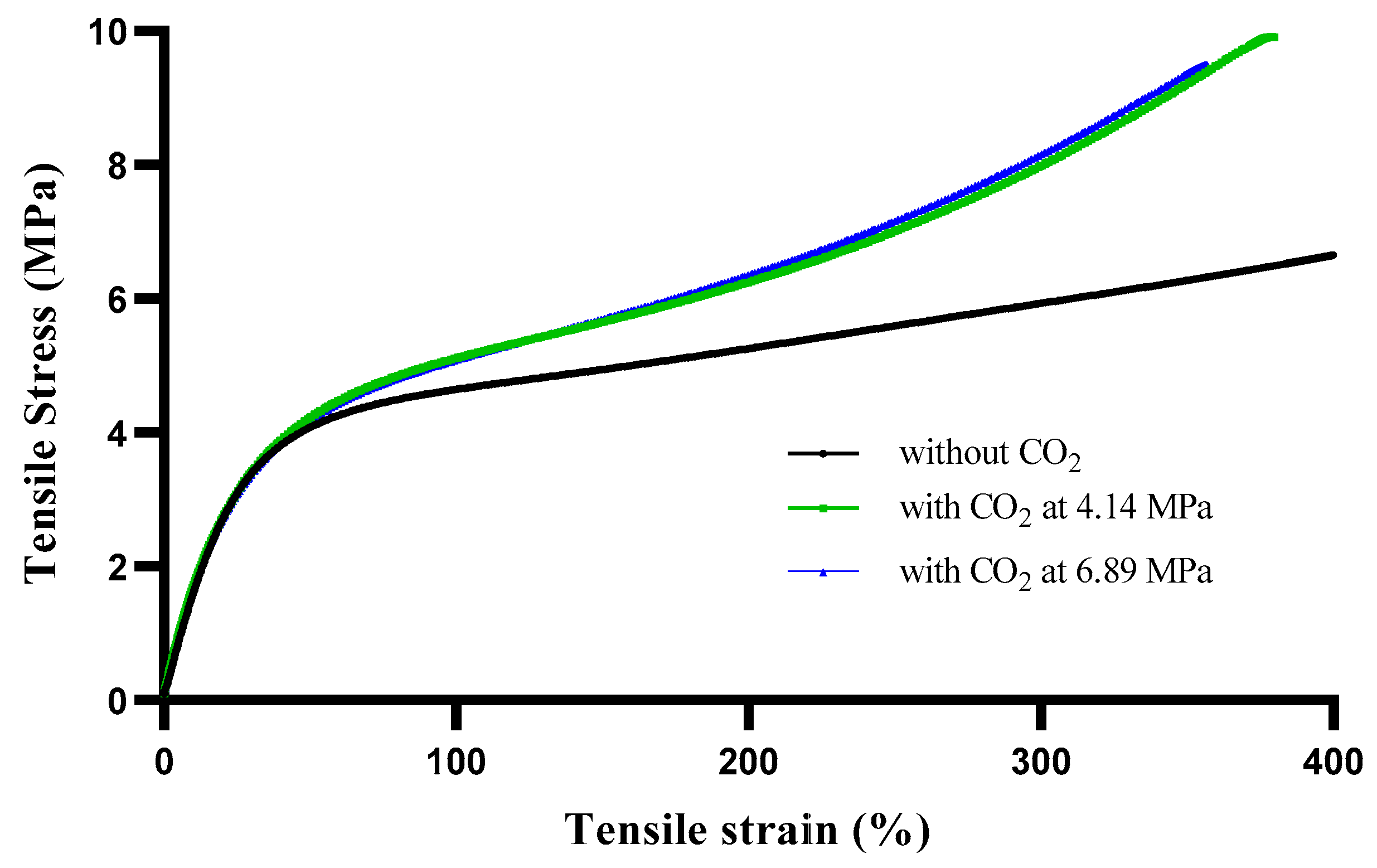
3.7. Mechanical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mailhe, M.; Aubry, C.; Brouqui, P.; Michelet, P.; Raoult, D.; Parola, P.; Lagier, J.-C. Complications of peripheral venous catheters (PVCs): The need to propose alternative route of administration. Int. J. Antimicrob. Agents 2020, 55, 105875. [Google Scholar] [CrossRef]
- Barde, M.; Davis, M.; Rangari, S.; Mendis, H.; De La Fuente, L.; Auad, M.L. Development of antimicrobial-loaded polyurethane films for drug-eluting catheters. J. Appl. Polym. Sci. 2018, 135, 8. [Google Scholar] [CrossRef]
- Zheng, Y.; Miao, J.; Zhang, F.; Cai, C.; Koh, A.; Simmons, T.J.; Mousa, S.A.; Linhardt, R.J. Surface modification of a polyethylene film for anticoagulant and antimicrobial catheter. React. Funct. Polym. 2016, 100, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Milne, M.; Bradbury, L. The Use of Ultrasound to Assess the Thrombogenic Properties of Teflon and Polyurethane Catheters for Short-Term Use in Systemically Healthy Horses. J. Equine Vet. Sci. 2009, 29, 833–841. [Google Scholar] [CrossRef]
- Phuengkham, H.; Nasongkla, N. Development of antibacterial coating on silicone surface via chlorhexidine-loaded nanospheres. J. Mater. Sci. Med. 2015, 26, 11. [Google Scholar] [CrossRef]
- Nozaki, S.; Hirai, T.; Higaki, Y.; Yoshinaga, K.; Kojio, K.; Takahara, A. Effect of chain architecture of polyol with secondary hydroxyl group on aggregation structure and mechanical properties of polyurethane elastomer. Polymer 2017, 116, 423–428. [Google Scholar] [CrossRef]
- Padsalgikar, A.D. 3–Speciality Plastics in Cardiovascular Applications. In Plastics in Medical Devices for Cardiovascular Applications; Padsalgikar, A.D., Ed.; William Andrew Publishing: Norwich, NY, USA, 2017; pp. 53–82. [Google Scholar] [CrossRef]
- Spontak, R.J.; Patel, N.P. Thermoplastic elastomers: Fundamentals and applications. Curr. Opin. Colloid Interface Sci. 2000, 5, 333–340. [Google Scholar] [CrossRef]
- Mrad, O.; Saunier, J.; Chodur, C.A.; Rosilio, V.; Agnely, F.; Aubert, P.; Vigneron, J.; Etcheberry, A.; Yagoubi, N. A comparison of plasma and electron beam-sterilization of PU catheters. Radiat. Phys. Chem. 2010, 79, 93–103. [Google Scholar] [CrossRef]
- Giles, H.F., Jr.; Mount, E.M., III; Wagner, J.R., Jr. Extrusion: The Definitive Processing Guide and Handbook, 2nd ed.; William Andrew: Norwich, NY, USA, 2013; pp. 1–620. [Google Scholar]
- Duroudier, J.-P. 5—Polymer Extruder Screw. In Fluid Transport; Duroudier, J.-P., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 163–195. [Google Scholar] [CrossRef]
- Huang, S.; O’Donnell, K.P.; de Vaux, S.D.; O’Brien, J.; Stutzman, J.; Williams, R.O., III. Processing thermally labile drugs by hot-melt extrusion: The lesson with gliclazide. Eur. J. Pharm. Biopharm. 2017, 119, 56–67. [Google Scholar] [CrossRef]
- Fleming, O.S.; Kazarian, S.G. Polymer Processing with Supercritical Fluids. In Supercrit Carbon Dioxide; Wiley Publishing: Hoboken, NJ, USA, 2005; pp. 205–238. [Google Scholar] [CrossRef]
- Cansell, F.; Aymonier, C.; Loppinet-Serani, A. Review on materials science and supercritical fluids. Curr. Opin. Solid State Mater. Sci. 2003, 7, 331–340. [Google Scholar] [CrossRef]
- Nalawade, S.P.; Picchioni, F.; Janssen, L.P.B.M. Supercritical carbon dioxide as a green solvent for processing polymer melts: Processing aspects and applications. Prog. Polym. Sci. 2006, 31, 19–43. [Google Scholar] [CrossRef]
- Higginbotham, C.L.; Yons, J.G.L.; Kennedy, J.E. 13-Polymer processing using supercritical fluids. In Advanced Polymer Processing Operations; Thomas, S., Weimin, Y., Eds.; Woodhead Publishing: Cambridge, UK, 2009; pp. 384–401. [Google Scholar] [CrossRef]
- Duarte, R.M.; Correia-Pinto, J.; Reis, R.L.; Duarte, A.R.C. Subcritical carbon dioxide foaming of polycaprolactone for bone tissue regeneration. J. Supercrit. Fluid. 2018, 140, 1–10. [Google Scholar] [CrossRef]
- Almutairi, M.; Srinivasan, P.; Zhang, P.; Austin, F.; Butreddy, A.; Alharbi, M.; Bandari, S.; Ashour, E.A.; Repka, M.A. Hot-Melt extrusion coupled with pressurized carbon dioxide for enhanced processability of pharmaceutical polymers and drug delivery applications–An integrated review. Int. J. Pharm. 2022, 629, 122291. [Google Scholar] [CrossRef] [PubMed]
- Snodgrass, J.M.; Pantelidis, D.; Jenkins, M.L.; Bravman, J.C.; Dauskardt, R.H. Subcritical debonding of polymer/silica interfaces under monotonic and cyclic loading. Acta Mater. 2002, 50, 2395–2411. [Google Scholar] [CrossRef]
- Poudel, A.; Karode, N.; Fitzhenry, L.; Kennedy, J.; Matthews, S.; Walsh, P.; Thomas, K.; Coffey, A. Investigation of the thermal, mechanical, electrical and morphological properties of supercritical carbon dioxide assisted extrusion of microphase-separated poly(styrene-ethylene/butylene-styrene). J. Supercrit. Fluid. 2017, 130, 1–9. [Google Scholar] [CrossRef]
- Verreck, G.; Decorte, A.; Li, H.; Tomasko, D.; Arien, A.; Peeters, J.; Rombaut, P.; Van den Mooter, G.; Brewster, M. The effect of pressurized carbon dioxide as a plasticizer and foaming agent on the hot melt extrusion process and extrudate properties of pharmaceutical polymers. J. Supercrit. Fluid. 2006, 38, 383–391. [Google Scholar] [CrossRef]
- Peng, H.-H.; Wang, Z.-D.; Guan, Y.-X.; Yao, S.-J. Supercritical CO2 assisted preparation of chitosan-based nano-in-microparticles with potential for efficient pulmonary drug delivery. J. CO2 Util. 2021, 46, 101486. [Google Scholar] [CrossRef]
- Amani, M.; Ardestani, N.S.; Majd, N.Y. Utilization of supercritical CO2 gas antisolvent (GAS) for production of Capecitabine nanoparticles as anti-cancer drug: Analysis and optimization of the process conditions. J. CO2 Util. 2021, 46, 101465. [Google Scholar] [CrossRef]
- Marković, D.; Milovanović, S.; De Clerck, K.; Zizovic, I.; Stojanović, D.; Radetić, M. Development of material with strong antimicrobial activity by high pressure CO2 impregnation of polyamide nanofibers with thymol. J. CO2 Util. 2018, 26, 19–27. [Google Scholar] [CrossRef]
- Verreck, G.; Decorte, A.; Heymans, K.; Adriaensen, J.; Liu, D.; Tomasko, D.; Arien, A.; Peeters, J.; Van den Mooter, G.; Brewster, M.E. Hot stage extrusion of p-amino salicylic acid with EC using CO2 as a temporary plasticizer. Int. J. Pharm. 2006, 327, 45–50. [Google Scholar] [CrossRef]
- Chauvet, M.; Sauceau, M.; Fages, J. Extrusion assisted by supercritical CO2: A review on its application to biopolymers. J. Supercrit. Fluid. 2017, 120, 408–420. [Google Scholar] [CrossRef]
- Zhang, J.; Martin, D.J.; Taran, E.; Thurecht, K.J.; Minchin, R.F. Effect of Supercritical Carbon Dioxide on the Loading and Release of Model Drugs from Polyurethane Films: Comparison with Solvent Casting, Macromol. Chem. Phys. 2014, 215, 54–64. [Google Scholar] [CrossRef]
- Winter, H. Three views of viscoelasticity for Cox–Merz materials. Rheol. Acta 2009, 48, 241–243. [Google Scholar] [CrossRef]
- Khan, M.; Sardar, H.; Gulzar, M.M.; Alshomrani, A.S. On multiple solutions of non-Newtonian Carreau fluid flow over an inclined shrinking sheet. Results Phys. 2018, 8, 926–932. [Google Scholar] [CrossRef]
- Xu, Z.-M.; Jiang, X.-L.; Liu, T.; Hu, G.-H.; Zhao, L.; Zhu, Z.-N.; Yuan, W.-K. Foaming of polypropylene with supercritical carbon dioxide. J. Supercrit. Fluid. 2007, 41, 299–310. [Google Scholar] [CrossRef]
- Doroudiani, S.; Park, C.B.; Kortschot, M.T. Effect of the crystallinity and morphology on the microcellular foam structure of semicrystalline polymers. Polym. Eng. Sci. 1996, 36, 2645–2662. [Google Scholar] [CrossRef]
- Xia, H.; Song, M. Preparation and characterization of polyurethane–carbon nanotube composites. Soft Matter. 2005, 1, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Karode, N.; Fitzhenry, L.; Poudel, A.; Walsh, P.; Matthews, S.; Coffey, A. Performance enhancement of PEBAX using supercritical fluid extrusion for biomedical applications. In Proceedings of the Conference: SPE ANTEC™ Indianapolis, Indianapolis, IN, USA, 23–25 May 2016. [Google Scholar]



| Properties | Test Method | Values |
|---|---|---|
| Shore hardness | ASTM D 2240 | 85-A |
| Glass transition temperature (Tg) | DSC | −47 °C |
| Vicat softening temperature | ASTM D 1525 | 81.7 °C |
| Melt flow rate, 190 ºC, 8700 g, g/10 min | ASTM D 1238 | 10 |
| Tensile modulus 50% elongation, MPa 100% elongation, MPa 300% elongation, MPa | ASTM D412 | 4.8 MPa 6.1 MPa 10.3 MPa |
| Ultimate tensile strength, MPa | ASTM D 412 | 28.9 MPa |
| Process Parameters | (1) Extrusion without CO2 (Control) | (2) Extrusion with CO2 @4.14 MPa | (3) Re-Extrusion with the Compounded Material from (2) | (4) Extrusion with CO2 @6.89 MPa | (5) Re-Extrusion with the Compounded Material from (4) |
|---|---|---|---|---|---|
| Temperature at zone 1 (°C) | 114 | 116 | 103 | 114 | 103 |
| Temperature at zone 2 (°C) | 170 | 170 | 150 | 170 | 150 |
| Temperature at zone 3 (°C) | 180 | 180 | 155 | 180 | 155 |
| Temperature at zone 4 (°C) | 185 | 185 | 160 | 185 | 160 |
| Temperature at melt (°C) | 188 | 186 | 163 | 186 | 162 |
| Temperature at die (°C) | 185 | 185 | 160 | 185 | 160 |
| Extruder torque (N/m) | 16 | 13 | 15 | 13 | 14 |
| Die pressure (MPa) | 2.76 | 1.65 | 2.21 | 1.65 | 2.21 |
| Screw speed (rpm) | 55 | 55 | 60 | 55 | 60 |
| CO2 pressure (MPa) | - | 4.14 | - | 6.89 | - |
| CO2 flow rate (ml/min) | - | 0.5 | - | 0.5 | - |
| CO2 temperature (°C) | - | 35 | - | 35 | - |
| Scenario | Average Weight of Extrudate (g) | % Increase |
|---|---|---|
| Without CO2 (control) | 8.33 ± 0.10 | - |
| With CO2 at 4.14 MPa | 10.31 ± 0.06 | 23.77% |
| With CO2 at 6.89 MPa | 10.53 ± 0.07 | 26.41% |
| Scenario | Zero-Shear Viscosity (Pa.s) | Regression (R2) | % Reduction |
|---|---|---|---|
| Without CO2 (control) | 690.407 | 0.9995 | - |
| With CO2 at 4.14 MPa | 439.374 | 0.9996 | 36.36% |
| With CO2 at 6.89 MPa | 413.900 | 0.9953 | 40.04% |
| Sample | Tg (°C) | Tm (°C) | Enthalpy (J/g) | Tc (°C) | Enthalpy (J/g) |
|---|---|---|---|---|---|
| Without CO2 (control) | −56.07 | 167.27 | 3.97 | 128.89 | 5.74 |
| With CO2 at 4.14 MPa | −61.65 | 161.16 | 2.37 | 76.79 | 4.74 |
| With CO2 at 6.89 MPa | −64.93 | 155.37 | 2.25 | 75.18 | 4.79 |
| Scenario | Tensile Modulus 50% Strain (%) | Tensile Modulus 100% Strain (%) | Tensile Modulus 300% Strain (%) | Final Tensile Strength (MPa) | Final Strain (%) |
|---|---|---|---|---|---|
| Without CO2 (control) | 4.08 ± 0.82 | 4.65 ± 0.30 | 5.93 ± 0.27 | 6.65 ± 0.39 | 415.25 ± 20.00 |
| With CO2 at 4.14 MPa | 4.09 ± 0.41 | 5.00 ± 0.50 | 7.71 ± 0.85 | 10.08 ± 0.91 | 428.72 ± 27.45 |
| With CO2 at 6.89 MPa | 4.15 ± 0.19 | 5.05 ± 0.24 | 7.71 ± 0.41 | 10.20 ± 0.86 | 422.86 ± 21.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baru, S.-i.; Matthews, S.; Marchese, E.; Walsh, P.; Coffey, A. The Effect of Sub- and Near-Critical Carbon Dioxide Assisted Manufacturing on Medical Thermoplastic Polyurethane. Polymers 2023, 15, 822. https://doi.org/10.3390/polym15040822
Baru S-i, Matthews S, Marchese E, Walsh P, Coffey A. The Effect of Sub- and Near-Critical Carbon Dioxide Assisted Manufacturing on Medical Thermoplastic Polyurethane. Polymers. 2023; 15(4):822. https://doi.org/10.3390/polym15040822
Chicago/Turabian StyleBaru, Sarn-ii, Siobhan Matthews, Eric Marchese, Philip Walsh, and Austin Coffey. 2023. "The Effect of Sub- and Near-Critical Carbon Dioxide Assisted Manufacturing on Medical Thermoplastic Polyurethane" Polymers 15, no. 4: 822. https://doi.org/10.3390/polym15040822
APA StyleBaru, S.-i., Matthews, S., Marchese, E., Walsh, P., & Coffey, A. (2023). The Effect of Sub- and Near-Critical Carbon Dioxide Assisted Manufacturing on Medical Thermoplastic Polyurethane. Polymers, 15(4), 822. https://doi.org/10.3390/polym15040822






