Bacterial Inhibition and Osteogenic Potentials of Sr/Zn Co-Doped Nano-Hydroxyapatite-PLGA Composite Scaffold for Bone Tissue Engineering Applications
Abstract
:1. Introduction
2. Methods
2.1. Preparation of Nano-Hydroxyapatite
2.2. Preparation of Zn/Sr-Substituted Nano-Hydroxyapatite
2.3. Preparation of Zn/Sr-nHAp-PLGA
2.4. Scaffold Fabrication
2.5. Assessment of Scaffolds’ Antibacterial Activity
2.6. Cell Culture
2.7. Scaffold Seeding
2.8. MTT Assay
2.9. Sr and Zn Ion Release Study
2.10. Statistical Analysis
3. Results and Discussion
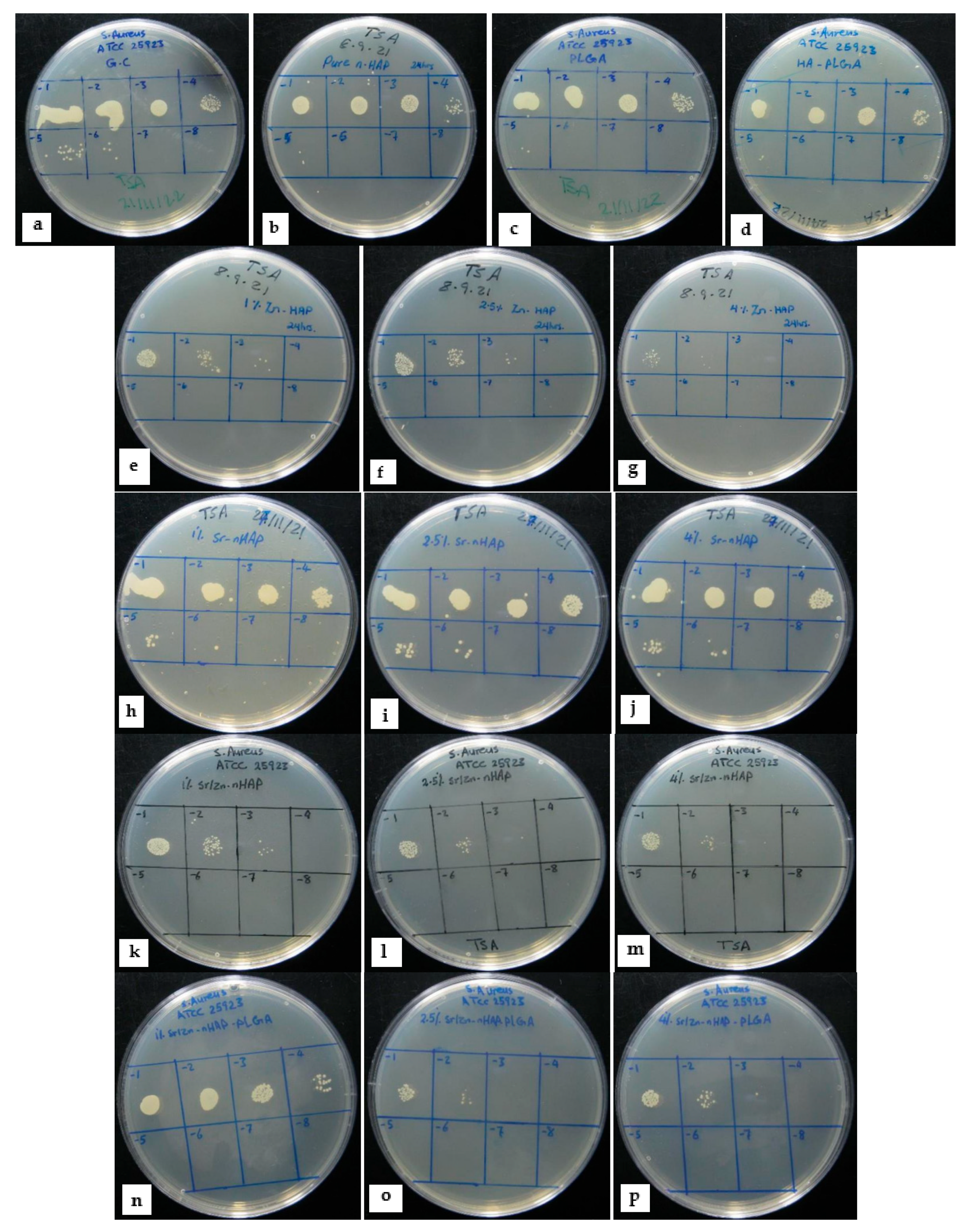
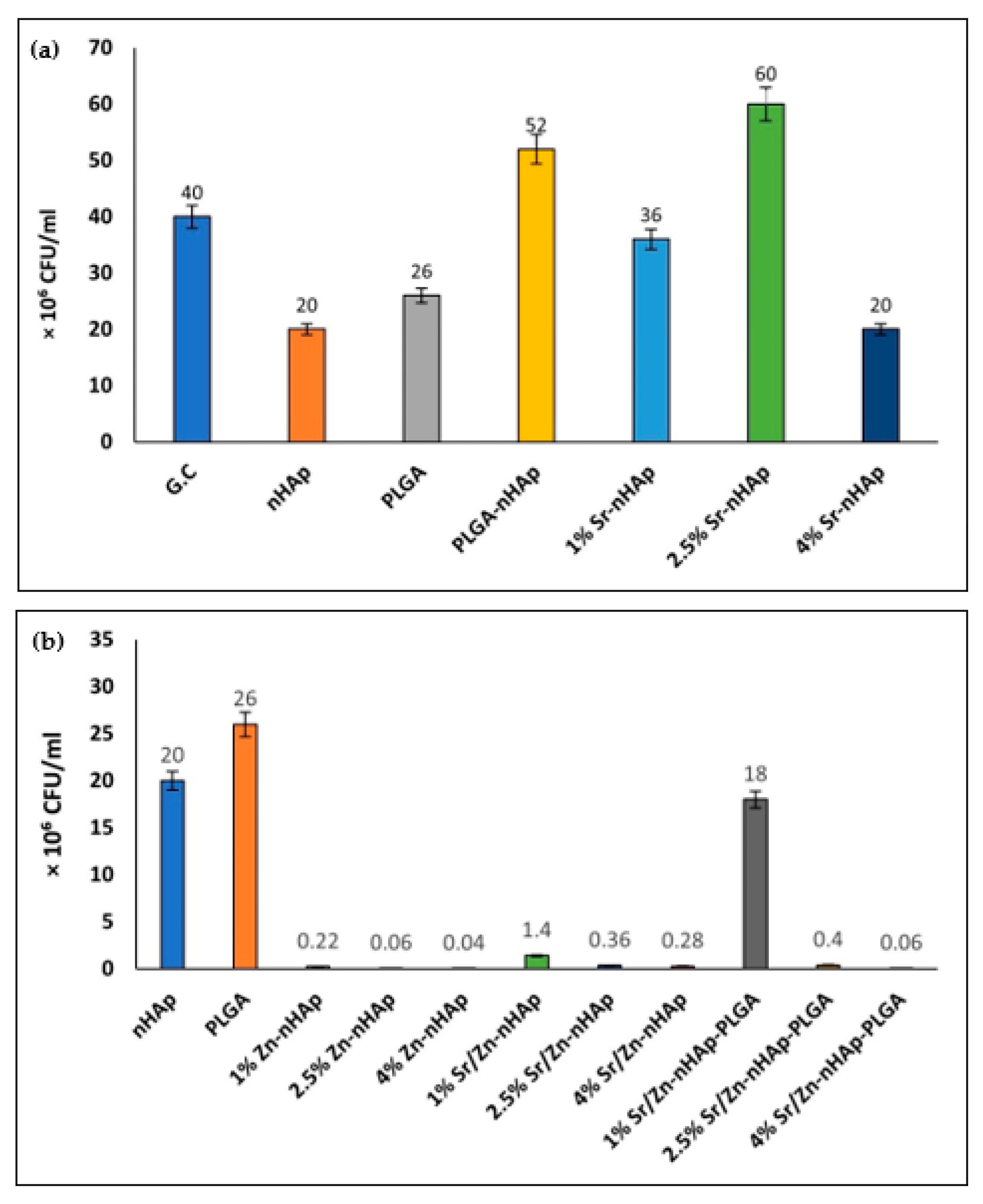
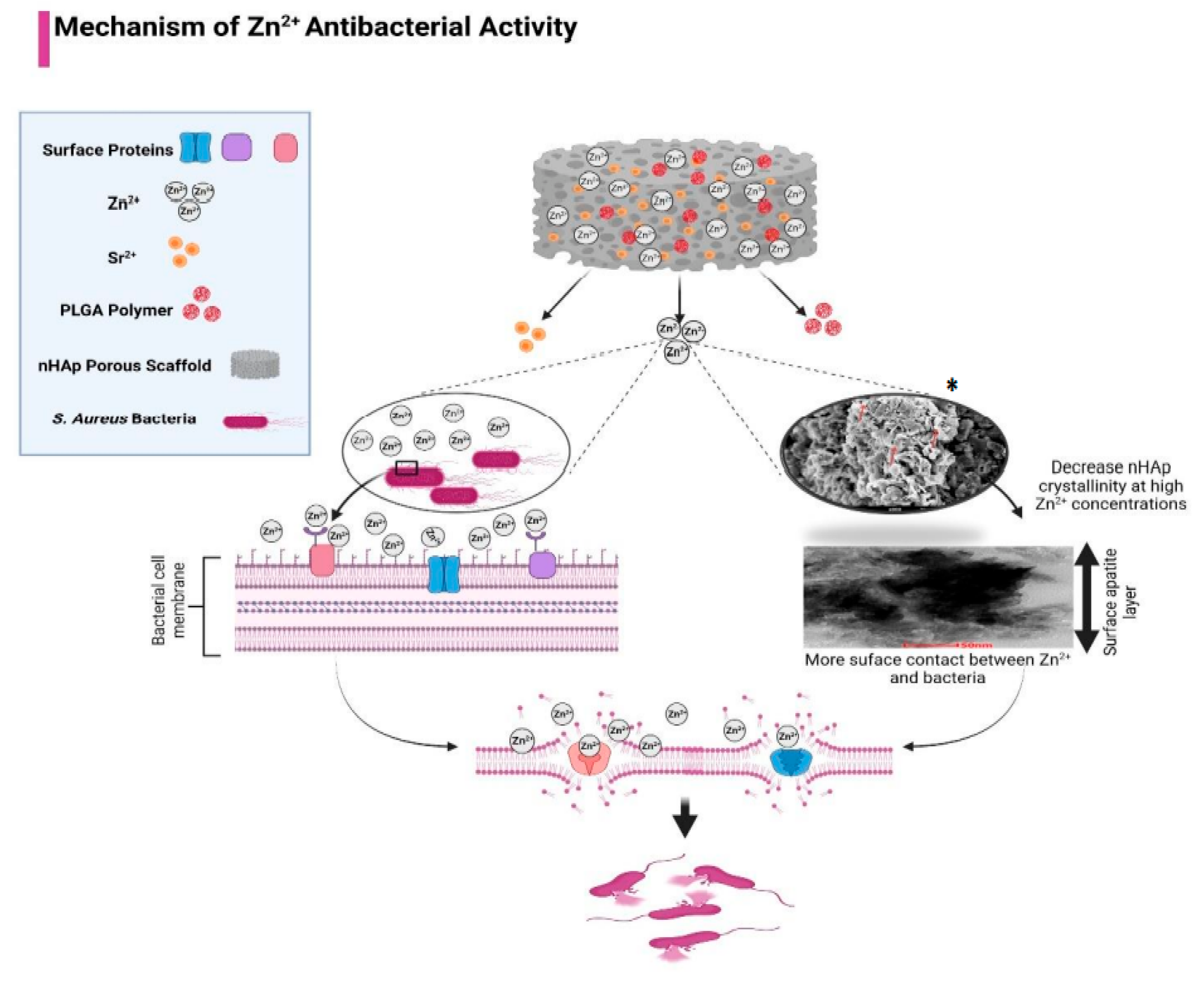
3.1. Scaffolds Antibacterial Activity
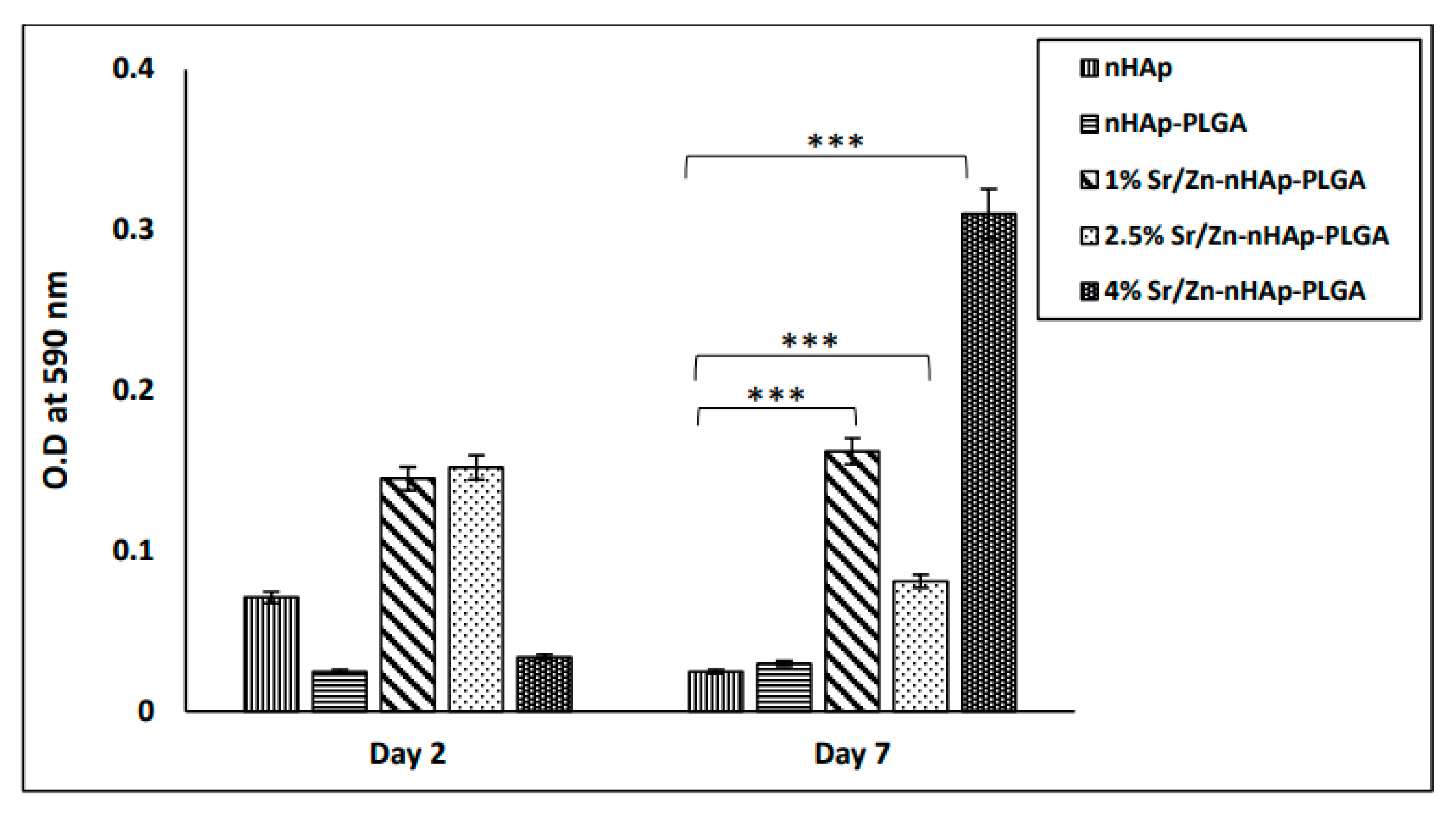
3.2. Cell Proliferation Using MTT Assay
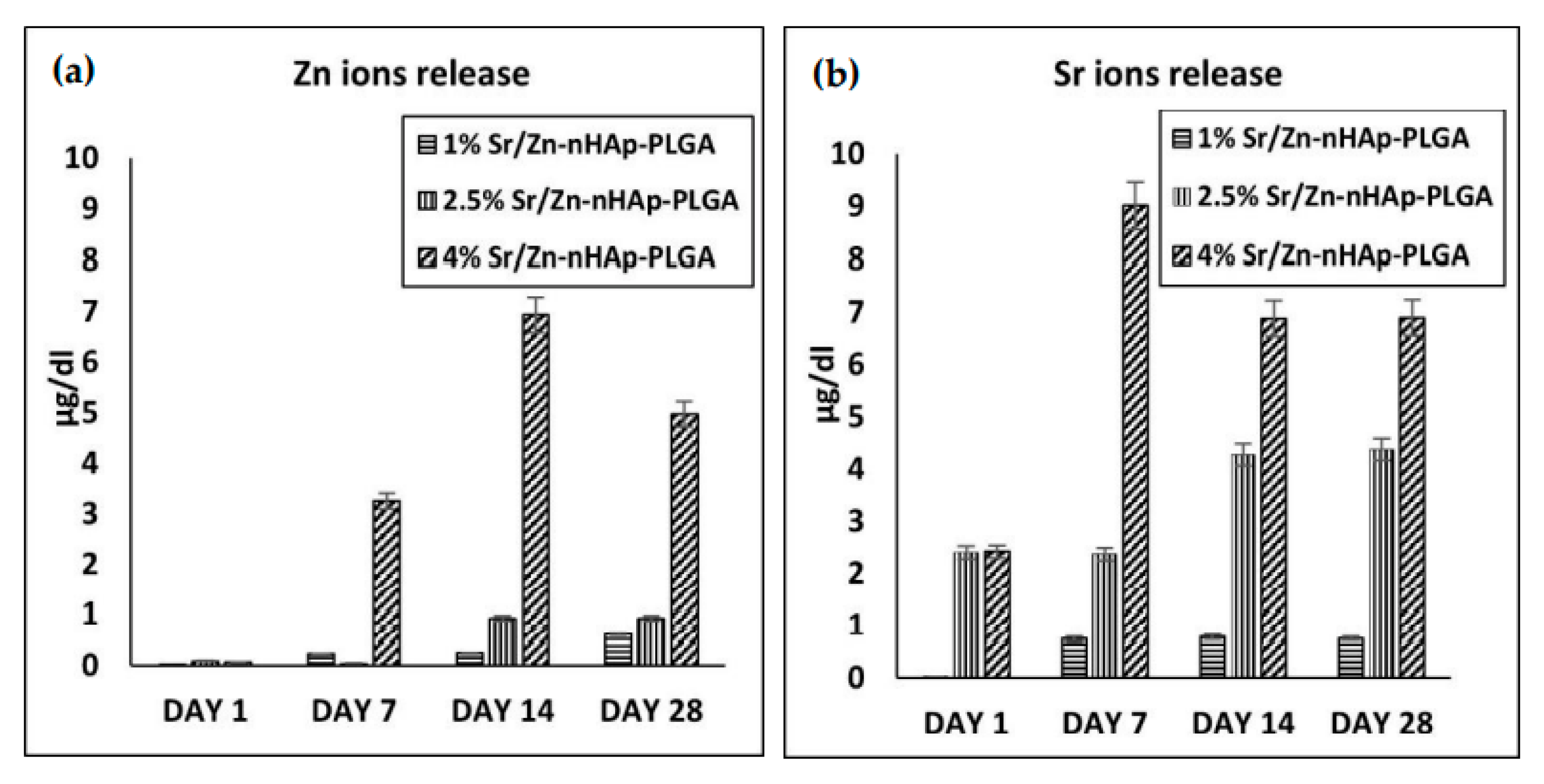
3.3. Sr and Zn Ion Release Using ICP-MS
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wippert, P.-M.; Rector, M.; Kuhn, G.; Wuertz-Kozak, K. Stress and Alterations in Bones: An Interdisciplinary Perspective. Front. Endocrinol. 2017, 8, 96. [Google Scholar] [CrossRef] [Green Version]
- Feng, X. Chemical and Biochemical Basis of Cell-Bone Matrix Interaction in Health and Disease. Curr. Chem. Biol. 2009, 3, 189–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuchs, R.K.; Thompson, W.R.; Warden, S.J. 2—Bone Biology. In Bone Repair Biomaterials, Woodhead Publishing Series in Biomaterials; 2nd ed.; Pawelec, K.M., Planell, J.A., Eds.; Woodhead Publishing: Sawston, UK, 2019; pp. 15–52. ISBN 978-0-08-102451-5. [Google Scholar]
- Offner, D.; de Grado, G.F.; Meisels, I.; Pijnenburg, L.; Fioretti, F.; Benkirane-Jessel, N.; Musset, A.-M. Bone Grafts, Bone Substitutes and Regenerative Medicine Acceptance for the Management of Bone Defects Among French Population: Issues about Ethics, Religion or Fear? Cell Med. 2019, 11, 2155179019857661. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Yeung, K.W.K. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact. Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef]
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S.P. Bone tissue engineering: Recent advances and challenges. Crit. Rev. Biomed. Eng. 2012, 40, 363–408. [Google Scholar] [CrossRef] [Green Version]
- Elsalanty, M.E.; Genecov, D.G. Bone grafts in craniofacial surgery. Craniomaxillofac. Trauma Reconstr. 2009, 2, 125–134. [Google Scholar] [CrossRef] [Green Version]
- Moore, W.R.; Graves, S.E.; Bain, G.I. Synthetic bone graft substitutes. ANZ J. Surg. 2001, 71, 354–361. [Google Scholar] [CrossRef]
- Bohner, M. Resorbable biomaterials as bone graft substitutes. Mater. Today 2010, 13, 24–30. [Google Scholar] [CrossRef]
- Zimmermann, C.E.; Gierloff, M.; Hedderich, J.; Açil, Y.; Wiltfang, J.; Terheyden, H. Biocompatibility of bone graft substitutes: Effects on survival and proliferation of porcine multilineage stem cells in vitro. Folia Morphol. 2011, 70, 154–160. [Google Scholar]
- Guo, L.; Liang, Z.; Yang, L.; Du, W.; Yu, T.; Tang, H.; Li, C.; Qiu, H. The role of natural polymers in bone tissue engineering. J. Control Release 2021, 338, 571–582. [Google Scholar] [CrossRef]
- Donnaloja, F.; Jacchetti, E.; Soncini, M.; Raimondi, M.T. Natural and Synthetic Polymers for Bone Scaffolds Optimization. Polymers 2020, 12, 905. [Google Scholar] [CrossRef] [Green Version]
- Baino, F.; Novajra, G.; Vitale-Brovarone, C. Bioceramics and Scaffolds: A Winning Combination for Tissue Engineering. Front. Bioeng. Biotechnol. 2015, 3, 202. [Google Scholar] [CrossRef] [Green Version]
- Kilpadi, K.L.; Chang, P.-L.; Bellis, S.L. Hydroxylapatite binds more serum proteins, purified integrins, and osteoblast precursor cells than titanium or steel. J. Biomed. Mater. Res. 2001, 57, 258–267. [Google Scholar] [CrossRef]
- Family, R.; Solati-Hashjin, M.; Nik, S.N.; Nemati, A. Surface modification for titanium implants by hydroxyapatite nanocomposite. Casp. J. Intern. Med. 2012, 3, 460–465. [Google Scholar]
- Bose, S.; Fielding, G.; Tarafder, S.; Bandyopadhyay, A. Understanding of dopant-induced osteogenesis and angiogenesis in calcium phosphate ceramics. Trends Biotechnol. 2013, 31, 594–605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boanini, E.; Gazzano, M.; Bigi, A. Ionic substitutions in calcium phosphates synthesized at low temperature. Acta Biomater. 2010, 6, 1882–1894. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, J.H.; Best, S.M. Calcium phosphate scaffolds for bone repair. JOM 2011, 63, 83–92. [Google Scholar] [CrossRef]
- Miyaji, F.; Kono, Y.; Suyama, Y. Formation and structure of zinc-substituted calcium hydroxyapatite. Mater. Res. Bull. 2005, 40, 209–220. [Google Scholar] [CrossRef]
- Murakami, M.; Hirano, T. Intracellular zinc homeostasis and zinc signaling. Cancer Sci. 2008, 99, 1515–1522. [Google Scholar] [CrossRef] [PubMed]
- Mikael, P.E.; Amini, A.R.; Basu, J.; Arellano-Jimenez, M.J.; Laurencin, C.T.; Sanders, M.M.; Carter, C.B.; Nukavarapu, S.P. Functionalized carbon nanotube reinforced scaffolds for bone regenerative engineering: Fabrication, in vitro and in vivo evaluation. Biomed. Mater. 2014, 9, 035001. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Weitzmann, M.N. Zinc stimulates osteoblastogenesis and suppresses osteoclastogenesis by antagonizing NF-κB activation. Mol. Cell. Biochem. 2011, 355, 179. [Google Scholar] [CrossRef]
- Grandjean-Laquerriere, A.; Laquerriere, P.; Jallot, E.; Nedelec, J.-M.; Guenounou, M.; Laurent-Maquin, D.; Phillips, T.M. Influence of the zinc concentration of sol–gel derived zinc substituted hydroxyapatite on cytokine production by human monocytes in vitro. Biomaterials 2006, 27, 3195–3200. [Google Scholar] [CrossRef] [Green Version]
- Predoi, D.; Iconaru, S.L.; Predoi, M.V.; Motelica-Heino, M.; Guegan, R.; Buton, N. Evaluation of Antibacterial Activity of Zinc-Doped Hydroxyapatite Colloids and Dispersion Stability Using Ultrasounds. Nanomaterials 2019, 9, 515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.-C.; Lee, Y.-T.; Huang, T.-C.; Lin, G.-S.; Chen, Y.-W.; Lee, B.-S.; Tung, K.-L. In Vitro Bioactivity and Antibacterial Activity of Strontium-, Magnesium-, and Zinc-Multidoped Hydroxyapatite Porous Coatings Applied via Atmospheric Plasma Spraying. ACS Appl. Bio Mater. 2021, 4, 2523–2533. [Google Scholar] [CrossRef]
- Ohtsu, N.; Kakuchi, Y.; Ohtsuki, T. Antibacterial effect of zinc oxide/hydroxyapatite coatings prepared by chemical solution deposition. Appl. Surf. Sci. 2018, 445, 596–600. [Google Scholar] [CrossRef]
- Charlena; Suparto, I.H.; Kurniawan, E. Synthesis and Characterization of Hydroxyapatite-Zinc Oxide (HAp-ZnO) as Antibacterial Biomaterial. IOP Conf. Ser. Mater. Sci. Eng. 2019, 599, 12011. [Google Scholar] [CrossRef]
- Arciola, C.R.; Campoccia, D.; Montanaro, L. Implant infections: Adhesion, biofilm formation and immune evasion. Nat. Rev. Microbiol. 2018, 16, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.; Monteiro, F.J.; Ferraz, M.P. Infection of orthopedic implants with emphasis on bacterial adhesion process and techniques used in studying bacterial-material interactions. Biomatter 2012, 2, 176–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tripathy, A.; Sen, P.; Su, B.; Briscoe, W.H. Natural and bioinspired nanostructured bactericidal surfaces. Adv. Colloid Interface Sci. 2017, 248, 85–104. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.S.; Kim, H.K.; Ghim, M.S.; Hong, M.W.; Kim, Y.Y.; Cho, Y.S. Evaluation of the antibacterial activity and cell response for 3D-printed polycaprolactone/ nanohydroxyapatite scaffold with zinc oxide coating. Polymers 2020, 12, 2193. [Google Scholar] [CrossRef] [PubMed]
- Lemire, J.A.; Harrison, J.J.; Turner, R.J. Antimicrobial activity of metals: Mechanisms, molecular targets and applications. Nat. Rev. Microbiol. 2013, 11, 371–384. [Google Scholar] [CrossRef]
- Saxena, V.; Hasan, A.; Pandey, L.M. Effect of Zn/ZnO integration with hydroxyapatite: A review. Mater. Technol. 2018, 33, 79–92. [Google Scholar] [CrossRef]
- Kao, Y.-Y.; Chen, Y.-C.; Cheng, T.-J.; Chiung, Y.-M.; Liu, P.-S. Zinc Oxide Nanoparticles Interfere with Zinc Ion Homeostasis to Cause Cytotoxicity. Toxicol. Sci. 2012, 125, 462–472. [Google Scholar] [CrossRef] [Green Version]
- Guo, D.; Bi, H.; Liu, B.; Wu, Q.; Wang, D.; Cui, Y. Reactive oxygen species-induced cytotoxic effects of zinc oxide nanoparticles in rat retinal ganglion cells. Toxicol. Vitro 2013, 27, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhao, S.; Xiao, W.; Cui, X.; Huang, W.; Rahaman, M.N.; Zhang, C.; Wang, D. Three-dimensional zinc incorporated borosilicate bioactive glass scaffolds for rodent critical-sized calvarial defects repair and regeneration. Colloids Surf. B Biointerfaces 2015, 130, 149–156. [Google Scholar] [CrossRef]
- Blaschko, S.D.; Chi, T.; Miller, J.; Flechner, L.; Fakra, S.; Kapahi, P.; Kahn, A.; Stoller, M.L. Strontium substitution for calcium in lithogenesis. J. Urol. 2013, 189, 735–739. [Google Scholar] [CrossRef] [Green Version]
- Cannata-Andía, J.B.; Rodríguez-García, M.; Gómez-Alonso, C. Action mechanism of strontium ranelate. Rev. Osteoporos. Metab. Miner. 2010, 2, 5–9. [Google Scholar]
- Chattopadhyay, N.; Quinn, S.J.; Kifor, O.; Ye, C.; Brown, E.M. The calcium-sensing receptor (CaR) is involved in strontium ranelate-induced osteoblast proliferation. Biochem. Pharmacol. 2007, 74, 438–447. [Google Scholar] [CrossRef]
- Liao, J.; Blake, G.M.; McGregor, A.H.; Patel, R. The effect of bone strontium on BMD is different for different manufacturers’ DXA Systems. Bone 2010, 47, 882–887. [Google Scholar] [CrossRef] [PubMed]
- Querido, W.; Rossi, A.L.; Farina, M. The effects of strontium on bone mineral: A review on current knowledge and microanalytical approaches. Micron 2016, 80, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Durmus, K.; Turgut, N.H.; Dogan, M.; Tuncer, E.; Ozer, H.; Altuntas, E.E.; Akyol, M. Histopathological evaluation of the effect of locally administered strontium on healing time in mandibular fractures: An experimental study. Adv. Clin. Exp. Med. 2017, 26, 1063–1067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Duliamy, M.J.; Ghaib, N.H.; Kader, O.A.; Abdullah, B.H. Enhancement of orthodontic anchorage and retention by the local injection of strontium: An experimental study in rats. Saudi Dent. J. 2015, 27, 22–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henriques Lourenço, A.; Neves, N.; Ribeiro-Machado, C.; Sousa, S.R.; Lamghari, M.; Barrias, C.C.; Trigo Cabral, A.; Barbosa, M.A.; Ribeiro, C.C. Injectable hybrid system for strontium local delivery promotes bone regeneration in a rat critical-sized defect model. Sci. Rep. 2017, 7, 5098. [Google Scholar] [CrossRef] [Green Version]
- Kołodziejska, B.; Stępień, N.; Kolmas, J. The influence of strontium on bone tissue metabolism and its application in osteoporosis treatment. Int. J. Mol. Sci. 2021, 22, 6564. [Google Scholar] [CrossRef] [PubMed]
- Chandran, S.; Shenoy, S.J.S.; Nair, R.P.; Varma, H.K.; John, A. Strontium Hydroxyapatite scaffolds engineered with stem cells aid osteointegration and osteogenesis in osteoporotic sheep model. Colloids Surf. B Biointerfaces 2018, 163, 346–354. [Google Scholar] [CrossRef]
- Yudaev, P.; Chuev, V.; Klyukin, B.; Kuskov, A.; Mezhuev, Y.; Chistyakov, E. Polymeric Dental Nanomaterials: Antimicrobial Action. Polymers 2022, 14, 864. [Google Scholar] [CrossRef]
- Yudaev, P.; Mezhuev, Y.; Chistyakov, E. Nanoparticle-Containing Wound Dressing: Antimicrobial and Healing Effects. Gels 2022, 8, 329. [Google Scholar] [CrossRef]
- Zhao, D.; Zhu, T.; Li, J.; Cui, L.; Zhang, Z.; Zhuang, X.; Ding, J. Poly(lactic-co-glycolic acid)-based composite bone-substitute materials. Bioact. Mater. 2021, 6, 346–360. [Google Scholar] [CrossRef]
- Gentile, P.; Chiono, V.; Carmagnola, I.; Hatton, P.V. An overview of poly(lactic-co-glycolic) Acid (PLGA)-based biomaterials for bone tissue engineering. Int. J. Mol. Sci. 2014, 15, 3640–3659. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Yan, Y.; Gao, J.; Li, Y.; Wang, R.; Wang, J.; Zou, Q.; Zuo, Y.; Zhu, M.; Li, J. 3D-printed hydroxyapatite microspheres reinforced PLGA scaffolds for bone regeneration. Biomater. Adv. 2022, 133, 112618. [Google Scholar] [CrossRef]
- Park, J.-W.; Hwang, J.-U.; Back, J.-H.; Jang, S.-W.; Kim, H.-J.; Kim, P.-S.; Shin, S.; Kim, T. High strength PLGA/Hydroxyapatite composites with tunable surface structure using PLGA direct grafting method for orthopedic implants. Compos. Part B Eng. 2019, 178, 107449. [Google Scholar] [CrossRef]
- Cheng, Y.; Qin, J.; Huang, Y.; Wang, T. The antimicrobial effects of PLGA microspheres containing the antimicrobial peptide OP-145 on clinically isolated pathogens in bone infections. Sci. Rep. 2022, 12, 14541. [Google Scholar] [CrossRef]
- Chen, L.; Shao, L.; Wang, F.; Huang, Y.; Gao, F. Enhancement in sustained release of antimicrobial peptide and BMP-2 from degradable three dimensional-printed PLGA scaffold for bone regeneration. RSC Adv. 2019, 9, 10494–10507. [Google Scholar] [CrossRef] [Green Version]
- McLaren, J.S.; White, L.J.; Cox, H.C.; Ashraf, W.; Rahman, C.V.; Blunn, G.W.; Goodship, A.E.; Quirk, R.A.; Shakesheff, K.M.; Bayston, R.; et al. A biodegradable antibiotic-impregnated scaffold to prevent osteomyelitis in a contaminated in vivo bone defect model. Eur. Cells Mater. 2014, 27, 332–349. [Google Scholar] [CrossRef]
- Cobb, L.H.; McCabe, E.M.; Priddy, L.B. Therapeutics and delivery vehicles for local treatment of osteomyelitis. J. Orthop. Res. 2020, 38, 2091–2103. [Google Scholar] [CrossRef] [PubMed]
- Sinulingga, K.; Sirait, M.; Siregar, N.; Doloksaribu, M.E. Investigation of Antibacterial Activity and Cell Viability of Ag/Mg and Ag/Zn Co-doped Hydroxyapatite Derived from Natural Limestone. ACS Omega 2021, 6, 34185–34191. [Google Scholar] [CrossRef]
- Shimabukuro, M. Antibacterial property and biocompatibility of silver, copper, and zinc in titanium dioxide layers incorporated by one-step micro-arc oxidation: A review. Antibiotics 2020, 9, 716. [Google Scholar] [CrossRef]
- Hassan, M.; Sulaiman, M.; Yuvaraju, P.D.; Galiwango, E.; Rehman, I.U.; Al-Marzouqi, A.H.; Khaleel, A.; Mohsin, S. Biomimetic PLGA/Strontium-Zinc Nano Hydroxyapatite Composite Scaffolds for Bone Regeneration. J. Funct. Biomater. 2022, 13, 13. [Google Scholar] [CrossRef] [PubMed]
- Anwar, A.; Kanwal, Q.; Akbar, S.; Munawar, A.; Durrani, A.; Hassan Farooq, M. Synthesis and characterization of pure and nanosized hydroxyapatite bioceramics. Nanotechnol. Rev. 2017, 6, 149–157. [Google Scholar] [CrossRef]
- Dou, L.; Zhang, Y.; Sun, H. Advances in Synthesis and Functional Modification of Nanohydroxyapatite. J. Nanomater. 2018, 2018, 3106214. [Google Scholar] [CrossRef] [Green Version]
- Ofudje, E.A.; Adeogun, A.I.; Idowu, M.A.; Kareem, S.O. Synthesis and characterization of Zn-Doped hydroxyapatite: Scaffold application, antibacterial and bioactivity studies. Heliyon 2019, 5, e01716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liuyun, J.; Chengdong, X.; Lixin, J.; Lijuan, X. Effect of hydroxyapatite with different morphology on the crystallization behavior, mechanical property and in vitro degradation of hydroxyapatite/poly(lactic-co-glycolic) composite. Compos. Sci. Technol. 2014, 93, 61–67. [Google Scholar] [CrossRef]
- Resmim, C.M.; Dalpasquale, M.; Vielmo, N.I.C.; Mariani, F.Q.; Villalba, J.C.; Anaissi, F.J.; Caetano, M.M.; Tusi, M.M. Study of physico-chemical properties and in vitro antimicrobial activity of hydroxyapatites obtained from bone calcination. Prog. Biomater. 2019, 8, 1–9. [Google Scholar] [CrossRef]
- Xu, K.; Yuan, Z.; Ding, Y.; He, Y.; Li, K.; Lin, C.; Tao, B.; Yang, Y.; Li, X.; Liu, P.; et al. Near-infrared light triggered multi-mode synergetic therapy for improving antibacterial and osteogenic activity of titanium implants. Appl. Mater. Today 2021, 24, 101155. [Google Scholar] [CrossRef]
- Kurzyk, A.; Ostrowska, B.; Święszkowski, W.; Pojda, Z. Characterization and Optimization of the Seeding Process of Adipose Stem Cells on the Polycaprolactone Scaffolds. Stem Cells Int. 2019, 2019, 1201927. [Google Scholar] [CrossRef]
- Sylvester, P.W. Optimization of the Tetrazolium Dye (MTT) Colorimetric Assay for Cellular Growth and Viability BT—Drug Design and Discovery: Methods and Protocols. In Drug Design and Discovery: Methods and Protocols; Satyanarayanajois, S.D., Ed.; Humana Press: Totowa, NJ, USA, 2011; pp. 157–168. ISBN 978-1-61779-012-6. [Google Scholar]
- Sari, M.; Hening, P.; Chotimah; Ana, I.D.; Yusuf, Y. Bioceramic hydroxyapatite-based scaffold with a porous structure using honeycomb as a natural polymeric Porogen for bone tissue engineering. Biomater. Res. 2021, 25, 2. [Google Scholar] [CrossRef]
- Ressler, A.; Ivanković, T.; Polak, B.; Ivanišević, I.; Kovačić, M.; Urlić, I.; Hussainova, I.; Ivanković, H. A multifunctional strontium/silver-co-substituted hydroxyapatite derived from biogenic source as antibacterial biomaterial. Ceram. Int. 2022, 48, 18361–18373. [Google Scholar] [CrossRef]
- Fielding, G.A.; Roy, M.; Bandyopadhyay, A.; Bose, S. Antibacterial and biological characteristics of silver containing and strontium doped plasma sprayed hydroxyapatite coatings. Acta Biomater. 2012, 8, 3144–3152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maqbool, M.; Nawaz, Q.; Rehman, M.A.U.; Cresswell, M.; Jackson, P.; Hurle, K.; Detsch, R.; Goldmann, W.H.; Shah, A.T.; Boccaccini, A.R. Synthesis, characterization, antibacterial properties, and in vitro studies of selenium and strontium co-substituted hydroxyapatite. Int. J. Mol. Sci. 2021, 22, 4246. [Google Scholar] [CrossRef]
- Stanić, V.; Dimitrijević, S.; Antić-Stanković, J.; Mitrić, M.; Jokić, B.; Plećaš, I.B.; Raičević, S. Synthesis, characterization and antimicrobial activity of copper and zinc-doped hydroxyapatite nanopowders. Appl. Surf. Sci. 2010, 256, 6083–6089. [Google Scholar] [CrossRef]
- Tank, K.P.; Chudasama, K.S.; Thaker, V.S.; Joshi, M.J. Pure and zinc doped nano-hydroxyapatite: Synthesis, characterization, antimicrobial and hemolytic studies. J. Cryst. Growth 2014, 401, 474–479. [Google Scholar] [CrossRef]
- Oshita, M.; Umeda, K.; Kataoka, M.; Azuma, Y.; Furuzono, T. Continuous antimicrobial mechanism of dispersible hydroxyapatite nanoparticles doped with zinc ions for percutaneous device coatings. J. Biomater. Appl. 2022, 37, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Zare, E.N.; Jamaledin, R.; Naserzadeh, P.; Afjeh-Dana, E.; Ashtari, B.; Hosseinzadeh, M.; Vecchione, R.; Wu, A.; Tay, F.R.; Borzacchiello, A.; et al. Metal-Based Nanostructures/PLGA Nanocomposites: Antimicrobial Activity, Cytotoxicity, and Their Biomedical Applications. ACS Appl. Mater. Interfaces 2020, 12, 3279–3300. [Google Scholar] [CrossRef] [PubMed]
- Valarmathi, N.; Sabareeswari, K.; Sumathi, S. Antimicrobial and larvicidal activity of zinc-substituted hydroxyapatite. Bull. Mater. Sci. 2020, 43, 218. [Google Scholar] [CrossRef]
- Chereddy, K.K.; Vandermeulen, G.; Préat, V. PLGA based drug delivery systems: Promising carriers for wound healing activity. Wound Repair Regen. 2016, 24, 223–236. [Google Scholar] [CrossRef]
- Kapoor, D.N.; Bhatia, A.; Kaur, R.; Sharma, R.; Kaur, G.; Dhawan, S. PLGA: A unique polymer for drug delivery. Ther. Deliv. 2015, 6, 41–58. [Google Scholar] [CrossRef]
- Ruirui, Z.; He, J.; Xu, X.; Li, S.; Peng, H.; Deng, Z.; Huang, Y. PLGA-based drug delivery system for combined therapy of cancer: Research progress. Mater. Res. Express 2021, 8, 122002. [Google Scholar] [CrossRef]
- Kattimani, V.S.; Kondaka, S.; Lingamaneni, K.P. Hydroxyapatite–-Past, Present, and Future in Bone Regeneration. Bone Tissue Regen. Insights 2016, 7, BTRI.S36138. [Google Scholar] [CrossRef] [Green Version]
- Ha, S.-W.; Jang, H.L.; Nam, K.T.; Beck, G.R. Nano-hydroxyapatite modulates osteoblast lineage commitment by stimulation of DNA methylation and regulation of gene expression. Biomaterials 2015, 65, 32–42. [Google Scholar] [CrossRef] [Green Version]
- Abe, Y.; Okazaki, Y.; Hiasa, K.; Yasuda, K.; Nogami, K.; Mizumachi, W.; Hirata, I. Bioactive Surface Modification of Hydroxyapatite. Biomed Res. Int. 2013, 2013, 626452. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Tang, P.; Ge, X.; Li, P.; Lv, C.; Wang, M.; Wang, K.; Fang, L.; Lu, X. Experimental and simulation studies of strontium/zinc-codoped hydroxyapatite porous scaffolds with excellent osteoinductivity and antibacterial activity. Appl. Surf. Sci. 2018, 462, 118–126. [Google Scholar] [CrossRef]
- Maleki-Ghaleh, H.; Siadati, M.H.; Fallah, A.; Koc, B.; Kavanlouei, M.; Khademi-Azandehi, P.; Moradpur-Tari, E.; Omidi, Y.; Barar, J.; Beygi-Khosrowshahi, Y.; et al. Antibacterial and cellular behaviors of novel zinc-doped hydroxyapatite/graphene nanocomposite for bone tissue engineering. Int. J. Mol. Sci. 2021, 22, 9564. [Google Scholar] [CrossRef]
- Xue, W.; Moore, J.L.; Hosick, H.L.; Bose, S.; Bandyopadhyay, A.; Lu, W.W.; Cheung, K.M.C.; Luk, K.D.K. Osteoprecursor cell response to strontium-containing hydroxyapatite ceramics. J. Biomed. Mater. Res. Part A 2006, 79A, 804–814. [Google Scholar] [CrossRef] [PubMed]
- Ahtzaz, S.; Nasir, M.; Shahzadi, L.; Amir, W.; Anjum, A.; Arshad, R.; Iqbal, F.; Chaudhry, A.A.; Yar, M.; ur Rehman, I. A study on the effect of zinc oxide and zinc peroxide nanoparticles to enhance angiogenesis-pro-angiogenic grafts for tissue regeneration applications. Mater. Des. 2017, 132, 409–418. [Google Scholar] [CrossRef]
- Ito, A.; Kawamura, H.; Otsuka, M.; Ikeuchi, M.; Ohgushi, H.; Ishikawa, K.; Onuma, K.; Kanzaki, N.; Sogo, Y.; Ichinose, N. Zinc-releasing calcium phosphate for stimulating bone formation. Mater. Sci. Eng. C 2002, 22, 21–25. [Google Scholar] [CrossRef]
- Kojima, C.; Watanabe, K.; Murata, H.; Nishio, Y.; Makiura, R.; Matsunaga, K.; Nakahira, A. Controlled release of DNA from zinc and magnesium ion-doped hydroxyapatites. Res. Chem. Intermed. 2019, 45, 23–32. [Google Scholar] [CrossRef]
- Sergi, R.; Bellucci, D.; Candidato, R.T.; Lusvarghi, L.; Bolelli, G.; Pawlowski, L.; Candiani, G.; Altomare, L.; De Nardo, L.; Cannillo, V. Bioactive Zn-doped hydroxyapatite coatings and their antibacterial efficacy against Escherichia coli and Staphylococcus aureus. Surf. Coatings Technol. 2018, 352, 84–91. [Google Scholar] [CrossRef]
- Fernandes, G.V.O.; Calasans-Maia, M.; Mitri, F.F.; Bernardo, V.G.; Rossi, A.; Almeida, G.D.S.; Granjeiro, J.M. Histomorphometric analysis of bone repair in critical size defect in rats calvaria treated with hydroxyapatite and zinc-containing hydroxyapatite 5%. Key Eng. Mater. 2009, 396–398, 15–18. [Google Scholar] [CrossRef]
- Popa, C.L.; Deniaud, A.; Michaud-Soret, I.; Guégan, R.; Motelica-Heino, M.; Predoi, D. Structural and Biological Assessment of Zinc Doped Hydroxyapatite Nanoparticles. J. Nanomater. 2016, 2016, 1062878. [Google Scholar] [CrossRef] [Green Version]
- Ullah, I.; Siddiqui, M.A.; Kolawole, S.K.; Liu, H.; Zhang, J.; Ren, L.; Yang, K. Synthesis, characterization and in vitro evaluation of zinc and strontium binary doped hydroxyapatite for biomedical application. Ceram. Int. 2020, 46, 14448–14459. [Google Scholar] [CrossRef]
- Chaudhry, A.A.; Khalid, H.; Zahid, M.; Ijaz, K.; Akhtar, H.; Younas, B.; Manzoor, F.; Iqbal, F.; Ur Rehman, I. Zinc containing calcium phosphates obtained via microwave irradiation of suspensions. Mater. Chem. Phys. 2022, 276, 124921. [Google Scholar] [CrossRef]
- Fu, Y.; Chen, D.; Zhang, J. In vitro study on the cytotoxicity of strontium substituted hydroxyapatite. Shanghai Kou Qiang Yi Xue 2002, 11, 229–232. [Google Scholar]
- Sun, L.; Li, T.; Yu, S.; Mao, M.; Guo, D. A Novel Fast-Setting Strontium-Containing Hydroxyapatite Bone Cement with a Simple Binary Powder System. Front. Bioeng. Biotechnol. 2021, 9, 643557. [Google Scholar] [CrossRef]
- Barman, N.; Salwa, M.; Ghosh, D.; Rahman, M.W.; Uddin, M.N.; Haque, M.A. Reference value for serum zinc level of adult population in Bangladesh. Electron. J. Int. Fed. Clin. Chem. Lab. Med. 2020, 31, 117–124. [Google Scholar]
- Somarouthu, S.; Ohh, J.; Shaked, J.; Cunico, R.L.; Yakatan, G.; Corritori, S.; Tami, J.; Foehr, E.D. Quantitative bioanalysis of strontium in human serum by inductively coupled plasma-mass spectrometry. Futur. Sci. OA 2015, 1, FSO76. [Google Scholar] [CrossRef] [PubMed]
- Grabska-Zielińska, S.; Sionkowska, A.; Carvalho, Â.; Monteiro, F.J. Biomaterials with Potential Use in Bone Tissue Regeneration—Collagen/Chitosan/Silk Fibroin Scaffolds Cross-Linked by EDC/NHS. Materials 2021, 14, 1105. [Google Scholar] [CrossRef]
- Hatton, J.; Davis, G.R.; Mourad, A.-H.I.; Cherupurakal, N.; Hill, R.G.; Mohsin, S. Fabrication of Porous Bone Scaffolds Using Alginate and Bioactive Glass. J. Funct. Biomater. 2019, 10, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Scaffolds | Sr (mol%) | Zn (mol%) | nHAp (mol%) | PLGA (mol%) |
|---|---|---|---|---|
| nHAp | 0 | 0 | 1 | 0 |
| PLGA-nHAp | 0 | 0 | 1 | 3 |
| 1% Sr/Zn-nHAp-PLGA | 1 | 1 | 1 | 3 |
| 2.5% Sr/Zn-nHAP-PLGA | 2.5 | 2.5 | 1 | 3 |
| 4% Sr/Zn-nHAp-PLGA | 4 | 4 | 1 | 3 |
| Composite Scaffolds | Growth Inhibition % |
|---|---|
| Pure nHAp | 0% |
| PLGA | 0% |
| PLGA-nHAp | 0% |
| 1%, 2.5%, 4% Sr-nHAp | 0% |
| 1% Zn-nHAp | 98.9 ± 0.5% |
| 2.5% Zn-nHAp | 99.7 ± 1.65% |
| 4% Zn-nHAp | 99.8 ± 0.1% |
| 1% Sr/Zn-nHAp | 93 ± 3.4% |
| 2.5% Sr/Zn-nHAp | 98.2 ± 0.4% |
| 4% Sr/Zn-nHAp | 98.6 ± 0.2% |
| 1% Zn/Sr-nHAp-PLGA | 10 ± 4.5% |
| 2.5% Zn/Sr-nHAp-PLGA | 98 ± 0.57% |
| 4% Zn/Sr-nHAp-PLGA | 99.7 ± 0.1% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassan, M.; Khaleel, A.; Karam, S.M.; Al-Marzouqi, A.H.; ur Rehman, I.; Mohsin, S. Bacterial Inhibition and Osteogenic Potentials of Sr/Zn Co-Doped Nano-Hydroxyapatite-PLGA Composite Scaffold for Bone Tissue Engineering Applications. Polymers 2023, 15, 1370. https://doi.org/10.3390/polym15061370
Hassan M, Khaleel A, Karam SM, Al-Marzouqi AH, ur Rehman I, Mohsin S. Bacterial Inhibition and Osteogenic Potentials of Sr/Zn Co-Doped Nano-Hydroxyapatite-PLGA Composite Scaffold for Bone Tissue Engineering Applications. Polymers. 2023; 15(6):1370. https://doi.org/10.3390/polym15061370
Chicago/Turabian StyleHassan, Mozan, Abbas Khaleel, Sherif Mohamed Karam, Ali Hassan Al-Marzouqi, Ihtesham ur Rehman, and Sahar Mohsin. 2023. "Bacterial Inhibition and Osteogenic Potentials of Sr/Zn Co-Doped Nano-Hydroxyapatite-PLGA Composite Scaffold for Bone Tissue Engineering Applications" Polymers 15, no. 6: 1370. https://doi.org/10.3390/polym15061370
APA StyleHassan, M., Khaleel, A., Karam, S. M., Al-Marzouqi, A. H., ur Rehman, I., & Mohsin, S. (2023). Bacterial Inhibition and Osteogenic Potentials of Sr/Zn Co-Doped Nano-Hydroxyapatite-PLGA Composite Scaffold for Bone Tissue Engineering Applications. Polymers, 15(6), 1370. https://doi.org/10.3390/polym15061370










