Epoxy Resins for Flooring Applications, an Optimal Host for Recycling Deactivated Cement Asbestos
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Mechanical Properties Determination (UTM) and Hardness
2.3. Thermal Properties Determination (DSC)
2.4. Composite Morphology
3. Results and Discussion
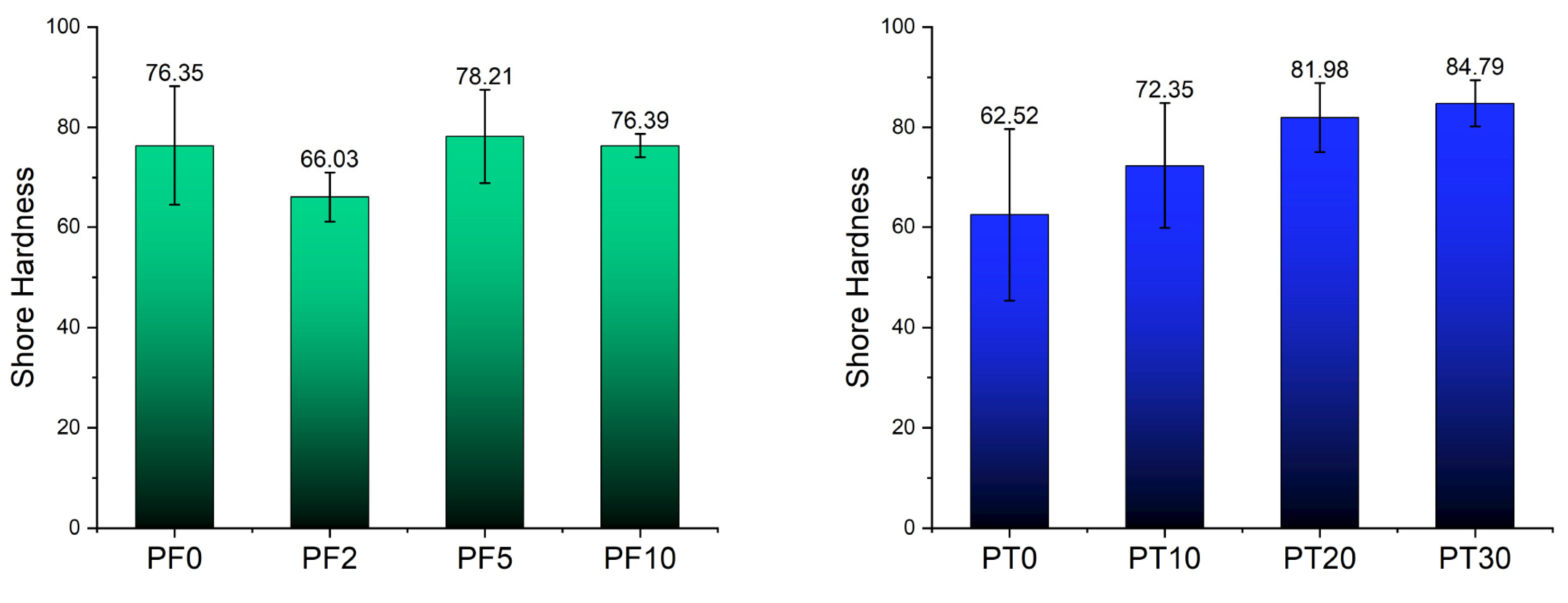
3.1. Mechanical Properties
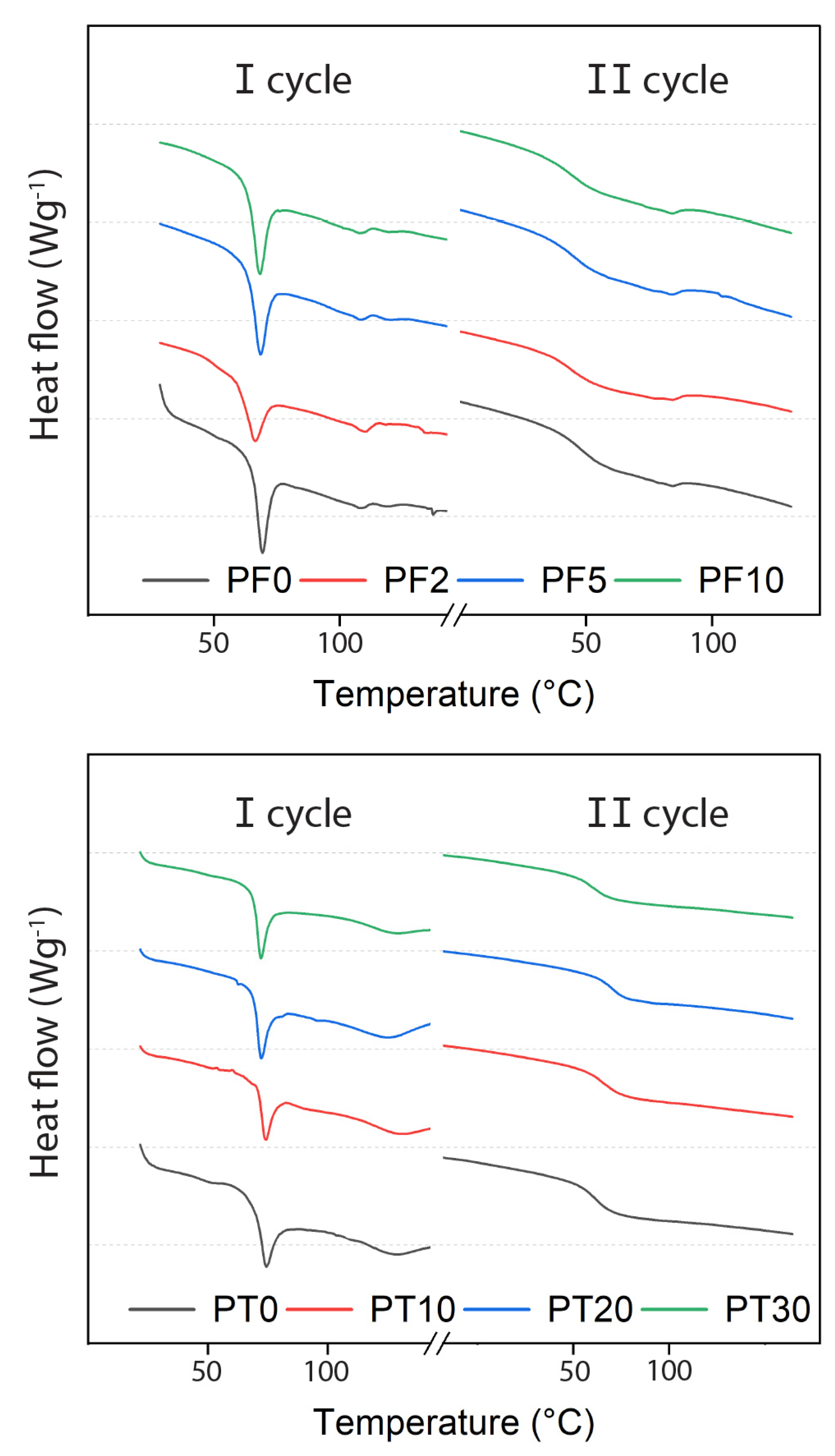
3.2. Thermal Properties
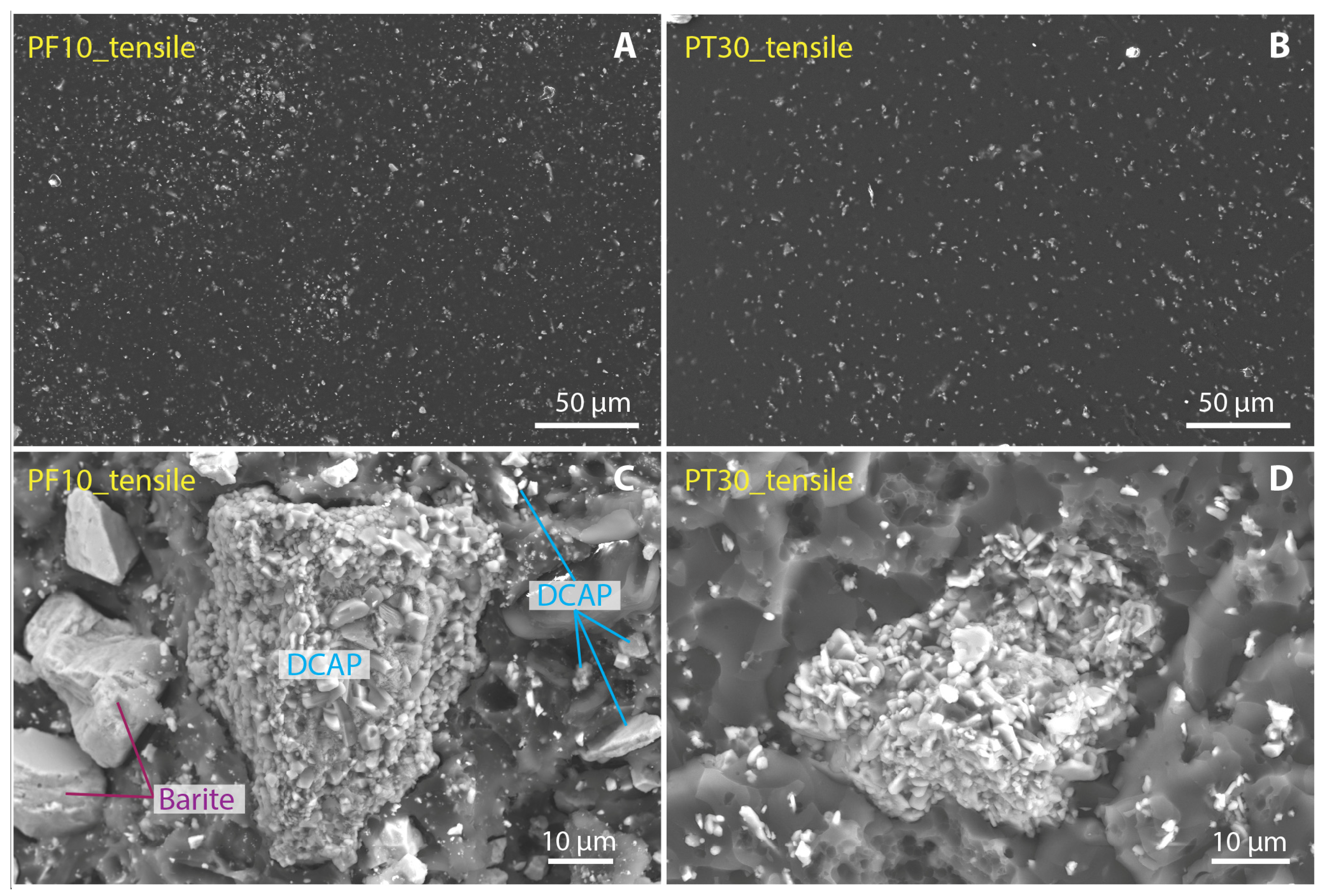
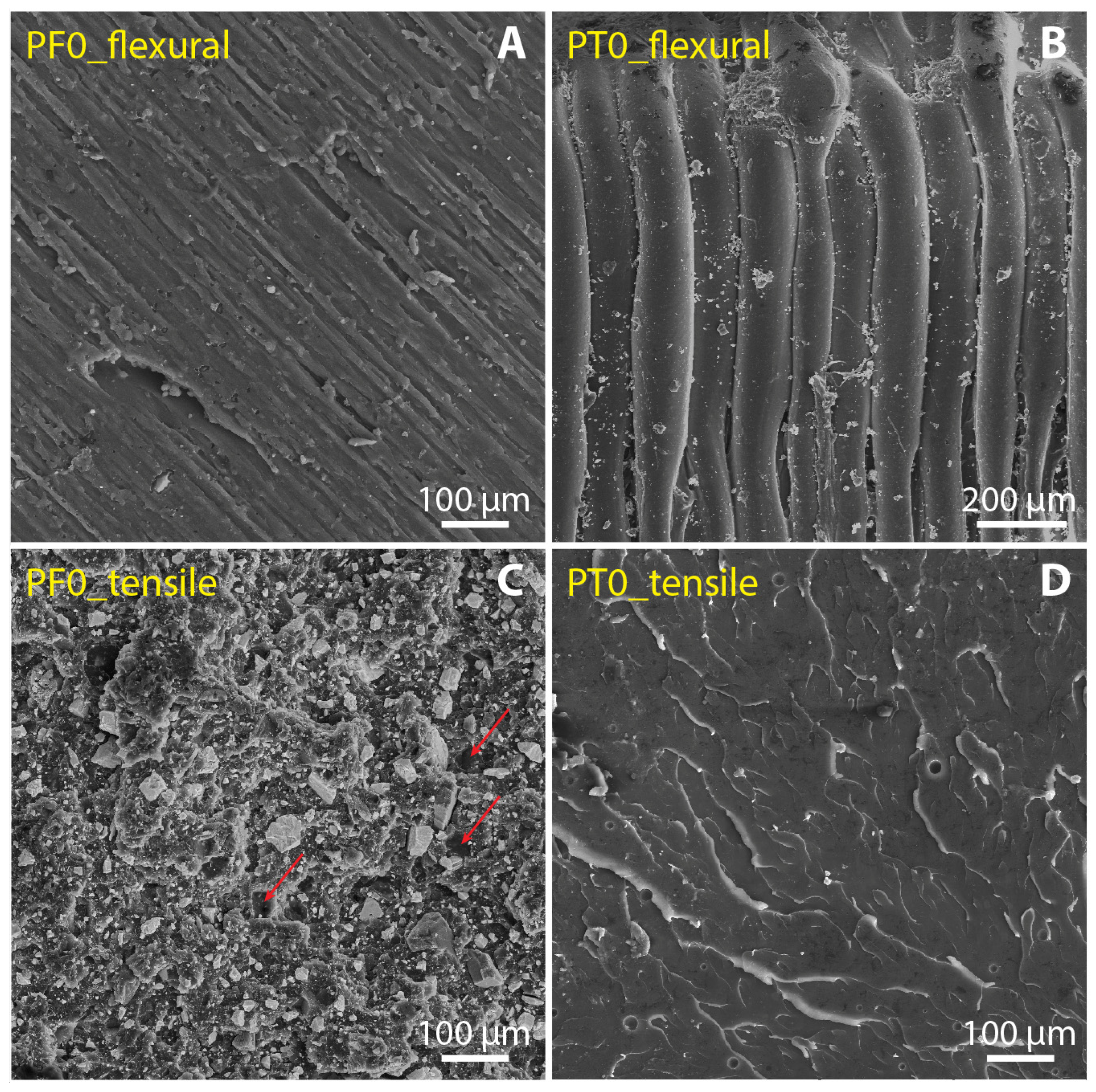
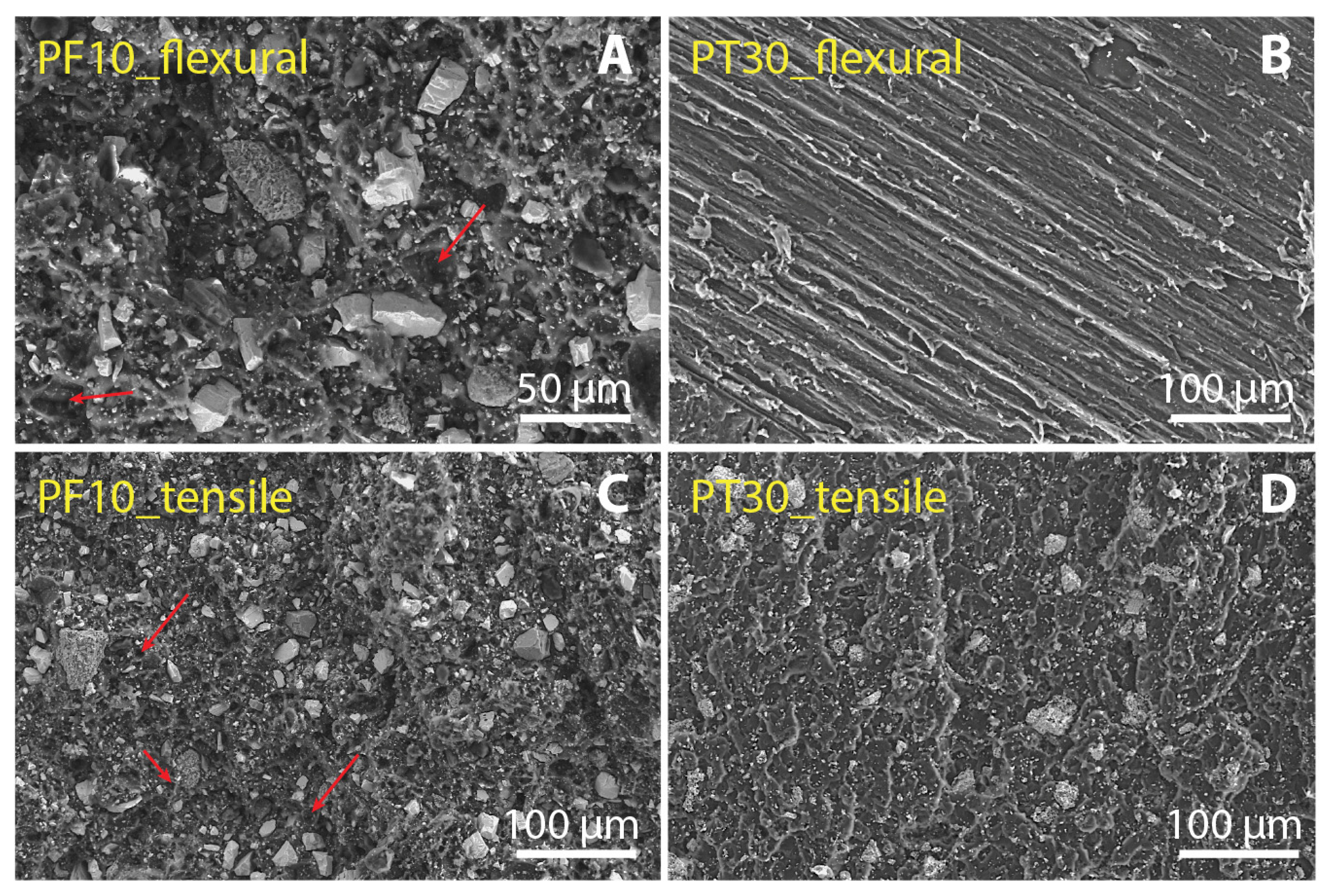
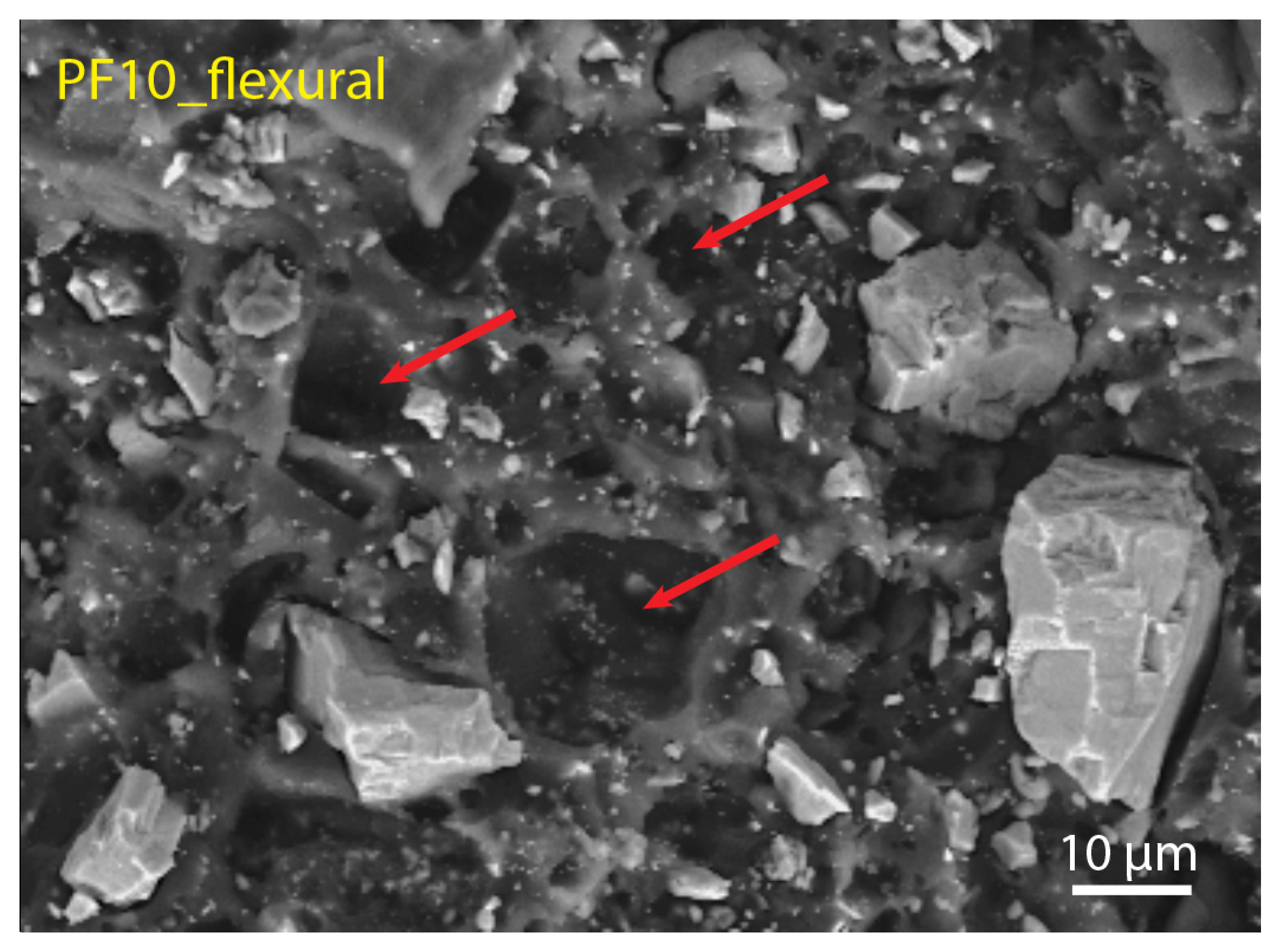
3.3. Filler Dispertion and Fracture Morphology
4. Conclusions
- (1)
- Cement–asbestos slates, a building material that still poses serious environmental and health hazards, can be detoxified by a sustainable thermal treatment, powdered and successfully mixed, potentially in large amounts, to commercial resin formulations of different compositions without any further surface treatment.
- (2)
- The addition of DCAP filler to an epoxy resin for flooring applications that already contained barite filler (PF resin) causes a slight (but still acceptable) worsening of the main mechanical properties (compressive, tensile, and flexural strengths) with increasing DCAP content. An exception is sample PF2, showing lower elasticity and higher plasticity. Repeated tests on this formulation confirmed previous results, suggesting that 2 wt% of DCAP added to the PF resin is a critical value worthy of further investigation. The other samples instead describe a trend in mechanical properties that encourages realistic applications where loading is 10% or more.
- (3)
- The addition of DCAP filler to the neat epoxy for flooring applications (PT resin) causes a slight decrease in the tensile and flexural strengths, with increasing DCAP content, while the compressive strength is almost unaffected, and the Shore hardness increases. Overall, the PT20 sample (20 wt% of DCAP added) shows the best technical properties.
- (4)
- The thermal behaviour of both PF and PT samples are only marginally affected by the addition of DCAP. However, the PT samples (i.e., those bearing DCAP exclusively) show an average Tg of ~59 °C, which is higher than the average Tg (~55 °C) of the PF samples, indicating that PT is a better product for flooring applications.
- (5)
- The comparison between PF and PT composites reveals that the main mechanical properties of the PT samples are significantly better than those of the PF0 sample, i.e., the filler-bearing sample of normal production, suggesting that DCAP can be advantageously used as a filler in substitution for commercial barite. In particular, the PT20 sample (20 wt% of DCAP filler) seems very promising for flooring applications.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Marian, N.M.; Giorgetti, G.; Magrini, C.; Capitani, G.C.; Galimberti, L.; Cavallo, A.; Salvini, R.; Vanneschi, C.; Viti, C. From Hazardous Asbestos Containing Wastes (ACW) to New Secondary Raw Material through a New Sustainable Inertization Process: A Multimethodological Mineralogical Study. J. Hazard. Mater. 2021, 413, 125419. [Google Scholar] [CrossRef]
- Vergani, F.; Galimberti, L.; Marian, N.M.; Giorgetti, G.; Viti, C.; Capitani, G.C. Thermal Decomposition of Cement–Asbestos at 1100 °C: How Much “Safe” Is “Safe”? J. Mater. Cycles Waste Manag. 2022, 24, 297–310. [Google Scholar] [CrossRef]
- Gualtieri, A.F. Mineral Fibres: Crystal Chemistry, Chemical-Physical Properties, Biological Interaction and Toxicity; Mineralogical Society: Chantilly, VA, USA, 2017. [Google Scholar]
- Bernasconi, A.; Pellegrino, L.; Vergani, F.; Campanale, F.; Marian, N.M.; Galimberti, L.; Perotti, M.; Viti, C.; Capitani, G. Recycling Detoxified Cement Asbestos Slates in the Production of Ceramic Sanitary Wares. Ceram. Int. 2022, 49, 1549–3100. [Google Scholar] [CrossRef]
- Gualtieri, A.F.; Giacobbe, C.; Sardisco, L.; Saraceno, M.; Lassinantti Gualtieri, M.; Lusvardi, G.; Cavenati, C.; Zanatto, I. Recycling of the Product of Thermal Inertization of Cement-Asbestos for Various Industrial Applications. Waste Manag. 2011, 31, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Gualtieri, A.F.; Tartaglia, A. Thermal Decomposition of Asbestos and Recycling in Traditional Ceramics. J. Eur. Ceram. Soc. 2000, 20, 1409–1418. [Google Scholar] [CrossRef]
- Ligabue, M.L.; Gualtieri, A.F.; Lassinantti Gualtieri, M.; Malferrari, D.; Lusvardi, G. Recycling of Thermally Treated Cement-Asbestos for the Production of Porcelain Stoneware Slabs. J. Clean. Prod. 2020, 247, 119084. [Google Scholar] [CrossRef]
- Ramakrishna, H.V.; Priya, S.P.; Rai, S.K. Flexural, Compression, Chemical Resistance, and Morphology Studies on Granite Powder-Filled Epoxy and Acrylonitrile Butadiene Styrene-Toughened Epoxy Matrices. J. Appl. Polym. Sci. 2007, 104, 171–177. [Google Scholar] [CrossRef]
- Mostovoi, A.S.; Kurbatova, E.A. Controlling the Properties of Epoxy Composites Filled with Brick Dust. Russ. J. Appl. Chem. 2017, 90, 267–276. [Google Scholar] [CrossRef]
- Mostovoy, A.S.; Nurtazina, A.S.; Burmistrov, I.N.; Kadykova, Y.A. Effect of Finely Dispersed Chromite on the Physicochemical and Mechanical Properties of Modified Epoxy Composites. Russ. J. Appl. Chem. 2018, 91, 1758–1766. [Google Scholar] [CrossRef]
- Sim, J.; Kang, Y.; Kim, B.J.; Park, Y.H.; Lee, Y.C. Preparation of Fly Ash/Epoxy Composites and Its Effects on Mechanical Properties. Polymers 2020, 12, 79. [Google Scholar] [CrossRef] [Green Version]
- Bekeshev, A.; Mostovoy, A.; Tastanova, L.; Kadykova, Y.; Kalganova, S.; Lopukhova, M. Reinforcement of Epoxy Composites with Application of Finely-Ground Ochre and Electrophysical Method of the Composition Modification. Polymers 2020, 12, 1437. [Google Scholar] [CrossRef] [PubMed]
- Bekeshev, A.; Mostovoy, A.; Kadykova, Y.; Akhmetova, M.; Tastanova, L.; Lopukhova, M. Development and Analysis of the Physicochemical and Mechanical Properties of Diorite-Reinforced Epoxy Composites. Polymers 2021, 13, 2421. [Google Scholar] [CrossRef]
- Friedrich, K.; Fakirov, S.; Zhang, Z. Polymer Composites: From Nano-to Macro-Scale; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2005; ISBN 0387241760. [Google Scholar]
- Vincent, S.R.; Jaafar, M.; Palaniandy, S. Properties of Calcium Carbonate/Mica and Calcium Farbonate/Talc Filled Polypropylene Composites. J. Eng. Sci. 2014, 10, 41. [Google Scholar]
- Nurdina, A.K.; Mariatti, M.; Samayamutthirian, P. Effect of Single-mineral Filler and Hybrid-mineral Filler Additives on the Properties of Polypropylene Composites. J. Vinyl Addit. Technol. 2009, 15, 20–28. [Google Scholar] [CrossRef]
- Molnár, S.; Pukanszky, B.; Hammer, C.O.; Maurer, F.H.J. Impact Fracture Study of Multicomponent Polypropylene Composites. Polymer 2000, 41, 1529–1539. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, Z.; Moon, K.; Wong, C. Glass Transition and Relaxation Behavior of Epoxy Nanocomposites. J. Polym. Sci. Part B 2004, 42, 3849–3858. [Google Scholar] [CrossRef]
- Kango, S.; Kalia, S.; Celli, A.; Njuguna, J.; Habibi, Y.; Kumar, R. Surface Modification of Inorganic Nanoparticles for Development of Organic–Inorganic Nanocomposites—A Review. Prog. Polym. Sci. 2013, 38, 1232–1261. [Google Scholar] [CrossRef]
- Amjad, A.; Abidin, M.; Alshahrani, H.; Rahman, A.A.A. Effect of Fibre Surface Treatment and Nanofiller Addition on the Mechanical Properties of Flax/PLA Fibre Reinforced Epoxy Hybrid Nanocomposite. Polymers 2021, 13, 3842. [Google Scholar] [CrossRef]
- Kumar, S.K.; Jouault, N.; Benicewicz, B.; Neely, T. Nanocomposites with Polymer Grafted Nanoparticles. Macromolecules 2013, 46, 3199–3214. [Google Scholar] [CrossRef]
- Jang, K.-S. Mineral Filler Effect on the Mechanics and Flame Retardancy of Polycarbonate Composites: Talc and Kaolin. e-Polymers 2016, 16, 379–386. [Google Scholar] [CrossRef]
- Nurdina, A.K.; Mariatti, M.; Samayamutthirian, P. Mechanical Properties and Morphology of Calcium Carbonate and Mica Filled Polypropylene Composites. Malays. J. Microsc. 2009, 5, 113–118. [Google Scholar]
- Uddin, F. Studies in Finishing Effects of Clay Mineral in Polymers and Synthetic Fibers. Adv. Mater. Sci. Eng. 2013, 2013, 243515. [Google Scholar] [CrossRef] [Green Version]
- Moreno, J.F.; da Silva, A.L.N.; da Silva, A.H.M.D.F.T.; de Sousa, A.M.F. Preparation and Characterization of Composites Based on Poly (Lactic Acid) and CaCO3 Nanofiller. In Proceedings of the AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2015; Volume 1664, p. 020008. [Google Scholar]
- Kulkarni, S.; Sharathchandra, S.; Sunil, D. On the Use of an Instrumented Set-up to Characterize the Impact Behaviour of an Epoxy System Containing Varying Fly Ash Content. Polym. Test. 2002, 21, 763–771. [Google Scholar]
- Pawar, M.J.; Patnaik, A.; Nagar, R. Experimental Investigation and Numerical Simulation of Granite Powder Filled Polymer Composites for Wind Turbine Blade: A Comparative Analysis. Polym. Compos. 2017, 38, 1335–1352. [Google Scholar] [CrossRef]
- Choudhary, M.; Singh, T.; Dwivedi, M.; Patnaik, A. Waste Marble Dust-Filled Glass Fiber-Reinforced Polymer Composite Part I: Physical, Thermomechanical, and Erosive Wear Properties. Polym. Compos. 2019, 40, 4113–4124. [Google Scholar] [CrossRef]
- Rajawat, A.S.; Singh, S.; Gangil, B.; Ranakoti, L.; Sharma, S.; Asyraf, M.R.M.; Razman, M.R. Effect of Marble Dust on the Mechanical, Morphological, and Wear Performance of Basalt Fibre-Reinforced Epoxy Composites for Structural Applications. Polymers 2022, 14, 1325. [Google Scholar] [CrossRef] [PubMed]
- Pegoretti, A.; Dorigato, A. Polymer Composites: Reinforcing Fillers. Encycl. Polym. Sci. Technol. 2002, 1–72. [Google Scholar] [CrossRef]
- Riley, A.M.; Paynter, C.D.; McGenity, P.M.; Adams, J.M. Factors Affecting the Impact Properties of Mineral Filled Polypropylene. Plast. Rubber Process. Appl. 1990, 14, 85–93. [Google Scholar]
- Wetzel, B.; Haupert, F.; Friedrich, K.; Zhang, M.Q.; Rong, M.Z. Impact and Wear Resistance of Polymer Nanocomposites at Low Filler Content. Polym. Eng. Sci. 2002, 42, 1919–1927. [Google Scholar] [CrossRef]
- Cipriano, T.F.; Silva, A.L.N.D.; Silva, A.H.M.D.F.T.D.; Sousa, A.M.F.D.; Silva, G.M.D.; Rocha, M.G. Thermal, Rheological and Morphological Properties of Poly (Lactic Acid)(PLA) and Talc Composites. Polímeros 2014, 24, 276–282. [Google Scholar]
- Mauri, M.; Floudas, G.; Simonutti, R. Local Order and Dynamics of Nanoconstrained Ethylene-Butylene Chain Segments in SEBS. Polymers 2018, 10, 655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suchitra, M.; Renukappa, N.M. The Thermal Properties of Glass Fiber Reinforced Epoxy Composites with and without Fillers. In Proceedings of the Macromolecular Symposia; Wiley Online Library: Hoboken, NJ, USA, 2016; Volume 361, pp. 117–122. [Google Scholar]
- Larsen, T.Ø.; Andersen, T.L.; Thorning, B.; Horsewell, A.; Vigild, M.E. Changes in the Tribological Behavior of an Epoxy Resin by Incorporating CuO Nanoparticles and PTFE Microparticles. Wear 2008, 265, 203–213. [Google Scholar] [CrossRef]
- Jakab, B.; Panaitescu, I.; Gamsjaeger, N. The Action of Fillers in the Enhancement of the Tribological Performance of Epoxy Composite Coatings. Polym. Test. 2021, 100, 107243. [Google Scholar] [CrossRef]
- Fernández-de-Alba, C.; Jimenez, A.M.; Abbasi, M.; Kumar, S.K.; Saalwächter, K.; Baeza, G.P. On the Immobilized Polymer Fraction in Attractive Nanocomposites: T g Gradient versus Interfacial Layer. Macromolecules 2021, 54, 10289–10299. [Google Scholar] [CrossRef]
- Siddique, S.; Smith, G.D.; Yates, K.; Mishra, A.K.; Matthews, K.; Csetenyi, L.J.; Njuguna, J. Structural and Thermal Degradation Behaviour of Reclaimed Clay Nano-Reinforced Low-Density Polyethylene Nanocomposites. J. Polym. Res. 2019, 26, 154. [Google Scholar] [CrossRef] [Green Version]



| Pavafloor H200/E | Pavatekno Gold 200 | ||
|---|---|---|---|
| # | DCAP | # | DCAP |
| PF0 | 0% | PT0 | 0% |
| PF2 | 2% | PT10 | 10% |
| PF5 | 5% | PT20 | 20% |
| PF10 | 10% | PT30 | 30% |
| EDXRF Chemical Analyses | XRPD Quantitative Phase Analysis | |||||
|---|---|---|---|---|---|---|
| Compound/wt% | Phase | Chemical Formula | Abundance (wt%) | |||
| Na2O | 0.17 | CaO | 47.17 | Akermanite | Ca2Mg(Si2O7) | 19.33 |
| MgO | 7.65 | TiO2 | 0.23 | Bredigite | Ca13.5Ba0.3Mg1.8Mn0.4Si9O32 | 19.25 |
| Al2O3 | 3.88 | MnO | 0.43 | Merwinite | Ca3Mg(SiO4)2 | 17.84 |
| SiO2 | 30.28 | Fe2O3 | 5.91 | Larnite | Ca2SiO4 | 3.67 |
| SO3 | 3.1 | LOI | 0.32 | Glass | 39.9 | |
| K2O | 0.42 | Sum | 99.56 | |||
| Compressive Strength | Tensile Strength | Flexural Strength | ||||||
|---|---|---|---|---|---|---|---|---|
| Ec (MPa) | σc (MPa) | ε (%) | Et (MPa) | σt (MPa) | ε (%) | σf (MPa) | Ef (MPa) | |
| PF0 | n = 5 | n = 4 | n = 5 | |||||
| Ave. | 839.2 | 100.9 | 48.5 | 614.1 | 21.6 | 5.2 | 39.2 | 1328.9 |
| St.dev. | 125.5 | 22.1 | 1.6 | 60.9 | 0.7 | 0.2 | 6.0 | 294.1 |
| PF2 | n = 5 | n = 5 | n = 5 | |||||
| Ave. | 143.4 | 112.7 | 56.8 | 256.0 | 9.2 | 33.9 | 18.0 | 484.8 |
| St.dev. | 24.8 | 18.8 | 1.7 | 41.8 | 0.9 | 4.5 | 1.5 | 39.6 |
| PF5 | n = 7 | n = 5 | n = 5 | |||||
| Ave. | 715.4 | 57.8 | 35.2 | 551.4 | 14.8 | 7.2 | 33.4 | 1074.0 |
| St.dev. | 90.6 | 28.2 | 10.3 | 110.5 | 2.6 | 1.5 | 3.7 | 196.6 |
| PF10 | n = 5 | n = 5 | n = 5 | |||||
| Ave. | 574.3 | 80.0 | 44.0 | 511.0 | 14.1 | 5.6 | 30.1 | 1004.1 |
| St.dev. | 164.8 | 22.0 | 4.3 | 120.9 | 2.3 | 1.2 | 5.3 | 249.5 |
| Compressive Strength | Tensile Strength | Flexural Strength | ||||||
|---|---|---|---|---|---|---|---|---|
| Ec (MPa) | σc (MPa) | ε (%) | Et (MPa) | σt (MPa) | ε (%) | σf (MPa) | Ef (MPa) | |
| PT0 | n = 5 | n = 4 | n = 5 | |||||
| Ave. | 1465.3 | 67.0 | 28.0 | 774.9 | 52.6 | 5.8 | 74.6 | 2033.4 |
| St.dev. | 160.8 | 4.5 | 19.5 | 182.3 | 4.3 | 0.3 | 8.3 | 338.5 |
| PT10 | n = 4 | n = 5 | n = 5 | |||||
| Ave. | 1238.0 | 61.6 | 22.4 | 752.1 | 51.4 | 5.1 | 65.6 | 1813.3 |
| St.dev. | 249.5 | 4.7 | 17.9 | 53.0 | 2.8 | 0.6 | 5.4 | 361.2 |
| PT20 | n = 4 | n = 5 | n = 4 | |||||
| Ave. | 1488.6 | 83.6 | 38.3 | 889.7 | 42.8 | 4.0 | 63.3 | 2212.3 |
| St.dev. | 160.9 | 10.9 | 21.7 | 84.7 | 3.5 | 0.4 | 4.1 | 190.5 |
| PT30 | n = 4 | n = 5 | n = 5 | |||||
| Ave. | 1361.0 | 67.0 | 35.2 | 839.9 | 42.2 | 3.9 | 49.9 | 1708.7 |
| St.dev. | 268.9 | 6.6 | 12.1 | 84.4 | 4.7 | 0.4 | 6.0 | 223.9 |
| n = 10 | PF0 | PF2 | PF5 | PF10 | PT0 | PT10 | PT20 | PT30 |
|---|---|---|---|---|---|---|---|---|
| Ave. | 76.4 | 66.0 | 78.2 | 76.4 | 62.5 | 72.4 | 82.0 | 84.8 |
| St.dev. | 11.9 | 5.0 | 9.3 | 2.3 | 17.2 | 12.5 | 6.9 | 4.6 |
| Pavafloor H200/E | Pavatekno Gold 200 | ||
|---|---|---|---|
| # | Tg (°C), II Cycle | # | Tg (°C), II Cycle |
| PF0 | 58.0 | PT0 | 58.6 |
| PF2 | 54.5 | PT10 | 61.5 |
| PF5 | 55.8 | PT20 | 59.0 |
| PF10 | 55.5 | PT30 | 58.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campanale, F.; Vergani, F.; Marian, N.M.; Viti, C.; Bianchi, A.; Ferrario, S.; Mauri, M.; Capitani, G. Epoxy Resins for Flooring Applications, an Optimal Host for Recycling Deactivated Cement Asbestos. Polymers 2023, 15, 1410. https://doi.org/10.3390/polym15061410
Campanale F, Vergani F, Marian NM, Viti C, Bianchi A, Ferrario S, Mauri M, Capitani G. Epoxy Resins for Flooring Applications, an Optimal Host for Recycling Deactivated Cement Asbestos. Polymers. 2023; 15(6):1410. https://doi.org/10.3390/polym15061410
Chicago/Turabian StyleCampanale, Fabrizio, Fabrizio Vergani, Narcisa Mihaela Marian, Cecilia Viti, Alberto Bianchi, Silvia Ferrario, Michele Mauri, and Giancarlo Capitani. 2023. "Epoxy Resins for Flooring Applications, an Optimal Host for Recycling Deactivated Cement Asbestos" Polymers 15, no. 6: 1410. https://doi.org/10.3390/polym15061410
APA StyleCampanale, F., Vergani, F., Marian, N. M., Viti, C., Bianchi, A., Ferrario, S., Mauri, M., & Capitani, G. (2023). Epoxy Resins for Flooring Applications, an Optimal Host for Recycling Deactivated Cement Asbestos. Polymers, 15(6), 1410. https://doi.org/10.3390/polym15061410








