Biodegradable Conducting Polymer-Based Composites for Biomedical Applications—A Review
Abstract
:1. Introduction
2. Biodegradable Materials
3. Biodegradable Polymers
Classification of Biodegradable Polymers
4. Applications of Biodegradable Conducting Polymer Based Composites
4.1. Tissue Engineering
4.2. Biomedical Implants
4.3. Antibacterial Therapy
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Qu, H.; Fu, H.; Han, Z.; Sun, Y. Biomaterials for bone tissue engineering scaffolds: A review. RSC Adv. 2019, 9, 26252–26262. [Google Scholar] [CrossRef]
- Basu, B.; Ghosh, S. Biomaterials for Musculoskeletal Regeneration; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Reis, L.A.; Chiu, L.L.Y.; Feric, N.; Fu, L.; Radisic, M. Biomaterials in myocardial tissue engineering. J. Tissue Eng. Regen. Med. 2016, 10, 11–28. [Google Scholar] [CrossRef] [PubMed]
- Mir, M.; Ali, M.N.; Barakullah, A.; Gulzar, A.; Arshad, M.; Fatima, S.; Asad, M. Synthetic polymeric biomaterials for wound healing: A review. Prog. Biomater. 2018, 7, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Rebelo, R.; Fernandes, M.; Fangueiro, R. Biopolymers in medical implants: A brief review. Procedia Eng. 2017, 200, 236–243. [Google Scholar] [CrossRef]
- Kabir, E.; Kaur, R.; Lee, J.; Kim, K.H.; Kwon, E.E. Prospects of biopolymer technology as an alternative option for non-degradable plastics and sustainable management of plastic wastes. J. Clean. Prod. 2020, 258, 120536. [Google Scholar] [CrossRef]
- Andreeben, C.; Steinbuchel, A. Recent developments in non-biodegradable biopolymers: Precursors, production processes, and future perspectives. Appl. Microbiol. Biotechnol. 2019, 103, 143–157. [Google Scholar] [CrossRef]
- George, A.; Sanjay, M.R.; Sriusk, R.; Parameswaranpillai, J.; Siengchin, S. A comprehensive review on chemical properties and applications of biopolymers and their composites. Int. J. Biol. Macromol. 2020, 154, 329–338. [Google Scholar] [CrossRef]
- Ribas, R.G.; Schatkoski, V.M.; do Amaral Montanheiro, T.L.; de Menezes, B.R.C.; Stegemann, C.; Leite, D.M.G.; Thim, G.P. Current advances in bone tissue engineering concerning ceramic and bioglass scaffolds: A review. Ceram. Int. 2019, 45, 21051–21061. [Google Scholar] [CrossRef]
- Cha, G.D.; Kang, D.; Lee, J.; Kim, D. Bioresorbable electronic implants: History, materials, fabrication, devices, and clinical applications. Adv. Healthc. Mater. 2019, 8, 1801660. [Google Scholar] [CrossRef]
- Prakasam, M.; Locs, J.; Salma-Ancane, K.; Loca, D.; Largeteau, A.; Berzina-Cimdina, L. Biodegradable materials and metallic implants—A review. J. Funct. Biomater. 2017, 8, 44. [Google Scholar] [CrossRef]
- Teo, A.J.T.; Mishra, A.; Park, I.; Kim, Y.J.; Park, W.T.; Yoon, Y.J. Polymeric biomaterials for medical implants and devices. ACS Biomater. Sci. Eng. 2016, 2, 454–472. [Google Scholar] [CrossRef] [PubMed]
- Kuncicka, L.; Kocich, R.; Lowe, T.C. Advances in metals and alloys for joint replacement. Prog. Mater. Sci. 2017, 88, 232–280. [Google Scholar] [CrossRef]
- Montero de Espinosa, L.; Meesorn, W.; Moatsou, D.; Weder, C. Bioinspired polymer systems with stimuli-responsive mechanical properties. Chem. Rev. 2017, 117, 12851–12892. [Google Scholar] [PubMed]
- Eliaz, N. Corrosion of metallic biomaterials: A review. Materials 2019, 12, 407. [Google Scholar] [CrossRef]
- Bharadwaj, A. An Overview on Biomaterials and Its Applications in Medical Science. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1116, 012178. [Google Scholar] [CrossRef]
- Ali, S.; Rani, A.M.A.; Baig, Z.; Ahmed, S.W.; Hussain, G.; Subramaniam, K.; Hastuty, S.; Rao, T.V. Biocompatibility and corrosion resistance of metallic biomaterials. Corros. Rev. 2020, 38, 381–402. [Google Scholar] [CrossRef]
- Manivasagam, G.; Dhinasekaran, D.; Rajamanickam, A. Biomedical Implants: Corrosion and its Prevention—A Review. Recent Pat. Corros. Sci. 2010, 2, 40–54. [Google Scholar] [CrossRef]
- Boni, R.; Ali, A.; Shavandi, A.; Clarkson, A.N. Current and novel polymeric biomaterials for neural tissue engineering. J. Biomed. Sci. 2018, 25, 90. [Google Scholar] [CrossRef] [PubMed]
- Hackett, A.J.; Malmstrom, J.; Travas-Sejdic, J. Functionalization of conducting polymers for biointerface applications. Prog. Polym. Sci. 2017, 70, 18–33. [Google Scholar] [CrossRef]
- Puiggali-Jou, A.; del Valle, L.J.; Aleman, C. Drug delivery systems based on intrinsically conducting polymers. J. Controlled Release 2019, 309, 244–264. [Google Scholar] [CrossRef]
- Jadoun, S.; Riaz, U.; Budhiraja, V. Biodegradable conducting polymeric materials for biomedical applications: A review. Med. Devices Sens. 2020, 4, 1–13. [Google Scholar] [CrossRef]
- Nair, L.S.; Laurencin, C.T. Biodegradable polymers as biomaterials. Prog. Polym. Sci. 2007, 32, 762–798. [Google Scholar] [CrossRef]
- Tian, H.; Tang, Z.; Zhuang, X.; Chen, X.; Jing, X. Biodegradable synthetic polymers: Preparation, functionalization and biomedical application. Prog. Polym. Sci. 2012, 37, 237–280. [Google Scholar]
- Kim, B.-S.; Baez, C.E.; Atala, A. Biomaterials for tissue engineering. World J. Urol. 2000, 18, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Bergsma, J.E.; Rozema, F.R.; Bos, R.R.M.; Van Rozendaal, A.W.M.; De Jong, W.H.; Teppema, J.S.; Joziasse, C.A.P. Biocompatibility and degradation mechanisms of predegraded and non-predegraded poly(lactide) implants: An animal study. J. Mater. Sci. Mater. Med. 1995, 6, 715–724. [Google Scholar] [CrossRef]
- Misra, S.K.; Valappil, S.P.; Roy, I.; Boccaccini, A.R. Polyhydroxyalkanoate (PHA)/Inorganic Phase Composites for Tissue Engineering Applications. Biomacromolecules 2006, 7, 2249–2258. [Google Scholar] [CrossRef] [PubMed]
- Amass, W.; Amass, A.; Tighe, B. A review of biodegradable polymers: Uses, current developments in the synthesis and characterization of biodegradable polyesters, blends of biodegradable polymers and recent advances in biodegradation studies. Polym Int. 1998, 47, 89–144. [Google Scholar] [CrossRef]
- Yang, Y.; Cheng, Y.; Deng, F.; Shen, L.; Zhao, Z.; Peng, S.; Shuai, C. A bifunctional bone scaffold combines osteogenesis and antibacterial activity via in situ grown hydroxyapatite and silver nanoparticle. Bio-Des. Manuf. 2021, 4, 452–468. [Google Scholar] [CrossRef]
- Saad, B.; Suter, U.W. Biodegradable polymeric materials. Encyclopedia Mater. Sci. Technol. 2001, 551–555. [Google Scholar]
- Malafaya, P.B.; Silva, G.A.; Reis, R.L. Natural–origin polymers as carriers and scaffolds for biomolecules and cell delivery in tissue engineering applications. Adv. Drug Deliv. Rev. 2007, 59, 207–233. [Google Scholar] [CrossRef]
- Pachence, J.M.; Bohrer, M.P.; Kohn, J. Principles of Tissue Engineering, 3rd ed.; Lanza, R., Langer, R., Vacanti, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; Volume 23, pp. 323–339. [Google Scholar]
- Middleton, J.C.; Tipton, A.J. Synthetic biodegradable polymers as medical devices. Med. Plast. Biomater. Mag. 1998, 3, 30. [Google Scholar]
- Navarro, M.; Michiardi, A.; Castano, O.; Planell, J.A. Biomaterials in orthopaedics. J. R. Soc. Interface 2008, 5, 1137–1158. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Wong, F.; McKay, I.; Rehman, I.U. Structural, mechanical, and biocompatibility analyses of a novel dental restorative nanocomposite. J. Appl. Polym. Sci. 2013, 127, 439–447. [Google Scholar] [CrossRef]
- Khan, A.F.; Afzal, A.; Chaudhary, A.A.; Saleem, M.; Shahzadi, L.; Jamal, A.; Yar, M.; Habib, A. (Hydroxypropyl) methylcellulose Mediated Synthesis of Highly Porous Composite Scaffolds for Trabecular Bone Repair Applications. Sci. Adv. Mater. 2015, 7, 1177–1186. [Google Scholar] [CrossRef]
- Khan, A.S.; Aziz, M.S.; Paul, D.; Wong, F.; Rehman, I.U. Synthesis and in-vitro analysis of degradative resistance of a novel bioactive composite. J. Bionanosci. 2008, 2, 75–88. [Google Scholar] [CrossRef]
- Gomes, M.E.; Reis, R. Biodegradable polymers and composites in biomedical applications: From catgut to tissue engineering. Part 2 Systems for temporary replacement and advanced tissue regeneration. Int. Mater. Rev. 2004, 49, 274–285. [Google Scholar]
- Sabir, M.; Xu, X.; Li, L. A review on biodegradable polymeric materials for bone tissue engineering applications. J. Mater. Sci. 2009, 44, 5713–5724. [Google Scholar]
- Gomes, M.E.; Reis, R. Biodegradable polymers and composites in biomedical applications: From catgut to tissue engineering. Part 1 Available systems and their properties. Int. Mater. Rev. 2004, 49, 261–273. [Google Scholar]
- Khan, A.S.; Aamer, S.; Chaudhry, A.A.; Wong, F.S.; Rehman, I.U. Synthesis and characterizations of a fluoride-releasing dental restorative material. Mater. Sci. Eng. C 2013, 33, 3458–3464. [Google Scholar] [CrossRef]
- Khan, A.S.; Ahmed, Z.; Edirisinghe, M.J.; Wong, F.S.L.; Rehman, I.U. Preparation and characterization of a novel bioactive restorative composite based on covalently coupled polyurethane–nanohydroxyapatite fibres. Acta Biomater. 2008, 4, 1275–1287. [Google Scholar] [CrossRef]
- Talal, A.; McKay, I.; Tanner, K.; Hughes, F.J. Effects of hydroxyapatite and PDGF concentrations on osteoblast growth in a nanohydroxyapatite-polylactic acid composite for guided tissue regeneration. J. Mater. Sci. Mater. Med. 2013, 24, 2211–2221. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Yang, J.; Bei, J.; Wang, S. A novel porous cells scaffold made of polylactide–dextran blend by combining phase-separation and particle-leaching techniques. Biomaterials 2002, 23, 4483–4492. [Google Scholar] [CrossRef] [PubMed]
- Maquet, V.; Boccaccini, A.R.; Pravata, L.; Notingher, I.; Jérôme, R. Porous poly(α-hydroxyacid)/Bioglass composite scaffolds for bone tissue engineering. I: Preparation and in vitro characterisation. Biomaterials 2004, 25, 4185–4194. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; Ma, P.X. Structure and properties of nano-hydroxyapatite/polymer composite scaffolds for bone tissue engineering. Biomaterials 2004, 25, 4749–4757. [Google Scholar] [CrossRef] [PubMed]
- Van Vlierberghe, S.; Dubruel, P.; Schacht, E. Biopolymer-based hydrogels as scaffolds for tissue engineering applications: A review. Biomacromolecules 2011, 12, 1387–1408. [Google Scholar] [CrossRef] [PubMed]
- Tate, M.L.K.; Falls, T.D.; McBride, S.H.; Atit, R.; Knothe, U.R. Mechanical modulation of osteochondroprogenitor cell fate. Int. J. Biochem. Cell. Biol. 2008, 40, 2720–2738. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, J.P.; Saeed, A.O.; Fernandez-Trillo, F.; Alexander, C. Synthetic polymers for biopharmaceutical delivery. Polym. Chem. 2011, 2, 48–59. [Google Scholar] [CrossRef]
- Cameron, D.J.A.; Shaver, M.P. Aliphatic polyester polymer stars: Synthesis, properties and applications in biomedicine and nanotechnology. Chem. Soc. Rev. 2011, 40, 1761–1776. [Google Scholar] [CrossRef] [PubMed]
- Hakkarainen, M.; Albertsson, A.C. Degradation products of aliphatic and aliphatic-aromatic polyesters. Adv. Polym. Sci. 2008, 211, 85–116. [Google Scholar]
- Torres, M.P.; Vogel, B.M.; Narasimhan, B.; Mallapragada, S.K. Synthesis and characterization of novel polyanhydrides with tailored erosion mechanisms. J. Biomed. Mater. Res. A 2006, 76A, 102–110. [Google Scholar] [CrossRef]
- Jain, J.P.; Chitkara, D.; Kumar, N. Polyanhydrides as localized drug delivery carrier: An update. Expert Opin. Drug Deliv. 2008, 5, 889–907. [Google Scholar] [CrossRef] [PubMed]
- Jain, J.P.; Modi, S.; Domb, A.J.; Kumar, N. Role of polyanhydrides as localized drug carriers. J. Controlled Release 2005, 103, 541–563. [Google Scholar] [CrossRef] [PubMed]
- Krol, P. Synthesis methods, chemical structures and phase structures of linear polyurethanes. Properties and applications of linear polyurethanes in polyurethane elastomers, copolymers and ionomers. Prog. Mater. Sci. 2007, 52, 915–1015. [Google Scholar] [CrossRef]
- Santerre, J.P.; Woodhouse, K.; Laroche, G.; Labow, R.S. Understanding the biodegradation of polyurethanes: From classical implants to tissue engineering materials. Biomaterials 2005, 26, 7457–7470. [Google Scholar] [CrossRef] [PubMed]
- Guelcher, S.A. Biodegradable polyurethanes: Synthesis and applications in regenerative medicine. Tissue Eng. Part B Rev. 2008, 14, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Y.; Doll, B.A.; Beckman, E.J.; Hollinger, J.O. Three-dimensional biocompatible ascorbic acidcontaining scaffold for bone tissue engineering. Tissue Eng. 2003, 9, 1143–1157. [Google Scholar] [CrossRef]
- Shi, Y.; Sun, X.; Zhang, L.; Sun, K.; Li, K.; Li, Y.; Zhang, Q. Fc-Modified exenatide-loaded nanoparticles for oral delivery to improve hypoglycemic effects in mice. Sci. Rep. 2018, 8, 726. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.; Dey, R.K. Development of docetaxel-loaded PEG–PLA nanoparticles using surfactant-free method for controlled release studies. Int. J. Polym. Mater. Polym. Biomater. 2018, 67, 535–542. [Google Scholar] [CrossRef]
- Canbolat, M.F.; Celebioglu, A.; Uyar, T. Drug delivery system based on cyclodextrin-naproxen inclusion complex incorporated in electrospun polycaprolactone nanofibers. Colloids Surf. B Biointerfaces 2014, 115, 15–21. [Google Scholar] [CrossRef]
- Bilthariya, U.; Jain, N.; Rajoriya, V.; Jain, A.K. Folate-Conjugated albumin nanoparticles for rheumatoid arthritis-targeted delivery of etoricoxib. Drug Dev. Ind. Pharm. 2015, 41, 95–104. [Google Scholar] [CrossRef]
- Sin, L.T. Overview of Poly (Lactic Acid). In Handbook of Biopolymers and Biodegradable Plastics: Properties, Processing and Applications; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Shuai, C.; He, C.; Peng, S.; Qi, F.; Wang, G.; Min, A.; Yang, W.; Wang, W. Mechanical Alloying of Immiscible Metallic Systems: Process, Microstructure, and Mechanism. Adv. Eng. Mater. 2021, 23, 2001098. [Google Scholar] [CrossRef]
- Hamad, K.; Kaseem, M.; Deri, F. Poly (lactic acid)/low density polyethylene polymer blends: Preparation and characterization. Asia-Pac. J. Chem. Eng. 2012, 7, S310–S316. [Google Scholar] [CrossRef]
- Niaounakis, M. Biopolymers Reuse, Recycling, and Disposal; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Legge, N.R. Thermoplastic elastomers. Rubber Chem. Technol. 1987, 60, 83–117. [Google Scholar] [CrossRef]
- Barkan, Y.; Levinman, M.; Veprinsky-Zuzuliya, I.; Tsach, T.; Merqioul, E.; Blum, G.; Domb, A.J.; Basu, A. Comparative evaluation of polycyanoacrylates. Acta Biomater. 2017, 48, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Mateo, J.M.; Sumera, F.C. In Vitro Controlled Drug Release of Anticancer Drugs Deguelin and Cisplatin by Lauric Acid Derived Polyanhydride as Carrier. Philipp. J. Sci. 2016, 145, 215–223. [Google Scholar]
- Svobodova, J.; Proks, V.; Karabiyik, O.; Calıkoglu Koyuncu, A.C.; Torun Kose, G.; Rypacek, F.; Studenovska, H. Poly (amino acid)-based fibrous scaffolds modified with surface-pendant peptides for cartilage tissue engineering. J. Tissue Eng. Regen. Med. 2017, 11, 831–842. [Google Scholar] [CrossRef]
- Hou, L.D.; Li, Z.; Pan, Y.; Sabir, M.; Zheng, Y.F.; Li, L. A review on biodegradable materials for cardiovascular stent application. Front. Mater. Sci. 2016, 10, 238–259. [Google Scholar] [CrossRef]
- Baillargeon, A.L.; Mequanint, K. Biodegradable polyphosphazene biomaterials for tissue engineering and delivery of therapeutics. BioMed Res. Int. 2014, 2014, 761373. [Google Scholar] [CrossRef]
- Diez-Pascual, A.M. Tissue Engineering Bionanocomposites Based on Poly (propylene fumarate). Polymers 2017, 9, 260. [Google Scholar] [CrossRef] [PubMed]
- Tyler, B.; Gullotti, D.; Mangraviti, A.; Utsuki, T.; Brem, H. Polylactic acid (PLA) controlled delivery carriers for biomedical applications. Adv. Drug Deliv. Rev. 2016, 107, 163–175. [Google Scholar] [CrossRef]
- Barouti, G.; Jaffredo, C.G.; Guillaume, S.M. Advances in drug delivery systems based on synthetic poly (hydroxybutyrate)(co) polymers. Prog. Polym. Sci. 2017, 73, 1–31. [Google Scholar] [CrossRef]
- Justinger, C.; Slotta, J.E.; Ningel, S.; Gräber, S.; Kollmar, O.; Schilling, M.K. Surgical-site infection after abdominal wall closure with triclosan-impregnated polydioxanone sutures: Results of a randomized clinical pathway facilitated trial (NCT00998907). Surgery 2013, 154, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Qi, F.; Wang, C.; Peng, S.; Shuai, C.; Yang, W.; Zhao, Z. A co-dispersed nanosystem of strontium-anchored reduced graphene oxide to enhance the bioactivity and mechanical property of polymer scaffolds. Mater. Chem. Front. 2021, 5, 2373–2386. [Google Scholar] [CrossRef]
- Armentano, I.; Dottori, M.; Fortunati, E.; Mattioli, S.; Kenny, J.M. Biodegradable polymer matrix nanocomposites for tissue engineering: A review. Polym. Degrad. Stab. 2010, 95, 2126–2146. [Google Scholar] [CrossRef]
- Almouemen, N.; Kelly, H.M.; O’Leary, C. Tissue engineering: Understanding the role of biomaterials and biophysical forces on cell functionality through computational and structural biotechnology analytical methods. Comput. Struct. Biotechnol. J. 2019, 17, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Broda, C.R.; Lee, J.Y.; Sirivisoot, S.; Schmidt, C.E.; Harrison, B.S. A chemically polymerized electrically conducting composite of polypyrrole nanoparticles and polyurethane for tissue engineering. J. Biomed. Mater. Res. A 2011, 98, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Ning, C.; Zhou, Z.; Tan, G.; Zhu, Y.; Mao, C. Electroactive polymers for tissue regeneration: Developments and perspectives. Prog. Polym. Sci. 2018, 81, 144–162. [Google Scholar] [CrossRef]
- Mostafavi, E.; Medina-Cruz, D.; Kalantari, K.; Taymoori, A.; Soltantabar, P.; Webster, T. Electroconductive nanobiomaterials for tissue engineering and regenerative medicine. Bioelectricity 2020, 2, 120–149. [Google Scholar] [CrossRef]
- Rivers, T.J.; Hudson, T.W.; Schmidt, C.E. Synthesis of a novel, biodegradable electrically conducting polymer for biomedical applications. Adv. Funct. Mater. 2002, 12, 33–37. [Google Scholar] [CrossRef]
- Schmidt, C.E.; Rivers, T.J. Biodegradable, Electrically Conducting Polymer for Tissue Engineering Applications; Springer: Berlin/Heidelberg, Germany, 2004. [Google Scholar]
- Huang, L.; Hu, J.; Lang, L.; Wang, X.; Zhang, P.; Jing, X.; MacDiarmid, A.G. Synthesis and characterization of electroactive and biodegradable ABA block copolymer of polylactide and aniline pentamer. Biomaterials 2007, 28, 1741–1751. [Google Scholar] [CrossRef]
- Subramanian, A.; Krishnan, U.M.; Sethuraman, S. Axially aligned electrically conducting biodegradable nanofibers for neural regeneration. J. Mater. Sci. Mater. Med. 2012, 23, 1797–1809. [Google Scholar] [CrossRef] [PubMed]
- Baheiraei, N.; Yeganeh, H.; Ai, J.; Gharibi, R.; Azami, M.; Faghihi, F. Synthesis, characterization and antioxidant activity of a novel electroactive and biodegradable polyurethane for cardiac tissue engineering application. Mater. Sci. Eng. C 2014, 44, 24–37. [Google Scholar] [CrossRef]
- Xie, M.; Wang, L.; Ge, J.; Guo, B.; Ma, P.X. Strong Electroactive Biodegradable Shape Memory Polymer Networks Based on Star-Shaped Polylactide and Aniline Trimer for Bone Tissue Engineering. ACS Appl. Mater. Interfaces 2015, 7, 6772–6781. [Google Scholar] [CrossRef]
- Song, F.; Jie, W.; Zhang, T.; Li, W.; Jiang, Y.; Wan, L.; Liu, B. Room-temperature fabrication of a three-dimensional reduced-graphene oxide/polypyrrole/hydroxyapatite composite scaffold for bone tissue engineering. RSC Adv. 2016, 6, 92804–92812. [Google Scholar] [CrossRef]
- Yang, S.; Jang, L.; Kim, S.; Yang, J.; Yang, K.; Cho, S.W.; Lee, J.Y. Polypyrrole/Alginate Hybrid Hydrogels: Electrically Conductive and Soft Biomaterials for Human Mesenchymal Stem Cell Culture and Potential Neural Tissue Engineering Applications. Macromol. Biosci. 2016, 16, 1653–1661. [Google Scholar] [CrossRef]
- Da Silva, A.C.; Semeano, A.T.S.; Dourado, A.H.B.; Ulrich, H.; Cordoba de Torresi, S.I. Novel Conducting and Biodegradable Copolymers with Noncytotoxic Properties toward Embryonic Stem Cells. ACS Omega 2018, 3, 5593–5604. [Google Scholar] [CrossRef]
- Xu, C.; Guan, S.; Wang, S.; Gong, W.; Liu, T.; Ma, X.; Sun, C. Biodegradable and electroconductive poly (3, 4-ethylenedioxythiophene)/carboxymethyl chitosan hydrogels for neural tissue engineering. Mater. Sci. Eng. C 2018, 84, 32–43. [Google Scholar] [CrossRef]
- Borah, R.; Upadhyay, J.; Acharjya, K. Functionalized polyaniline: Chitosan nanocomposites as a potential biomaterial. Mater. Today: Proc. 2020, 32, 334–343. [Google Scholar]
- Maziz, A.; Ozgur, E.; Bergaud, C.; Uzun, L. Progress in conducting polymers for biointerfacing and biorecognition applications. Sens. Actuators Rep. 2021, 3, 100035. [Google Scholar] [CrossRef]
- Boutry, C.M.; Sun, W.; Strunz, T.; Chandrahalim, H.; Hierold, C. Development and characterization of biodegradable conductive polymers for the next generation of RF bio-resonators. In Proceedings of the 2010 IEEE International Frequency Control Symposium, Newport Beach, CA, USA, 1–4 June 2010. [Google Scholar]
- Lu, Y.; Li, T.; Zhao, X.; Li, M.; Cao, Y.; Yang, H.; Duan, Y.Y. Electrodeposited polypyrrole/carbon nanotubes composite films electrodes for neural interfaces. Biomaterials 2010, 31, 5169–5181. [Google Scholar] [CrossRef] [PubMed]
- Wan, P.; Yuan, C.; Tan, L.; Li, Q.; Yang, K. Fabrication and evaluation of bioresorbable PLLA/magnesium and PLLA/magnesium fluoride hybrid composites for orthopedic implants. Compos. Sci. Technol. 2014, 98, 36–43. [Google Scholar] [CrossRef]
- Ghaziof, S.; Mehdikhani-Nahrkhalaji, M. Preparation, characterization, mechanical properties and electrical conductivity assessment of novel polycaprolactone/multi-wall carbon nanotubes nanocomposites for myocardial tissue engineering. Acta Phys. Pol. A 2017, 131, 428–431. [Google Scholar] [CrossRef]
- Wang, H.; Chiang, P.; Tzeng, J.; Wu, T.; Pan, Y.; Chang, W.; Huang, H. In vitro biocompatibility, radiopacity, and physical property tests of nano-Fe3O4 incorporated poly-L- lactide bone screws. Polymers 2017, 9, 191. [Google Scholar] [CrossRef] [PubMed]
- Loffler, S.; Melican, K.; Nilsson, K.P.R.; Richter Dahlfors, A. Organic bioelectronics in medicine. J. Intern. Med. 2017, 282, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Kim, Y.S.; Tillman, B.W.; Yeo, W.H.; Chun, Y. Advances in materials for recent low-profile implantable bioelectronics. Materials 2018, 11, 522. [Google Scholar] [CrossRef] [PubMed]
- Do, A.V.; Smith, R.; Acri, T.M.; Geary, S.M.; Salem, A.K. 3D printing technologies for 3D scaffold engineering. Functional 3D Tissue Engineering Scaffolds. Intech Open 2018, 203–234. [Google Scholar]
- Eslami, H.; Lisar, H.A.; Kashi, T.S.J.; Tahriri, M.; Ansari, M.; Rafiei, T.; Bastami, F.; Shahin-Shamsabadi, A.; Abbas, F.M.; Tayebi, L. Poly (lactic-co-glycolic acid) (PLGA)/TiO2 nanotube bioactive composite as a novel scaffold for bone tissue engineering: In vitro and in vivo studies. Biologicals 2018, 53, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.K. Conductive Polymer-Based Functional Structures for Neural Therapeutic Applications. Conjugated Polymers for Biological and Biomedical Applications. Conjug. Polym. Biol. Biomed. Appl. 2018, 243–267. [Google Scholar]
- Barua, E.; Deoghare, A.B.; Chatterjee, S.; Sapkal, P. Effect of ZnO reinforcement on the compressive properties, in vitro bioactivity, biodegradability and cytocompatibility of bone scaffold developed from bovine bone-derived HAp and PMMA. Ceram. Int. 2019, 45, 20331–20345. [Google Scholar] [CrossRef]
- Wu, B.; Cao, B.; Taylor, I.M.; Woeppel, K.; Cui, X.T. Facile synthesis of a 3,4-ethylene- dioxythiophene (EDOT) derivative for ease of bio-functionalization of the conducting polymer PEDOT. Front. Chem. 2019, 7, 178. [Google Scholar] [CrossRef]
- Gupta, S.; Sharma, A.; Verma, R.S. Polymers in Biosensor Devices for Cardiovascular Applications. Curr. Opin. Biomed. Eng. 2019, 13, 69–75. [Google Scholar] [CrossRef]
- Jayaram, A.K.; Pitsalidis, C.; Tan, E.; Moysidou, C.M.; De Volder, M.F.L.; Kim, J.S.; Owens, R.M. 3D Hybrid Scaffolds Based on PEDOT:PSS/MWCNT Composites. Front. Chem. 2019, 7, 363. [Google Scholar] [CrossRef] [PubMed]
- Mohammadalizadeh, Z.; Karbasi, S.; Arasteh, S. Physical, mechanical and biological evaluation of poly (3-hydroxybutyrate)-chitosan/MWNTs as a novel electrospun scaffold for cartilage tissue engineering applications. Polym. Plast. Tech. Mater. 2020, 59, 417–429. [Google Scholar] [CrossRef]
- Guo, Y.; Jia, S.; Qiao, L.; Su, Y.; Rui, G.; Li, G.; Lian, J. A Multifunctional Polypyrrole/Zinc Oxide Composite Coating on Biodegradable Magnesium Alloys for Orthopedic Implants. Colloids Surf. B 2020, 194, 111186. [Google Scholar] [CrossRef] [PubMed]
- Feng, P.; Kong, Y.; Liu, M.; Peng, S.; Shuai, C. Dispersion strategies for low-dimensional nanomaterials and their application in biopolymer implants. Mater. Today Nano 2021, 15, 100127. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, K.; Liu, Y.; Zhang, C.; Wang, B. Using Wet Electrospun PCL/Gelatin/CNT Yarns to Fabricate Textile-Based Scaffolds for Vascular Tissue Engineering. ACS Biomater. Sci. Eng. 2021, 7, 2627–2637. [Google Scholar] [CrossRef]
- Nouri, A.; Shirvan, A.R.; Li, Y.; Wen, C. Additive manufacturing of metallic and polymeric load-bearing biomaterials using laser powder bed fusion: A review. J. Mater. Sci. Technol. 2021, 94, 196–215. [Google Scholar] [CrossRef]
- Bhushan, J.; Grover, V. Additive manufacturing: Current concepts, methods, and applications in oral health care. In Biomanufacturing; Springer International Publishing: Cham, Switzerland, 2019; pp. 103–122. [Google Scholar]
- Mohan, D.; Teong, Z.K.; Bakir, A.N.; Sajab, M.S.; Kaco, H. Extending cellulose-based polymers application in additive manufacturing technology: A review of recent approaches. Polymers 2020, 12, 1876. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.A. Direct ink writing of 3D functional materials. Adv. Funct. Mater. 2006, 16, 2193–2204. [Google Scholar] [CrossRef]
- Bikas, H.; Stavropoulos, P.; Chryssolouris, G. Additive manufacturing methods and modeling approaches: A critical review. Int. J. Adv. Manuf. Technol. 2016, 83, 389–405. [Google Scholar] [CrossRef]
- Park, S.; Shou, W.; Makatura, L.; Matusik, W.; Fu, K.K. 3D printing of polymer composites: Materials, processes, and applications. Matter 2022, 5, 43–76. [Google Scholar] [CrossRef]
- Spirescu, V.A.; Chircov, C.; Grumezescu, A.M.; Andronescu, E. Polymeric nanoparticles for antimicrobial therapies: An up-to-date overview. Polymers 2021, 13, 724. [Google Scholar] [CrossRef] [PubMed]
- Mao, Z.; Li, Y.; Yang, Y.; Fang, Z.; Chen, X.; Wang, Y.; Kang, J.; Qu, X.; Yuan, W.; Dai, K.; et al. Osteoinductivity and Antibacterial Properties of Strontium Ranelate-Loaded Poly(Lactic-co-Glycolic Acid) Microspheres With Assembled Silver and Hydroxyapatite Nanoparticles. Front. Pharmacol. 2018, 9, 368. [Google Scholar] [CrossRef] [PubMed]
- Ejaz, S.; Ihsan, A.; Noor, T.; Shabbir, S.; Imran, M. Mannose functionalized chitosan nanosystems for enhanced antimicrobial activity against multidrug resistant pathogens. Polym. Test. 2020, 91, 106814. [Google Scholar] [CrossRef]
- Kritchenkov, A.S.; Egorov, A.R.; Artemjev, A.A.; Kritchenkov, I.S.; Volkova, O.V.; Kurliuk, A.V.; Shakola, T.V.; Rubanik, V.V.; Rubanik, V.V.; Tskhovrebov, A.G.; et al. Ultrasound-assisted catalyst-free thiol-yne click reaction in chitosan chemistry: Antibacterial and transfection activity of novel cationic chitosan derivatives and their based nanoparticles. Int. J. Biol. Macromol. 2020, 143, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, F.; Liu, Q.; Du, J. Antibacterial polymeric nanostructures for biomedical applications. Chem. Commun. 2014, 50, 14482–14493. [Google Scholar] [CrossRef] [PubMed]
- Ucak, S.; Sudagidan, M.; Borsa, B.A.; Mansuroglu, B.; Ozalp, V. CInhibitory effects of aptamer targeted teicoplanin encapsulated PLGA nanoparticles for Staphylococcus aureus strains. World J. Microbiol. Biotechnol. 2020, 36, 69. [Google Scholar] [CrossRef]
- Deepika, M.S.; Thangam, R.; Sheena, T.S.; Vimala, R.T.V.; Sivasubramanian, S.; Jeganathan, K.; Thirumurugan, R. Dual drug loaded PLGA nanospheres for synergistic efficacy in breast cancer therapy. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 103, 109716. [Google Scholar] [CrossRef]
- Durak, S.; Arasoglu, T.; Ates, S.C.; Derman, S. Enhanced antibacterial and antiparasitic activity of multifunctional polymeric nanoparticles. Nanotechnology 2020, 31, 175705. [Google Scholar] [CrossRef]
- Da Costa, D.; Exbrayat-Heritier, C.; Rambaud, B.; Megy, S.; Terreux, R.; Verrier, B.; Primard, C. Surface charge modulation of rifampicin-loaded PLA nanoparticles to improve antibiotic delivery in Staphylococcus aureus biofilms. J. Nanobiotechnol. 2021, 19, 12. [Google Scholar] [CrossRef]
- Vrouvaki, I.; Koutra, E.; Kornaros, M.; Avgoustakis, K.; Lamari, F.N.; Hatziantoniou, S. Polymeric nanoparticles of pistacia lentiscus var. chia essential oil for cutaneous applications. Pharmaceutics 2020, 12, 353. [Google Scholar] [CrossRef] [PubMed]
- Nam, H.; An, T.; Lim, G. Cell Behaviour on a Polyaniline Nanoprotrusion Structure Surface. Nanoscale Res. Lett. 2014, 9, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Hussain, E.; Ahtesham, A.; Shahadat, M.; Ibrahim, M.N.M.; Ismail, S. Recent advances of Clay/polymer-based nanomaterials for the treatment of environmental contaminants in wastewater: A review. J. Environ. Chem. Eng. 2024, 12, 112401. [Google Scholar] [CrossRef]
- Sen, T.; Mishra, S.; Shimpi, N.G. Synthesis and Sensing Applications of Polyaniline Nanocomposites: A Review. RSC Adv. 2016, 6, 42196–42222. [Google Scholar] [CrossRef]
- Qi, K.; Cheng, B.; Yu, J.; Ho, W. Review on the Improvement of the Photocatalytic and Antibacterial Activities of ZnO. J. Alloys Compd. 2017, 727, 792–820. [Google Scholar] [CrossRef]
- Sumitha, M.S.; Shalumon, K.T.; Sreeja, V.N.; Jayakumar, R.; Nair, S.V.; Menon, D. Biocompatible and Antibacterial Nanofibrous Poly(ϵ-caprolactone)-Nanosilver Composite Scaffolds for Tissue Engineering Applications. J. Macromol. Sci. A 2012, 49, 131–138. [Google Scholar] [CrossRef]
- Cabuk, M.; Alan, Y.; Yavuz, M.; Unal, H.I. Synthesis, characterization and antimicrobial activity of biodegradable conducting polypyrrole-graft-chitosan copolymer. Appl. Surf. Sci. 2014, 318, 168–175. [Google Scholar] [CrossRef]
- Ebrahimiasl, S.; Zakaria, A.; Kassim, A.; Norleha Basri, S. Novel conductive polypyrrole/zinc oxide/chitosan bionanocomposite: Synthesis, characterization, antioxidant, and antibacterial activities. Int. J. Nanomed. 2014, 10, 217–227. [Google Scholar] [CrossRef]
- Eren, O.; Ucar, N.; Onen, A.; Kizildag, N.; Karacan, I. Synergistic Effect of Polyaniline, Nanosilver, and Carbon Nanotube Mixtures on the Structure and Properties of Polyacrylonitrile Composite Nanofiber. J. Compos. Mater. 2016, 50, 2073–2086. [Google Scholar] [CrossRef]
- Shoja, M.; Shameli, K.; Ahmad, M.B.; Kalantari, K. Preparation, characterization and antibacterial properties of polycaprolactone/ZnOmicrocomposites. Dig. J. Nanomater. Biostruct. 2015, 10, 169–178. [Google Scholar]
- Hou, Y.; Feng, J.; Wang, Y.; Li, L. Enhanced Antibacterial Activity of Ag-Doped ZnO/Polyaniline Nanocomposites. J. Mater. Sci. Mater. Electron. 2016, 27, 6615–6622. [Google Scholar] [CrossRef]
- Kumar, S.; Raj, S.; Jain, S.; Chatterjee, K. Multifunctional biodegradable polymer nanocomposite incorporating graphene-silver hybrid for biomedical applications. Mater. Des. 2016, 108, 319–332. [Google Scholar] [CrossRef]
- Shahadat, M.; Khan, M.Z.; Rupani, P.F.; Embrandiri, A.; Sultana, S.; Ahammad, S.Z.; Ali, S.W.; Sreekrishnan, T.R. A Critical Review on the Prospect of Polyaniline-Grafted Biodegradable Nanocomposite. Adv. Colloid Interface Sci. 2017, 249, 2–16. [Google Scholar] [CrossRef] [PubMed]
- Zare, E.N.; Makvandi, P.; Ashtari, B.; Rossi, F.; Motahari, A.; Perale, G. Progress in Conductive Polyaniline-Based Nanocomposites for Biomedical Applications: A Review. J. Med. Chem. 2019, 63, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Masim, F.C.P.; Tsai, C.H.; Lin, Y.F.; Fu, M.L.; Liu, M.; Kang, F.; Wang, Y.F. Synergistic Effect of PANI−ZrO2 Composite as Antibacterial, Anti-Corrosion, and Phosphate Adsorbent Material: Synthesis, Characterization and Applications. Environ. Technol. 2019, 40, 226–238. [Google Scholar] [CrossRef] [PubMed]
- Abou Hammad, A.B.; Abd El-Aziz, M.E.; Hasanin, M.S.; Kamel, S. A novel electromagnetic biodegradable nanocomposite based on cellulose, polyaniline, and cobalt ferrite nanoparticles. Carbohydr. Polym. 2019, 216, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, B.; Pirsa, S.; Alizadeh, M. Preparing chitosan–polyaniline nanocomposite film and examining its mechanical, electrical, and antimicrobial properties. Polym. Polym. Compos. 2019, 27, 1–11. [Google Scholar] [CrossRef]
- Munoz-Escobar, A.; de Ruiz-Baltazar, A.J.; Reyes-Lopez, S.Y. Novel Route of Synthesis of PCL-CuO NPs Composites with Antimicrobial Properties. Dose-Response 2019, 17, 1–11. [Google Scholar] [CrossRef]
- Youssef, A.M.; El Aziz, M.E.A.; Abd El-Sayed, E.S.; Moussa, M.A.; Turky, G.; Kamel, S. Rational design and electrical study of conducting bionanocomposites hydrogel based on chitosan and silver nanoparticles. Int. J. Biol. Macromol. 2019, 140, 886–894. [Google Scholar] [CrossRef]
- Talebi, A.; Labbaf, S.; Karimzadeh, F. A conductive film of chitosan-polycaprolcatone- polypyrrole with potential in heart patch application. Polym. Test. 2019, 75, 254–261. [Google Scholar] [CrossRef]
- Mirmohseni, A.; Rastgar, M.; Olad, A. PANI-chitosan-TiO2 ternary nanocomposite and its effectiveness on antibacterial and antistatic behavior of epoxy coating. J. Appl. Polym. Sci. 2019, 136, 47629. [Google Scholar] [CrossRef]
- Balitaan, J.N.I.; Martin, G.A.V.; Santiago, K.S. Revamping squid gladii to biodegradable composites: In situ grafting of polyaniline to β-chitin and their antibacterial activity. J. Bioact. Compat. Polym. 2020, 36, 13–28. [Google Scholar] [CrossRef]
- Golbaten-Mofrad, H.; Seyfi Sahzabi, A.; Seyfikar, S.; Salehi, M.H.; Goodarzi, V.; Wurm, F.R.; Jafari, S.H. Facile template preparation of novel electroactive scaffold composed of polypyrrole-coated poly(glycerol-sebacate-urethane) for tissue engineering applications. Eur. Polym. J. 2021, 159, 110749. [Google Scholar] [CrossRef]
- Unnikrishnan, G.; Joy, A.; Megha, M.; Thomas, J.; Haris, M.; Kolanthai, E.; Muthuswamy, S. Preparation and characterizations of antibacterial and electroactive polymeric composites for wound healing applications. Polym. Compos. 2024, 45, 267–285. [Google Scholar] [CrossRef]
- Hasan, P.M.Z.; Saini, S.; Melaibari, A.A.; Leel, N.S.; Darwesh, R.; Quraishi, A.M.; Singh, J.; Kuznetsov, A.E.; Hashmi, S.Z.; Dalela, S.; et al. Tunable optical and structural characteristics with improved electrical properties of (PVA-GO-CuO) eco-friendly-polymer nanocomposites and their DFT study. Diam. Relat. Mater. 2023, 140, 110425. [Google Scholar] [CrossRef]
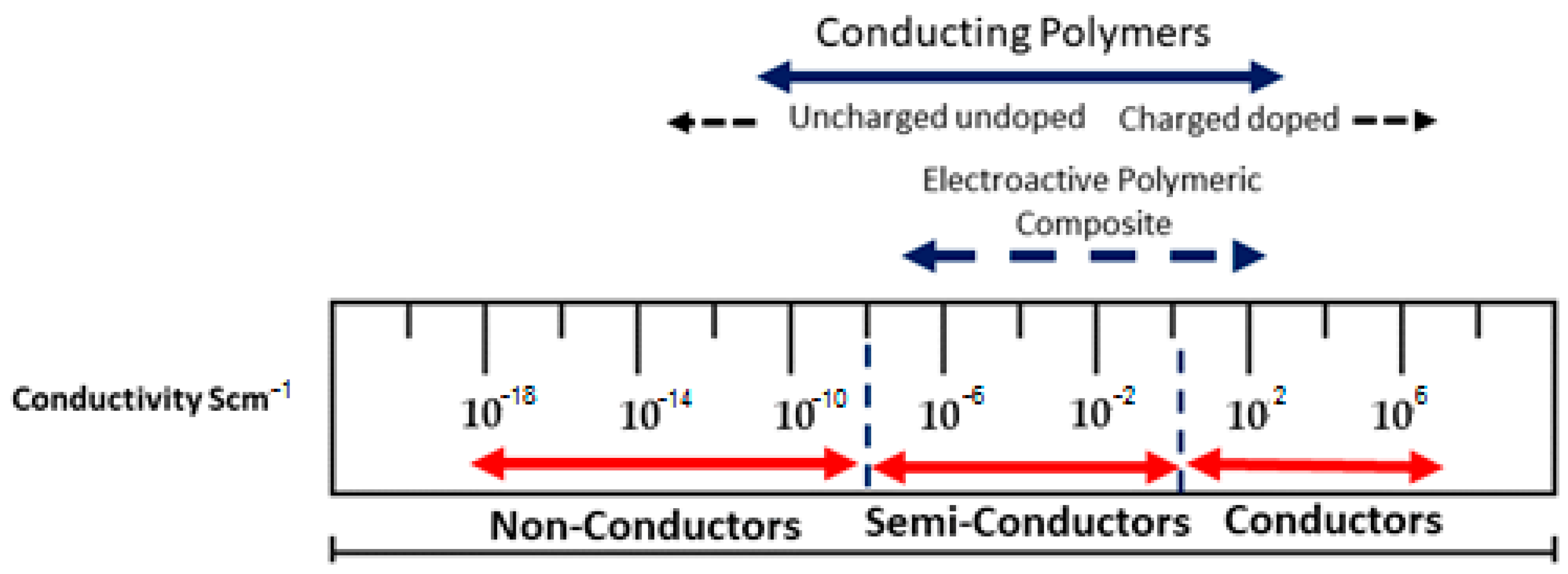
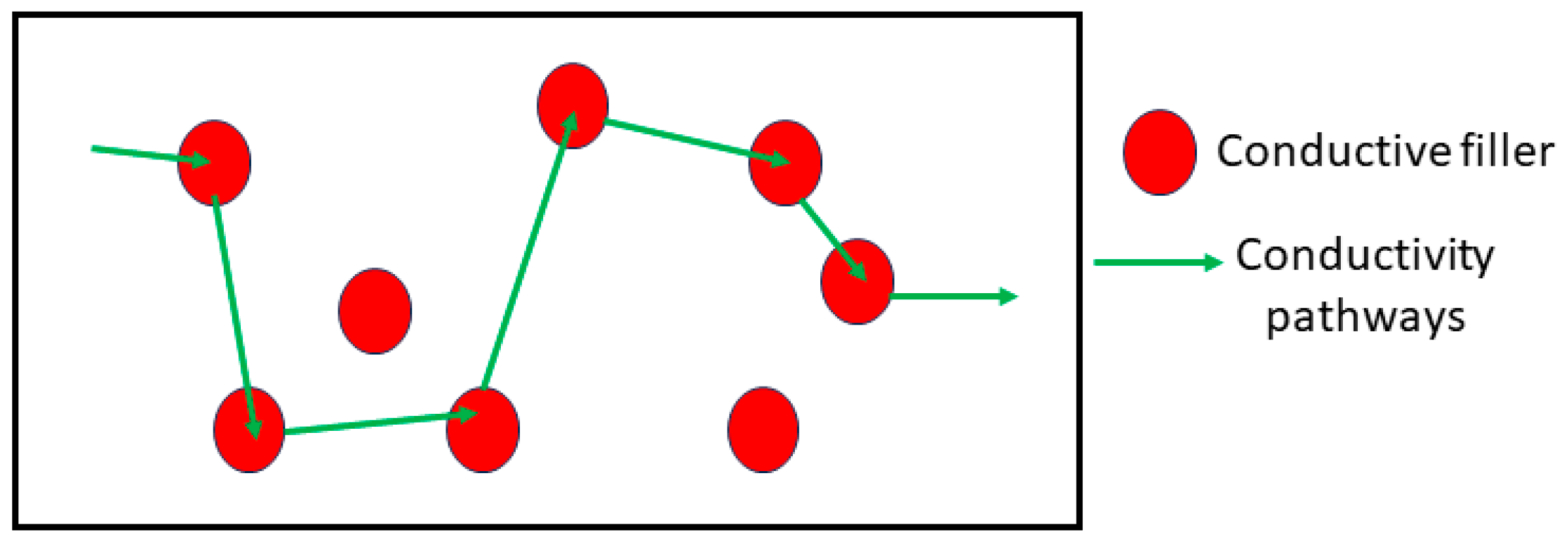
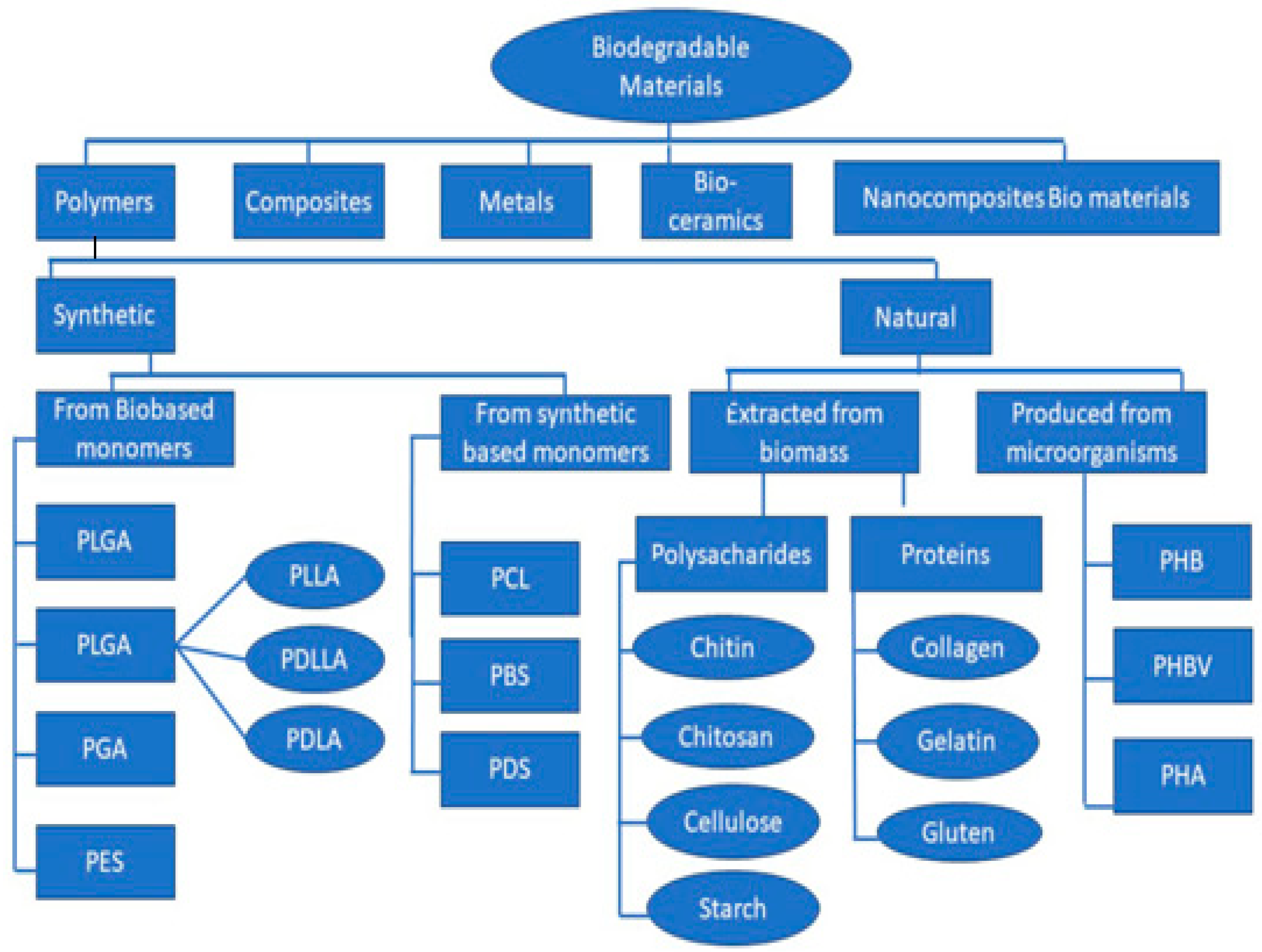
| S.No. | Polymers | Area of Applications | Ref. |
|---|---|---|---|
| 1 | Polyethylene glycol/PLGA | Improved oral administration for enhanced cellular absorption and hypoglycemic impact. | [59] |
| 2 | PLA (Polylactic acid) | Biomaterials find applications in various fields, including elevated apoptosis and cytotoxicity, barrier membranes, drug delivery, orthopedic applications, guided tissue regeneration (in dental applications), stents, staples, sutures, and tissue engineering. | [60,61] |
| 3 | Albumin | Folate-conjugated albumin enhances the potential for activated macrophage cells to reach their target. | [62] |
| 4 | Polycaprolactones (PCL) | Biomaterials are crucial in various fields, including orthopedics, guided tissue regeneration in dentistry, Ethicon’s implantable contraception, Monocryl suture, Capronor, barrier membranes, drug delivery, stents, and tissue engineering. | [63,64] |
| 5 | Polybutyrate adipate terephthalate (PBAT) | Applications of packaging include the utilization of bottles. | [65] |
| 6 | Polyesteramides (PEA) | Hydrogels find applications in drug delivery, tissue engineering, and smart materials, particularly temperature-sensitive ones. | [66] |
| 7 | Polyhydroxyvalerate (PHV) | Cons: Limited biodegradability ranging from three to twelve months for products such as Paper Mate, BioTuf, Rubbermaid, Calphalon, agricultural film, dung bags, and client packing materials. | [67] |
| 8 | Poly(alkylenealkanoate)s (PBS) | Injection molding is commonly used to produce single-use items like forks and spoons, as well as textiles, fishing equipment, and plant pots. | [68] |
| 9 | Thermoplastic starch (TPS) | Applications of packaging | [69] |
| 10 | Polycyanoacrylates | Drug delivery and adhesives | [70] |
| 11 | Polyanhydrides | Bioactive substance delivery | [71] |
| 12 | Poly(amino acids) | Drug delivery, tissue engineering, and orthopedic applications are the areas where these technologies find significant utilization. | [72] |
| 13 | Poly(ortho ester) | Drug delivery in the context of stents involves the controlled release or administration of medications directly to the target site where the stent is placed. | [73] |
| 14 | Polyphosphazenes | These technologies are crucial in skeletal reconstruction, drug delivery, and blood-contacting devices, among other applications. | [74] |
| 15 | Poly(propylene fumarate) | Utilizing medical procedures, equipment, or therapies specifically designed for the musculoskeletal system, consisting of bones, joints, ligaments, tendons, and muscles, is referred to as orthopedic applications. | [75] |
| 16 | Polyhydroxybutyrate (PHB) | Biomaterials are crucial in various fields, including orthopedics, guided tissue regeneration in dentistry, barrier membranes, drug delivery, stents, sutures, and tissue engineering. | [76] |
| 17 | Polydioxanone | Medical materials and interventions find applications in wound clips, sutures, and fracture fixation in non-load-bearing bones. | [77] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, T.; Vadivel, G.; Ramasamy, B.; Murugesan, G.; Sebaey, T.A. Biodegradable Conducting Polymer-Based Composites for Biomedical Applications—A Review. Polymers 2024, 16, 1533. https://doi.org/10.3390/polym16111533
Khan T, Vadivel G, Ramasamy B, Murugesan G, Sebaey TA. Biodegradable Conducting Polymer-Based Composites for Biomedical Applications—A Review. Polymers. 2024; 16(11):1533. https://doi.org/10.3390/polym16111533
Chicago/Turabian StyleKhan, Tabrej, Gayathri Vadivel, Balan Ramasamy, Gowtham Murugesan, and Tamer A. Sebaey. 2024. "Biodegradable Conducting Polymer-Based Composites for Biomedical Applications—A Review" Polymers 16, no. 11: 1533. https://doi.org/10.3390/polym16111533
APA StyleKhan, T., Vadivel, G., Ramasamy, B., Murugesan, G., & Sebaey, T. A. (2024). Biodegradable Conducting Polymer-Based Composites for Biomedical Applications—A Review. Polymers, 16(11), 1533. https://doi.org/10.3390/polym16111533








