The Potential of Sugarcane Waste-Derived Cellulose Fibres as Haemostatic Agents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
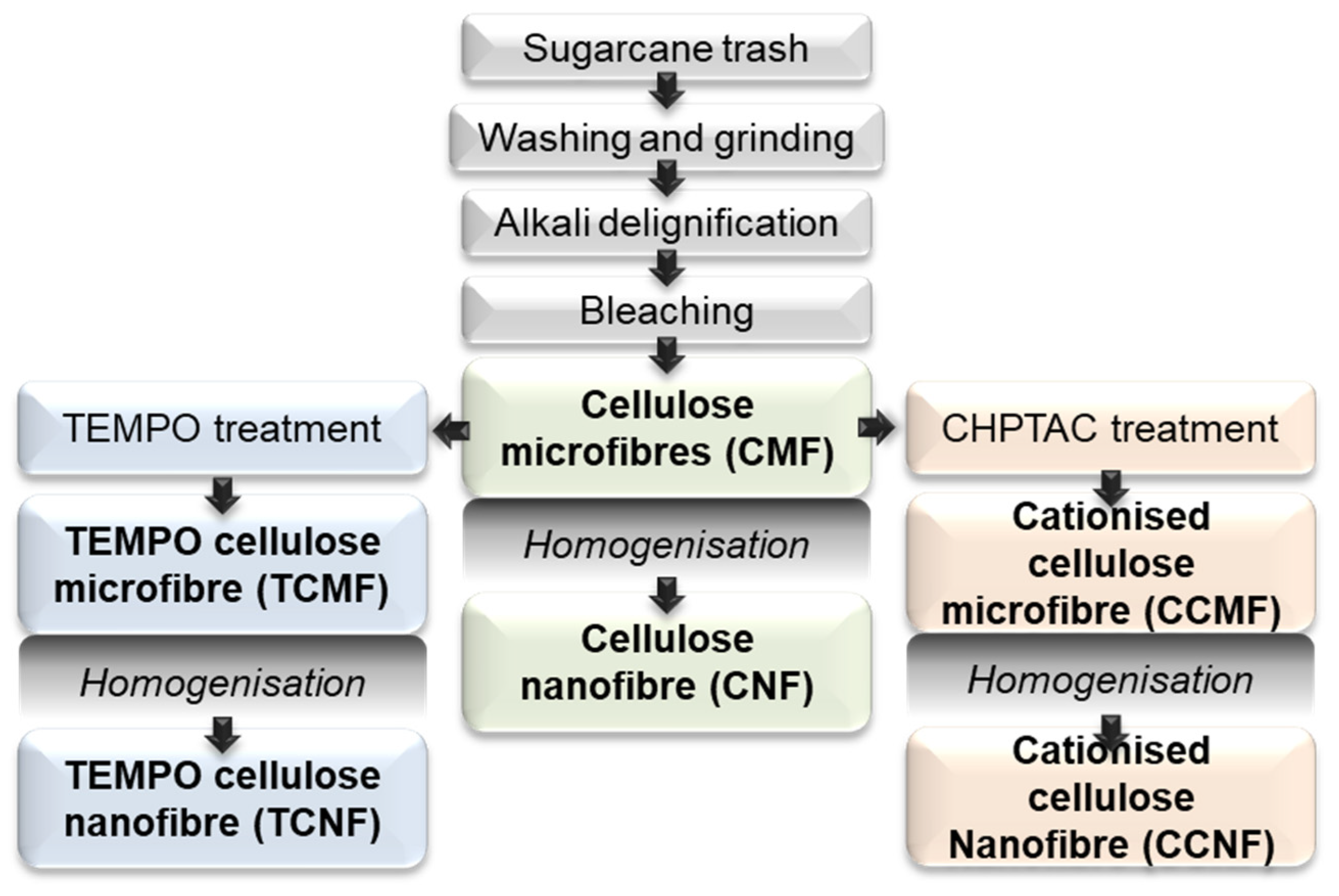
2.2. Pulping and Chemical Treatment Methods
2.3. Cellulose Nanofibrillation Using High-Pressure Homogeniser
2.4. Characterisation
3. Results and Discussion
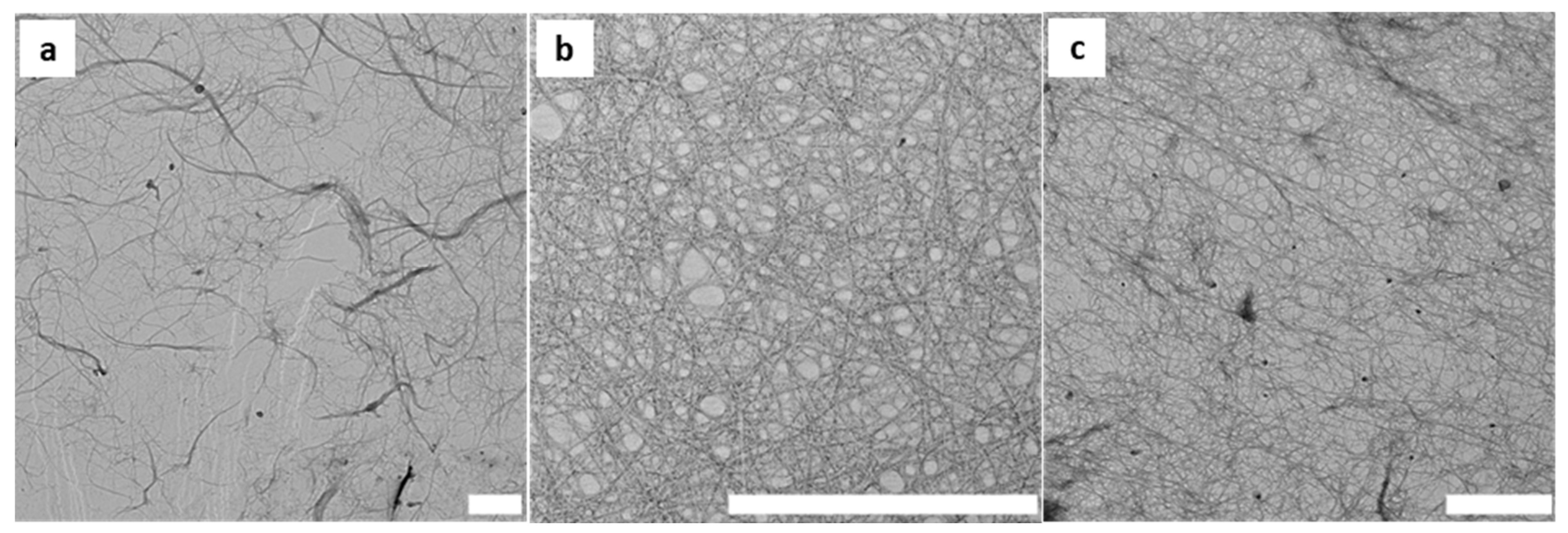
3.1. Preparation and Characterisation of Cellulose Fibres
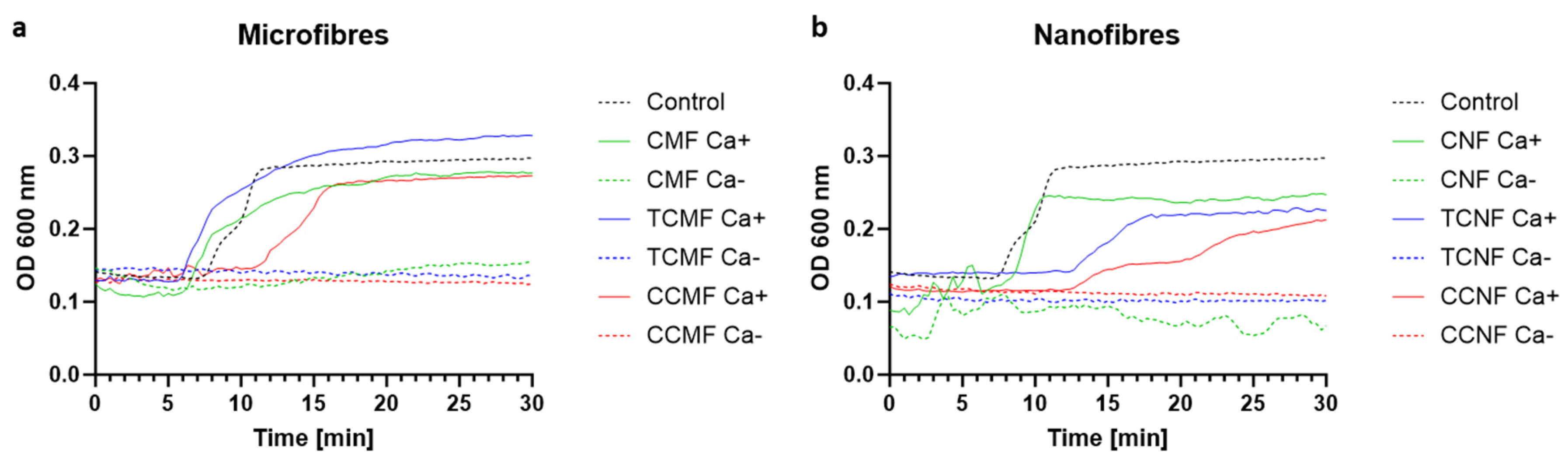
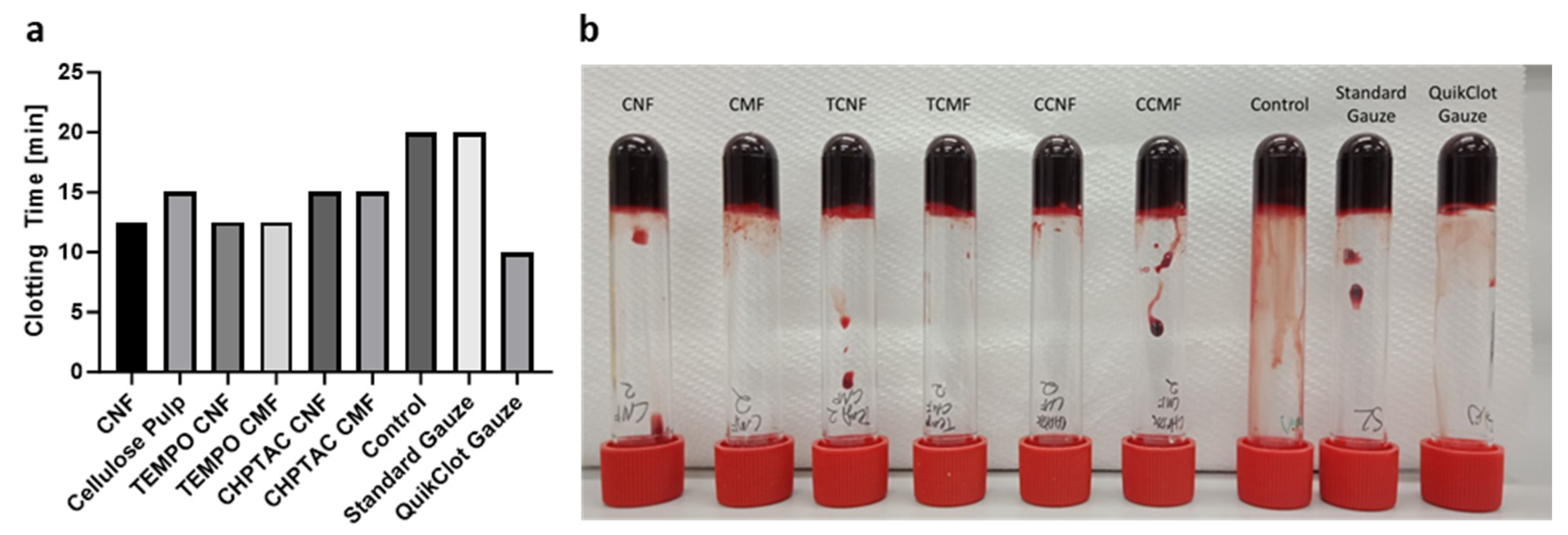
3.2. Characterisation of Cellulose Fibres as Haemostatic Agents
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kauvar, D.S.; Lefering, R.; Wade, C.E. Impact of Hemorrhage on Trauma Outcome: An Overview of Epidemiology, Clinical Presentations, and Therapeutic Considerations. J. Trauma Acute Care Surg. 2006, 60, S3–S11. [Google Scholar] [CrossRef] [PubMed]
- Palsson, B.O.; Bhatia, S.N. Tissue Engineering; Pearson Prentice Hall: Upper Saddle River, NJ, USA, 2004. [Google Scholar]
- Smith, S.A.; Travers, R.J.; Morrissey, J.H. How it all starts: Initiation of the clotting cascade. Crit. Rev. Biochem. Mol. Biol. 2016, 50, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.T. Hemostatic agents for prehospital hemorrhage control: A narrative review. Mil. Med. Res. 2020, 7, 13. [Google Scholar] [CrossRef]
- State of Queensland QAS. Clinical Practice Procedures: Trauma/Haemostatic-QuikClot®Combat Gauze 2018. Available online: https://www.ambulance.qld.gov.au/docs/clinical/cpp/CPP_Haemostatic_QuikClot.pdf (accessed on 1 November 2023).
- Mohamed, E.; Wang, Y.; Crispin, P.J.; Fitzgerald, A.; Dahlstrom, J.E.; Fowler, S.; Nisbet, D.R.; Tsuzuki, T.; Coupland, L.A. Superior Hemostatic and Wound-Healing Properties of Gel and Sponge Forms of Nonoxidized Cellulose Nanofibers: In Vitro and In Vivo Studies. Macromol. Biosci. 2022, 22, 2200222. [Google Scholar] [CrossRef] [PubMed]
- Bennett, B.L.; Littlejohn, L. Review of new topical hemostatic dressings for combat casualty care. Mil. Med. 2014, 179, 497–514. [Google Scholar] [CrossRef] [PubMed]
- Kheirabadi, B. Evaluation of topical hemostatic agents for combat wound treatment. US Army Med. Dep. J. 2011, 2, 25–37. [Google Scholar]
- Gordy, S.D.; Rhee, P.; Schreiber, M.A. Military applications of novel hemostatic devices. Expert Rev. Med. Devices 2011, 8, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Khoshmohabat, H.; Paydar, S.; Kazemi, H.M.; Dalfardi, B. Overview of Agents Used for Emergency Hemostasis. Trauma Mon. 2016, 21, e26023. [Google Scholar] [CrossRef] [PubMed]
- Littlejohn, L.F.; Devlin, J.J.; Kircher, S.S.; Lueken, R.; Melia, M.R.; Johnson, A.S. Comparison of Celox-A, ChitoFlex, WoundStat, and combat gauze hemostatic agents versus standard gauze dressing in control of hemorrhage in a swine model of penetrating trauma. Acad. Emerg. Med. 2011, 18, 340–350. [Google Scholar] [CrossRef]
- Kragh, J.F.; Aden, J.K.; Steinbaugh, J.; Bullard, M.; Dubick, M.A. Gauze vs XSTAT in wound packing for hemorrhage control. Am. J. Emerg. Med. 2015, 33, 974–976. [Google Scholar] [CrossRef]
- Wang, Y.-W.; Liu, C.-C.; Cherng, J.-H.; Lin, C.-S.; Chang, S.-J.; Hong, Z.-J.; Liu, C.-C.; Chiu, Y.-K.; Hsu, S.-D.; Chang, H. Biological Effects of Chitosan-Based Dressing on Hemostasis Mechanism. Polymers 2019, 11, 1906. [Google Scholar] [CrossRef]
- Subhedar, A.; Bhadauria, S.; Ahankari, S.; Kargarzadeh, H. Nanocellulose in biomedical and biosensing applications: A review. Int. J. Biol. Macromol. 2021, 166, 587–600. [Google Scholar] [CrossRef] [PubMed]
- Celma, G. Nanocellulose Hydrogels for Topical Wound-Care Applications: A Study on Human Skin Interactions; Uppsala University: Uppsala, Sweden, 2017. [Google Scholar]
- Guzdek-Zając, K.; Krajcer, A.; Lewandowska-Łańcucka, J. Bioactive moist bionanocellulose-based wound dressing material. Appl. Surf. Sci. 2020, 516, 146108. [Google Scholar] [CrossRef]
- Li, T.; Chen, C.; Brozena, A.H.; Zhu, J.Y.; Xu, L.; Driemeier, C.; Dai, J.; Rojas, O.J.; Isogai, A.; Wågberg, L.; et al. Developing fibrillated cellulose as a sustainable technological material. Nature 2021, 590, 47–56. [Google Scholar] [CrossRef]
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose nanomaterials review: Structure, properties and nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lu, Y.-T.; Zeng, K.; Heinze, T.; Groth, T.; Zhang, K. Recent Progress on Cellulose-Based Ionic Compounds for Biomaterials. Adv. Mater. 2021, 33, 2000717. [Google Scholar] [CrossRef]
- Yuan, H.; Chen, L.; Hong, F.F. A Biodegradable Antibacterial Nanocomposite Based on Oxidized Bacterial Nanocellulose for Rapid Hemostasis and Wound Healing. ACS Appl. Mater. Interfaces 2020, 12, 3382–3392. [Google Scholar] [CrossRef] [PubMed]
- Ibne Mahbub, M.S.; Sultana, T.; Gwon, J.-G.; Lee, B.-T. Fabrication of thrombin loaded TEMPO-oxidized cellulose nanofiber-gelatin sponges and their hemostatic behavior in rat liver hemorrhage model. J. Biomater. Sci. Polym. Ed. 2022, 33, 499–516. [Google Scholar] [CrossRef]
- Tripathi, G.; Gwon, J.; Lee, B.T. Nano cellulose-laden alginate/chitosan sponge with enhanced biological and hemostatic behavior. J. Biomater. Sci. Polym. Ed. 2023, 34, 1813–1823. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Li, M.; Yang, Q.; Wan, G.; Li, Y.; Li, N.; Tang, K. Morphology-controllable cellulose/chitosan sponge for deep wound hemostasis with surfactant and pore-foaming agent. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 118, 111408. [Google Scholar] [CrossRef]
- Biranje, S.S.; Sun, J.; Cheng, L.; Cheng, Y.; Shi, Y.; Yu, S.; Jiao, H.; Zhang, M.; Lu, X.; Han, W.; et al. Development of Cellulose Nanofibril/Casein-Based 3D Composite Hemostasis Scaffold for Potential Wound-Healing Application. ACS Appl. Mater. Interfaces 2022, 14, 3792–3808. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, E.; Coupland, L.A.; Crispin, P.J.; Fitzgerald, A.; Nisbet, D.R.; Tsuzuki, T. Non-oxidized cellulose nanofibers as a topical hemostat: In vitro thromboelastometry studies of structure vs function. Carbohydr. Polym. 2021, 265, 118043. [Google Scholar] [CrossRef] [PubMed]
- Isogai, A.; Saito, T.; Fukuzumi, H. TEMPO-oxidized cellulose nanofibers. Nanoscale 2011, 3, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Aguado, R.; Murtinho, D.; Valente, A.J.M.; Sousa, A.P.M.D.; Ferreira, P.J.T. A review on cationic starch and nanocellulose as paper coating components. Int. J. Biol. Macromol. 2020, 162, 578–598. [Google Scholar] [CrossRef] [PubMed]
- Amiralian, N.; Annamalai, P.K.; Memmott, P.; Taran, E.; Schmidt, S.; Martin, D.J. Easily deconstructed, high aspect ratio cellulose nanofibres from Triodia pungens; an abundant grass of Australia’s arid zone. RSC Adv. 2015, 5, 32124–32132. [Google Scholar] [CrossRef]
- Saito, T.; Kimura, S.; Nishiyama, Y.; Isogai, A. Cellulose Nanofibers Prepared by TEMPO-Mediated Oxidation of Native Cellulose. Biomacromolecules 2007, 8, 2485–2491. [Google Scholar] [CrossRef]
- Saito, T.; Kuramae, R.; Wohlert, J.; Berglund, L.A.; Isogai, A. An Ultrastrong Nanofibrillar Biomaterial: The Strength of Single Cellulose Nanofibrils Revealed via Sonication-Induced Framentation. Biomacromolucules 2013, 14, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Habibi, Y.; Chanzy, H.; Vignon, M.R. TEMPO-mediated surface oxidation of cellulose whiskers. Cellulose 2006, 13, 679–687. [Google Scholar] [CrossRef]
- Rol, F.; Saini, S.; Meyer, V.; Petit-Conil, M.; Bras, J. Production of cationic nanofibrils of cellulose by twin-screw extrusion. Ind. Crop. Prod. 2019, 137, 81–88. [Google Scholar] [CrossRef]
- Segal, L.G.J.M.A.; Creely, J.J.; Martin, A.E., Jr.; Conrad, C.M. An Empirical Method for Estimating the Degree of Crystallinity of Native Cellulose Using the X-Ray Diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- French, A.D. Idealized powder diffraction patterns for cellulose polymorphs. Cellulose 2014, 21, 885–895. [Google Scholar] [CrossRef]
- Sheltami, R.M.; Abdullah, I.; Ahmad, I.; Dufresne, A.; Kargarzadeh, H. Extraction of cellulose nanocrystals from mengkuang leaves (Pandanus tectorius). Carbohydr. Polym. 2012, 88, 772–779. [Google Scholar] [CrossRef]
- Qing, Y.; Sabo, R.; Zhu, J.Y.; Agarwal, U.; Cai, Z.; Wu, Y. A comparative study of cellulose nanofibrils disintegrated via multiple processing approaches. Carbohydr. Polym. 2013, 97, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Bose, E.; Hravnak, M. Thromboelastography: A Practice Summary for Nurse Practitioners Treating Hemorrhage. J. Nurse Pract. 2015, 11, 702–709. [Google Scholar] [CrossRef]
- Gorbet, M.B.; Sefton, M.V. Biomaterial-associated thrombosis: Roles of coagulation factors, complement, platelets and leukocytes. Biomaterials 2004, 25, 5681–5703. [Google Scholar] [CrossRef]




| Treatment | Clot Initiation R (min) | Maximum Amplitude MA (mm) | Time to Reach Maximum Amplitude TMA (min) |
|---|---|---|---|
| Cellulose fibre dispersions at 0.25 wt.% | |||
| Control | 14.9 | 53.9 | 45.5 |
| CMF | 8.7 | 63.1 | 38.6 |
| TCMF | 10 | 55.0 | 46.1 |
| CCMF | 25.4 | 56.2 | 66.5 |
| CNF | 12.8 | 59.0 | 40.0 |
| TCNF | 11.7 | 57.1 | 44.7 |
| CCNF | 16.2 | 56.8 | 46.5 |
| Freeze-dried cellulose fibres and gauzes | |||
| Control | 13.7 | 52.8 | 45.9 |
| CMF | 3.8 | 56.6 | 32.3 |
| TCMF | 8.1 | 60.6 | 28.7 |
| CCMF | 10.3 | 56.5 | 40.1 |
| CNF | 5.9 | 54.7 | 33.2 |
| TCNF | 9.6 | 60 | 32.2 |
| CCNF | 13.8 | 52.2 | 44.4 |
| Standard gauze | 12.9 | 50.5 | 43.2 |
| QuikClot® gauze | 3.1 | 62.2 | 26.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malone, S.; Yegappan, R.; Kijas, A.W.; Gemmell, A.; Rowan, A.E.; Rajah, D.; Kim, M.; Lauko, J.; Amiralian, N. The Potential of Sugarcane Waste-Derived Cellulose Fibres as Haemostatic Agents. Polymers 2024, 16, 1654. https://doi.org/10.3390/polym16121654
Malone S, Yegappan R, Kijas AW, Gemmell A, Rowan AE, Rajah D, Kim M, Lauko J, Amiralian N. The Potential of Sugarcane Waste-Derived Cellulose Fibres as Haemostatic Agents. Polymers. 2024; 16(12):1654. https://doi.org/10.3390/polym16121654
Chicago/Turabian StyleMalone, Siobhan, Ramanathan Yegappan, Amanda W. Kijas, Anna Gemmell, Alan E. Rowan, Divya Rajah, Minjun Kim, Jan Lauko, and Nasim Amiralian. 2024. "The Potential of Sugarcane Waste-Derived Cellulose Fibres as Haemostatic Agents" Polymers 16, no. 12: 1654. https://doi.org/10.3390/polym16121654





