Biocompatible Preparation of Beta-Lactoglobulin/Chondroitin Sulfate Carrier Nanoparticles and Modification of Their Colloidal and Hydropathic Properties by Tween 80
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Sample Preparation
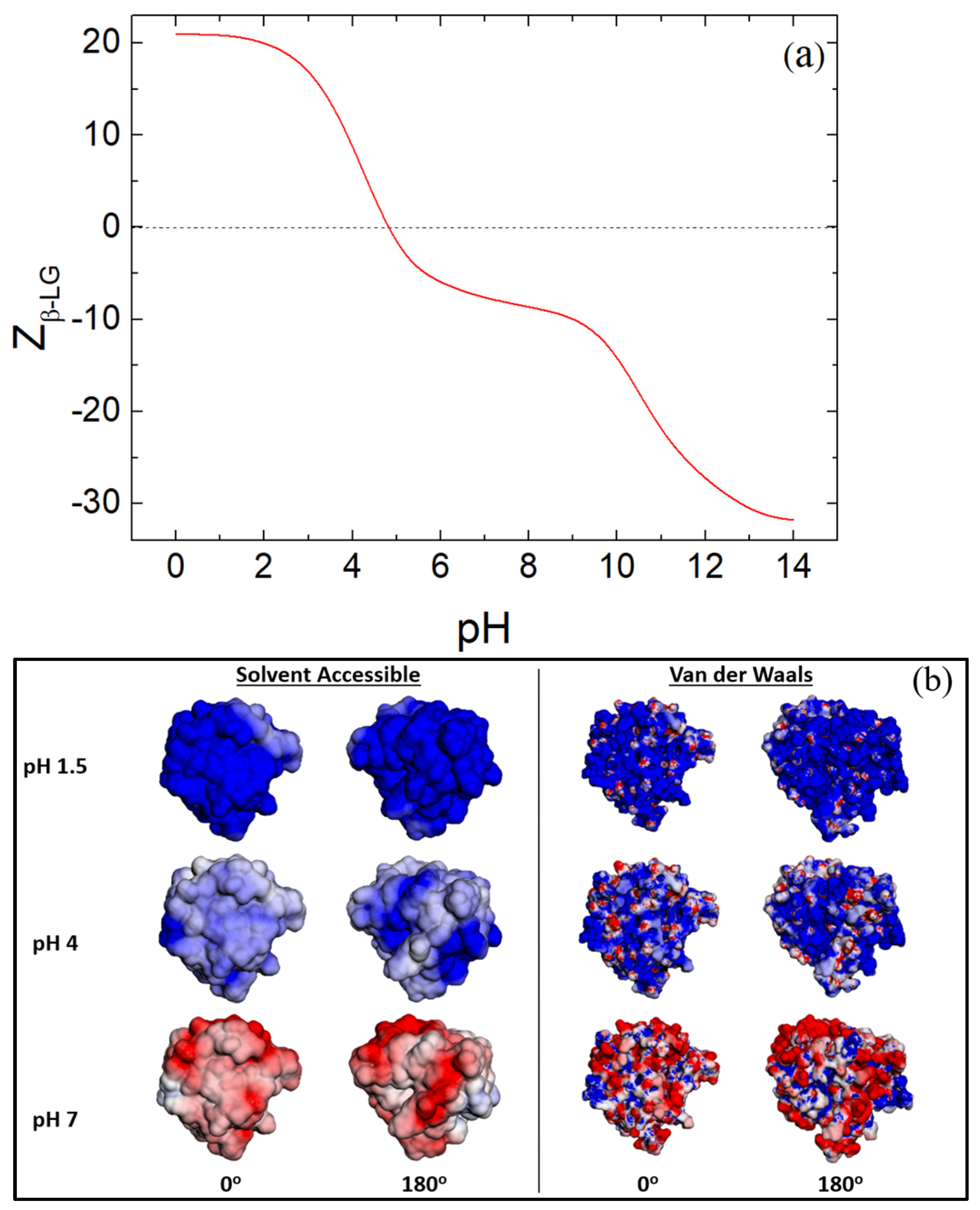
2.2. Surface Charge, Hydropathy and Secondary Structure Calculations Based on the Crystal Structure of β-LG
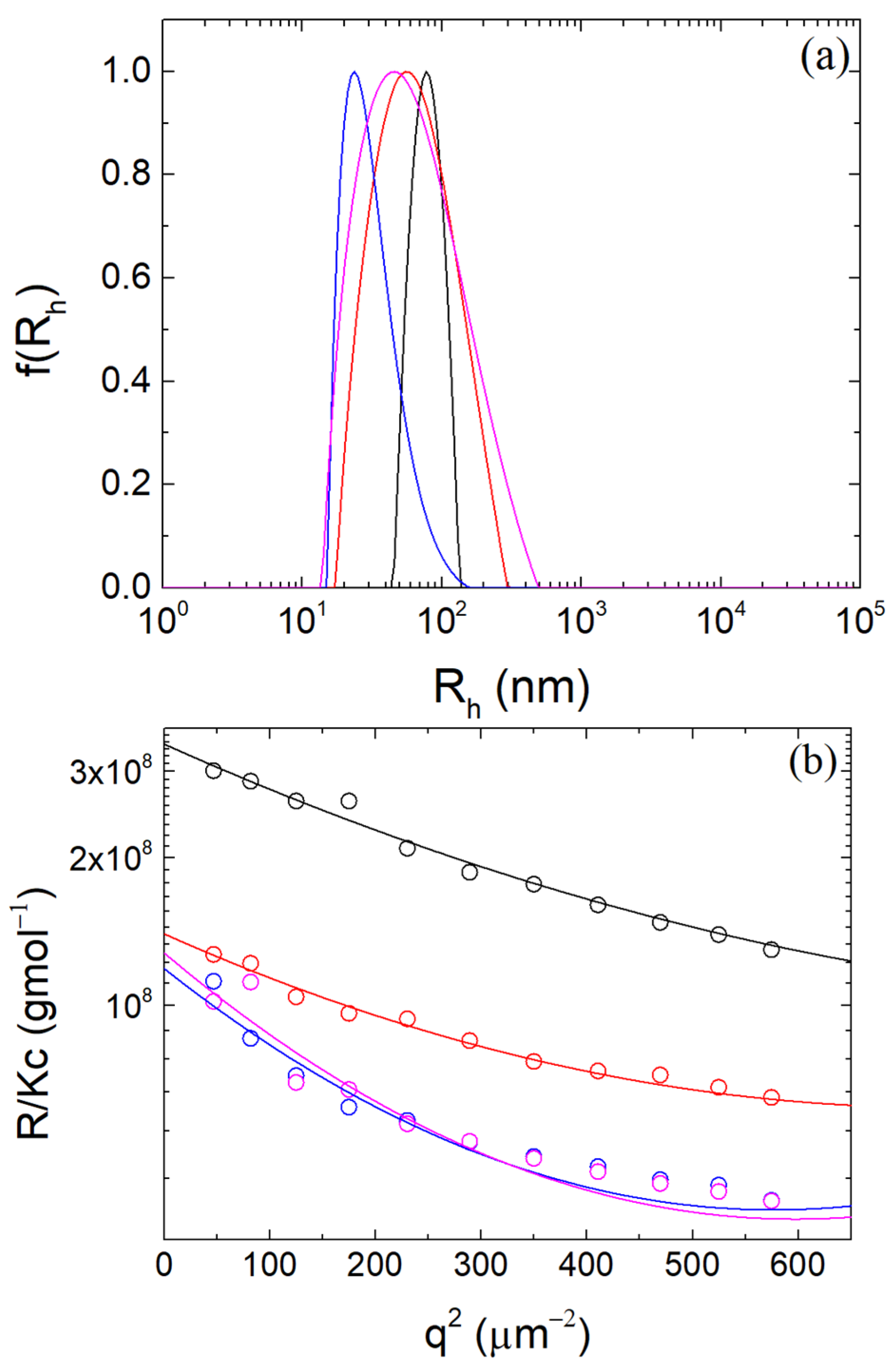
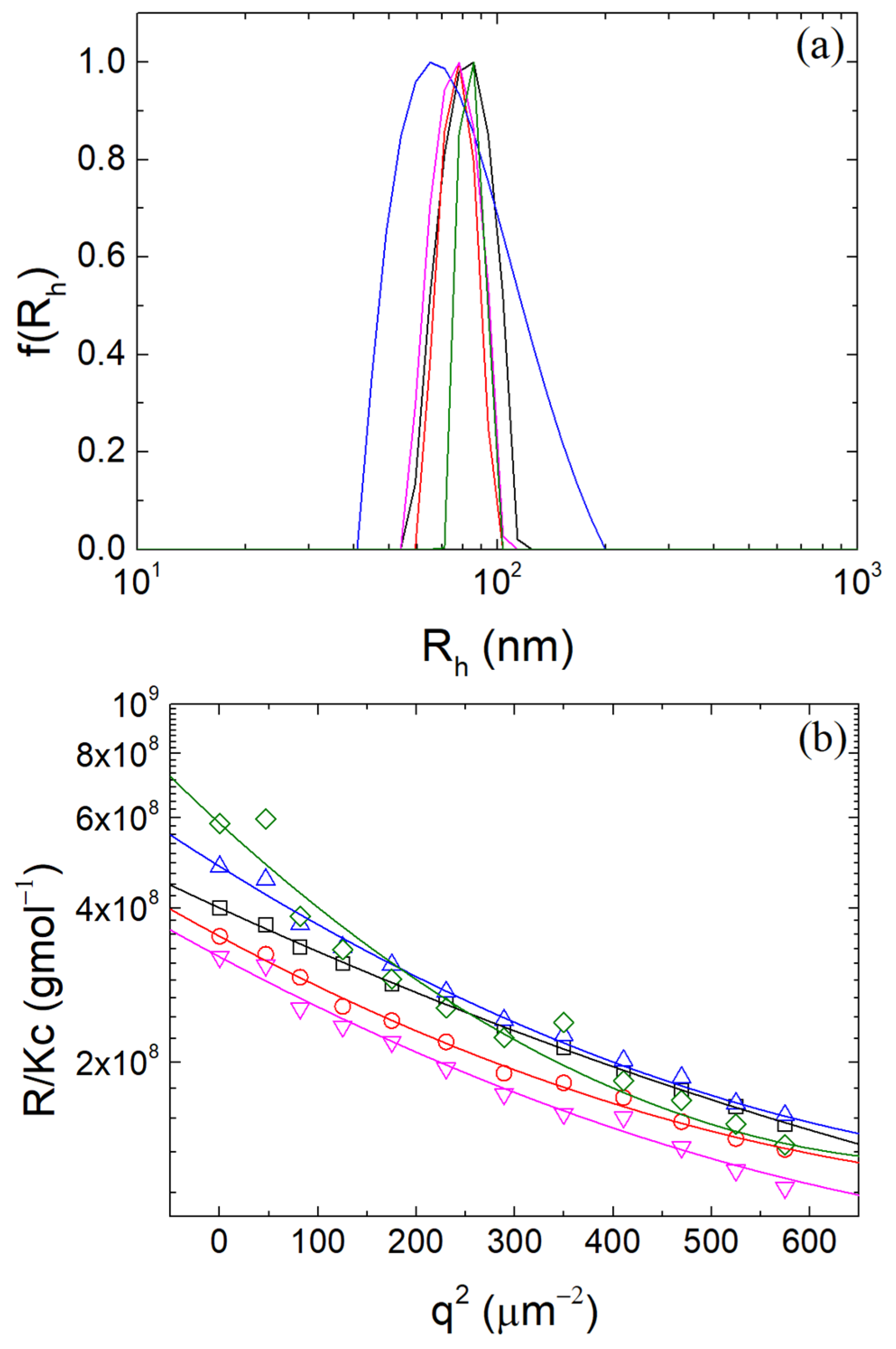
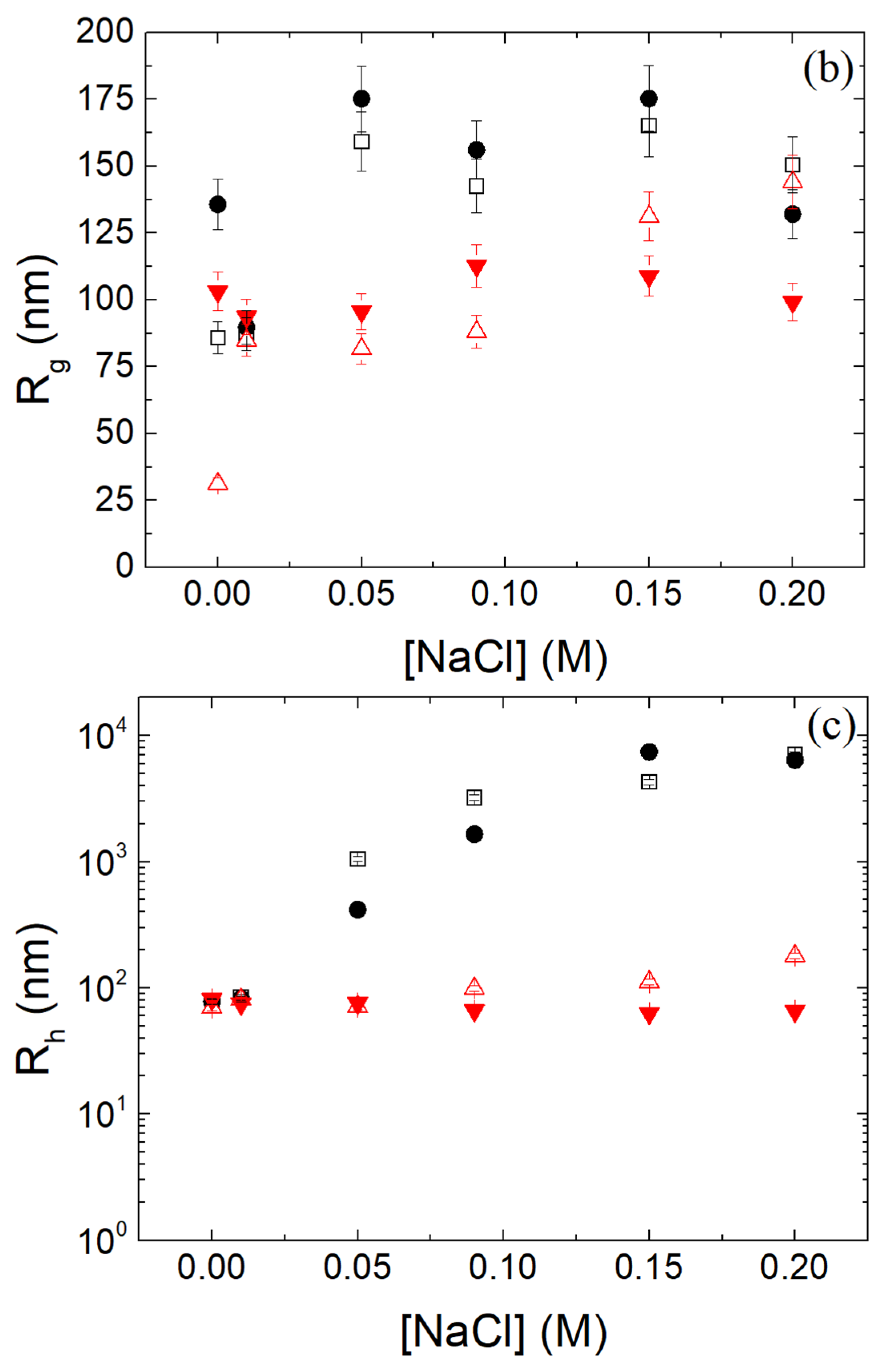
2.3. Static and Dynamic Light Scattering
2.4. Electrophoretic Light Scattering
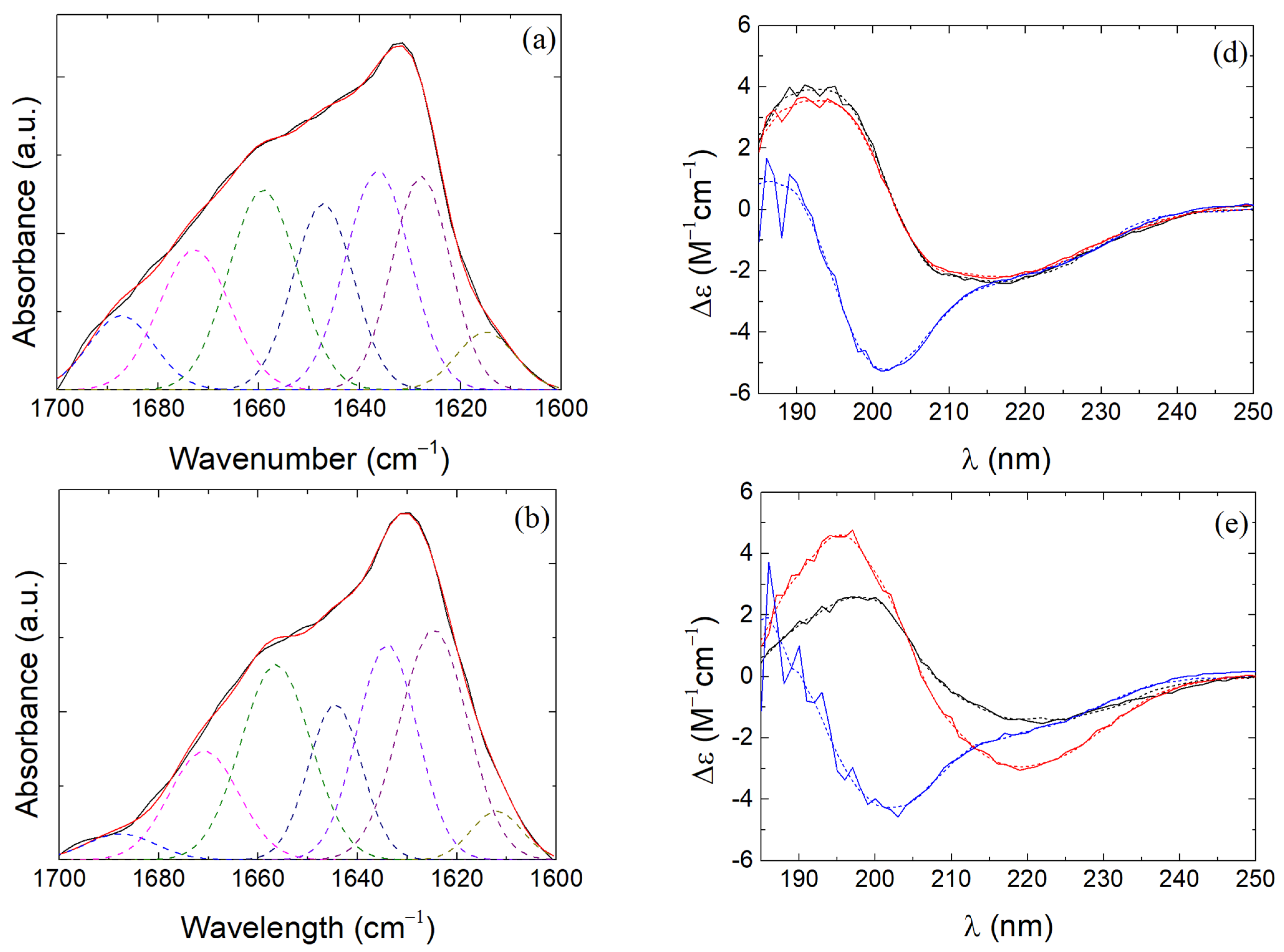
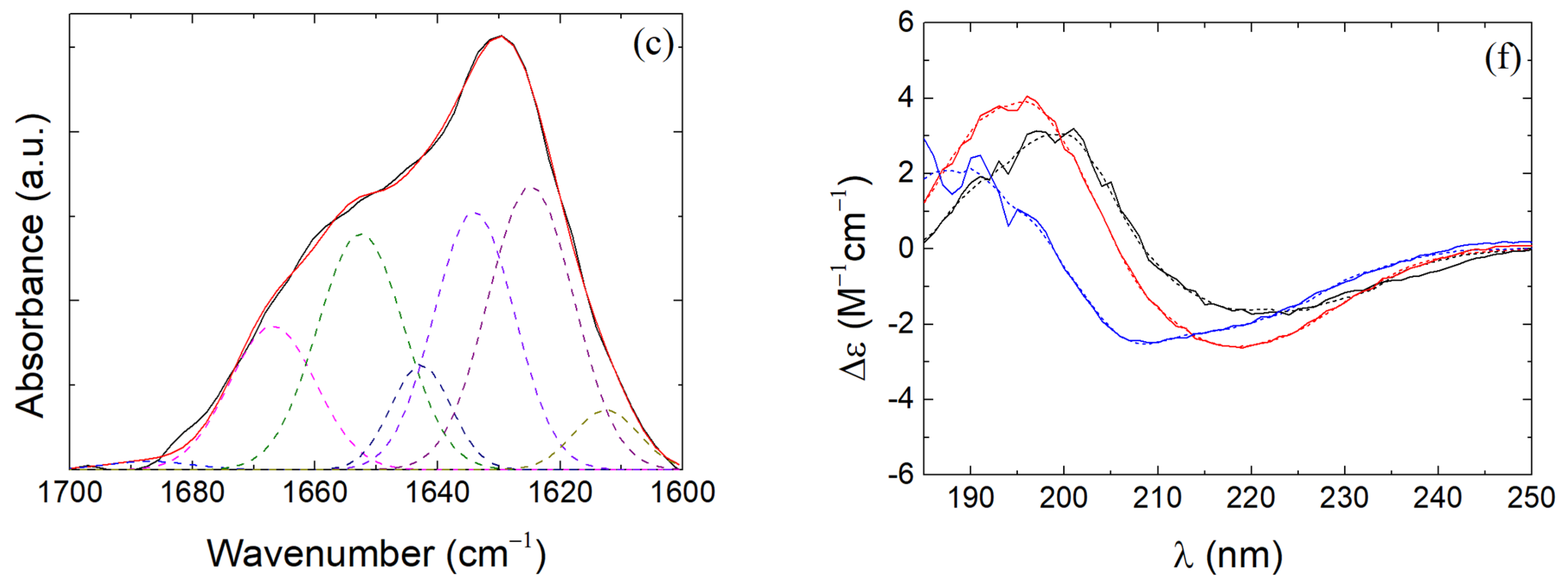
2.5. Fourier-Transform Infrared Spectroscopy
2.6. Fluorescence Spectroscopy
2.7. Circular Dichroism
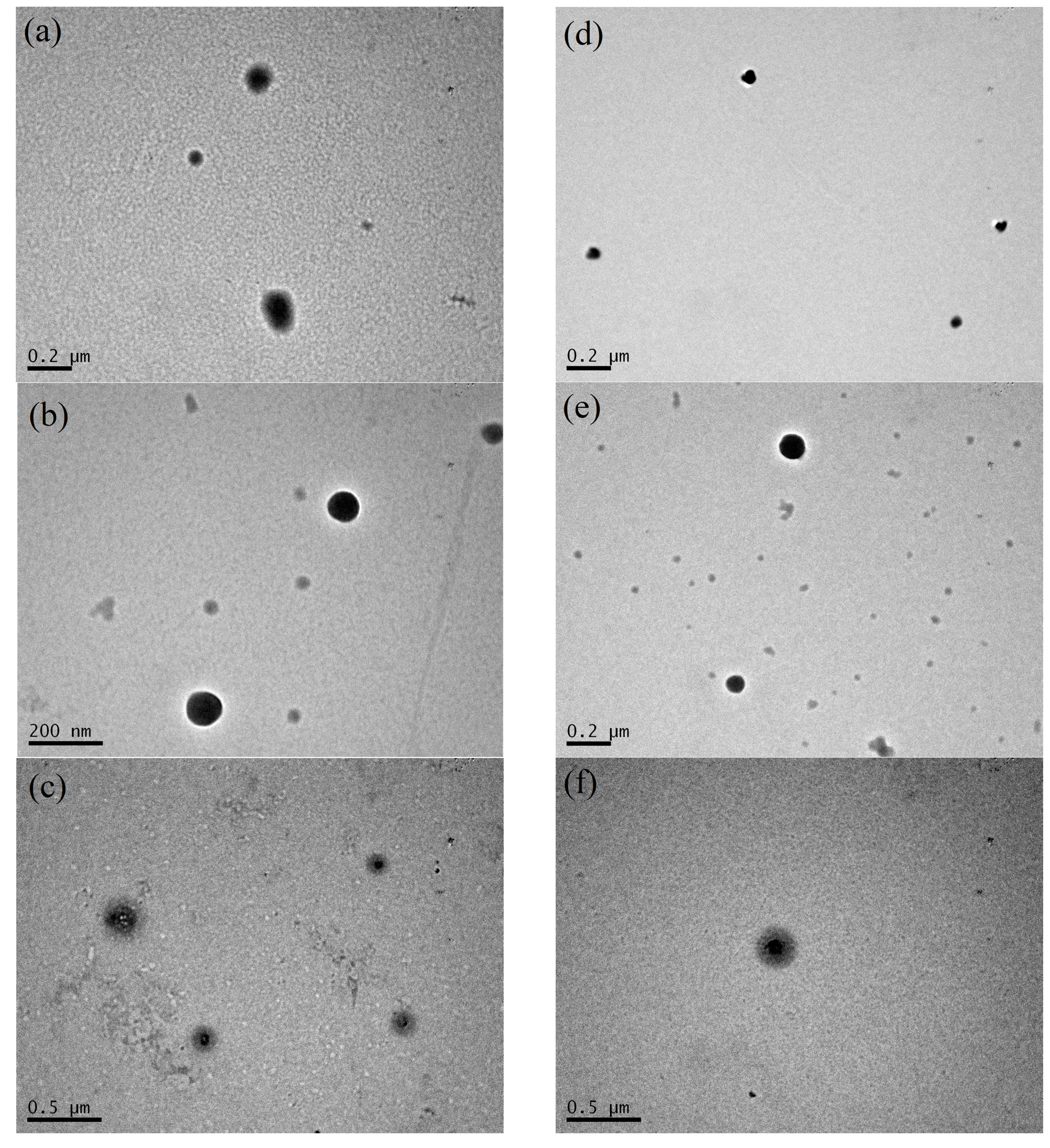
2.8. Transmission Electron Microscopy
3. Results and Discussion
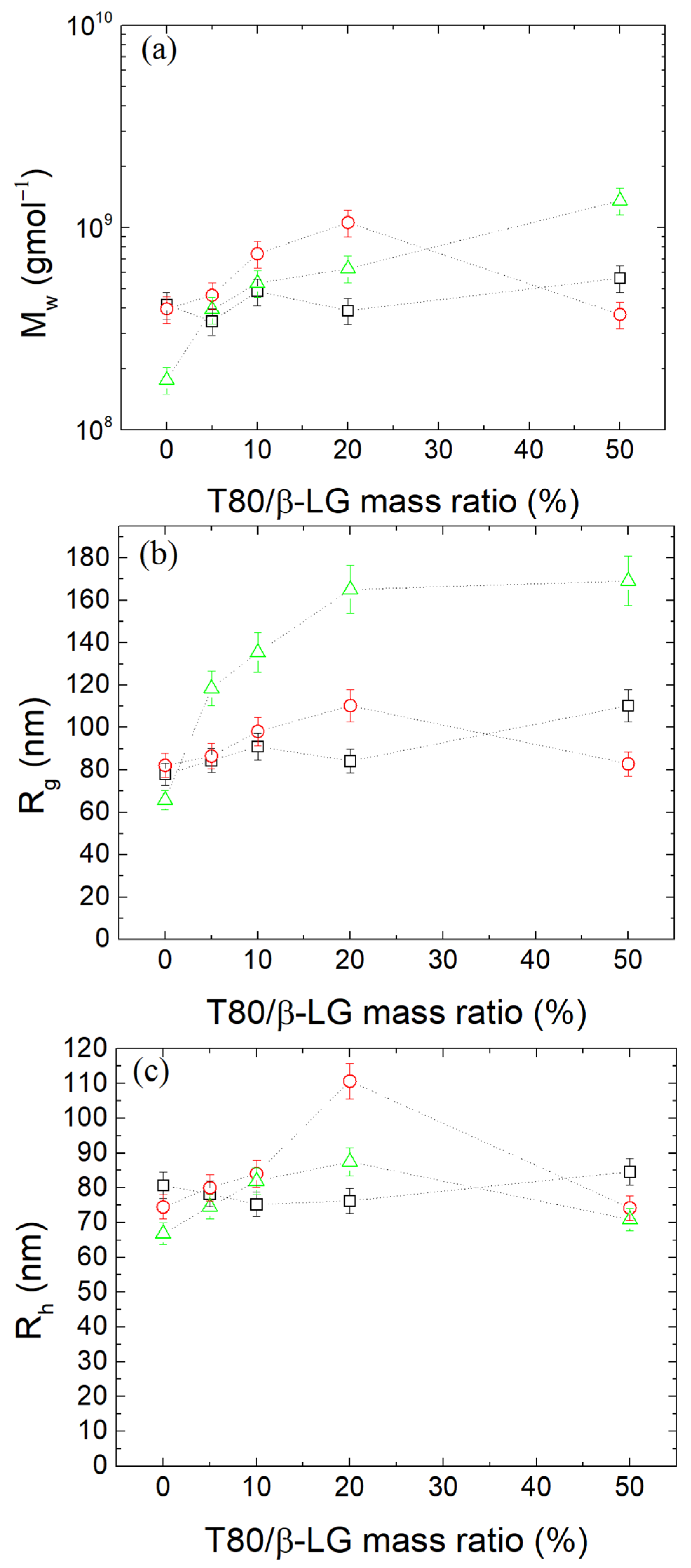
3.1. Formation of β-LG/CS Complexes and Effect of T80
3.2. Thermal Stabilization and Morphology of β-LG/CS and β-LG/CS/T80 NPs
3.3. Zeta Potential of β-LG/CS and β-LG/CS/T80 NPs
3.4. β-LG Conformation in β-LG/CS and β-LG/CS/T80 NPs
3.5. Tryptophan Endogenous Fluorescence of Pure β-LG and in Its Complexes upon Titration of CS and T80
3.6. Hydrophobicity of β-LG/CS and β-LG/CS/T80 NPs
3.7. Stability of β-LG/CS and β-LG/CS/T80 NPs in Salt and Biological Fluids and over Time
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jawahar, N.; Meyyanathan, S. Polymeric nanoparticles for drug delivery and targeting: A comprehensive review. Int. J. Health Allied Sci. 2012, 1, 217–223. [Google Scholar] [CrossRef]
- Karabasz, A.; Bzowska, M.; Szczepanowicz, K. Biomedical Applications of Multifunctional Polymeric Nanocarriers: A Review of Current Literature. Int. J. Nanomed. 2020, 15, 8673–8696. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, A.; Carreiró, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Venkatesh, D.N.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; et al. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef] [PubMed]
- Elezaby, R.S.; Gad, H.A.; Metwally, A.A.; Geneidi, A.S.; Awad, G.A. Self-assembled amphiphilic core-shell nanocarriers in line with the modern strategies for brain delivery. J. Controlled Release 2017, 261, 43–61. [Google Scholar] [CrossRef] [PubMed]
- Papagiannopoulos, A.; Meristoudi, A.; Pispas, S.; Radulescu, A. Micelles from HOOC-PnBA-b-PAA-C12 H15 Diblock Amphiphilic Polyelectrolytes as Protein Nanocarriers. Biomacromolecules 2016, 17, 3816–3827. [Google Scholar] [CrossRef]
- Blocher, W.C.; Perry, S.L. Complex coacervate-based materials for biomedicine. WIREs Nanomed. Nanobiotechnol. 2017, 9, e1442. [Google Scholar] [CrossRef] [PubMed]
- Comert, F.; Malanowski, A.J.; Azarikia, F.; Dubin, P.L. Coacervation and precipitation in polysaccharide–protein systems. Soft Matter 2016, 12, 4154–4161. [Google Scholar] [CrossRef] [PubMed]
- Cooper, C.L.; Dubin, P.L.; Kayitmazer, A.B.; Turksen, S. Polyelectrolyte–protein complexes. Curr. Opin. Colloid Interface Sci. 2005, 10, 52–78. [Google Scholar] [CrossRef]
- Zheng, J.; Van Der Meeren, P.; Sun, W. New insights into protein–polysaccharide complex coacervation: Dynamics, molecular parameters, and applications. Aggregate 2024, 5, e449. [Google Scholar] [CrossRef]
- Jones, O.G.; Decker, E.A.; McClements, D.J. Comparison of protein–polysaccharide nanoparticle fabrication methods: Impact of biopolymer complexation before or after particle formation. J. Colloid Interface Sci. 2010, 344, 21–29. [Google Scholar] [CrossRef]
- Jones, O.G.; McClements, D.J. Biopolymer Nanoparticles from Heat-Treated Electrostatic Protein–Polysaccharide Complexes: Factors Affecting Particle Characteristics. J. Food Sci. 2010, 75, N36–N43. [Google Scholar] [CrossRef] [PubMed]
- McKee, T.; McKee, J.R. Biochemistry: The Molecular Basis of Life, 7th ed.; Oxford University Press: New York, NY, USA, 2020. [Google Scholar]
- Cortés-Morales, E.A.; Mendez-Montealvo, G.; Velazquez, G. Interactions of the molecular assembly of polysaccharide-protein systems as encapsulation materials. A review. Adv. Colloid Interface Sci. 2021, 295, 102398. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Huang, Q. Assembly of Protein–Polysaccharide Complexes for Delivery of Bioactive Ingredients: A Perspective Paper. J. Agric. Food Chem. 2019, 67, 1344–1352. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Jiao, Y.; Wang, Y.; Zhou, C.; Zhang, Z. Polysaccharides-based nanoparticles as drug delivery systems. Adv. Drug Deliv. Rev. 2008, 60, 1650–1662. [Google Scholar] [CrossRef] [PubMed]
- Miyake, M.; Yamashita, Y. Molecular Structure and Phase Behavior of Surfactants. In Cosmetic Science and Technology; Elsevier: New York, NY, USA, 2017; pp. 389–414. [Google Scholar] [CrossRef]
- Nakama, Y. Surfactants. In Cosmetic Science and Technology; Elsevier: New York, NY, USA, 2017; pp. 231–244. [Google Scholar] [CrossRef]
- Le Maux, S.; Bouhallab, S.; Giblin, L.; Brodkorb, A.; Croguennec, T. Bovine β-lactoglobulin/fatty acid complexes: Binding, structural, and biological properties. Dairy Sci. Technol. 2014, 94, 409–426. [Google Scholar] [CrossRef] [PubMed]
- Roth-Walter, F.; Pacios, L.F.; Gomez-Casado, C.; Hofstetter, G.; Roth, G.A.; Singer, J.; Diaz-Perales, A.; Jensen-Jarolim, E. The Major Cow Milk Allergen Bos d 5 Manipulates T-Helper Cells Depending on Its Load with Siderophore-Bound Iron. PLoS ONE 2014, 9, e104803. [Google Scholar] [CrossRef]
- Van Den Akker, C.C.; Schleeger, M.; Bonn, M.; Koenderink, G.H. Structural Basis for the Polymorphism of β-Lactoglobulin Amyloid-Like Fibrils. In Bio-Nanoimaging; Elsevier: New York, NY, USA, 2014; pp. 333–343. [Google Scholar] [CrossRef]
- Sawyer, L.; Kontopidis, G. The core lipocalin, bovine β-lactoglobulin. Biochim. Biophys. Acta BBA—Protein Struct. Mol. Enzymol. 2000, 1482, 136–148. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Harris, P.; Kaur, A.; Pastrana, L.; Jauregi, P. Characterisation of β-lactoglobulin nanoparticles and their binding to caffeine. Food Hydrocoll. 2017, 71, 85–93. [Google Scholar] [CrossRef]
- Li, L.; Chunta, S.; Zheng, X.; He, H.; Wu, W.; Lu, Y. β-Lactoglobulin stabilized lipid nanoparticles enhance oral absorption of insulin by slowing down lipolysis. Chin. Chem. Lett. 2024, 35, 108662. [Google Scholar] [CrossRef]
- Rubinstein, A.; Nakar, D.; Sintov, A. Chondroitin sulfate: A potential biodegradable carrier for colon-specific drug delivery. Int. J. Pharm. 1992, 84, 141–150. [Google Scholar] [CrossRef]
- McAtee, C.O.; Barycki, J.J.; Simpson, M.A. Emerging Roles for Hyaluronidase in Cancer Metastasis and Therapy. In Advances in Cancer Research; Elsevier: New York, NY, USA, 2014; Volume 123, pp. 1–34. [Google Scholar] [CrossRef]
- Schieber, A.; Lopes-Lutz, D. Analytical Methods—Functional Foods and Dietary Supplements. In Comprehensive Biotechnology; Elsevier: New York, NY, USA, 2011; pp. 487–499. [Google Scholar] [CrossRef]
- Pigman, W.; Horton, D. , The Carbohydrates. 1B, 2nd ed.; Academic Press: New York, NY, USA, 1980. [Google Scholar]
- Henrotin, Y.; Mathy, M.; Sanchez, C.; Lambert, C. Chondroitin sulfate in the treatment of osteoarthritis: From in vitro studies to clinical recommendations. Ther. Adv. Musculoskelet. Dis. 2010, 2, 335–348. [Google Scholar] [CrossRef]
- Klecker, C.; Nair, L.S. Matrix Chemistry Controlling Stem Cell Behavior. In Biology and Engineering of Stem Cell Niches; Elsevier: New York, NY, USA, 2017; pp. 195–213. [Google Scholar] [CrossRef]
- Yamakoshi, Y. Dental and Oral Biology, Biochemistry. In Reference Module in Biomedical Sciences; Elsevier: New York, NY, USA, 2014; p. B9780128012383000374. [Google Scholar] [CrossRef]
- Mehmood, T.; Ahmed, A. Tween 80 and Soya-Lecithin-Based Food-Grade Nanoemulsions for the Effective Delivery of Vitamin D. Langmuir 2020, 36, 2886–2892. [Google Scholar] [CrossRef]
- Ravichandran, V.; Lee, M.; Nguyen Cao, T.G.; Shim, M.S. Polysorbate-Based Drug Formulations for Brain-Targeted Drug Delivery and Anticancer Therapy. Appl. Sci. 2021, 11, 9336. [Google Scholar] [CrossRef]
- Ponphaiboon, J.; Limmatvapirat, S.; Limmatvapirat, C. Development and Evaluation of a Stable Oil-in-Water Emulsion with High Ostrich Oil Concentration for Skincare Applications. Molecules 2024, 29, 982. [Google Scholar] [CrossRef]
- Liu, H.; Jin, Y.; Menon, R.; Laskowich, E.; Bareford, L.; De Vilmorin, P.; Kolwyck, D.; Yeung, B.; Yi, L. Characterization of Polysorbate 80 by Liquid Chromatography-Mass Spectrometry to Understand Its Susceptibility to Degradation and Its Oxidative Degradation Pathway. J. Pharm. Sci. 2022, 111, 323–334. [Google Scholar] [CrossRef]
- Ron, N.; Zimet, P.; Bargarum, J.; Livney, Y.D. Beta-lactoglobulin–polysaccharide complexes as nanovehicles for hydrophobic nutraceuticals in non-fat foods and clear beverages. Int. Dairy J. 2010, 20, 686–693. [Google Scholar] [CrossRef]
- Dai, L.; Wei, Y.; Sun, C.; Mao, L.; McClements, D.J.; Gao, Y. Development of protein-polysaccharide-surfactant ternary complex particles as delivery vehicles for curcumin. Food Hydrocoll. 2018, 85, 75–85. [Google Scholar] [CrossRef]
- Fuenzalida, J.P.; Xiong, T.; Moerschbacher, B.M.; Ostermeier, M.; Goycoolea, F.M. Protein-surfactant-polysaccharide nanoparticles increase the catalytic activity of an engineered β-lactamase maltose-activated switch enzyme. bioRxiv 2019. [Google Scholar] [CrossRef]
- Berman, H.M. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef]
- Dolinsky, T.J.; Nielsen, J.E.; McCammon, J.A.; Baker, N.A. PDB2PQR: An automated pipeline for the setup of Poisson-Boltzmann electrostatics calculations. Nucleic Acids Res. 2004, 32, W665–W667. [Google Scholar] [CrossRef]
- Jurrus, E.; Engel, D.; Star, K.; Monson, K.; Brandi, J.; Felberg, L.E.; Brookes, D.H.; Wilson, L.; Chen, J.; Liles, K.; et al. Improvements to the APBS biomolecular solvation software suite. Protein Sci. 2018, 27, 112–128. [Google Scholar] [CrossRef] [PubMed]
- Hebditch, M.; Warwicker, J. Web-based display of protein surface and pH-dependent properties for assessing the developability of biotherapeutics. Sci. Rep. 2019, 9, 1969. [Google Scholar] [CrossRef] [PubMed]
- Klose, D.P.; Wallace, B.A.; Janes, R.W. 2Struc: The secondary structure server. Bioinformatics 2010, 26, 2624–2625. [Google Scholar] [CrossRef] [PubMed]
- Kabsch, W.; Sander, C. Dictionary of protein secondary structure: Pattern recognition of hydrogen-bonded and geometrical features. Biopolymers 1983, 22, 2577–2637. [Google Scholar] [CrossRef] [PubMed]
- Chu, B. Laser Light Scattering: Basic Principles and Practise; Courier Corporation: New York, NY, USA, 2007. [Google Scholar]
- Minton, A.P. Recent applications of light scattering measurement in the biological and biopharmaceutical sciences. Anal. Biochem. 2016, 501, 4–22. [Google Scholar] [CrossRef] [PubMed]
- Vlassi, E.; Papagiannopoulos, A. Nanoformulation of fibrinogen by thermal stabilization of its electrostatic complexes with hyaluronic acid. Int. J. Biol. Macromol. 2020, 158, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Jiang, F.; Cong, H.; Zhu, S. Molecular weight determination of low molecular weight hyaluronic acid and chondroitin sulfate by gel permeation chromatography. Carbohydr. Polym. 2023, 311, 120488. [Google Scholar] [CrossRef] [PubMed]
- Borsali, R.; Pecora, R. Soft-Matter Characterization; Springer: New York, NY, USA, 2008. [Google Scholar]
- Øgendal, L. Light Scattering a Brief Introduction; University of Copenhagen: Copenhagen, Denmark, 2019. [Google Scholar]
- Papagiannopoulos, A.; Vlassi, E. Stimuli-responsive nanoparticles by thermal treatment of bovine serum albumin inside its complexes with chondroitin sulfate. Food Hydrocoll. 2019, 87, 602–610. [Google Scholar] [CrossRef]
- Berne, B.J.; Pecora, R. Dynamic Light Scattering: With Applications to Chemistry, Biology, and Physics; Dover Publications Inc.: New York, NY, USA, 2000. [Google Scholar]
- Alexander, M.; Dalgleish, D.G. Dynamic Light Scattering Techniques and Their Applications in Food Science. Food Biophys. 2006, 1, 2–13. [Google Scholar] [CrossRef]
- Hunter, R.J. Zeta Potential in Colloid Science: Principles and Applications, New paperback ed.; Colloid science; Academic Press: London, UK; San Diego, CA, USA, 1988. [Google Scholar]
- Ohshima, H. Electrophoretic Mobility of a Spherical Colloidal Particle in a Salt-Free Medium. J. Colloid Interface Sci. 2002, 248, 499–503. [Google Scholar] [CrossRef]
- Papagiannopoulos, A.; Sklapani, A. Xanthan-based polysaccharide/protein nanoparticles: Preparation, characterization, encapsulation and stabilization of curcumin. Carbohydr. Polym. Technol. Appl. 2021, 2, 100075. [Google Scholar] [CrossRef]
- Micsonai, A.; Wien, F.; Kernya, L.; Lee, Y.-H.; Goto, Y.; Réfrégiers, M.; Kardos, J. Accurate secondary structure prediction and fold recognition for circular dichroism spectroscopy. Proc. Natl. Acad. Sci. USA 2015, 112, E3095–E3103. [Google Scholar] [CrossRef] [PubMed]
- Matsunami, H.; Kikuchi, R.; Ogawa, K.; Kokufuta, E. Light scattering study of complex formation between protein and polyelectrolyte at various ionic strengths. Colloids Surf. B Biointerfaces 2007, 56, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.C.; Chen, W.L.; Mao, S.J.T. Antioxidant nature of bovine milk beta-lactoglobulin. J. Dairy Sci. 2007, 90, 547–555. [Google Scholar] [CrossRef]
- Nunes, C.S.; Rufato, K.B.; Souza, P.R.; De Almeida, E.A.M.S.; Da Silva, M.J.V.; Scariot, D.B.; Nakamura, C.V.; Rosa, F.A.; Martins, A.F.; Muniz, E.C. Chitosan/chondroitin sulfate hydrogels prepared in [Hmim][HSO4] ionic liquid. Carbohydr. Polym. 2017, 170, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Hassan, M.; Islam, A.; Ahmad, F. A Review of Methods Available to Estimate Solvent-Accessible Surface Areas of Soluble Proteins in the Folded and Unfolded States. Curr. Protein Pept. Sci. 2014, 15, 456–476. [Google Scholar] [CrossRef] [PubMed]
- Müller, A.; Burchard, W. Structure formation of surfactants in concentrated sulphuric acid: A light scattering study. Colloid Polym. Sci. 1995, 273, 866–875. [Google Scholar] [CrossRef]
- Schmitz, K.S.; Wang, B.; Kokufuta, E. Mechanism of Microgel Formation via Cross-Linking of Polymers in Their Dilute Solutions: Mathematical Explanation with Computer Simulations. Macromolecules 2001, 34, 8370–8377. [Google Scholar] [CrossRef]
- Bide, Y.; Fashapoyeh, M.A.; Shokrollahzadeh, S. Structural investigation and application of Tween 80-choline chloride self-assemblies as osmotic agent for water desalination. Sci. Rep. 2021, 11, 17068. [Google Scholar] [CrossRef]
- Brändén, C.-I.; Tooze, J. Introduction to Protein Structure, 2nd ed.; Garland Pub: New York, NY, USA, 1999. [Google Scholar]
- Whitford, D. Proteins: Structure and Function; John Wiley & Sons: Hoboken, NJ, USA, 2005. [Google Scholar]
- Loveday, S.M. β-Lactoglobulin heat denaturation: A critical assessment of kinetic modelling. Int. Dairy J. 2016, 52, 92–100. [Google Scholar] [CrossRef]
- Iurciuc-Tincu, C.-E.; Cretan, M.S.; Purcar, V.; Popa, M.; Daraba, O.M.; Atanase, L.I.; Ochiuz, L. Drug Delivery System Based on pH-Sensitive Biocompatible Poly(2-vinyl pyridine)-b-poly(ethylene oxide) Nanomicelles Loaded with Curcumin and 5-Fluorouracil. Polymers 2020, 12, 1450. [Google Scholar] [CrossRef] [PubMed]
- Rohman, N.; Ahmed, K.; Skelton, A.A.; Mohiuddin, T.; Khan, I.; Selvaraj, R.; Yamin, M. Theoretical insights and implications of pH-dependent drug delivery systems using silica and carbon nanotube. J. Mol. Graph. Model. 2023, 125, 108609. [Google Scholar] [CrossRef] [PubMed]
- McSwiney, M.; Singh, H.; Campanella, O.H. Thermal aggregation and gelation of bovine β-lactoglobulin. Food Hydrocoll. 1994, 8, 441–453. [Google Scholar] [CrossRef]
- Xiong, Y.L.; Dawson, K.A.; Wan, L. Thermal Aggregation of β-Lactoglobulin: Effect of pH, Ionic Environment, and Thiol Reagent. J. Dairy Sci. 1993, 76, 70–77. [Google Scholar] [CrossRef]
- Lapanje, S.; Poklar, N. Calorimetric and circular dichroic studies of the thermal denaturation of β-lactoglobulin. Biophys. Chem. 1989, 34, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, L.; Sarangi, J.; Misra, P.K. Organization of Amphiphiles, Part 1: Evidence in Favor of Pre-micellar Aggregates through Fluorescence Spectroscopy. Bull. Chem. Soc. Jpn. 2002, 75, 859–865. [Google Scholar] [CrossRef]
- Lombardo, D.; Munaò, G.; Calandra, P.; Pasqua, L.; Caccamo, M.T. Evidence of pre-micellar aggregates in aqueous solution of amphiphilic PDMS–PEO block copolymer. Phys. Chem. Chem. Phys. 2019, 21, 11983–11991. [Google Scholar] [CrossRef] [PubMed]
- Adabi, M.; Naghibzadeh, M.; Adabi, M.; Zarrinfard, M.A.; Esnaashari, S.S.; Seifalian, A.M.; Faridi-Majidi, R.; Tanimowo Aiyelabegan, H.; Ghanbari, H. Biocompatibility and nanostructured materials: Applications in nanomedicine. Artif. Cells Nanomedicine Biotechnol. 2017, 45, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Tatur, S.; Maccarini, M.; Barker, R.; Nelson, A.; Fragneto, G. Effect of Functionalized Gold Nanoparticles on Floating Lipid Bilayers. Langmuir 2013, 29, 6606–6614. [Google Scholar] [CrossRef]
- Roach, P.; Farrar, D.; Perry, C.C. Interpretation of Protein Adsorption: Surface-Induced Conformational Changes. J. Am. Chem. Soc. 2005, 127, 8168–8173. [Google Scholar] [CrossRef]
- Tsutsumi, M.; Otaki, J.M. Parallel and Antiparallel β-Strands Differ in Amino Acid Composition and Availability of Short Constituent Sequences. J. Chem. Inf. Model. 2011, 51, 1457–1464. [Google Scholar] [CrossRef]
- Albani, J.-R. Sub-Structures Formed in the Excited State are Responsible for Tryptophan Residues Fluorescence in β-Lactoglobulin. J. Fluoresc. 2011, 21, 1683–1687. [Google Scholar] [CrossRef]
- Otteson, E.W.; Welch, W.H.; Kozel, T.R. Protein-polysaccharide interactions. A monoclonal antibody specific for the capsular polysaccharide of Cryptococcus neoformans. J. Biol. Chem. 1994, 269, 1858–1864. [Google Scholar] [CrossRef]
- Instrumentation for Fluorescence Spectroscopy. In Principles of Fluorescence Spectroscopy; Springer: Boston, MA, USA, 2006; pp. 27–61. [CrossRef]
- Mohammadi, F.; Bordbar, A.-K.; Divsalar, A.; Mohammadi, K.; Saboury, A.A. Analysis of Binding Interaction of Curcumin and Diacetylcurcumin with Human and Bovine Serum Albumin Using Fluorescence and Circular Dichroism Spectroscopy. Protein J. 2009, 28, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Barik, A.; Priyadarsini, K.I.; Mohan, H. Photophysical Studies on Binding of Curcumin to Bovine Serum Albumin. Photochem. Photobiol. 2003, 77, 597. [Google Scholar] [CrossRef]
- Rabe, M.; Kerth, A.; Blume, A.; Garidel, P. Albumin displacement at the air–water interface by Tween (Polysorbate) surfactants. Eur. Biophys. J. 2020, 49, 533–547. [Google Scholar] [CrossRef] [PubMed]
- Garidel, P.; Hoffmann, C.; Blume, A. A thermodynamic analysis of the binding interaction between polysorbate 20 and 80 with human serum albumins and immunoglobulins: A contribution to understand colloidal protein stabilisation. Biophys. Chem. 2009, 143, 70–78. [Google Scholar] [CrossRef]
- Piñeiro, L.; Novo, M.; Al-Soufi, W. Fluorescence emission of pyrene in surfactant solutions. Adv. Colloid Interface Sci. 2015, 215, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Dong, D.C.; Winnik, M.A. The P y Scale of Solvent Polarities. Solvent Effects on The Vibronic Fine Structure of Pyrene Fluorescence and Empirical Correlations with E T and Y VALUES. Photochem. Photobiol. 1982, 35, 17–21. [Google Scholar] [CrossRef]
- Matulis, D.; Baumann, C.G.; Bloomfield, V.A.; Lovrien, R.E. 1-Anilino-8-naphthalene sulfonate as a protein conformational tightening agent. Biopolymers 1999, 49, 451–458. [Google Scholar] [CrossRef]



| Composition of Secondary Structure Elements | Three State Composition (%) | Analysis vs. DSSP | DSSP |
|---|---|---|---|
| Helix | 16.04 | Similarity% | 100 |
| Sheet | 40.74 | Matthews CC | 1 |
| Other | 43.20 |
| Assignment | Β-Sheet/Β-Turn | A-Helix | Random Coil | Intramolecular β-Sheet | Others |
|---|---|---|---|---|---|
| Wavenumbers (cm−1) | 1685–1663 | 1655–1650 | 1648–1644 | 1632–1621 | 1639–1635 1616–1600 |
| Pure β-LG | 21.3% | 19.6% | 16.2% | 17.8% | 25.1% |
| β-LG/CS (NoTT) | 15.4% | 22.0% | 13.9% | 20.3% | 28.5% |
| β-LG/CS (TT) | 15.1% | 21.8% | 13.3% | 25.0% | 24.7% |
| β-LG/CS/T80 (NoTT) | 18.5% | 22.2% | 14.1% | 19.7% | 25.5% |
| β-LG/CS/T80 (TT) | 14.8% | 23.0% | 7.1% | 27.3% | 27.8% |
| Pure β-LG | A-Helix | Β-Sheet | Β-Turn | Irregular/Others |
|---|---|---|---|---|
| Untreated/pH 4 | 14.6% | 35.0% | 10.3% | 40.1% |
| Treated/pH 4 | 13.1% | 35.4% | 10.9% | 40.7% |
| Treated/pH 7 | 13.2% | 22.3% | 14.6% | 49.8% |
| β-LG/CS NPs | α-helix | β-sheet | β-turn | Irregular/Others |
| Untreated/pH 4 | 3.1% | 36.4% | 15.1% | 45.3% |
| Treated/pH 4 | 9.8% | 30.0% | 10.7% | 49.6% |
| Treated/pH 7 | 9.2% | 28.0% | 17.5% | 45.3% |
| β-LG/CS/T80 NPs | α-helix | β-sheet | β-turn | Irregular/Others |
| Untreated/pH 4 | 5.9% | 37.4% | 13.5% | 43.2% |
| Treated/pH 4 | 10.4% | 33.6% | 12.4% | 43.4% |
| Treated/pH 7 | 16.1% | 26.7% | 13.5% | 43.7% |
| Parameter/Sample | Pure β-LG | β-LG/CS NPs |
|---|---|---|
| KSV (104 M−1) | 0.211 ± 0.032 | 0.383 ± 0.104 |
| KA (104 M−1) | 4.96 ± 0.64 | 0.451 ± 0.586 |
| n | 1.32 ± 0.20 | 1.00 ± 0.27 |
| State | Sample/Parameter | I1/I3 | So (105 CPS·mL/mg) |
|---|---|---|---|
| Before TT | Pure β-LG | 1.60 ± 0.08 | 144 ± 9 |
| Pure β-LG/T80 | 1.27 ± 0.07 | 454 ± 20 | |
| β-LG/CS NPs | 1.36 ± 0.07 | 28.0 ± 0.9 | |
| β-LG/CS/T80 NPs | 1.32 ± 0.07 | 127 ± 7 | |
| After TT | Pure β-LG | 1.54 ± 0.08 | 128 ± 19 |
| Pure β-LG/T80 | 1.34 ± 0.07 | 414 ± 40 | |
| β-LG/CS NPs | 1.30 ± 0.07 | 334 ± 9 | |
| β-LG/CS/T80 NPs | 1.31 ± 0.07 | 422 ± 33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pispas, I.; Spiliopoulos, N.; Papagiannopoulos, A. Biocompatible Preparation of Beta-Lactoglobulin/Chondroitin Sulfate Carrier Nanoparticles and Modification of Their Colloidal and Hydropathic Properties by Tween 80. Polymers 2024, 16, 1995. https://doi.org/10.3390/polym16141995
Pispas I, Spiliopoulos N, Papagiannopoulos A. Biocompatible Preparation of Beta-Lactoglobulin/Chondroitin Sulfate Carrier Nanoparticles and Modification of Their Colloidal and Hydropathic Properties by Tween 80. Polymers. 2024; 16(14):1995. https://doi.org/10.3390/polym16141995
Chicago/Turabian StylePispas, Ioannis, Nikolaos Spiliopoulos, and Aristeidis Papagiannopoulos. 2024. "Biocompatible Preparation of Beta-Lactoglobulin/Chondroitin Sulfate Carrier Nanoparticles and Modification of Their Colloidal and Hydropathic Properties by Tween 80" Polymers 16, no. 14: 1995. https://doi.org/10.3390/polym16141995
APA StylePispas, I., Spiliopoulos, N., & Papagiannopoulos, A. (2024). Biocompatible Preparation of Beta-Lactoglobulin/Chondroitin Sulfate Carrier Nanoparticles and Modification of Their Colloidal and Hydropathic Properties by Tween 80. Polymers, 16(14), 1995. https://doi.org/10.3390/polym16141995







