Preparation of N-Halamine Gelatin Sponge and Its Application in the Treatment of Skin Infection
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Measurements
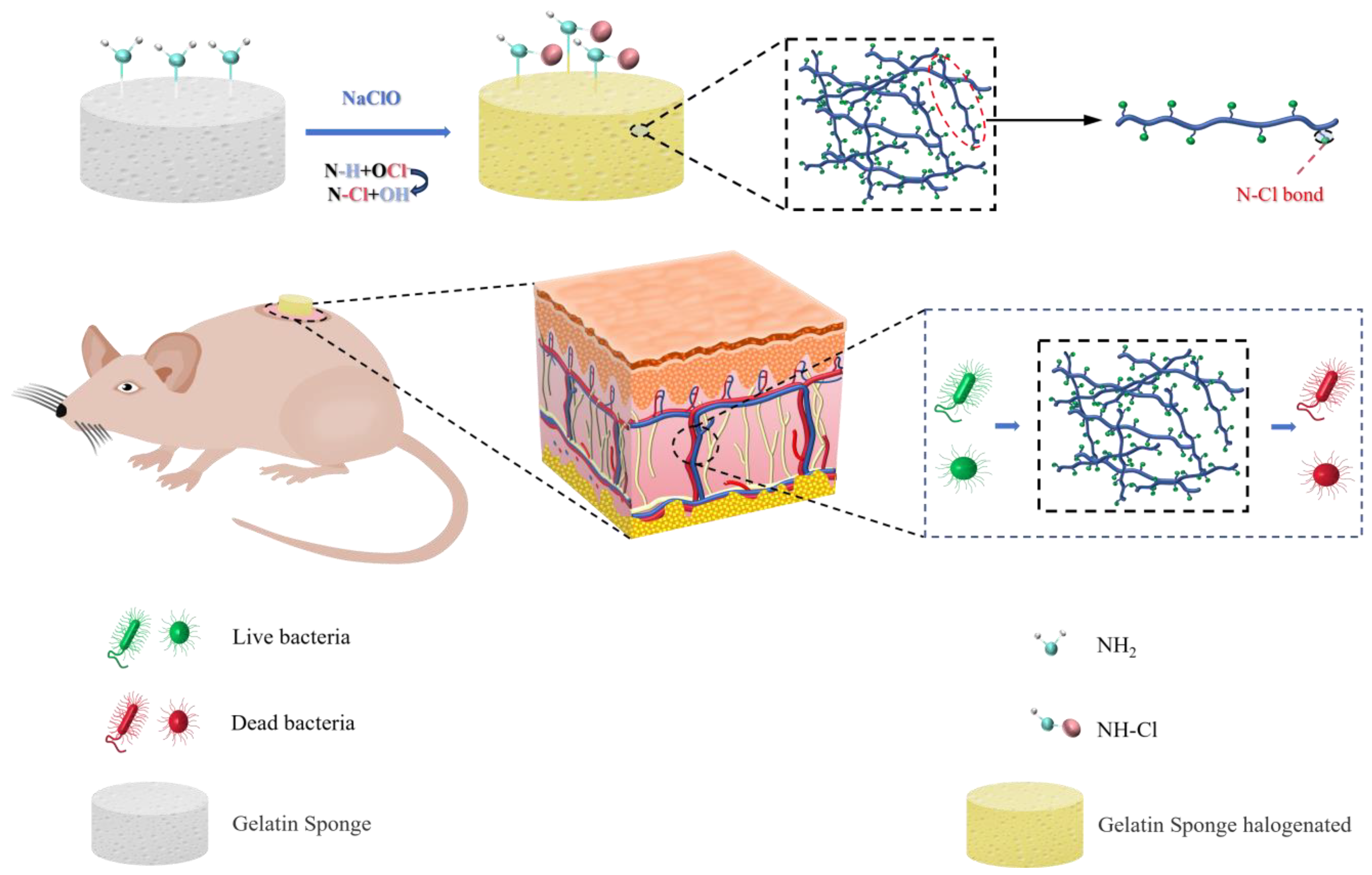
2.3. Synthesis of Protein N-Halamines
2.4. Mechanical Properties Test
2.5. Characterization
2.6. Determination of GS-Cl Samples’ Chlorine Content
2.7. In Vitro Antibacterial Test
2.8. Biocompatibility Assay
2.9. In Vivo Wound Healing Study
3. Results and Discussion
3.1. The Influence of Chlorination Time and NaClO Concentrations on the Chlorine Contents
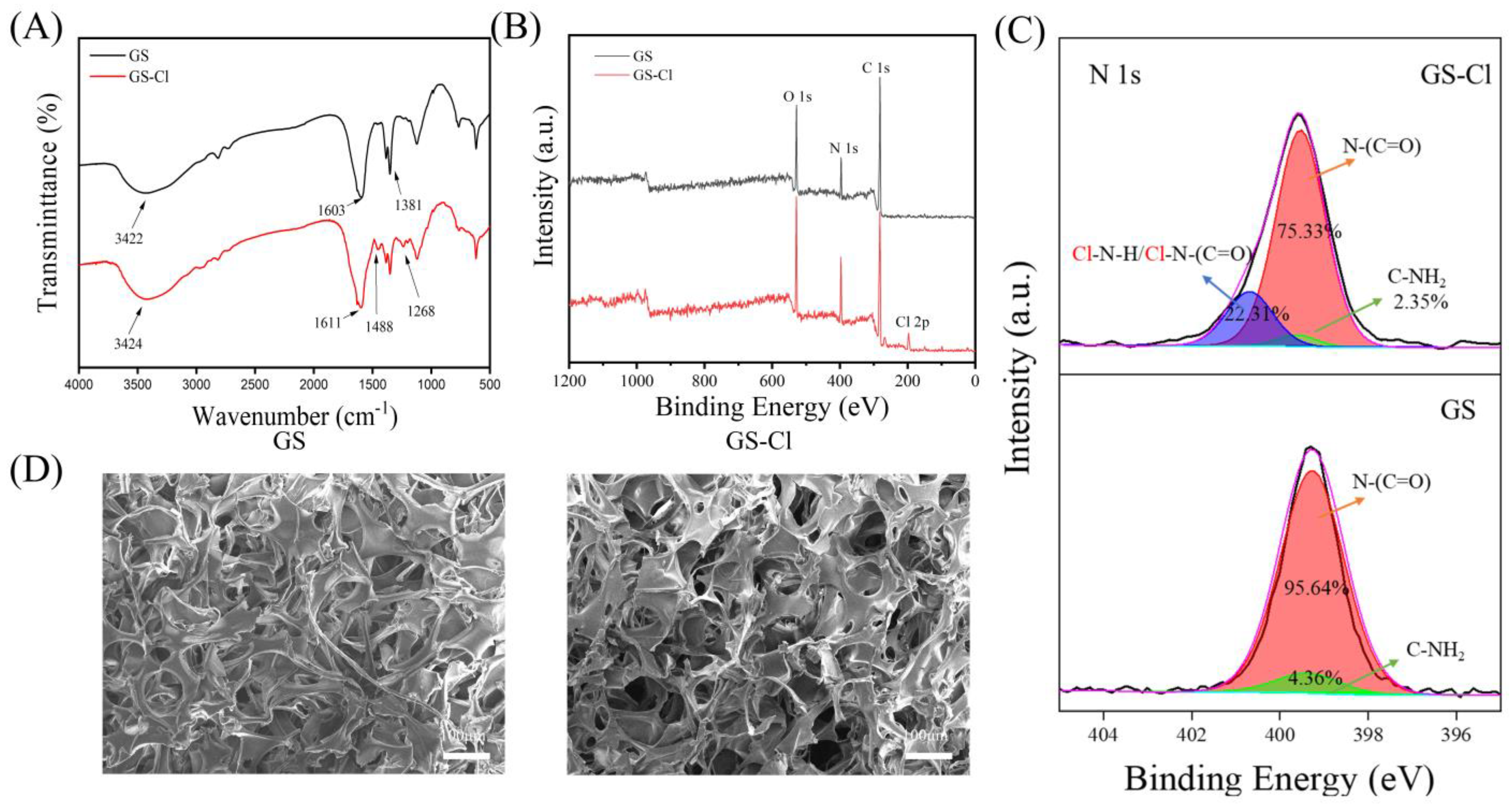
3.2. FTIR Analysis
3.3. XPS Analysis
3.4. SEM Analysis
3.5. Mechanical and Stability Studies
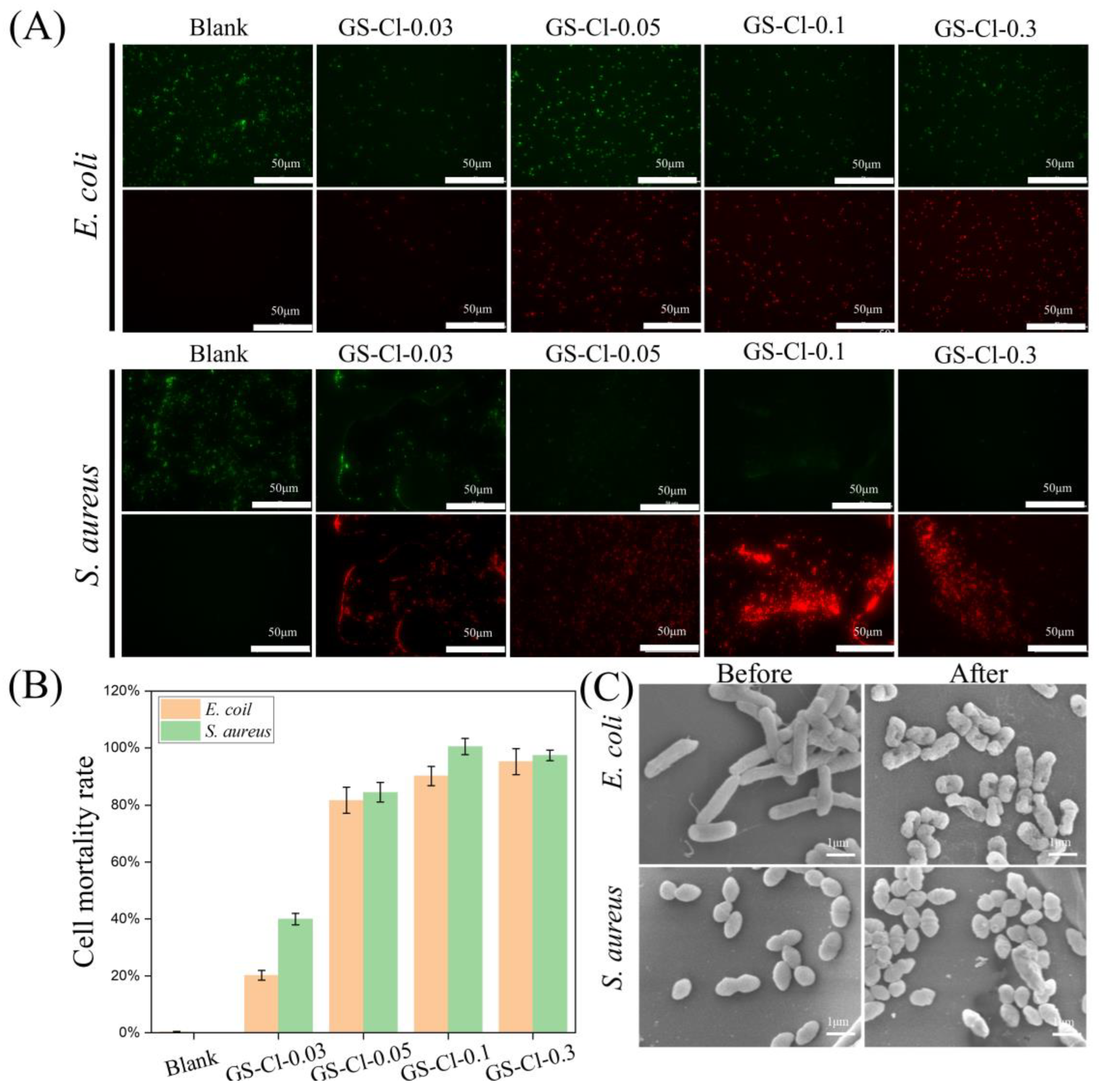
3.6. Antibacterial Activity
3.7. Biocompatibility
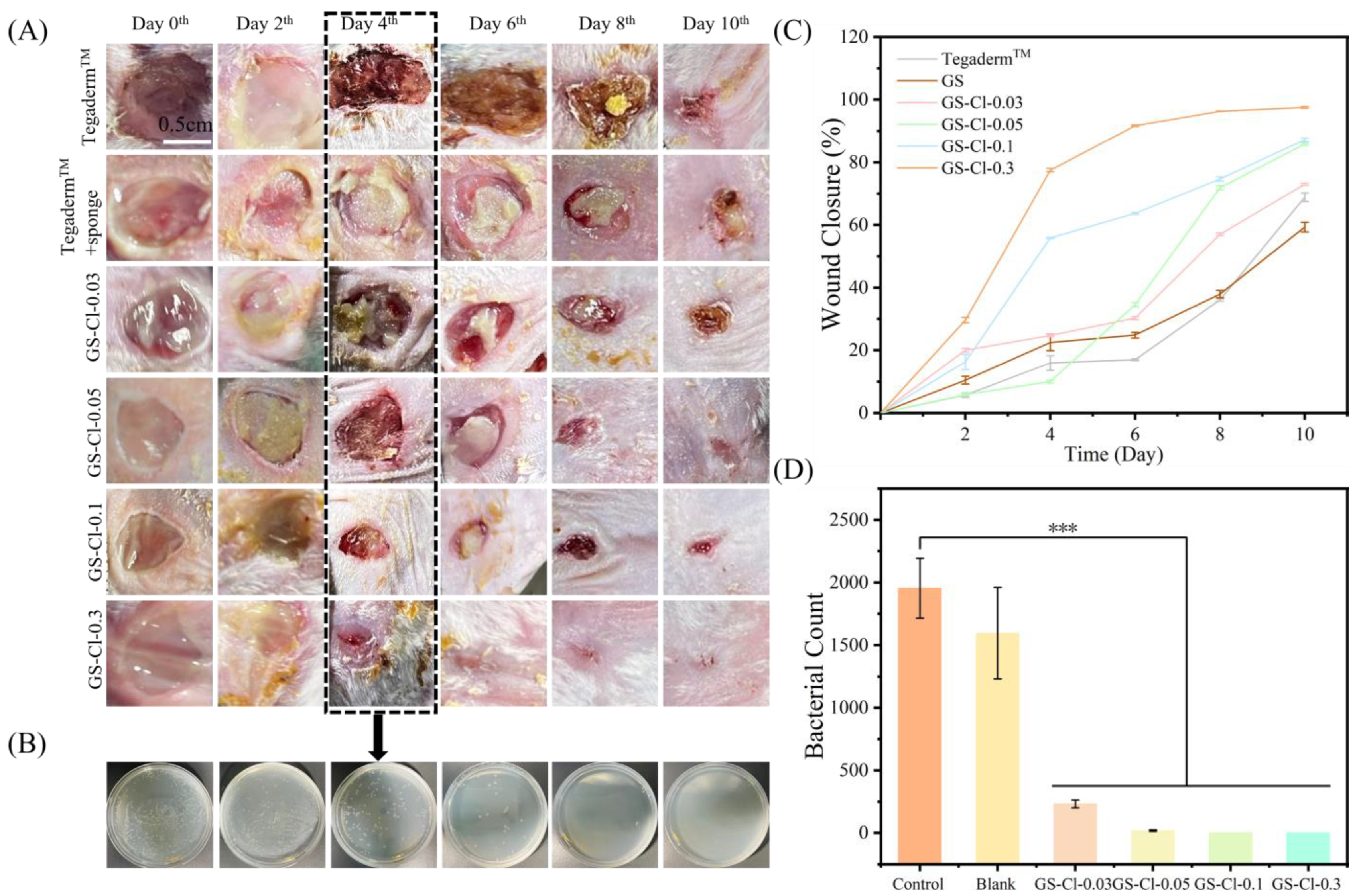
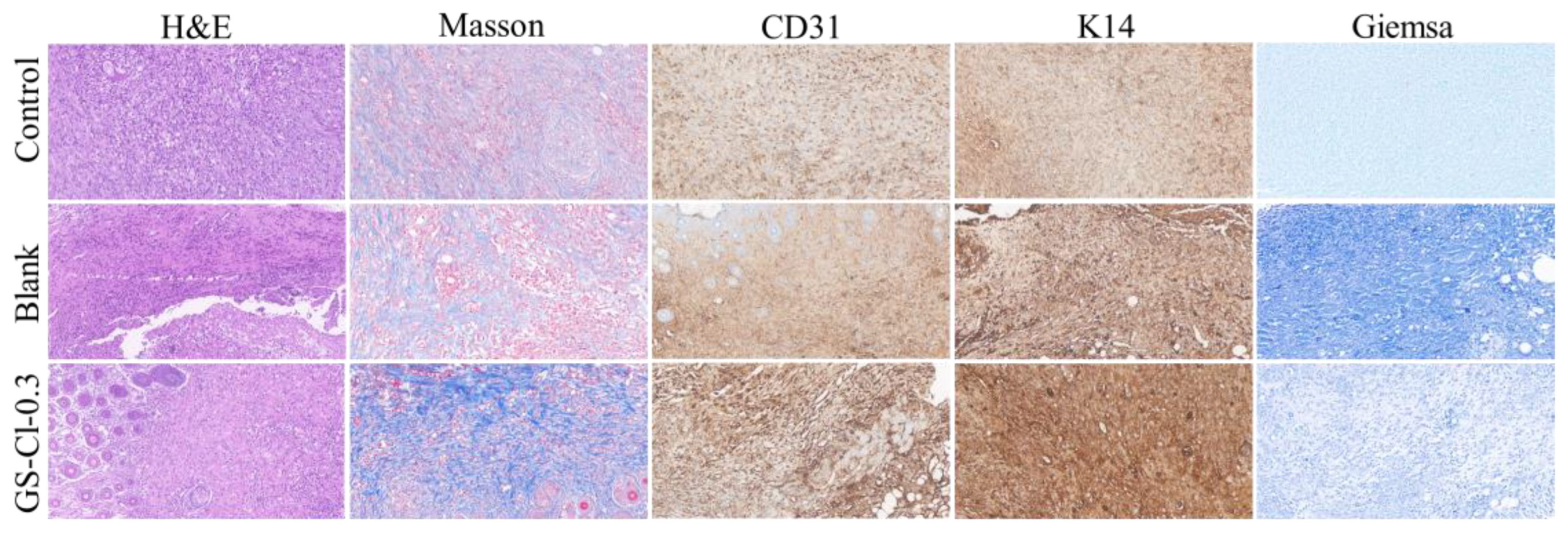
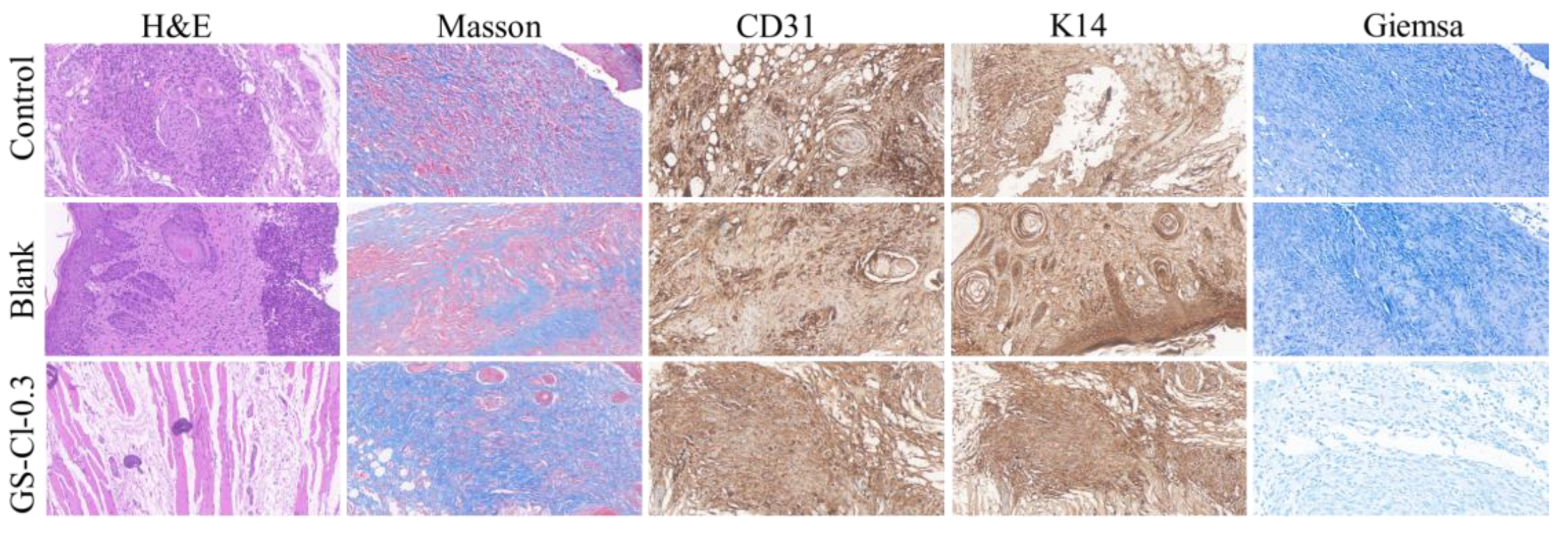
3.8. In Vivo Infectious Wound Healing Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ding, Y.-W.; Wang, Z.-Y.; Ren, Z.-W.; Zhang, X.-W.; Wei, D.-X. Advances in modified hyaluronic acid-based hydrogels for skin wound healing. Biomater. Sci. 2022, 10, 3393–3409. [Google Scholar] [CrossRef] [PubMed]
- Mardina, Z.; Venezuela, J.; Maher, C.; Shi, Z.; Dargusch, M.S.; Atrens, A. Design, mechanical and degradation requirements of biodegradable metal mesh for pelvic floor reconstruction. Biomater. Sci. 2022, 10, 3371–3392. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liu, X.; Peng, X.; Zheng, Y.; Cheng, Z.; Sun, S.; Ding, Q.; Liu, W.; Ding, C. A poloxamer/hyaluronic acid/chitosan-based thermosensitive hydrogel that releases dihydromyricetin to promote wound healing. Int. J. Biol. Macromol. 2022, 216, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Roberts, K.D.; Zhu, Y.; Azad, M.A.K.; Han, M.-L.; Wang, J.; Wang, L.; Yu, H.H.; Horne, A.S.; Pinson, J.-A.; Rudd, D.; et al. A synthetic lipopeptide targeting top-priority multidrug-resistant Gram-negative pathogens. Nat. Commun. 2022, 13, 1625. [Google Scholar] [CrossRef]
- Suo, H.; Hussain, M.; Wang, H.; Zhou, N.; Tao, J.; Jiang, H.; Zhu, J. Injectable and pH-Sensitive Hyaluronic Acid-Based Hydrogels with On-Demand Release of Antimicrobial Peptides for Infected Wound Healing. Biomacromolecules 2021, 22, 3049–3059. [Google Scholar] [CrossRef]
- Mei, L.; Zhang, D.; Shao, H.; Hao, Y.; Zhang, T.; Zheng, W.; Ji, Y.; Ling, P.; Lu, Y.; Zhou, Q. Injectable and Self-Healing Probiotics-Loaded Hydrogel for Promoting Superbacteria-Infected Wound Healing. ACS Appl. Mater Inter. 2022, 14, 20538–20550. [Google Scholar] [CrossRef]
- Li, J.; Ren, S.; Qiu, X.; Zhao, S.; Wang, R.; Wang, Y. Electroactive Ultrafiltration Membrane for Simultaneous Removal of Antibiotic, Antibiotic Resistant Bacteria, and Antibiotic Resistance Genes from Wastewater Effluent. Environ. Sci. Technol. 2022, 56, 15120–15129. [Google Scholar] [CrossRef]
- Kumar, S.B.; Arnipalli, S.R.; Ziouzenkova, O. Antibiotics in Food Chain: The Consequences for Antibiotic Resistance. Antibiotics 2020, 9, 688. [Google Scholar] [CrossRef]
- Kumar, M.; Sarma, D.K.; Shubham, S.; Kumawat, M.; Verma, V.; Nina, P.B.; JP, D.; Kumar, S.; Singh, B.; Tiwari, R.R. Futuristic Non-antibiotic Therapies to Combat Antibiotic Resistance: A Review. Front. Microbiol. 2021, 12, 609459. [Google Scholar] [CrossRef]
- Li, W.; Li, Y.; Sun, P.; Zhang, N.; Zhao, Y.; Qin, S.; Zhao, Y. Antimicrobial peptide-modified silver nanoparticles for enhancing the antibacterial efficacy. RSC Adv. 2020, 10, 38746–38754. [Google Scholar] [CrossRef]
- Ahani, E.; Montazer, M.; Mianehro, A.; Samadi, N.; Toliyat, T.; Mahmoudi Rad, M. Preparation of long-lasting antibacterial wound dressing through diffusion of cationic-liposome-encapsulated polyhexamethylene biguanide. React. Funct. Polym. 2021, 169, 105092. [Google Scholar] [CrossRef]
- Cao, S.; Xu, G.; Li, Q.; Zhang, S.; Yang, Y.; Chen, J. Double crosslinking chitosan sponge with antibacterial and hemostatic properties for accelerating wound repair. Compos. Part B-Eng. 2022, 234, 109746. [Google Scholar] [CrossRef]
- Gao, Y.; Song, N.; Liu, W.; Dong, A.; Wang, Y.-J.; Yang, Y.-W. Construction of Antibacterial N-Halamine Polymer Nanomaterials Capable of Bacterial Membrane Disruption for Efficient Anti-Infective Wound Therapy. Macromol. Biosci. 2019, 19, 1800453. [Google Scholar] [CrossRef]
- Riaz Ahmed, K.B.; Nagy, A.M.; Brown, R.P.; Zhang, Q.; Malghan, S.G.; Goering, P.L. Silver nanoparticles: Significance of physicochemical properties and assay interference on the interpretation of in vitro cytotoxicity studies. Toxicol. Vitr. 2017, 38, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Röhner, E.; Seeger, J.B.; Hoff, P.; Dähn-Wollenberg, S.; Perka, C.; Matziolis, G. Toxicity of Polyhexanide and Hydrogen Peroxide on Human Chondrocytes In Vitro. Orthopedics 2011, 34, e290–e294. [Google Scholar] [CrossRef] [PubMed]
- Yabes, J.M.; White, B.K.; Murray, C.K.; Sanchez, C.J.; Mende, K.; Beckius, M.L.; Zera, W.C.; Wenke, J.C.; Akers, K.S. In Vitro activity of Manuka Honey and polyhexamethylene biguanide on filamentous fungi and toxicity to human cell lines. Med. Mycol. 2016, 55, 334–343. [Google Scholar]
- Kim, S.-H.; Semenya, D.; Castagnolo, D. Antimicrobial drugs bearing guanidine moieties: A review. Eur. J. Med. Chem. 2021, 216, 113293. [Google Scholar] [CrossRef]
- Jiao, Y.; Liu, Q.; Chen, J. Construction of N-halamine biocompatible multilayers onto BMP2 loaded titanium nanotubes for bacterial infection inhibition and osteogenic effect improvement. Mater. Lett. 2020, 267, 127526. [Google Scholar] [CrossRef]
- Chen, Z.; Sun, Y. N-Halamine-Based Antimicrobial Additives for Polymers: Preparation, Characterization, and Antimicrobial Activity. Ind. Eng. Chem. Res. 2006, 45, 2634–2640. [Google Scholar] [CrossRef]
- Ren, X.; Kocer, H.B.; Worley, S.D.; Broughton, R.M.; Huang, T.S. Rechargeable biocidal cellulose: Synthesis and application of 3-(2,3-dihydroxypropyl)-5,5-dimethylimidazolidine-2,4-dione. Carbohyd. Polym. 2009, 75, 683–687. [Google Scholar] [CrossRef]
- Padmanabhuni, R.V.; Luo, J.; Cao, Z.; Sun, Y. Preparation and Characterization of N-Halamine-Based Antimicrobial Fillers. Ind. Eng. Chem. Res. 2012, 51, 5148–5156. [Google Scholar] [CrossRef] [PubMed]
- Cerkez, I.; Kocer, H.B.; Worley, S.D.; Broughton, R.M.; Huang, T.S. Epoxide tethering of polymeric N-halamine moieties. Cellulose 2012, 19, 959–966. [Google Scholar] [CrossRef]
- Ma, Y.; Wisuthiphaet, N.; Nitin, N.; Sun, G. A Novel N-Halamine Biocidal Nanofibrous Membrane for Chlorine Rechargeable Rapid Water Disinfection Applications. ACS Appl. Mater. Inter. 2021, 13, 41056–41065. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Liu, M.; Ding, R.; Liang, M.; Huang, L.; Yu, J.; Si, Y. Rechargeable Antibacterial Polysulfonamide-Based N-Halamine Nanofibrous Membranes for Bioprotective Applications. ACS Appl. Bio Mater. 2019, 2, 3668–3677. [Google Scholar] [CrossRef]
- Choi, J.; Moon, D.S.; Ryu, S.G.; Lee, B.; Ying, W.B.; Lee, K.J. N-chloro hydantoin functionalized polyurethane fibers toward protective cloth against chemical warfare agents. Polymer 2018, 138, 146–155. [Google Scholar] [CrossRef]
- Zheng, Y.; Pan, N.; Liu, Y.; Ren, X. Novel porous chitosan/N-halamine structure with efficient antibacterial and hemostatic properties. Carbohyd. Polym. 2021, 253, 117205. [Google Scholar] [CrossRef]
- Moore, G.W.K.; Howell, S.E.L.; Brady, M.; Xu, X.; McNeil, K. Anomalous collapses of Nares Strait ice arches leads to enhanced export of Arctic sea ice. Nat. Commun. 2021, 12, 1. [Google Scholar] [CrossRef]
- Shoulders, M.D.; Raines, R.T. Collagen Structure and Stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef]
- Hagen, M.L.; Harper, J.B.; Croft, A.K. Recent advances in the use of ionic liquids as solvents for protein-based materials and chemistry. Curr. Opin. Green Sust. 2022, 36, 100637. [Google Scholar] [CrossRef]
- Wang, Y.; Katyal, P.; Montclare, J.K. Protein-Engineered Functional Materials. Adv. Healthc. Mater. 2019, 8, 1801374. [Google Scholar] [CrossRef]
- Jao, D.; Xue, Y.; Medina, J.; Hu, X. Protein-Based Drug-Delivery Materials. Materials 2017, 10, 517. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Kai, C.; Liu, B.; Zhang, S.; Wei, W.; Xu, X.; Zhou, Z. Preparation, characterization and antibacterial properties of cellulose membrane containing N-halamine. Cellulose 2019, 26, 5621–5633. [Google Scholar] [CrossRef]
- Wen, Y.; Yu, B.; Zhu, Z.; Yang, Z.; Shao, W. Synthesis of Antibacterial Gelatin/Sodium Alginate Sponges and Their Antibacterial Activity. Polymers 2020, 12, 1926. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, J.; Xue, J.; Qin, H.; Wang, Y.; Wang, Y.; Cheng, Y.; Ma, Y.; Zhang, X.; Gong, C.; Zhao, G. Preparation of N-Halamine Gelatin Sponge and Its Application in the Treatment of Skin Infection. Polymers 2024, 16, 2579. https://doi.org/10.3390/polym16182579
Zhu J, Xue J, Qin H, Wang Y, Wang Y, Cheng Y, Ma Y, Zhang X, Gong C, Zhao G. Preparation of N-Halamine Gelatin Sponge and Its Application in the Treatment of Skin Infection. Polymers. 2024; 16(18):2579. https://doi.org/10.3390/polym16182579
Chicago/Turabian StyleZhu, Jiahao, Jiageng Xue, Huaiying Qin, Yiqing Wang, Yefan Wang, Yidan Cheng, Yingxia Ma, Xiaoyun Zhang, Chenliang Gong, and Guanghui Zhao. 2024. "Preparation of N-Halamine Gelatin Sponge and Its Application in the Treatment of Skin Infection" Polymers 16, no. 18: 2579. https://doi.org/10.3390/polym16182579







