Polymer Bionanocomposites Based on a P3BH/Polyurethane Matrix with Organomodified Montmorillonite—Mechanical and Thermal Properties, Biodegradability, and Cytotoxicity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Nanobiocomposite Preparation
2.3. Analytical Methods
2.3.1. Scanning Electron Microscopy
2.3.2. SAXS Analysis
2.3.3. Fourier Transformation Infrared Spectroscopy
2.3.4. Thermogravimetric Analysis
2.3.5. Differential Scanning Calorimetry (DSC) Analysis
2.3.6. Strength Tests
2.3.7. Biodegradability Testing
2.3.8. Elemental Analysis
2.3.9. Cell Culture
2.3.10. In Vitro Cytotoxicity Tests
2.3.11. Statistical Analysis
3. Results and Discussion
3.1. Results of SAXS Measurements
3.2. Results of FTIR Analysis
3.3. Results of SEM Analysis
3.4. Mechanical Properties the Obtained Hybrid Nanobiocomposites
3.5. Thermal Properties of the Obtained Nanobiocomposites
3.6. Thermal Parameters from DSC Measurements
3.7. Biodegradability of P3HB and Its Composition and Hybrid Nanocomposites
3.8. In Vitro Cytotoxicity Studies
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Howarth, G. Polyurethanes, polyurethane dispersions and polyureas: Past, present and future. Surf. Coatings Int. B Coatings Trans. 2003, 86, 111–118. [Google Scholar] [CrossRef]
- Szycher, M.; Siciliano, A.A.; Reed, A.M. Polyurethanes in medical devices. Med. Des. Mat. 1991, 1, 18–25. [Google Scholar]
- Krol, P. Linear Polyurethanes: Synthesis Methods, Chemical Structures, Properties and Applications; Brill Academic Publishers: Leiden, The Netherlands, 2008. [Google Scholar]
- Ismail, E.A.; Motawie, A.M.; Sadek, E.M. Synthesis and characterization of polyurethane coatings based on soybean oil–polyester polyols. Egypt J. Pet. 2011, 20, 1–8. [Google Scholar] [CrossRef]
- Zia, K.M.; Zuber, M.; Saif, M.J.; Jawaid, M.; Mahmood, K.; Shahid, M.; Ahmad, M.N. Chitin based polyurethanes using hydroxyl terminated polybutadiene, part III: Surface characteristics. Int. J. Biol. Macromol. 2013, 62, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Zia, K.M.; Bhatti, H.N.; Ahmad Bhatti, I. Methods for polyurethane and polyurethane composites, recycling and recovery: A review. React. Funct. Polym. 2007, 67, 675–692. [Google Scholar] [CrossRef]
- Ionescu, M. Chemistry and Technology of Polyols for Polyurethanes; Rapra Technology: Shrewsbury, UK; Polymer International: Shawbury, UK, 2007; Volume 56. [Google Scholar]
- Rafiee, Z.; Keshavarz, V. Synthesis and characterization of polyurethane/microcrystalline cellulose bionanocomposites. Prog. Org. Coatings 2015, 86, 190–193. [Google Scholar] [CrossRef]
- Prisacariu, C. Polyurethane Elastomers: From Morphology to Mechanical Aspects; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Chattopadhyay, D.K.; Raju, K.V.S.N. Structural engineering of polyurethane coatings for high performance applications. Prog. Polym. Sci. 2007, 32, 352–418. [Google Scholar] [CrossRef]
- Zia, K.M.; Anjum, S.; Zuber, M.; Mujahid, M.; Jamil, T. Synthesis and molecular characterization of chitosan based polyurethane elastomers using aromatic diisocyanate. Int. J. Biol. Macromol. 2014, 66, 26–32. [Google Scholar] [CrossRef]
- Akindoyo, J.O.; Beg, M.D.H.; Ghazali, S.; Islam, M.R.; Jeyaratnam, N.; Yuvaraj, A.R. Polyurethane types, synthesis and applications—A review. RSC Adv. 2016, 6, 114453–114482. [Google Scholar] [CrossRef]
- Lelah, M.D.; Cooper, S.I. Polyurethane in Medicine; CRC Press: Boca Raton, FL, USA, 1986; pp. 35–71. [Google Scholar]
- Hung, K.-C.; Tseng, C.-S.; Dai, L.-G.; Hsu, S. Water-based polyurethane 3D printed scaffolds with controlled release function for customized cartilage tissue engineering. Biomaterials 2016, 83, 156–168. [Google Scholar] [CrossRef]
- Poussard, L.; Burel, F.; Couvercelle, J.-P.; Merhi, Y.; Tabrizian, M.; Bunel, C. Hemocompatibilty of new ionic polyurethanes: Influence of carboxylic group insertion modes. Biomaterials 2004, 25, 3473–3483. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-H.; Yao, C.-H.; Chuang, W.-Y.; Young, T.-H. Development of biodegradable polyesterurethane membranes with different surface morphologies for the culture of osteoblasts. J. Biomed. Mater. Res. 2000, 51, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Hu, Y.; Li, J.; Li, B. Chitosan/phosvitin antibacterial films fabricated via layer-by-layer deposition. Int. J. Biol. Macromol. 2014, 64, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hong, Q.; Chen, Y.; Lian, X.; Xiong, Y. Surface properties of polyurethanes modified by bioactive polysaccharide-based polyelectrolyte multilayers. Colloids Surf. B Biointerfaces 2012, 100, 77–83. [Google Scholar] [CrossRef]
- Solanki, A.; Mehta, J.; Thakore, S. Structure–property relationships and biocompatibility of carbohydrate crosslinked polyurethanes. Carbohydr. Polym. 2014, 110, 338–344. [Google Scholar] [CrossRef]
- Solanki, A.; Thakore, S. Cellulose crosslinked pH-responsive polyurethanes for drug delivery: α-hydroxy acids as drug release modifiers. Int. J. Biol. Macromol. 2015, 80, 683–691. [Google Scholar] [CrossRef]
- Kara, F.; Aksoy, E.A.; Yuksekdag, Z.; Hasirci, N.; Aksoy, S. Synthesis and surface modification of polyurethanes with chitosan for antibacterial properties. Carbohydr. Polym. 2014, 112, 39–47. [Google Scholar] [CrossRef]
- Baheiraei, N.; Yeganeh, H.; Ai, J.; Gharibi, R.; Azami, M.; Faghihi, F. Synthesis, characterization and antioxidant activity of a novel electroactive and biodegradable polyurethane for cardiac tissue engineering application. Mater. Sci. Eng. C 2014, 44, 24–37. [Google Scholar] [CrossRef]
- Gao, Z.; Peng, J.; Zhong, T.; Sun, J.; Wang, X.; Yue, C. Biocompatible elastomer of waterborne polyurethane based on castor oil and polyethylene glycol with cellulose nanocrystals. Carbohydr. Polym. 2012, 87, 2068–2075. [Google Scholar] [CrossRef]
- Krzykowska, B.; Czerniecka-Kubicka, A.; Białkowska, A.; Bakar, M.; Kovarova, M.; Sedlarik, V.; Hanusova, D.; Zarzyka, I. Biopolymer Compositions Based on Poly(3-hydroxybutyrate) and Linear Polyurethanes with Aromatic Rings-Preparation and Properties Evaluation. Polymers 2024, 16, 1618. [Google Scholar] [CrossRef]
- PN-EN ISO 527:1998; Plastics—Determination of Mechanical Properties in Static Tension—General Principles. Polish Stand-ArdsInstitution: Warszawa, Poland, 1998.
- PN-EN ISO 6506-1:2006; Metallic Materials—Brinell Hardness Test—Part 1: Test Method. Polish Stand-ArdsInstitution: Warszawa, Poland, 2006.
- PN-EN ISO 527-1; Determination of Mechanical Properties under Static Stretching. Polish Stand-ArdsInstitution: Warszawa, Poland, 2013.
- ISO 8256-A-4J; Plastics—Determination of Tensile-Impact Strength. ISO: Geneva, Switzerland, 2004.
- ISO 17556:2012; Plastics—Determination of the Ultimate Aerobic Biodegradability of Plastic Materials in Soil by Measuring the Oxygen Demand in Respirometer or the Amount of Carbon Dioxide Evolved. ISO: Geneva, Switzerland, 2012.
- ISO 11274; Soil Quality—Determination of the Water Retention Characteristics—Laboratory Methods. International Organization for Standardization: Geneva, Switzerland, 2019.
- ISO 10390; Soil Quality—Determination of pH. ISO: Geneva, Switzerland, 2021.
- Park, J.-C.; Park, B.J.; Lee, D.H.; Suh, H.; Kim, D.-G.; Kwon, O.-H. Evaluation of the cytotoxicity of polyetherurethane (PU) film containing zinc diethyldithiocarbamate on various cell lines. Yonsei Med. J. 2002, 43, 518–526. [Google Scholar] [CrossRef] [PubMed]
- US Pharmacopeia. Chapter 87: Biological Reactivity Test, In Vitro. Available online: https://www.drugfuture.com/pharmacopoeia/usp32/pub/data/v32270/usp32nf27s0_c87.html (accessed on 13 August 2024).
- ISO 10993-12; Biological Evaluation of Medical Devices-Part 12: Sample Preparation and Reference Materials. ISO: Geneva, Switzerland, 2021.
- García-Quiles, L.; Cuello, A. F’.; Castell, P. Sustainable Materials with Enhanced Mechanical Properties Based on Industrial Polyhydroxyalkanoates Reinforced with Organomodified Sepiolite and Montmorillonite. Polymers 2019, 11, 696. [Google Scholar] [CrossRef] [PubMed]
- Jost, V.; Kopitzky, R. Blending of Polyhydroxybutyrate-co-valerate with Polylactic Acid for Packaging Applications—Reflections on Miscibility and Effects on the Mechanical and Barrier Properties. Chem. Biochem. Eng. Q. 2015, 29, 221–246. [Google Scholar] [CrossRef]
- Jin, P.; Pang, A.; Yang, R.; Guo, X.; He, J.; Zhai, J. Study on Mechanical Properties of Polyurethane Cross-Linked P(E-co-T)/PEG Blended Polyether Elastomer. Polymers 2022, 14, 5419. [Google Scholar] [CrossRef]
- Yeo, J.C.C.; Muiruri, J.K.; Thitsartarn, W.; Li, Z.; He, C. Recent advances in the development of biodegradable PHB-based toughening materials: Approaches, advantages and applications. Mater. Sci. Eng. C 2018, 92, 1092–1116. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, M.; Pokhrel, D.; Coats, E.R.; Guho, N.M.; McDonald, A.G. Effect of 3-Hydroxyvalerate Content on Thermal, Mechanical, and Rheological Properties of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) Biopolymers Produced from Fermented Dairy Manure. Polymers 2022, 14, 4140. [Google Scholar] [CrossRef]
- Iron, R.; Mehdikhani, M.; Naghashzargar, E.; Karbasi, S.; Semnani, D. Effects of nano-bioactive glass on structural, mechanical and bioactivity properties of Poly (3-hydroxybutyrate) electrospun scaffold for bone tissue engineering applications. Mater. Tech. 2019, 34, 540–548. [Google Scholar] [CrossRef]
- Corre, Y.M.; Bruzaud, S.; Audic, J.L.; Grohens, Y. Morphology and functional properties of commercial polyhydroxyalkanoates: A comprehensive and comparative study. Polym. Test. 2012, 31, 226–235. [Google Scholar] [CrossRef]
- Botana, A.; Mollo, M.; Eisenberg, P.; Torres Sanchez, R.M. Effect of modified montmorillonite on biodegradable PHB nanocomposites. Appl. Clay Sci. 2010, 47, 263–270. [Google Scholar] [CrossRef]
- Paşcu, E.I.; Stokes, J.; McGuinness, G.B. Electrospun composites of PHBV, silk fibroin and nano-hydroxyapatite for bone tissue engineering. Mater. Sci. Eng. C 2013, 33, 4905–4916. [Google Scholar] [CrossRef]
- Chandar, J.V.; Shanmugan, S.; Mutharasu, D.; Azlan, A.A. Poly (3-hydroxybutyrate-co-15 mol% 3hydroxyhexanoate)/ZnO nanocomposites by solvent casting method: A study of optical, surface, and thermal properties. Mater. Res. Express 2017, 4, 015301. [Google Scholar] [CrossRef]
- Colombo, I.; Sangiovanni, E.; Maggio, R.; Mattozzi, C.; Zava, S.; Corbett, Y.; Fumagalli, M.; Carlino, C.; Corsetto, P.A.; Scaccabarozzi, D.; et al. HaCaT Cells as a Reliable In Vitro Differentiation Model to Dissect the Inflammatory/Repair Response of Human Keratinocytes. Mediat. Inflamm. 2017, 2017, 7435621. [Google Scholar] [CrossRef]
- Hoh, A.; Maier, K. Comparative Cytotoxicity Test with Human Keratinocytes, HaCaT Cells, and Skin Fibroblasts to Investigate Skin-Irritating Substances. In Cell and Tissue Culture Models in Dermatological Research; Bernd, A., Bereiter-Hahn, J., Hevert, F., Holzmann, H., Eds.; Springer: Berlin/Heidelberg, Germany, 1993; pp. 341–347. [Google Scholar] [CrossRef]
- Freier, T.; Kunze, C.; Nischan, C.; Kramer, S.; Sternberg, K.; Saß, M.; Hopt, U.T.; Schmitz, K.-P. In vitro and in vivo degradation studies for development of a biodegradable patch based on poly(3-hydroxybutyrate). Biomaterials 2002, 23, 2649–2657. [Google Scholar] [CrossRef]
- García-Cerna, S.; Sánchez-Pacheco, U.; Meneses-Acosta, A.; Rojas-García, J.; Campillo-Illanes, B.; Segura-González, D.; Peña-Malacara, C. Evaluation of Poly-3-Hydroxybutyrate (P3HB) Scaffolds Used for Epidermal Cells Growth as Potential Biomatrix. Polymers 2022, 14, 4021. [Google Scholar] [CrossRef] [PubMed]
- Hamada, H.; Isaksson, M.; Bruze, M.; Engfeldt, M.; Liljelind, I.; Axelsson, S.; Jönsson, B.; Tinnerberg, H.; Zimerson, E. Dermal uptake study with 4,4′-diphenylmethane diisocyanate led to active sensitization. Contact Dermat. 2012, 66, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Król, P.; Uram, Ł.; Król, B.; Pielichowska, K.; Walczak, M. Study of chemical, physico-mechanical and biological properties of 4,4′-methylenebis(cyclohexyl isocyanate)-based polyurethane films. Mater. Sci. Eng. C 2018, 93, 483–494. [Google Scholar] [CrossRef]
- Wagner, A.; Eldawud, R.; White, A.; Agarwal, S.; Stueckle, T.A.; Sierros, K.A.; Rojanasakul, Y.; Gupta, R.K.; Dinu, C.Z. Toxicity evaluations of nanoclays and thermally degraded byproducts through spectroscopical and microscopical approaches. Biochim. Biophys. Acta BBA-Gen. Subj. 2017, 1861, 3406–3415. [Google Scholar] [CrossRef]
- Maisanaba, S.; Gutiérrez-Praena, D.; Pichardo, S.; Moreno, F.J.; Jordá, M.; Cameán, A.M.; Aucejo, S.; Jos, Á. Toxic effects of a modified montmorillonite clay on the human intestinal cell line Caco-2. J. Appl. Toxicol. 2014, 34, 714–725. [Google Scholar] [CrossRef]


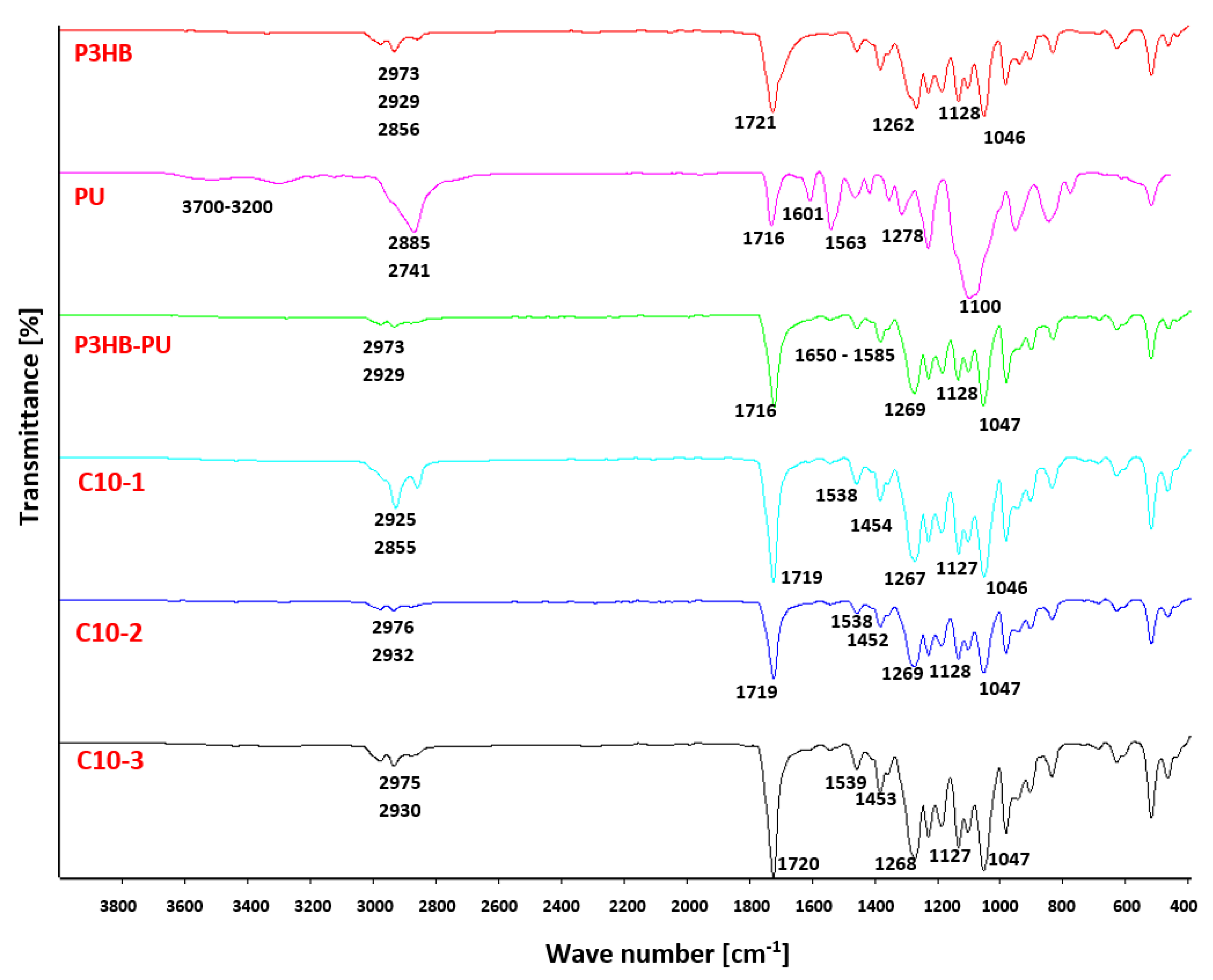
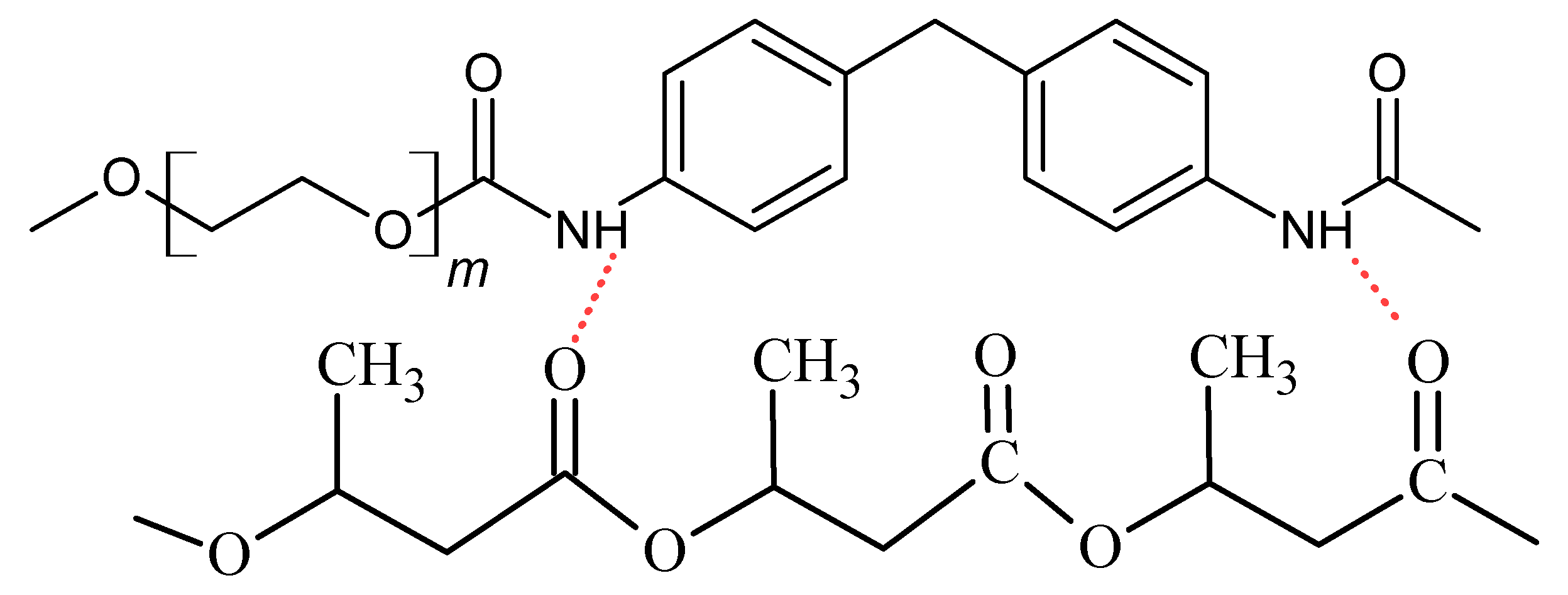


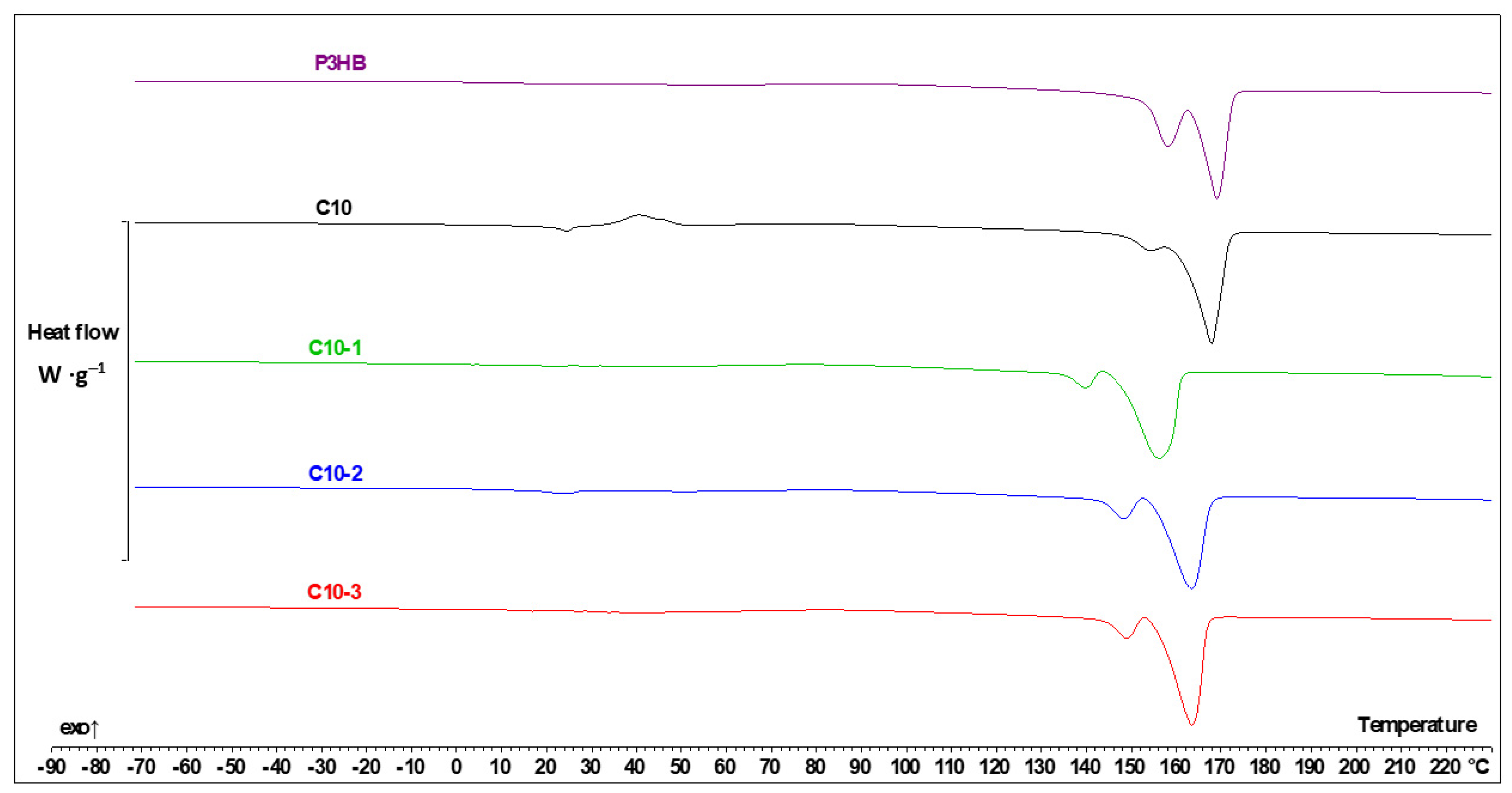

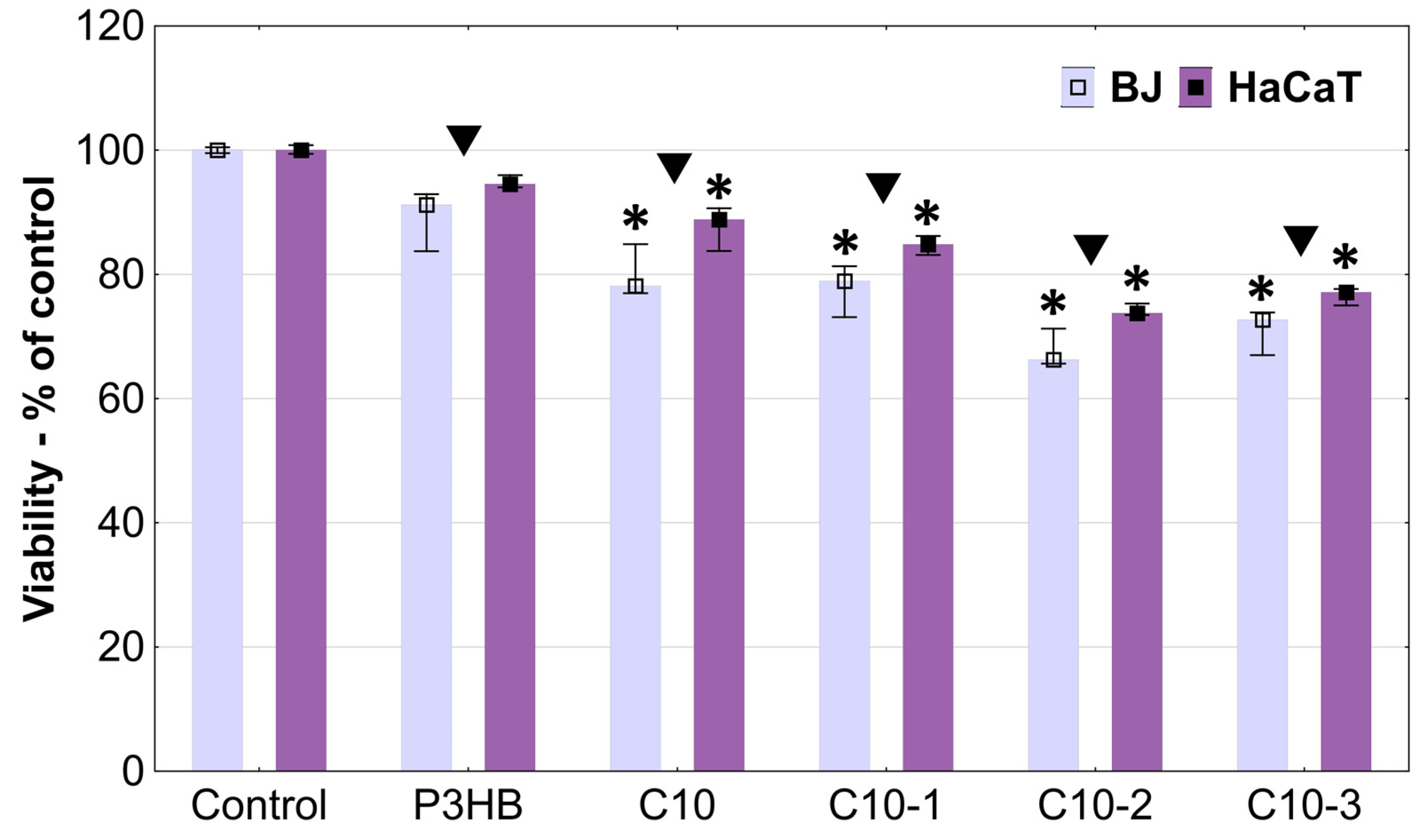
| Content (m/m%) | Sample Designation | ||
|---|---|---|---|
| P3HB | PU | Cloisite® | |
| 90 | 10 | 0 | C10 |
| 89 | 10 | 1 | C10-1 |
| 88 | 10 | 2 | C10-2 |
| 87 | 10 | 3 | C10-3 |
| Sample | Ton (°C) | T5% (°C) | T10% (°C) | T50% (°C) | Tmax (°C) | Residue at 600 °C (%) |
|---|---|---|---|---|---|---|
| P3HB | 221.1 | 236.2 | 245.6 | 281.2 | 295.7 | 1.70 |
| C10 | 252.1 | 268.8 | 295.1 | 295.6 | 294.5 | 2.82 |
| C10-1 | 252.2 | 272.8 | 292.5 | 293.3 | 293.7 | 1.38 |
| C10-2 | 254.8 | 273.5 | 295.2 | 284.5 | 295.2 | 1.97 |
| C10-3 | 252.3 | 272.3 | 294.8 | 283.8 | 293.3 | 2.59 |
| Sample | Tg1, (°C) | ΔCp (J·g−1·°C−1) | Tg2, (°C) | ΔCp (J·g−1·°C−1) | Tm1, (°C) | ΔHf1 (J·g−1) | Tcc, (°C) | ΔHcc (J·g−1) | Tm2, (°C) | Tm3, (°C) | ΔHf (J·g−1) | Tc, (°C) | ΔHc, (J·g−1) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P3HB | ----- | ----- | 5.5 | 0.148 | ----- | ----- | 89.9 | -4.76 | 157.5 | 167.8 | 97.4 | 85.7 | −78.6 |
| PU | −38.4 | 0.145 | ----- | ----- | 54.9 | 111.3 | ----- | ----- | ------ | ----- | ----- | 23.9 | −124.5 |
| C10 | −42.9 | 0.045 | −2.9 | 0.086 | 24.2 | 1.35 | 40.5 | −24.4 | 153.8 | 166.7 | 82.1 | 63.5 | −43.8 |
| C10-1 | −26.2 | 0.079 | ----- | ----- | 21.9 | 0.37 | 79.9 | −8.22 | 139.3 | 155.3 | 79.7 | 59.8 | −59.2 |
| C10-2 | −45.0 | 0.050 | ----- | ----- | 23.5 | 2.18 | 84.8 | −9.62 | 147.7 | 162.0 | 75.2 | 66.4 | −56.7 |
| C10-3 | −43.8 | 0.039 | ----- | ----- | 44.4 | 1.47 | 85.7 | −12.1 | 148.5 | 162.3 | 77.5 | 70.2 | −58.1 |
| Sample | Sample Mass (g) | TOD (mg/L) | Determined BOD (mg/L) | BOD of Sample (mg/L) | Dt—Degree of Biodegradability (%) |
|---|---|---|---|---|---|
| P3HB | 0.25 | 49.84 | 60.00 | 31.50 | 63.21 |
| C10 | 0.15 | 84.08 | 70.00 | 41.50 | 49.36 |
| C10-1 | 0.25 | 50.20 | 50.70 | 22.20 | 44.22 |
| C10-2 | 0.23 | 54.31 | 50.60 | 22.10 | 40.70 |
| C10-3 | 0.24 | 51.79 | 48.50 | 20.00 | 38.62 |
| Sample | C (%) | H (%) | O (%) Calculated | Si (%) Calculated | N (%) | |||
|---|---|---|---|---|---|---|---|---|
| Calculated | Determined | Calculated | Determined | Calculated | Determined | |||
| P3HB | 55.81 | 55.95 | 6.98 | 6.93 | 37.24 | 0.00 | 0.00 | 0.79 |
| C10 | 55.65 | 55.62 | 7.15 | 7.07 | 36.40 | 0.00 | 0.80 | 0.82 |
| C10-1 | 55.60 | 55.40 | 6.99 | 7.04 | 36.55 | 0.47 | 0.42 | 0.83 |
| C10-2 | 55.04 | 55.04 | 6.92 | 7.02 | 36.71 | 0.93 | 0.42 | 0.80 |
| C10-3 | 54.48 | 54.73 | 6.85 | 6.99 | 36.87 | 1.40 | 0.42 | 0.76 |
| Grade | Reactivity | Description of Reactivity Zone |
|---|---|---|
| 0 | None | No detectable zone around or under specimen |
| 1 | Slight | Some malformed or degenerated cells under specimen |
| 2 | Mild | Zone limited to area under specimen and less than 0.45 cm beyond specimen |
| 3 | Moderate | Zone extends 0.45–1.0 cm beyond specimen |
| 4 | Severe | Zone extends more than 1.0 cm beyond specimen |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krzykowska, B.; Uram, Ł.; Frącz, W.; Kovářová, M.; Sedlařík, V.; Hanusova, D.; Kisiel, M.; Paciorek-Sadowska, J.; Borowicz, M.; Zarzyka, I. Polymer Bionanocomposites Based on a P3BH/Polyurethane Matrix with Organomodified Montmorillonite—Mechanical and Thermal Properties, Biodegradability, and Cytotoxicity. Polymers 2024, 16, 2681. https://doi.org/10.3390/polym16182681
Krzykowska B, Uram Ł, Frącz W, Kovářová M, Sedlařík V, Hanusova D, Kisiel M, Paciorek-Sadowska J, Borowicz M, Zarzyka I. Polymer Bionanocomposites Based on a P3BH/Polyurethane Matrix with Organomodified Montmorillonite—Mechanical and Thermal Properties, Biodegradability, and Cytotoxicity. Polymers. 2024; 16(18):2681. https://doi.org/10.3390/polym16182681
Chicago/Turabian StyleKrzykowska, Beata, Łukasz Uram, Wiesław Frącz, Miroslava Kovářová, Vladimir Sedlařík, Dominika Hanusova, Maciej Kisiel, Joanna Paciorek-Sadowska, Marcin Borowicz, and Iwona Zarzyka. 2024. "Polymer Bionanocomposites Based on a P3BH/Polyurethane Matrix with Organomodified Montmorillonite—Mechanical and Thermal Properties, Biodegradability, and Cytotoxicity" Polymers 16, no. 18: 2681. https://doi.org/10.3390/polym16182681
APA StyleKrzykowska, B., Uram, Ł., Frącz, W., Kovářová, M., Sedlařík, V., Hanusova, D., Kisiel, M., Paciorek-Sadowska, J., Borowicz, M., & Zarzyka, I. (2024). Polymer Bionanocomposites Based on a P3BH/Polyurethane Matrix with Organomodified Montmorillonite—Mechanical and Thermal Properties, Biodegradability, and Cytotoxicity. Polymers, 16(18), 2681. https://doi.org/10.3390/polym16182681










