Status of Polymer Fused Deposition Modeling (FDM)-Based Three-Dimensional Printing (3DP) in the Pharmaceutical Industry
Abstract
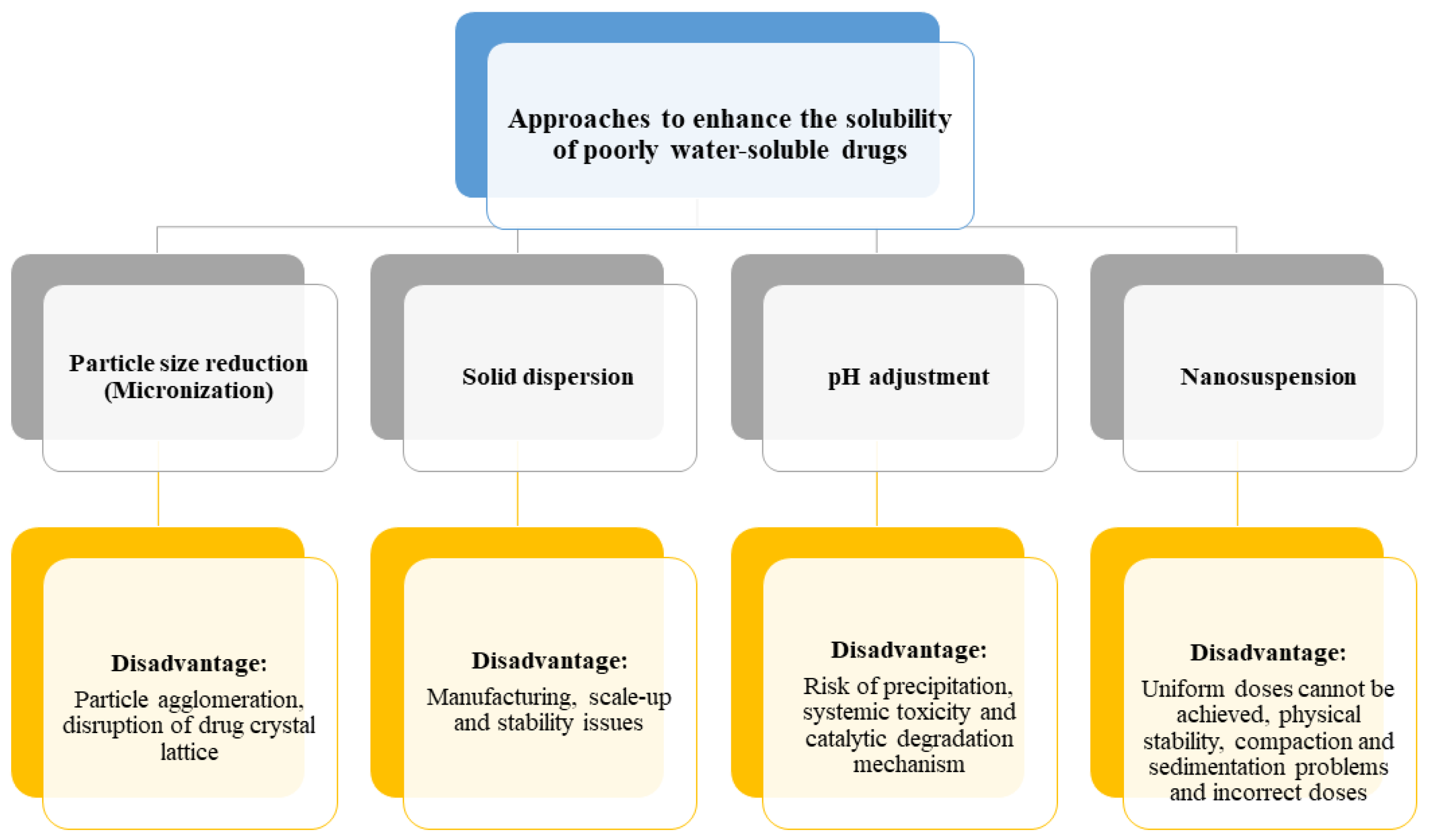
1. Introduction
2. Key Elements of Fused Deposition Modeling (FDM)
3. Polymers Utilized in FDM-Based 3DP
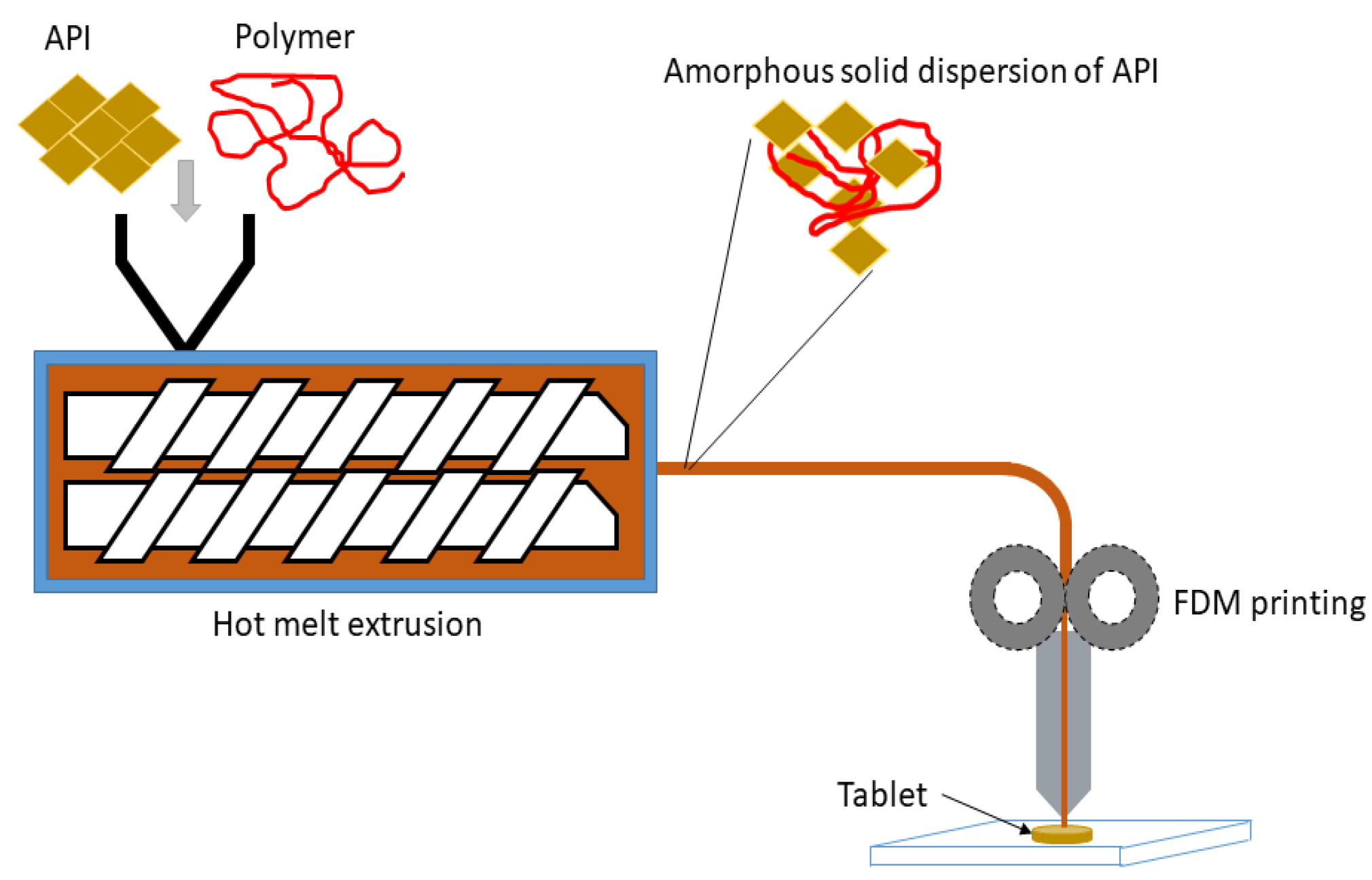
4. FDM 3D Printing and Amorphous Solid Dispersions (ASDs)
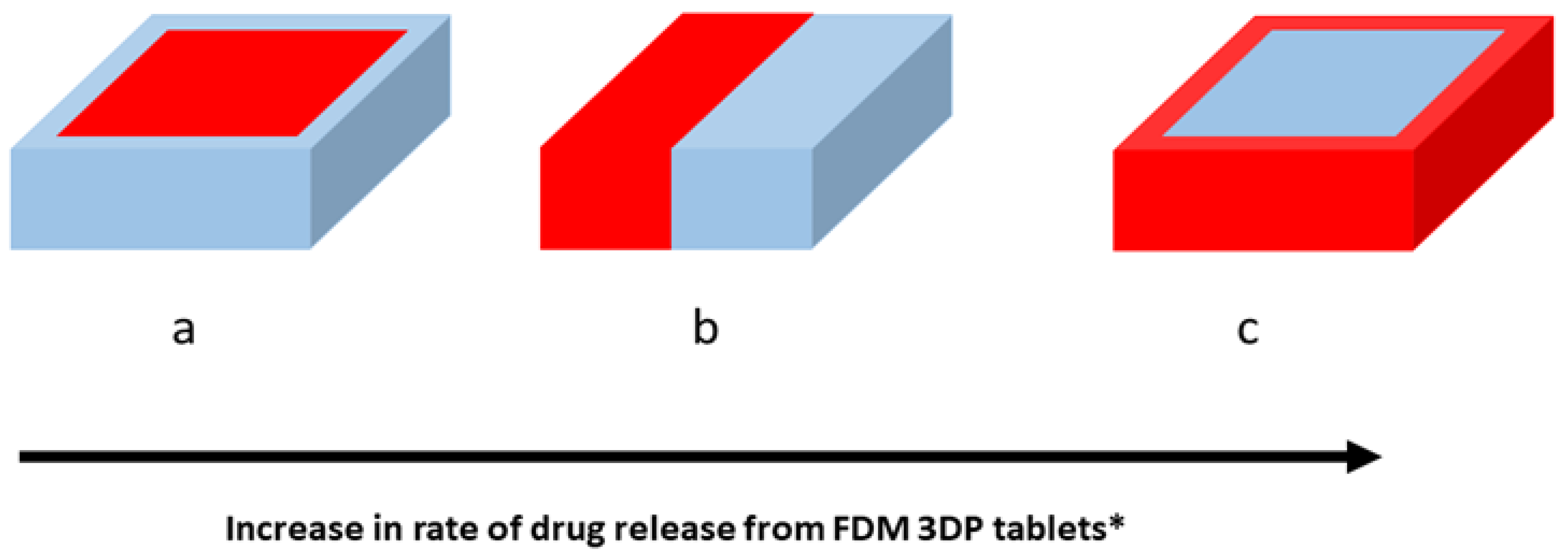
5. FDM-Printed Tablets for Targeted Gastrointestinal Drug Delivery
6. FDM-Based Bioprinting of Implant Transplantation Devices and Prosthetics
7. Challenges
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Mwema, F.M.; Akinlabi, E.T. Basics of Fused Deposition Modelling (FDM). In Fused Deposition Modeling; Springer Nature: Berlin/Heidelberg, Germany, 2020; pp. 1–15. [Google Scholar] [CrossRef]
- Gibson, I.; Rosen, D.; Stucker, B. Additive Manufacturing Technologies; Springer: New York, NY, USA, 2015. [Google Scholar] [CrossRef]
- Yan, Q.; Dong, H.; Su, J.; Han, J.; Song, B.; Wei, Q.; Shi, Y. A Review of 3D Printing Technology for Medical Applications. Engineering 2018, 4, 729–742. [Google Scholar] [CrossRef]
- Shahrubudin, N.; Lee, T.C.; Ramlan, R. An Overview on 3D Printing Technology: Technological, Materials, and Applications. Procedia Manuf. 2019, 35, 1286–1296. [Google Scholar] [CrossRef]
- Banks, J. Adding Value in Additive Manufacturing: Researchers in the United Kingdom and Europe Look to 3D Printing for Customization. IEEE Pulse 2013, 4, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Mertz, L. Dream It, Design It, Print It in 3-D: What Can 3-D Printing Do for You? IEEE Pulse 2013, 4, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Ratri, M.C.; Choe, G.; Nam, M.; Cho, D.; Shin, K. Three-Dimensional, Printed Water-Filtration System for Economical, on-Site Arsenic Removal. PLoS ONE 2020, 15, e0231475. [Google Scholar] [CrossRef] [PubMed]
- Rankin, T.M.; Giovinco, N.A.; Cucher, D.J.; Watts, G.; Hurwitz, B.; Armstrong, D.G. Dimensional Printing Surgical Instruments: Are We There Yet? J. Surg. Res. 2014, 189, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Ursan, I.D.; Chiu, L.; Pierce, A. Three-Dimensional Drug Printing: A Structured Review. J. Am. Pharm. Assoc. 2013, 53, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Soh, S. Printing Tablets with Fully Customizable Release Profiles for Personalized Medicine. Adv. Mater. 2015, 27, 7847–7853. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Yu, J.; Wang, C.; Nguyen, N.; Walker, G.M.; Buse, J.B.; Gu, Z. Microneedles Integrated with Pancreatic Cells and Synthetic Glucose-Signal Amplifiers for Smart Insulin Delivery. Adv. Mater. 2016, 28, 3115–3121. [Google Scholar] [CrossRef] [PubMed]
- Vukicevic, M.; Mosadegh, B.; Min, J.K.; Little, S.H. Cardiac 3D Printing and its Future Directions. JACC Cardiovasc. Imaging 2017, 10, 171–184. [Google Scholar] [CrossRef]
- Thawani, J.; Randazzo, M.; Pisapia, J.; Singh, N. 3D printing in neurosurgery: A systematic review. Surg. Neurol. Int. 2016, 7, 801–809. [Google Scholar] [CrossRef]
- Crafts, T.D.; Ellsperman, S.E.; Wannemuehler, T.J.; Bellicchi, T.D.; Shipchandler, T.Z.; Mantravadi, A.V. Three-Dimensional Printing and Its Applications in Otorhinolaryngology–Head and Neck Surgery. Otolaryngol. Neck Surg. 2016, 156, 999–1010. [Google Scholar] [CrossRef] [PubMed]
- Guibert, N.; Mhanna, L.; Didier, A.; Moreno, B.; Leyx, P.; Plat, G.; Mazieres, J.; Hermant, C. Integration of 3D Printing and Additive Manufacturing in the Interventional Pulmonologist’s Toolbox. Respir. Med. 2018, 134, 139–142. [Google Scholar] [CrossRef]
- Williams, C.; James, A.; Chae, M.P.; Hunter-Smith, D.J. 3D Printing in Clinical Podiatry: A Pilot Study and Review. J. Foot Ankle Res. 2015, 8, O41. [Google Scholar] [CrossRef]
- Jeon, H.; Kang, K.; Park, S.; Kim, W.D.; Paik, S.S.; Lee, S.-H.; Jeong, J.; Choi, D. Generation of Multilayered 3D Structures of HepG2 Cells Using a Bio-printing Technique. Gut Liver 2017, 11, 121–128. [Google Scholar] [CrossRef]
- Su, S.; Moran, K.; Robar, J.L. Design and Production of 3D Printed Bolus for Electron Radiation Therapy. J. Appl. Clin. Med. Phys. 2014, 15, 194–211. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Xu, Y.; Kwok, P.C.L.; Kang, L. Pharmaceutical Applications of 3D Printing. Addit. Manuf. 2020, 34, 101209. [Google Scholar] [CrossRef]
- Mohammed, A.; Elshaer, A.; Sareh, P.; Elsayed, M.; Hassanin, H. Additive Manufacturing Technologies for Drug Delivery Applications. Int. J. Pharm. 2020, 580, 119245. [Google Scholar] [CrossRef]
- Reddy, C.V.; Balamuralidhara, V.; Venkatesh, M.; Kumar, T.P. First FDA Approved 3D Printed Drug Paved New Path for Increased Precision in Patient Care. Appl. Clin. Res. Clin. Trials Regul. Aff. 2020, 7, 93–103. [Google Scholar] [CrossRef]
- Wright, D.; Begent, D.; Crawford, H. Medication Management of Adults with Swallowing Difficulties. In Consensus Guideline on the Gedication Ganagement of Adults with Swallowing Difficulties; Mebendium Gr. Publication Ltd.: Buckinghamshire, UK, 2017. [Google Scholar]
- Pamudji, J.S.; Mauludin, R.; Nurhabibah. Influence of β-Cyclodextrin on Cefixime Stability in Liquid Suspension Dosage Form. Procedia Chem. 2014, 13, 119–127. [Google Scholar] [CrossRef]
- Everett, H. Triastek Receives FDA IND Clearance for 3D Printed Drug to Treat Rheumatoid Arthritis. Available online: https://3dprintingindustry.com/news/triastek-receives-fda-ind-clearance-for-3d-printed-drug-to-treat-rheumatoid-arthritis-184159/ (accessed on 22 January 2024).
- Sigfridsson, K.; Lundqvist, A.J.; Strimfors, M. Particle Size Reduction for Improvement of Oral Absorption of the Poorly Soluble Drug UG558 in Rats during Early Development. Drug Dev. Ind. Pharm. 2009, 35, 1479–1486. [Google Scholar] [CrossRef] [PubMed]
- Doke, V.V.; Khutle, N.M.; Sharma, M.; Gupta, K. Solubility Enhancement of Poorly Soluble Drug Ezetimibe by Developing Self Nano Emulsifying Drug Delivery System. Indian J. Sci. Technol. 2022, 15, 1504–1516. [Google Scholar] [CrossRef]
- Putra, O.D.; Umeda, D.; Fujita, E.; Haraguchi, T.; Uchida, T.; Yonemochi, E.; Uekusa, H. Solubility Improvement of Benexate through Salt Formation Using Artificial Sweetener. Pharmaceutics 2018, 10, 64. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, A.N.; Nayal, S. A Review: Hydrotropy a Solubility Enhancing Technique. Pharma Innov. J. 2019, 8, 1149–1153. [Google Scholar]
- Hart, M.L. Brief Overview of Various Approaches to Enhance Drug Solubility. J. Dev. Drugs 2013, 2, 1149–1153. [Google Scholar] [CrossRef]
- Dalvi, P.B.; Gerange, A.B.; Ingale, P.R. Solid dispersion: Strategy to enhance solubility. J. Drug Deliv. Ther. 2015, 5, 20–28. [Google Scholar] [CrossRef]
- Vemula, V.R.; Lagishetty, V.; Lingala, S. ChemInform Abstract: Solubility Enhancement Techniques. Int. J. Pharm. Sci. Rev. Res. 2010, 5, 41–51. [Google Scholar] [CrossRef]
- Pawar, S.S.; Dahifale, B.R.; Nagargoje, S.P.; Shendge, R.S. Nanosuspension Technologies for Delivery of Drugs. Nanosci. Nanotech. Res. 2017, 4, 59–66. [Google Scholar]
- Trenfield, S.J.; Januskaite, P.; Goyanes, A.; Wilsdon, D.; Rowland, M.; Gaisford, S.; Basit, A.W. Prediction of Solid-State Form of SLS 3D Printed Medicines Using NIR and Raman Spectroscopy. Pharmaceutics 2022, 14, 589. [Google Scholar] [CrossRef] [PubMed]
- Prasad, L.K.; Smyth, H. 3D Printing Technologies for Drug Delivery: A Review. Drug Dev. Ind. Pharm. 2015, 42, 1019–1031. [Google Scholar] [CrossRef] [PubMed]
- Kamran, M.; Saxena, A. A Comprehensive Study on 3D Printing Technology. MIT Int. J. Mech. Eng. 2016, 6, 63–69. [Google Scholar]
- Patil, H.; Tiwari, R.V.; Repka, M.A. Hot-Melt Extrusion: From Theory to Application in Pharmaceutical Formulation. AAPS PharmSciTech 2016, 17, 20–42. [Google Scholar] [CrossRef]
- Zema, L.; Melocchi, A.; Maroni, A.; Gazzaniga, A. Three-Dimensional Printing of Medicinal Products and the Challenge of Personalized Therapy. J. Pharm. Sci. 2017, 106, 1697–1705. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, R.; Thakkar, R.; Pillai, A.; Ashour, E.A.; Repka, M.A. Systematic Screening of Pharmaceutical Polymers for Hot Melt Extrusion Processing: A Comprehensive Review. Int. J. Pharm. 2020, 576, 118989. [Google Scholar] [CrossRef] [PubMed]
- Melocchi, A.; Briatico-Vangosa, F.; Uboldi, M.; Parietti, F.; Turchi, M.; von Zeppelin, D.; Maroni, A.; Zema, L.; Gazzaniga, A.; Zidan, A. Quality Considerations on the Pharmaceutical Applications of Fused Deposition Modeling 3D Printing. Int. J. Pharm. 2020, 592, 119901. [Google Scholar] [CrossRef] [PubMed]
- Skowyra, J.; Pietrzak, K.; Alhnan, M.A. Fabrication of Extended-Release Patient-Tailored Prednisolone Tablets via Fused Deposition Modelling (FDM) 3D Printing. Eur. J. Pharm. Sci. 2015, 68, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Pietrzak, K.; Isreb, A.; Alhnan, M.A. A Flexible-Dose Dispenser for Immediate and Extended Release 3D Printed Tablets. Eur. J. Pharm. Biopharm. 2015, 96, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Friel, R.J. Power Ultrasonics for Additive Manufacturing and Consolidating of Materials. In Power Ultrasonics; Elsevier: Amsterdam, The Netherlands, 2015; pp. 313–335. [Google Scholar] [CrossRef]
- Praveena, B.A.; Lokesh, N.; Buradi, A.; Santhosh, N.; Praveena, B.L.; Vignesh, R. Comprehensive Review of Emerging Additive Manufacturing (3D Printing Technology): Methods, Materials, Applications, Challenges, Trends and Future Potential. Mater. Today Proc. 2021, 52, 1309–1313. [Google Scholar] [CrossRef]
- Zarek, M.; Layani, M.; Cooperstein, I.; Sachyani, E.; Cohn, D.; Magdassi, S. 3D Printing of Shape Memory Polymers for Flexible Electronic Devices. Adv. Mater. 2015, 28, 4449–4454. [Google Scholar] [CrossRef] [PubMed]
- Kristiawan, R.B.; Imaduddin, F.; Ariawan, D.; Ubaidillah; Arifin, Z. A Review on the Fused Deposition Modeling (FDM) 3D Printing: Filament Processing, Materials, and Printing Parameters. Open Eng. 2021, 11, 639–649. [Google Scholar] [CrossRef]
- Maniruzzaman, M.; Boateng, J.S.; Snowden, M.J.; Douroumis, D. A Review of Hot-Melt Extrusion: Process Technology to Pharmaceutical Products. ISRN Pharm. 2012, 2012, 436763. [Google Scholar] [CrossRef]
- Thanawuth, K.; Sutthapitaksakul, L.; Konthong, S.; Suttiruengwong, S.; Huanbutta, K.; Dass, C.R.; Sriamornsak, P. Impact of Drug Loading Method on Drug Release from 3D-Printed Tablets Made from Filaments Fabricated by Hot-Melt Extrusion and Impregnation Processes. Pharmaceutics 2021, 13, 1607. [Google Scholar] [CrossRef]
- Long, J.; Gholizadeh, H.; Lu, J.; Bunt, C.; Seyfoddin, A. Application of Fused Deposition Modelling (FDM) Method of 3D Printing in Drug Delivery. Curr. Pharm. Des. 2017, 23, 433–439. [Google Scholar] [CrossRef]
- Chai, X.; Chai, H.; Wang, X.; Yang, J.; Li, J.; Zhao, Y.; Cai, W.; Tao, T.; Xiang, X. Fused Deposition Modeling (FDM) 3D Printed Tablets for Intragastric Floating Delivery of Domperidone. Sci. Rep. 2017, 7, 2829. [Google Scholar] [CrossRef]
- Ponsar, H.; Wiedey, R.; Quodbach, J. Hot-Melt Extrusion Process Fluctuations and Their Impact on Critical Quality Attributes of Filaments and 3D-Printed Dosage Forms. Pharmaceutics 2020, 12, 511. [Google Scholar] [CrossRef]
- Venâncio, N.; Pereira, G.G.; Pinto, J.F.; Fernandes, A.I. Influence of the Infill Geometry of 3D-Printed Tablets on Drug Dissolution. In Proceedings of the CiiEM 2021, Bucharest, Romania, 14–15 October 2021; p. 15. [Google Scholar]
- Gallas, M.; Boulet, P.; de Margerie, V. Extrusion for pharma applications: An update. SPE Polym. 2023, 4, 16–23. [Google Scholar] [CrossRef]
- Gadgeppa, B.O.; Tukaram, M.S.; Baburo, G.; Sidram, G.P.; Gaurav, A.; Agarwal, S.; Jyoti, G.; Singal, R. Formulation and Evaluation of 3D Printed Pregabalin Tablets Targeted for Neuropathic Pain by Qbd Approach for Personalized Medicine. Int. J. Pharma Bio Sci. 2021, 11, P1–P13. [Google Scholar] [CrossRef]
- Kollamaram, G.; Croker, D.M.; Walker, G.M.; Goyanes, A.; Basit, A.W.; Gaisford, S. Low Temperature Fused Deposition Modeling (FDM) 3D Printing of Thermolabile Drugs. Int. J. Pharm. 2018, 545, 144–152. [Google Scholar] [CrossRef]
- Aguilar-De-Leyva, Á.; Linares, V.; Casas, M.; Caraballo, I. 3D Printed Drug Delivery Systems Based on Natural Products. Pharmaceutics 2020, 12, 620. [Google Scholar] [CrossRef] [PubMed]
- Eleftheriadis, G.K.; Katsiotis, C.S.; Andreadis, D.A.; Tzetzis, D.; Ritzoulis, C.; Bouropoulos, N.; Kanellopoulou, D.; Andriotis, E.G.; Tsibouklis, J.; Fatouros, D.G. Inkjet Printing of a Thermolabile Model Drug onto FDM-Printed Substrates: Formulation and Evaluation. Drug Dev. Ind. Pharm. 2020, 46, 1253–1264. [Google Scholar] [CrossRef] [PubMed]
- Alhijjaj, M.; Belton, P.; Qi, S. An investigation into the use of polymer blends to improve the printability of and regulate drug release from pharmaceutical solid dispersions prepared via fused deposition modeling (FDM) 3D printing. Eur. J. Pharm. Biopharm. 2016, 108, 111–125. [Google Scholar] [CrossRef]
- Mohapatra, S.; Kar, R.K.; Biswal, P.K.; Bindhani, S. Approaches of 3D Printing in Current Drug Delivery. Sens. Int. 2021, 3, 100146. [Google Scholar] [CrossRef]
- Paul, A.; Sreedevi, K.; Sharma, S.S.; Anjana, V.N. Polylactic Acid (PLA) BT—Handbook of Biopolymers; Thomas, S., Ajitha, A.R., Jose Chirayil, C., Thomas, B., Eds.; Springer Nature: Singapore, 2022; pp. 1–33. [Google Scholar] [CrossRef]
- Durand, B.; Marchand, C. Smart Features in Fibrous Implantable Medical Devices. In Smart Textiles and Their Applications; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar] [CrossRef]
- Melocchi, A.; Parietti, F.; Maroni, A.; Foppoli, A.; Gazzaniga, A.; Zema, L. Hot-Melt Extruded Filaments Based on Pharmaceutical Grade Polymers for 3D Printing by Fused Deposition Modeling. Int. J. Pharm. 2016, 509, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Rastpeiman, S.; Panahi, Z.; Akrami, M.; Haririan, I.; Asadi, M. Facile Fabrication of an Extended-Release Tablet of Ticagrelor Using Three Dimensional Printing Technology. J. Biomed. Mater. Res. Part A 2024, 112, 20–30. [Google Scholar] [CrossRef]
- Abdollahi, A.; Ansari, Z.; Akrami, M.; Haririan, I.; Dashti-Khavidaki, S.; Irani, M.; Kamankesh, M.; Ghobadi, E. Additive Manufacturing of an Extended-Release Tablet of Tacrolimus. Materials 2023, 16, 4927. [Google Scholar] [CrossRef]
- Asadi, M.; Salehi, Z.; Akrami, M.; Hosseinpour, M.; Jockenhövel, S.; Ghazanfari, S. 3D Printed PH-Responsive Tablets Containing N-Acetylglucosamine-Loaded Methylcellulose Hydrogel for Colon Drug Delivery Applications. Int. J. Pharm. 2023, 645, 123366. [Google Scholar] [CrossRef] [PubMed]
- Walden, D.M.; Bundey, Y.; Jagarapu, A.; Antontsev, V.; Chakravarty, K.; Varshney, J. Molecular Simulation and Statistical Learning Methods toward Predicting Drug–Polymer Amorphous Solid Dispersion Miscibility, Stability, and Formulation Design. Molecules 2021, 26, 182. [Google Scholar] [CrossRef]
- Chiou, W.L.; Riegelman, S. Pharmaceutical Applications of Solid Dispersion Systems. J. Pharm. Sci. 1971, 60, 1281–1302. [Google Scholar] [CrossRef]
- Parulski, C.; Gresse, E.; Jennotte, O.; Felten, A.; Ziemons, E.; Lechanteur, A.; Evrard, B. Fused Deposition Modeling 3D Printing of Solid Oral Dosage Forms Containing Amorphous Solid Dispersions: How to Elucidate Drug Dissolution Mechanisms through Surface Spectral Analysis Techniques? Int. J. Pharm. 2022, 626, 122157. [Google Scholar] [CrossRef]
- Kissi, E.O.; Nilsson, R.; Nogueira, L.P.; Larsson, A.; Tho, I. Influence of Drug Load on the Printability and Solid-State Properties of 3D-Printed Naproxen-Based Amorphous Solid Dispersion. Molecules 2021, 26, 4492. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.K.; Maniruzzaman, M.; Nokhodchi, A. Development and Optimisation of Novel Polymeric Compositions for Sustained Release Theophylline Caplets (PrintCap) via FDM 3D Printing. Polymers 2019, 12, 27. [Google Scholar] [CrossRef] [PubMed]
- Lasprilla, A.J.R.; Martinez, G.A.R.; Lunelli, B.H.; Jardini, A.L.; Filho, R.M. Poly-Lactic Acid Synthesis for Application in Biomedical Devices—A review. Biotechnol. Adv. 2012, 30, 321–328. [Google Scholar] [CrossRef]
- Da Silva, D.; Kaduri, M.; Poley, M.; Adir, O.; Krinsky, N.; Shainsky-Roitman, J.; Schroeder, A. Biocompatibility, Biodegradation and Excretion of Polylactic Acid (PLA) in Medical Implants and Theranostic Systems. Chem. Eng. J. 2018, 340, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Tagami, T.; Nagata, N.; Hayashi, N.; Ogawa, E.; Fukushige, K.; Sakai, N.; Ozeki, T. Defined Drug Release from 3D-Printed Composite Tablets Consisting of Drug-Loaded Polyvinylalcohol and a Water-Soluble or Water-Insoluble Polymer Filler. Int. J. Pharm. 2018, 543, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Jamróz, W.; Kurek, M.; Czech, A.; Szafraniec, J.; Gawlak, K.; Jachowicz, R. 3D Printing of Tablets Containing Amorphous Aripiprazole by Filaments Co-Extrusion. Eur. J. Pharm. Biopharm. 2018, 131, 44–47. [Google Scholar] [CrossRef] [PubMed]
- Fuenmayor, E.; Forde, M.; Healy, A.V.; Devine, D.M.; Lyons, J.G.; McConville, C.; Major, I. Material Considerations for Fused-Filament Fabrication of Solid Dosage Forms. Pharmaceutics 2018, 10, 44. [Google Scholar] [CrossRef] [PubMed]
- Kukkonen, J.; Ervasti, T.; Laitinen, R. Production and Characterization of Glibenclamide Incorporated PLA Filaments for 3D Printing by Fused Deposition Modeling. J. Drug Deliv. Sci. Technol. 2022, 77, 103843. [Google Scholar] [CrossRef]
- Goyanes, A.; Allahham, N.; Trenfield, S.J.; Stoyanov, E.; Gaisford, S.; Basit, A.W. Direct Powder Extrusion 3D Printing: Fabrication of Drug Products Using a Novel Single-Step Process. Int. J. Pharm. 2019, 567, 118471. [Google Scholar] [CrossRef]
- Gottschalk, N.; Bogdahn, M.; Quodbach, J. 3D Printing of Amorphous Solid Dispersions: A Comparison of Fused Deposition Modeling and Drop-on-Powder Printing. Int. J. Pharm. X 2023, 5, 100179. [Google Scholar] [CrossRef]
- Lee, V.H.; Yamamoto, A. Penetration and Enzymatic Barriers to Peptide and Protein Absorption. Adv. Drug Deliv. Rev. 1989, 4, 171–207. [Google Scholar] [CrossRef]
- Krishna, R.; Garg, A.; Jin, B.; Keshavarz, S.S.; Bieberdorf, F.A.; Chodakewitz, J.; Wagner, J.A. Assessment of a Pharmacokinetic and Pharmacodynamic Interaction between Simvastatin and Anacetrapib, a Potent Cholesteryl Ester Transfer Protein (CETP) Inhibitor, in Healthy Subjects. Br. J. Clin. Pharmacol. 2009, 67, 520–526. [Google Scholar] [CrossRef]
- Nikam, V.K.; Kotade, K.B.; Gaware, V.M.; Dolas, R.T.; Dhamak, K.; Somwanshi, S.; Khadse, A.; Kashid, V. Eudragit a Versatile Polymer: A Review. Pharmacologyonline 2011, 1, 152–164. [Google Scholar]
- Tambuwala, M.M.; Charbe, N.B.; McCarron, P.; Lane, M. Application of Three-Dimensional Printing for Colon Targeted Drug Delivery Systems. Int. J. Pharm. Investig. 2017, 7, 47–59. [Google Scholar] [CrossRef]
- Ali, H.; Kurokawa, S.; Shehab, E.; Mukhtarkhanov, M. Development of a Large-Scale Multi-Extrusion FDM Printer, and Its Challenges. Int. J. Lightweight Mater. Manuf. 2023, 6, 198–213. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, M.; Chohan, J.S. The Role of Additive Manufacturing for Biomedical Applications: A Critical Review. J. Manuf. Process. 2021, 64, 828–850. [Google Scholar] [CrossRef]
- Ventola, C.L. Medical Applications for 3D Printing: Current and Projected Uses. Pharm. Ther. 2014, 39, 704–711. [Google Scholar]
- Akmal, J.S.; Salmi, M.; Mäkitie, A.; Björkstrand, R.; Partanen, J. Implementation of Industrial Additive Manufacturing: Intelligent Implants and Drug Delivery Systems. J. Funct. Biomater. 2018, 9, 41. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Zheng, Q.; Sun, W.; Xu, H.; Yang, X. Levofloxacin Implants with Predefined Microstructure Fabricated by Three-Dimensional Printing Technique. Int. J. Pharm. 2007, 339, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Halliday, A.J.; Moulton, S.E.; Wallace, G.G.; Cook, M.J. Novel Methods of Antiepileptic Drug Delivery—Polymer-Based Implants. Adv. Drug Deliv. Rev. 2012, 64, 953–964. [Google Scholar] [CrossRef] [PubMed]
- Tack, P.; Victor, J.; Gemmel, P.; Annemans, L. 3D-Printing Techniques in a Medical Setting: A Systematic Literature Review. Biomed. Eng. Online 2016, 15, 115. [Google Scholar] [CrossRef] [PubMed]
- Wake, N.; Alexander, A.E.; Christensen, A.M.; Liacouras, P.C.; Schickel, M.; Pietila, T.; Matsumoto, J. Creating Patient-Specific Anatomical Models for 3D Printing and AR/VR: A Supplement for the 2018 Radiological Society of North America (RSNA) Hands-on Course. 3D Print. Med. 2019, 5, 17. [Google Scholar] [CrossRef] [PubMed]
- Pati, F.; Song, T.-H.; Rijal, G.; Jang, J.; Kim, S.W.; Cho, D.-W. Ornamenting 3D Ornamenting 3D Printed Scaffolds with Cell-Laid Extracellular Matrix for Bone Tissue Regeneration. Biomaterials 2015, 37, 230–241. [Google Scholar] [CrossRef]
- Tam, M.D.; Laycock, S.D.; Bell, D.G.; Chojnowski, A. 3-D Printout of a DICOM File to Aid Surgical Planning in a 6 Year Old Patient with a Large Scapular Osteochondroma Complicating Congenital Diaphyseal Aclasia. J. Radiol. Case Rep. 2012, 6, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Goyanes, A.; Det-Amornrat, U.; Wang, J.; Basit, A.W.; Gaisford, S. 3D Scanning and 3D Printing as Innovative Technologies for Fabricating Personalized Topical Drug Delivery Systems. J. Control. Release 2016, 234, 41–48. [Google Scholar] [CrossRef]
- Kwak, M.K.; Jeong, H.; Suh, K.Y. Rational Design and Enhanced Biocompatibility of a Dry Adhesive Medical Skin Patch. Adv. Mater. 2011, 23, 3949–3953. [Google Scholar] [CrossRef] [PubMed]
- Sodian, R.; Weber, S.; Markert, M.; Rassoulian, D.; Kaczmarek, I.; Lueth, T.C.; Reichart, B.; Daebritz, S. Stereolithographic Models for Surgical Planning in Congenital Heart Surgery. Ann. Thorac. Surg. 2007, 83, 1854–1857. [Google Scholar] [CrossRef]
- Kim, B.-S.; Mooney, D.J. Development of Biocompatible Synthetic Extracellular Matrices for Tissue Engineering. Trends Biotechnol. 1998, 16, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Bártolo, P.J.; Chua, C.K.; Almeida, H.A.; Chou, S.M.; Lim, A.S.C. Biomanufacturing for Tissue Engineering: Present and Future Trends. Virtual Phys. Prototyp. 2009, 4, 203–216. [Google Scholar] [CrossRef]
- Zadpoor, A.A. Design for Additive Bio-Manufacturing: From Patient-Specific Medical Devices to Rationally Designed Meta-Biomaterials. Int. J. Mol. Sci. 2017, 18, 1607. [Google Scholar] [CrossRef]
- Van Noort, R. The Future of Dental Devices Is Digital. Dent. Mater. 2012, 28, 3–12. [Google Scholar] [CrossRef]
- Gargiulo, P.; Árnadóttir, Í.; Gíslason, M.; Edmunds, K.; Ólafsson, I. New Directions in 3D Medical Modeling: 3D-Printing Anatomy and Functions in Neurosurgical Planning. J. Health Eng. 2017, 2017, 1439643. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Zhang, Q.; Li, X.; Zhao, C.; Quan, X.; Zhao, R.; Chen, Z.; Li, Y. Preliminary Application of a Multi-Level 3D Printing Drill Guide Template for Pedicle Screw Placement in Severe and Rigid Scoliosis. Eur. Spine J. 2017, 26, 1684–1689. [Google Scholar] [CrossRef]
- Oberoi, G.; Nitsch, S.; Edelmayer, M.; Janjić, K.; Müller, A.S.; Agis, H. 3D Printing—Encompassing the Facets of Dentistry. Front. Bioeng. Biotechnol. 2018, 6, 172. [Google Scholar] [CrossRef]
- Hao, W.; Liu, Y.; Zhou, H.; Chen, H.; Fang, D. Preparation and Characterization of 3D Printed Continuous Carbon Fiber Reinforced Thermosetting Composites. Polym. Test. 2018, 65, 29–34. [Google Scholar] [CrossRef]
- Wu, D.; Thames, J.L.; Rosen, D.W.; Schaefer, D. Enhancing the Product Realization Process with Cloud-Based Design and Manufacturing Systems. J. Comput. Inf. Sci. Eng. 2013, 13, 041004. [Google Scholar] [CrossRef]
- Wu, D.; Rosen, D.W.; Wang, L.; Schaefer, D. Cloud-Based Design and Manufacturing: A New Paradigm in Digital Manufacturing and Design Innovation. Comput. Des. 2015, 59, 1–14. [Google Scholar] [CrossRef]
- Akilbekova, D.; Mektepbayeva, D. 5—Patient Specific in Situ 3D Printing. In 3D Printing in Medicine; Kalaskar, D.M.B.T., Ed.; Woodhead Publishing: Sawston, UK, 2017; pp. 91–113. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, Z.; Li, X.; Chen, Y.; Zhang, S.; Du, M.; Li, J. Custom Prosthetic Reconstruction for Proximal Tibial Osteosarcoma with Proximal Tibiofibular Joint Involved. Surg. Oncol. 2008, 17, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.S.; Lightdale-Miric, N. Advances in 3D-Printed Pediatric Prostheses for Upper Extremity Differences. JBJS 2016, 98, 1320–1326. [Google Scholar] [CrossRef]
- Salmi, M. Additive Manufacturing Processes in Medical Applications. Materials 2021, 14, 191. [Google Scholar] [CrossRef]
- Rosicky, J.; Grygar, A.; Chapcak, P.; Bouma, T.; Rosicky, J. Application of 3D Scanning in Prosthetic and Orthotic Clinical Practice. In Proceedings of the 7th International Conference on 3D Body Scanning Technologies, Lugano, Switzerland, 30 November–1 December 2016; pp. 88–97. [Google Scholar]
- Bhushan, B.; Caspers, M. An Overview of Additive Manufacturing (3D Printing) for Microfabrication. Microsyst. Technol. 2017, 23, 1117–1124. [Google Scholar] [CrossRef]
- Weller, C.; Kleer, R.; Piller, F.T. Economic Implications of 3D Printing: Market Structure Models in Light of Additive Manufacturing Revisited. Int. J. Prod. Econ. 2015, 164, 43–56. [Google Scholar] [CrossRef]
- Kleer, R.; Piller, F.T. Modeling Benefits of Local Production by Users. In Academy of Management Proceedings; Academy of Management: Briarcliff Manor, NY, USA, 2013. [Google Scholar] [CrossRef]
- Brabazon, P.G.; MacCarthy, B.; Woodcock, A.; Hawkins, R.W. Mass Customization in the Automotive Industry: Comparing Interdealer Trading and Reconfiguration Flexibilities in Order Fulfillment. Prod. Oper. Manag. 2010, 19, 489–502. [Google Scholar] [CrossRef]
- Boschetto, A.; Bottini, L. Accuracy Prediction in Fused Deposition Modeling. Int. J. Adv. Manuf. Technol. 2014, 73, 913–928. [Google Scholar] [CrossRef]
- Goyanes, A.; Buanz, A.B.; Hatton, G.B.; Gaisford, S.; Basit, A.W. 3D Printing of Modified-Release Aminosalicylate (4-ASA and 5-ASA) Tablets. Eur. J. Pharm. Biopharm. 2015, 89, 157–162. [Google Scholar] [CrossRef]
- Griffey, J. The Types of 3-D Printing. Libr. Technol. Rep. 2014, 50, 8–12. [Google Scholar]
- Serajuddin, A. Challenges, Current Status and Emerging Strategies in the Development of Rapidly Dissolving FDM 3D-Printed Tablets: An Overview and Commentary. ADMET 2023, 11, 33–55. [Google Scholar] [CrossRef]
- Chung, S.; Srinivasan, P.; Zhang, P.; Bandari, S.; Repka, M.A. Development of Ibuprofen Tablet with Polyethylene Oxide Using Fused Deposition Modeling 3D-Printing Coupled with Hot-Melt Extrusion. J. Drug Deliv. Sci. Technol. 2022, 76, 103716. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iqbal, H.; Fernandes, Q.; Idoudi, S.; Basineni, R.; Billa, N. Status of Polymer Fused Deposition Modeling (FDM)-Based Three-Dimensional Printing (3DP) in the Pharmaceutical Industry. Polymers 2024, 16, 386. https://doi.org/10.3390/polym16030386
Iqbal H, Fernandes Q, Idoudi S, Basineni R, Billa N. Status of Polymer Fused Deposition Modeling (FDM)-Based Three-Dimensional Printing (3DP) in the Pharmaceutical Industry. Polymers. 2024; 16(3):386. https://doi.org/10.3390/polym16030386
Chicago/Turabian StyleIqbal, Heba, Queenie Fernandes, Sourour Idoudi, Renuka Basineni, and Nashiru Billa. 2024. "Status of Polymer Fused Deposition Modeling (FDM)-Based Three-Dimensional Printing (3DP) in the Pharmaceutical Industry" Polymers 16, no. 3: 386. https://doi.org/10.3390/polym16030386
APA StyleIqbal, H., Fernandes, Q., Idoudi, S., Basineni, R., & Billa, N. (2024). Status of Polymer Fused Deposition Modeling (FDM)-Based Three-Dimensional Printing (3DP) in the Pharmaceutical Industry. Polymers, 16(3), 386. https://doi.org/10.3390/polym16030386







