Abstract
FVPT1, a novel heteropolysaccharide, was purified from the fruiting body of Flammulina velutipes using magnetic-field-assisted three-phase partitioning and gel permeation chromatography. The structure was characterized using monosaccharide composition and methylation analysis, infrared spectroscopy and nuclear magnetic resonance (NMR). The FVPT1 (~1.64 × 104 Da) was composed of L-fucose, D-galactose, D-glucose and D-mannose at a molar ratio of 1.0:3.5:1.0:1.4. The polysaccharide repeating unit of FVPT1 was established with methylation analyses and NMR spectroscopy. Moreover, a zebrafish larva hyperlipidemia model test demonstrated that FVPT1 can show appreciable lipid-lowering effects. In addition, the FVPT1 exhibited remarkable immunoregulatory activity by increasing nitric oxide, interleukin (IL)-1β and IL-1 secretion in macrophages. Therefore, these results suggest that FVPT1 has the potential to be developed into a new immune or hypolipidemic health product.
1. Introduction
Flammulina velutipes, also known as the golden needle mushroom, ranks fourth in the world in terms of the production and consumption of edible mushrooms [1]. F. velutipes is rich in carbohydrates, protein and vitamins, which makes it popular among consumers in Asia, especially China and Japan. The polysaccharides, glycoproteins, phenols and sesquiterpenes isolated from F. velutipes have multiple pharmacological activities, such as antitumor, anti-inflammatory, antioxidant, hypolipidemic, immunity-regulation and other health-promotion effects [2,3,4,5,6,7,8]. F. velutipes’ polysaccharides, as some of its most important active components, have been studied for many years [9,10]. The polysaccharides extracted from F. velutipes using different methods have had remarkable therapeutic effects and undetected toxicity, which has attracted widespread attention [11,12]. Many studies have shown that different extraction methods can affect extraction yield, structural characteristics and biological activity. As described by Guo et al. [13], microwave-assisted extraction of polysaccharides has more obvious antioxidant activity than do hot-water and ultrasound-assisted extraction. Another study showed that the microwave extraction of Cordyceps gunnii mycelia polysaccharides demonstrated the highest yield and antitumor activity, as well as the largest macromolecular polysaccharide ratio [14]. At present, a variety of extraction methods of F. velutipes polysaccharides, mainly including hot-water and microwave-assisted, ultrasonic-assisted and enzyme-assisted extraction, have been widely used in research. For example, Zhang et al. [15] found that the maximum extraction yield of F. velutipes polysaccharides arrived at 8.33% with 620 W for 20 min at 45 °C. Although traditional extraction methods are relatively simple, their shortcomings, such as time-consuming treatment and complicated separation and purification steps, subsequently cannot be ignored. As a new method of polysaccharide extraction, three-phase partitioning (TPP) has become more and more widely used due to its high efficiency and semipurification characteristics.
As a normal method of separation and purification of proteins and oils, three-phase partitioning technology (TPP) is a new, safe and green technology including salting out, isoelectric point precipitation and solvent precipitation using t-butanol and ammonium sulfate solution successively to obtain the upper organic phase, the intermediate protein phase of the interfacial precipitate and the lower aqueous phase [16,17,18]. Currently, TPP has also been widely applied as a more efficient and fast extraction technology to extract polysaccharides such as those of Corbicula fluminea, xylanase isolated from Bacillus oceanisediminis strain SJ3 and those of aloe [19,20,21]. In addition, the magnetic-field-enhanced extraction method called “green separation technology” uses its field to enhance the chemical separation process. It can use the special energy generated by the magnetic field to change the properties of diamagnetic substances by changing their microstructure. In a research paper about magnetic-field-assisted solvent extraction, published by Palyska [22], D-2EHPA (di-2-ethylhexyl phosphoric acid) was used to extract Cu2+, and the distribution ratio of copper increased 160 times after the magnetization of the extractant. Another article found that a variable magnetic-field-assisted extraction technique made it possible to extract more mineral components, caffeine and polyphenols from dry black and green tea at a frequency of 50 Hz compared to conventional extraction methods [23]. Therefore, magnetic-field-assisted three-phase partitioning was used to separate F. velutipes polysaccharides in this study in hopes of obtaining new F. velutipes polysaccharides with higher yields and purity.
In this study, the structure of F. velutipes polysaccharide FVPTI, extracted using magnetic-field-assisted TPP and gel permeation chromatography, was analyzed using monosaccharide composition and methylation analyses, as well as NMR. In addition, the lipid-lowering and immunological activities of FVPT1 are also discussed. This study will provide a theoretical basis for the future large-scale industrial production of this polysaccharide as well as active polysaccharide materials for the pharmaceutical, the food and other industries.
2. Materials and Methods
2.1. Materials and Chemicals
Fresh F. velutipes fruiting bodies were collected from Jiangsu Chinagreen Biotechnology Co., Ltd. (Suqian, China). Standard monosaccharides were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). Zebrafish larva AB strains (Nanjing YSY Biotech Company Ltd. (Nanjing, China)), egg yolk powder (Shangdong Xitang Company (Jinan, China)), and an ELISA kit for cytokine assays (Becton, Dickinson and Company (New York, NY, USA)) were also used.
2.2. Isolation and Purification of F. velutipes Polysaccharides Using Magnetic-Field-Assisted Three-Phase Partitioning
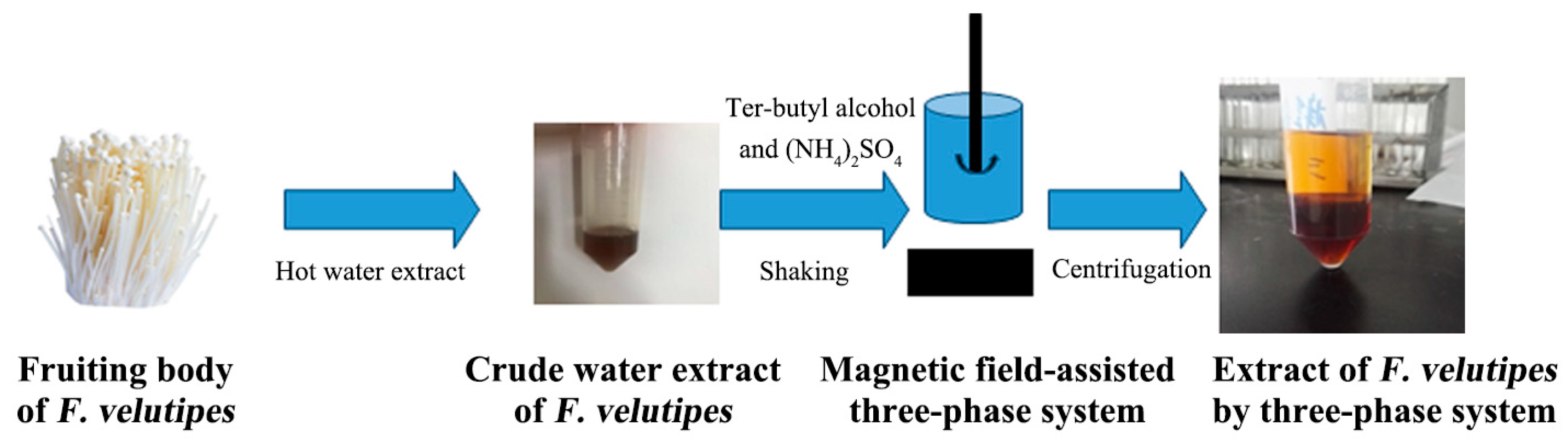
The fruiting body of F. velutipes was washed, dried, crushed and sieved at 100 mesh. The powder of the F. velutipes was suspended in 500 mL of 95% ethanol and extracted for 1 h with an SB25-12D ultrasonic generator (Ningbo Scientz Biotechnology Co., Ltd. (Ningbo, China))at a 40 kHz frequency three times and hot water extraction three times, each time for 2 h. Certain amounts of solid ammonium sulfate (mass fraction 10–60%) and tert-butyl alcohol (5–30 mL) were added to the water extract at room temperature. The extraction tube was placed under a magnetic field, stirred at a certain rotation speed of 100 rpm for 30 min and then used to form three clear phases at 2500 rpm for 5 min. The lower aqueous phase was dialyzed, concentrated and dried to obtain purified extracted F. velutipes polysaccharides (Figure 1).
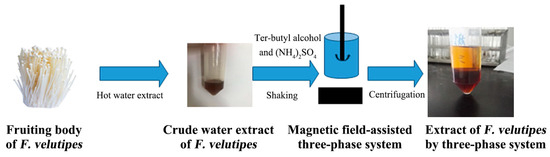
Figure 1.
Extraction and separation steps for polysaccharides from F. velutipes using the three-phase partitioning (TPP) system.
Further purification used gel permeation chromatography (GPC) (high-resolution Sephacryl S-300 column) (XK16 × 100 cm) (GE Healthcare (Cardiff, UK)), and filtered, distilled water was used as the eluent. Two peaks were detected using a refractive index detector (RID-10A, Shimadzu Corporation (Kyoto, Japan)), and the first peak was collected and designated as FVPT1.
2.3. Monosaccharide Composition Analysis
The sample (2 mg) was hydrolyzed completely at 110 °C using 2 M TFA (Trifluoroacetic acid) for 4 h. High-performance anion exchange chromatography (HPAEC) equipped with a CarboPac Dionex LC30 ™ PA20 column (3 mm × 150 mm) (Thermo Fisher Scientific Inc. (Waltham, MA, USA)), eluted with 2 mM of NaOH and 0.05–0.2 M NaAc (0.45 mL/min), was used to detect sugar composition with a pulse amperometric detector (Dionex), using monosaccharides as standards.
2.4. Determination of Purity and Molecular Weight
The purity and molecular weight of the sample was analyzed with high-performance liquid size exclusion chromatography (HPSEC) equipped with a refractive index detector (RI) and TSK PWXL 6000 and 3000 gel filtration columns; eluted with a PB buffer at 0.5 mL/min; and calibrated with pullulan standards of P5 (6200 Da), P10 (10,000 Da), P20 (21,700 Da), P100 (113,000 Da) and P200 (200,000 Da) (Shodex, Tokyo, Japan) at 35 °C.
2.5. Fourier-Transform Infrared (FTIR) Spectroscopy
The FVPT1 was ground with KBr powder and made into KBr discs after being mixed evenly for transformation infrared spectra analysis in a Perkin–Elmer 599B FTIR spectrophotometer (Waltham, MA, USA) in the wavenumber region of 4000–400 cm−1 at a 4 cm−1 resolution, with 32 sample scans [24].
2.6. Methylation Analysis
Methylation analysis of the FVPT1 (2 mg) was conducted according to a previous study [25]. The methylated polysaccharide was then converted into partially methylated alditol acetates (PMAAs) using hydrolysis, reduction with sodium borodeuteride and acetylation. Glycosidic linkage analysis was performed via a GC-MS system (Thermo Finnigan TRACE 2000/MS) (Thermo Fisher Scientific Inc. (Waltham, MA, USA)) equipped with a DB-5 MS column (30 m × 0.25 mm, 0.25 µm and 0.2 mm film thicknesses). The temperature was programmed from 180 to 270 °C at 20 °C/min and held at 270 °C for 25 min [26]. The individual peaks of the PMAA and fragmentation patterns were identified using their mass spectra and relative retention times in the NIST 2011 database of GC-MS. Percentages of methylated sugars were estimated as ratios of the peak areas.
2.7. Nuclear Magnetic Resonance (NMR) Analysis
The FVPT1 was dissolved with D2O and lyophilized in a vacuum freeze dryer to facilitate deuterium exchange. The deuterium-exchanged FVPT1 (40 mg) was dissolved in 0.5 mL of 99.96% D2O for NMR. 1H,13C, Nuclear Overhauser Effect Spectroscopy (NOESY), heteronuclear multiple quantum coherence (HMQC) and 1H-detected heteronuclear multiple-bond correlation (HMBC) NMR spectra were recorded at 27 °C on a Bruker Avance III 600 MHz NMR spectrometer (Bruker Corporation (Billerica, MA, USA)). 1H chemical shifts were referenced to residual HDO, with δ 4.78 ppm (27 °C) as the internal standard. 13C chemical shifts were determined in relation to DSS (δ 0.00 ppm) calibrated externally. The 1H-1H-correlated spectroscopy (COSY) and HMQC were used to assign signals. HMBC and NOESY were used to assign inter-residue linkages and sequences.
2.8. Determination of NO from Macrophages
RAW264.7 mouse macrophages in the logarithmic growth phase were diluted into 2 × 105 cells/well with the colorless medium DMEM, and the number of cells per well was as similar as possible. After 4 h in the incubator, samples were added to the cells, followed by observation of the adherence of the cells under the microscope and discarding of the supernatant. The 5 mg/mL F. velutipes polysaccharide solution was diluted to 100, 200 and 500 μg/mL with RPMI-1640. Then, 96-well plates were added to 200 μL of each well. The negative and positive control media, containing 5% PBSs and 10 μg/mL of LPSs (lipopolysaccharides), respectively, were set up with six replicates each. After 48 h of incubation at a constant temperature with a 5% carbon dioxide incubator atmosphere, 100 μL of supernatant was taken and detected with a Griess experiment to determine NO levels, as has been described previously [27].
2.9. Detection of Cytokine Secretion in Macrophages Using ELISA
IL-1 and IL-1β released by macrophages were detected with an ELISA kit. The macrophage suspension was diluted to 2 × 106 cells/mL and cultured in a 96-well plate with an FVPT1 solution at a concentration of 200 μg/mL in each well. RPMI-1640 media containing 0.5% PBS and 10 μg/mL of LPS were used as negative and positive controls, respectively, followed by the setup of three replicates. The levels of IL-1 and IL-1β in the supernatant were determined using ELISA, with reference to the operation steps of the kit instructions after culturing for 4, 8 and 10 days at 37 °C and 5% CO2, respectively.
2.10. Lipid-Lowering Activity of FVPT1
2.10.1. Zebrafish Larva Hyperlipidemia Model
The embryos of zebrafish of the AB strain were obtained from Nanjing YSY Biotech Company Ltd. (Nanjing, China) and incubated in clean fish water with oxygen for 48 h at 28 °C. The model of hyperlipidemia was established by giving yolk powder as a high-cholesterol diet (HCD) feed to the zebrafish larvae for six days [28]. The zebrafish larvae were divided into a control group, a high-cholesterol group and a sample group (100, 200 and 400 μg/mL of FVPT1, respectively) at a density of 10 zebrafish per group, placed in 12-well plates containing 2 mL of fish water per hole for treatment. In the high-cholesterol group, the yolk powder was used as HCD feed for the zebrafish larvae, with a final concentration of 0.1%, while the control group was not fed. From the 12th hour of feeding the zebrafish larvae with the high-cholesterol egg yolk diet, the lipids in the larvae began to accumulate rapidly. Soaking zebrafish larvae with egg yolk powder for 48 h can induce a large amount of lipid accumulation in the larvae and establish a hyperlipidemia model. In the high-cholesterol polysaccharide group, the polysaccharide and egg yolk powder were added into the fish water to soak the zebrafish larvae for 48 h. The lipid accumulation in the larvae was observed using oil red staining.
2.10.2. Lipid Oil Red Staining in Zebrafish Larvae
An oil-red-O dye solution was configured according to instructions and slowly filtered with qualitative filter paper. The prepared solution was used up within 2 h. The zebrafish larvae were immobilized with polyformaldehyde (4%) for 1 h and then washed with PBS 2–3 times, followed by dehydration with methanol solvent in gradients of 25%, 50%, 75% and 100%, successively. After oil-red-O dyeing for 2 h, 100%, 75%, 50% and 25% gradient methanol solvent were used for gradient elution, followed by washing with PBS. Finally, fluorescence microscopy was used to observe and take photos.
2.10.3. Intensity Quantification
After the oil-red-O staining, lipid accumulation could be clearly observed in the blood vessels and stomachs of the zebrafish larvae. Image-Pro Plus (IPP) analysis software (version 6.0) was used to determine the integrated optical density (IOD) value of the dyed lipids in the zebrafish, to quantitatively evaluate the level of lipid accumulation in vivo and then to analyze the inhibitory activity of the polysaccharide samples on the lipid accumulation in the larvae [29]. The normalized degree of lipid accumulation in vivo (%) = (IODpolysaccharide group − IODcontrol group)/(IODhyperlipidemia group − IODcontrol group).
2.11. Statistical Analysis
All experiments were performed in triplicate, and the data are expressed as means ± standard deviations (SDs). One-way analysis of variance (ANOVA) and the LSD test were used for intergroup comparisons. All variables were given Levene’s test for normality and homogeneous variance. If necessary, Tamhane’s T2 test was performed. p < 0.05 and p < 0.01 were significant and very significant, respectively.
3. Results and Discussion
3.1. Purification Results of FVPT1
The yield, polysaccharide content and protein content of the F. velutipes polysaccharide obtained using the magnetic-field-assisted three-phase partitioning were 2.3%, 55.53% and 12.19%, respectively. The polysaccharide content of the FVPT1 obtained after purification with gel-permeation chromatography reached 90.1%, and the moisture content was 2.91%.
3.2. Composition and Structural Characterization of FVPT1
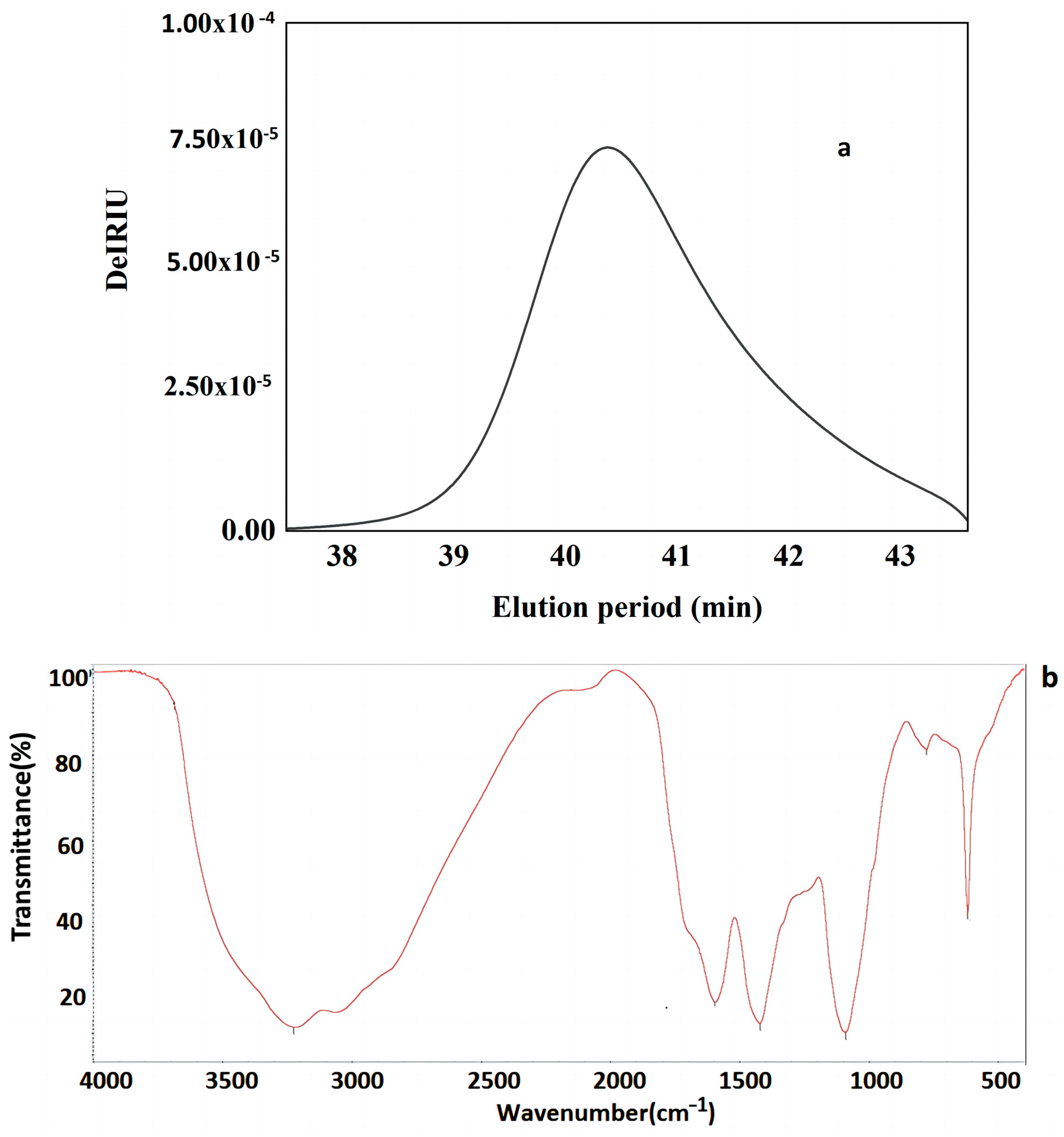
The total polysaccharide content and protein content of the FVPT1 were 90.1% and 5.20%, respectively. A single symmetrical peak (Figure 2a) in the HPSEC profile indicated that FVPT1 is a homogeneous polysaccharide, with ~1.64 × 104 Da and galactose (Gal), mannose (Man), fucose (Fuc) and glucose (Glc) at a molar ratio of 3.5:1.4:1:1. In previous studies, FVPA1, FVPA2 and FVPB2 were obtained from F. velutipes using traditional extraction, separation and purification methods [8,10,27]. The three polysaccharides had different molecular weights and did not contain proteins. The monosaccharide composition of FVPA2 did not contain glucose.
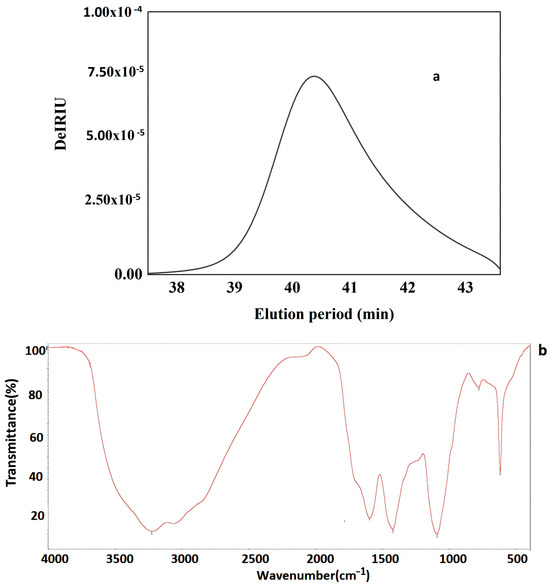
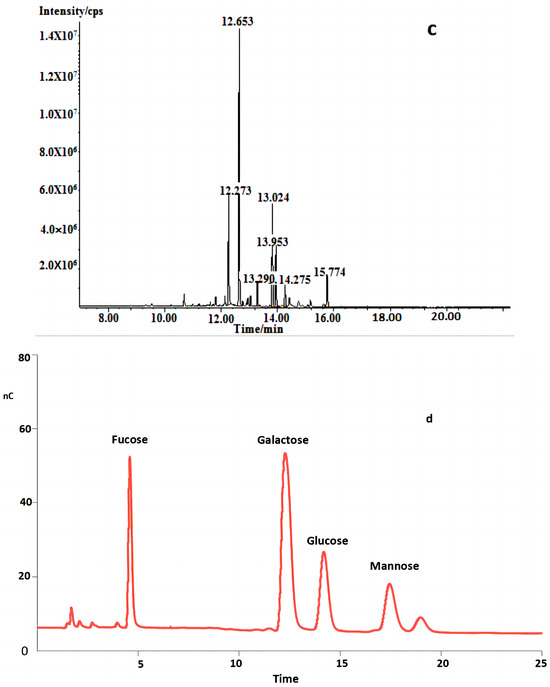
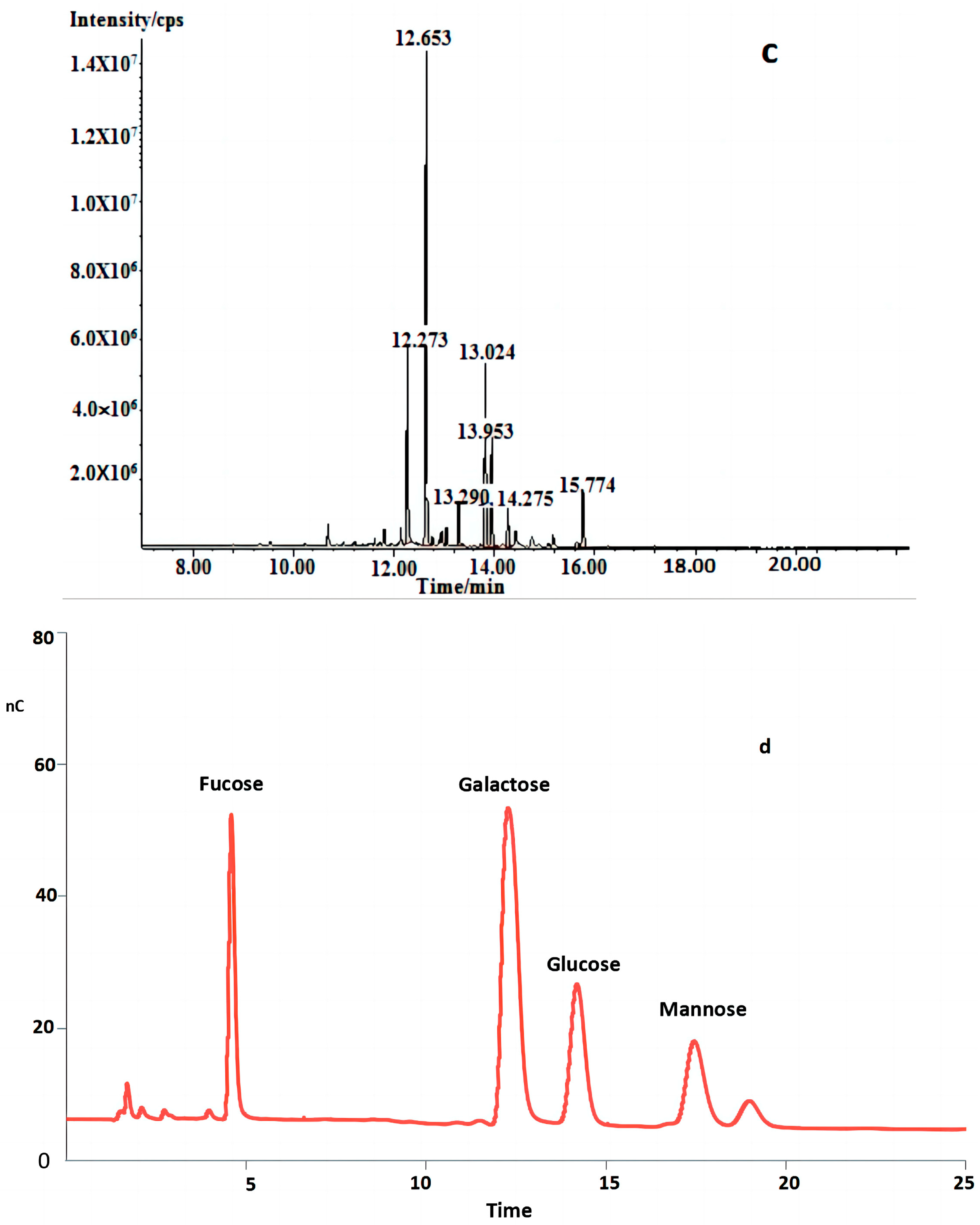
Figure 2.
(a) HPLC profiles of FVPT1 (black line represents FVPT1). (b) FT-IR spectrum of FVPT1. (c) Total ion spectrum of FVPT1. (d) Spectrum of HPAEC of FVPT1.
Figure 2b shows that the FVPT1 had three major absorption peaks at 3416, 1651 and 1453 cm−1. The 3416 cm−1 peak indicates the polysaccharide’s hydroxyl stretching vibration. The strong absorption peak at 1615 cm−1 and the weak peak at 1453 cm−1 illustrate the characteristics of the polysaccharide. No uronic acids were present because of the absent peaks at 1730 cm−1 [30].
In Table 1 and Figure 2c,d, the GC-MS data indicate that the FVPT1 had a branched structure, including mannopyranose residue and glucopyranose residue, at the terminals; a main chain structure including 1,2-di-substituted fucose, 1,4,6-tri-substituted galactose, 1,3,6-tri-substituted galactose, 1,2-di-substituted fucose, 1,4-di-substituted galactose and 1,3-di-substituted glucose; and a fucose residue at the other terminal.

Table 1.
Methylation analysis data for FVPT1.
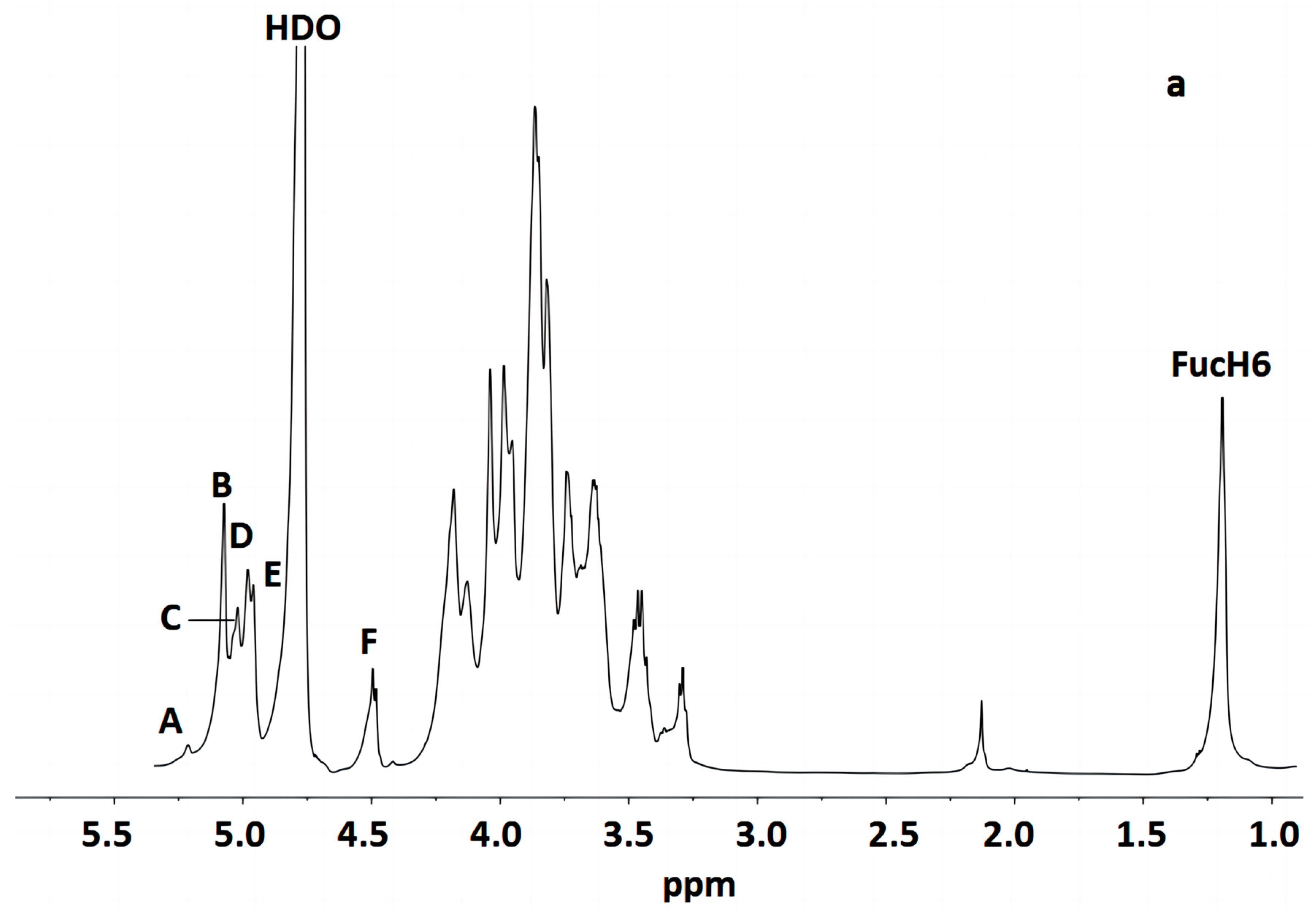
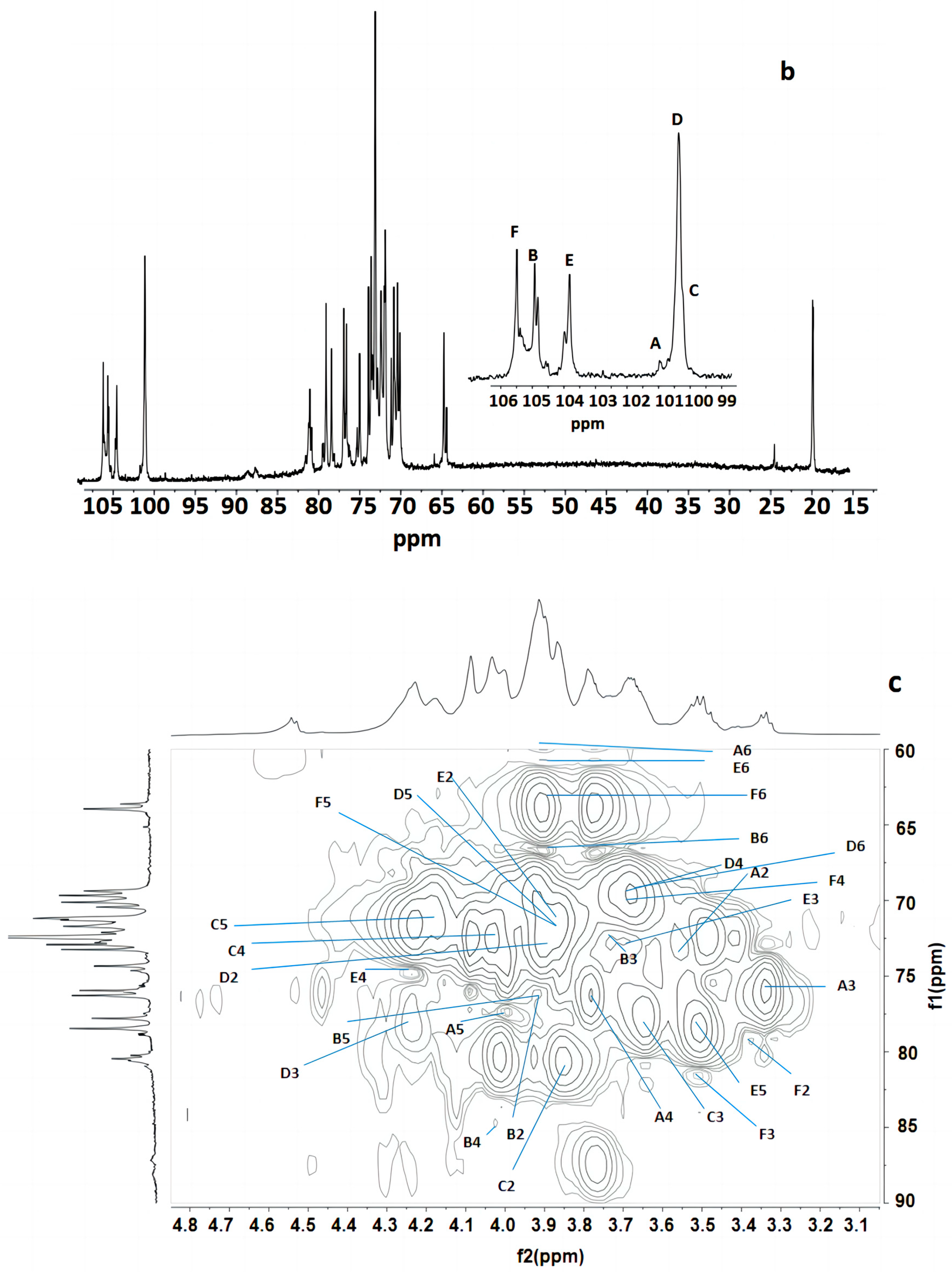
Figure 3 indicates that the polysaccharide structure of the FVPT1 included six sugar residues, named A–F, at δ 5.26 (single broad peak (br.s)), δ 5.12 (br.s), δ 5.07 (br.s), δ 5.03 (br.s), 5.01 (br.s) and δ 4.55 (double peaks (d), JH-1, H-2 = 6 Hz), respectively, corresponding to the signals at δ 101.55, 105.29, 100.73, 100.81, 104.20 and 105.87, respectively. A special signal at 1.24 (JH-5, H-6 = 6.42 Hz) showed the CH3-C group of the Fuc 1H signal, corresponding to the signals at C-6 of Fuc at δ 18.81.
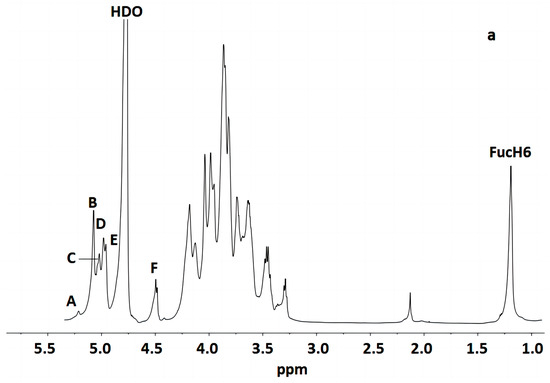
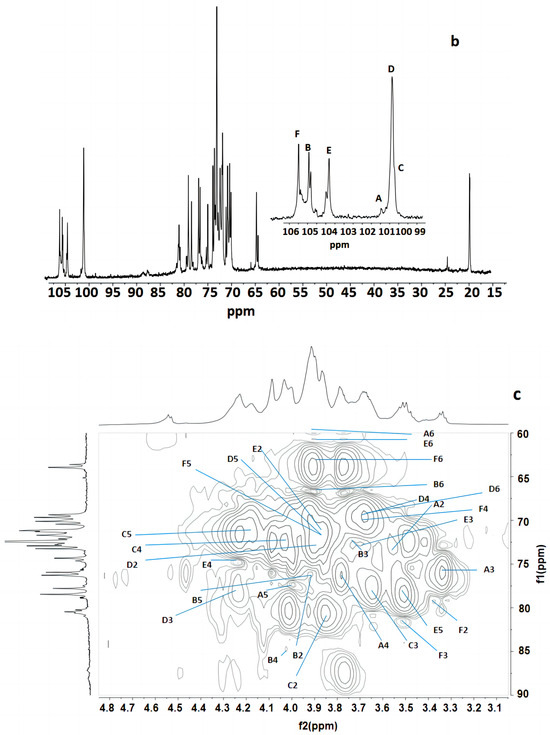
Figure 3.
NMR of FVPT1 in deuterium oxide (D2O) at 27 °C. (a) The 600 MHz 1H NMR spectrum of FVPT1. The anomeric protons are labeled as (A)–(F). (b) The 150 MHz 13C NMR spectrum of the FVPT1 polysaccharide in D2O at 27 °C. (c) HMQC of the FVPT1 polysaccharide in D2O at 27 °C.
The identities of monosaccharide residues A–F depended on the NMR, according to the chemical shifts and anomeric configurations of the six sugar residues in the FVPT1 structure.
Table 2 shows that the spin system for three sugar residues with the galacto configuration, A, B and D, was identified using the H-1/H-2 up to H-4 and H-6/H-5, H-4 correlations found in the 1H-1H COSY and TOCSY spectra. The downfield shifts of C-4 (δ 79.33) indicated that A was 1, 4- α-D-Galp. C-4 (δ 84.70) and C-6 (δ 67.48) indicated that residue B was 1, 4, 6-α-D-Galp. C-3 (δ 78.49) and C-6 (δ 69.32) indicated that residue D was (1, 3, 6)-α-D-Galp.

Table 2.
1H and 13C NMR chemical shifts (ppm) of FVPT1 at 27 °C.
Residue C has special signals at 1.24 (1H NMR) and δ 18.81 (1C NMR), indicating having α- fucose residue ether with δ 5.07, in addition to its small JH-1, H-2 value and GC-MS data. The C-2 (δ 87.43) carbon signals, compared with standard values, indicated that residue C was 1, 2-α-L-Fucp.
Small values of JH-1, H-2 and JH-2, H-3, a large value of JH-4, H-5 and a small JH-1, H-2 value have identified that residue E was of D-mannosyl. That JC-1, H-1 = 170 Hz in HMBC indicated the presence of an α- Mannose configuration [31]. Except for C-1 (δ 104.20), no carbon signal was evident within the δ 76–88 range, indicating that E was a terminal α-D-mannopyranose [32].
The anomeric signal at δ 4.55 and the large value of JH-1, H-2 indicated that F was a β-linked residue. Proton chemical shifts from H-2 to H-6 were assigned from the COSY and HMQC spectra. Large values of JH-2, H-3 and JH-3, H-4 (9 Hz) and the typical H-1, H-2 and H-4 intracorrelations in the NOESY spectrum pointed out that residue F was D-glucopyranose [8]. C-3 (δ 81.66) carbon indicated that F was a 1, 3-link β-D Glcp.
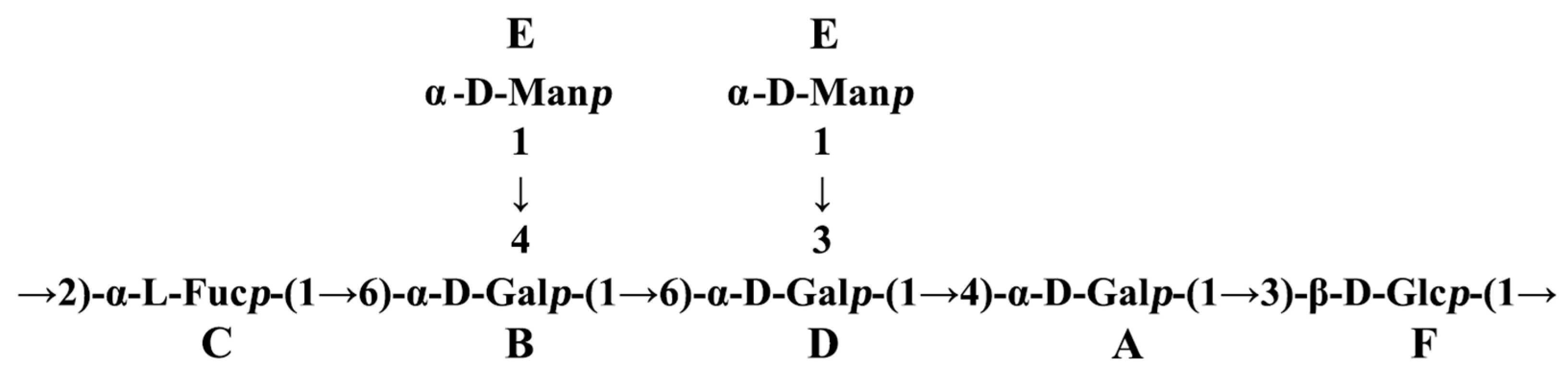
The sequence of the glycosyl residues was determined from the NOESY studies, followed by confirmation with HMBC experiments (Table 3 and Table 4). Based on the data presented above, the polysaccharide FVPT1 has the following repeating unit (Figure 4):

Table 3.
Interglycosidic correlations from of FVPT1.

Table 4.
Interglycosidic correlations of FVPT1.

Figure 4.
Repeating unit of FVPT1.
3.3. The Enhancement of FVPT1 to NO Release
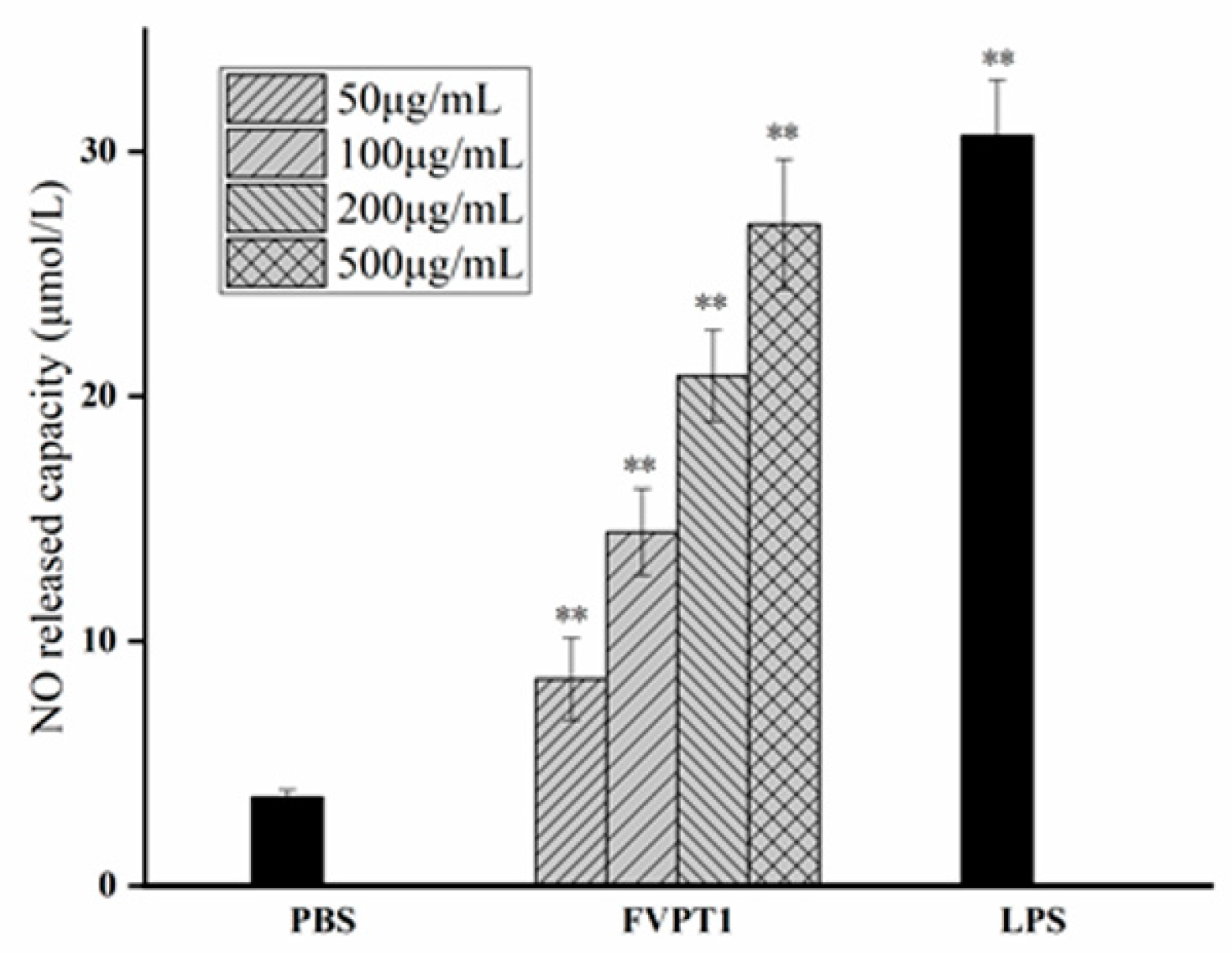
As a key signaling molecule and an important transduction factor in the immune system, NO’s content can be evaluated in the RNI levels of samples due to its relatively stable properties with RNI metabolites. As shown in Figure 5, FVPT1 could stimulate the level of NO in a dose-dependent manner at a certain concentration of 50–500 μg/mL compared to the negative control. Moreover, the 500 μg/mL FVPT1 showed obvious activation ability in NO production.
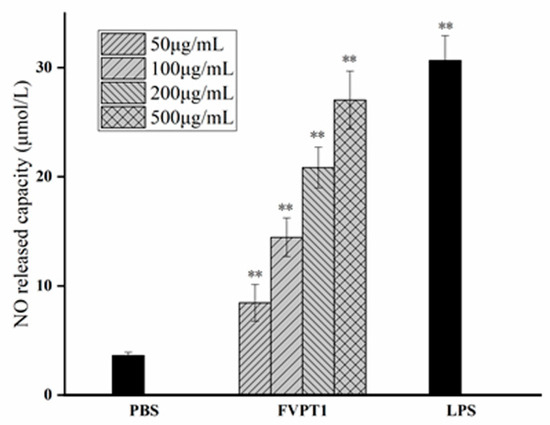
Figure 5.
The enhancement of FVPT1 in NO release. The Raw264.7 cells were treated with FVPT1 (50, 100, 200 and 500 μg/mL) or 10 μg/mL of LPS for 48 h. ** p < 0.01 vs. the PBS group.
3.4. Effect on Cytokines Produced by Mouse Macrophages Activated by FVPT1
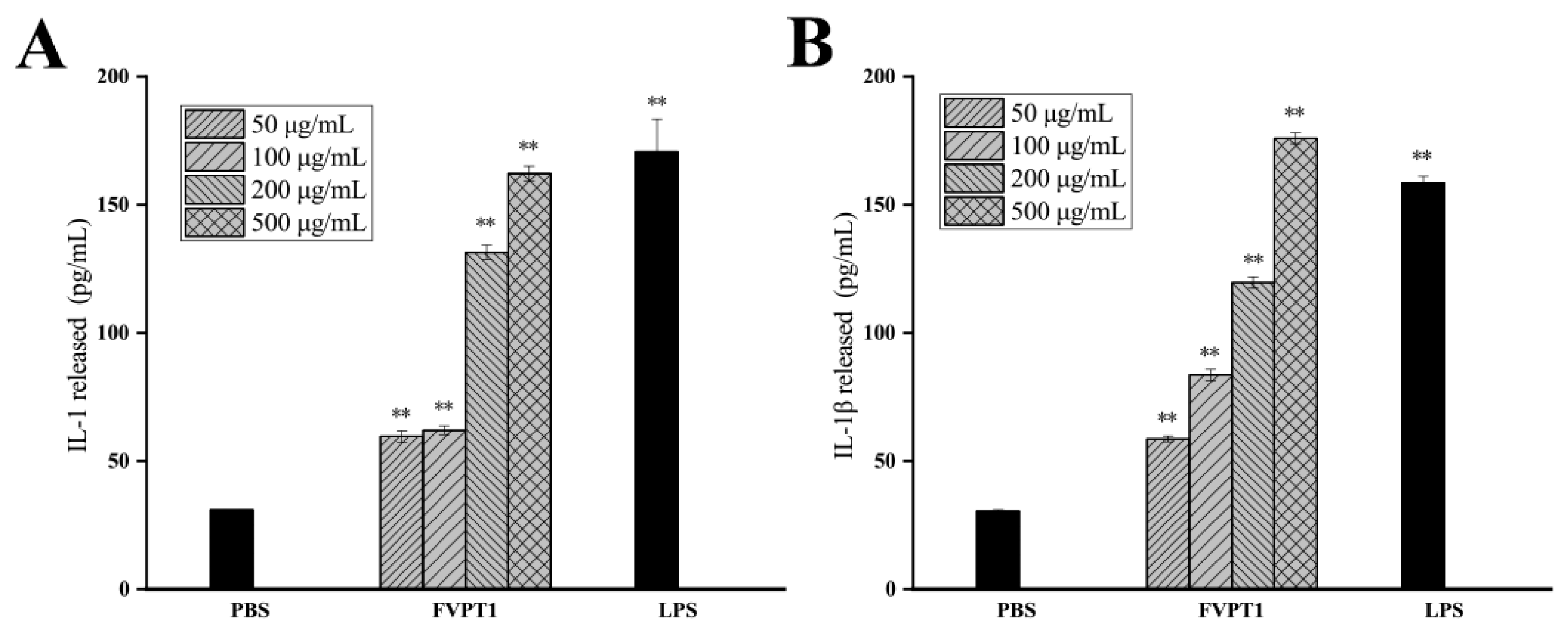
Activated macrophages can not only produce inflammatory mediators but also secrete cytokines such as IL-1 and IL-1β, which regulate local microenvironments and resist the invasion of adverse factors. An ELISA kit was used to detect the effect of FVPT1 on promoting macrophages to produce the aforementioned four cytokines, with the results showing that cocultures of FVPT1 could stimulate macrophages to secrete the IL-1β and IL-1 cytokines. Levels of IL-1β and IL-1 cytokines can be significantly promoted by a concentration of 500 μg/mL of FVPT1 (Figure 6). The results showed that FVPT1 could activate macrophages and secrete IL-1 and IL-1β cytokines in large quantities. In previous studies, FVPA1, FVPA2 and FVPB2 have been obtained from F. velutipes using traditional extraction, separation and purification methods [8,10,27]. Similarly to the in vitro immune efficacy of FVPT1, FVPA1 could stimulate Raw264.7 macrophages to secrete NO [10]. However, FVPA2 and FVPB2 could act on B cells or NK cells in vitro rather than on macrophages [8,27].
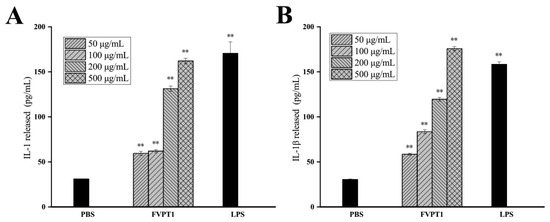
Figure 6.
The promotion effects of FVPT1 on the cytokines released by RAW264.7. (A) Detection of IL-1 secretion with ELISA assay. (B) Detection of IL-1β with ELISA assay. ** p < 0.01 vs. the PBS group.
3.5. Lipid-Decreasing Effect of FVPT1 In Vivo
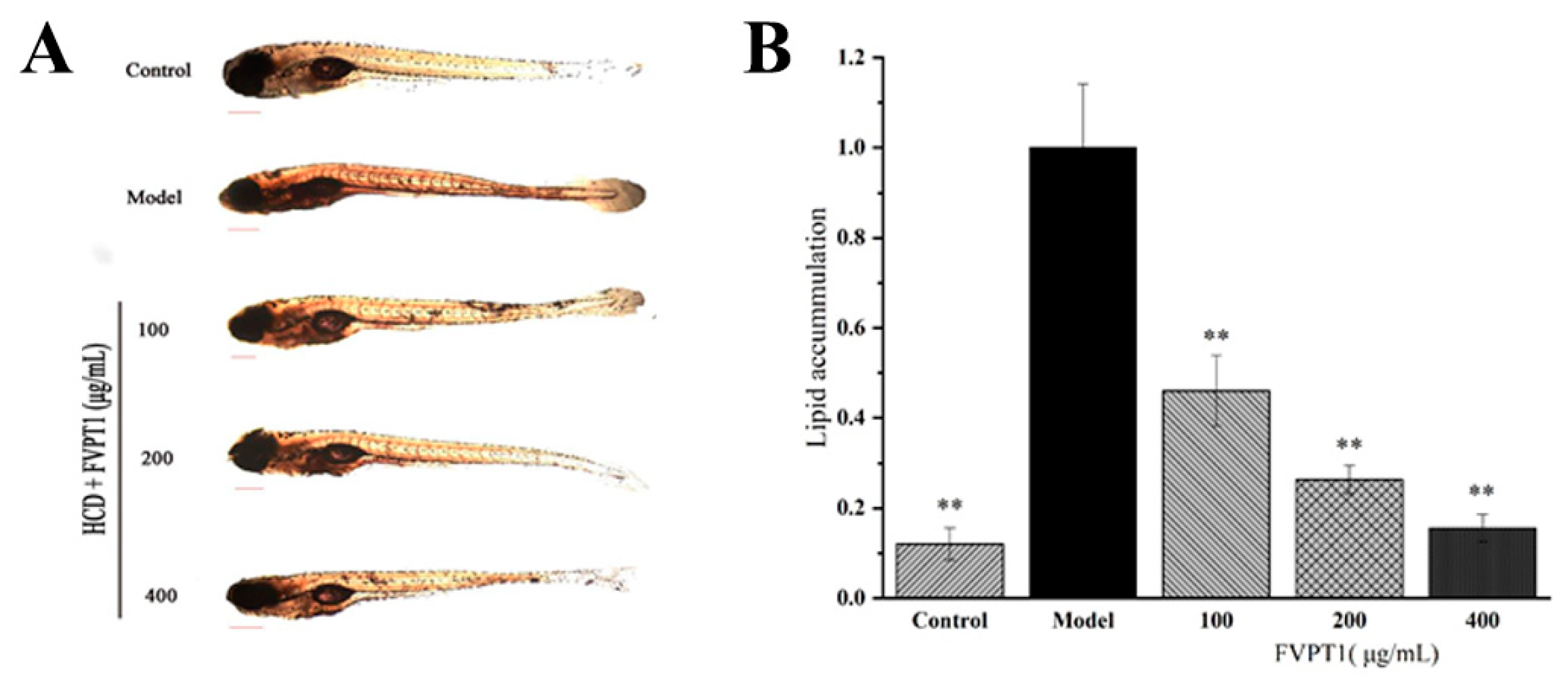
In recent years, zebrafish have often been used as models to clarify mechanisms of pharmacological action [33]. Previous studies have shown that the polysaccharides isolated from Agaricus bisporus and Pleurotus eryngii demonstrate significant blood lipid-lowering activity in zebrafish models [28,34]. Obviously, FVPT1 reduced the lipid content in the zebrafish larvae in our hyperlipidemia model as expected. The 100, 200 and 400 μg/mL FVPT1 lowered the lipid content to 53.89%, 73.76% and 83.89%, respectively, compared with 100% in the model group of zebrafish fed on yolk powder as a high-cholesterol diet (Figure 7). The results of the in vitro experiment suggested that FVPT1 has a good inhibitory effect on lipid accumulation.
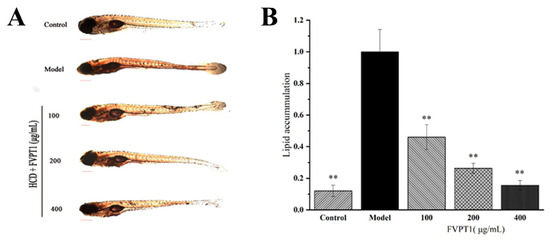
Figure 7.
The lipid-decreasing effect of FVPT1 in vivo. (A) Representative photos of the whole-body stable lipid appearance of zebrafish larvae treated with FVPT1 at different concentrations (100, 200 and 400 μg/mL). (B) Lipid content was normalized to IOD data through blood vessels. ** p < 0.01 vs. the model group.
4. Conclusions
In this study, FVPT1 with the molecular weight of ~1.64 × 104 Da was separated using magnetic-field-assisted TPP and purified with S-300 (16 mm × 100 cm). Monosaccharide composition analysis showed that FVPT1 is composed of L-fucose, D-galactose, D-glucose and D-mannose at a molar ratio of 1.0:3.5:1.0:1.4. Methylation analysis and NMR spectroscopy established the polysaccharide repeating unit of FVPT1 and revealed that it was a new polysaccharide. Although several polysaccharide structures from F. velutipes have been reported recently, the polysaccharide moiety of FVPT1 represents a previously undocumented novel structure. In addition, the results of biological activity experiments have shown that FVPT1 exhibits good immunomodulatory activity by increasing nitric oxide, interleukin (IL)-1β and IL-1 secretion in macrophages and good hypolipidemic activity via appreciable lipid-lowering effects in a zebrafish larva hyperlipidemia model test. Research is ongoing in our laboratory to further characterize the nature of this polysaccharide and to investigate structure–activity relationships.
Author Contributions
Conceptualization, H.Z.; methodology, H.L.; formal analysis, W.W.; resources, D.Y.; data curation, Y.Y. and Z.F.; writing—original draft, W.J.; project administration, J.Z.; funding acquisition, Z.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the SAAS Program for Excellent Research Team, grant number G2022003, and the APC was funded by the Aggregation Plan of Thousand Leading Talents in Suqian, Jiangsu Province in 2020.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
Authors Yangchao Yu, Zhan Feng and Hewen Li have been employed by the company Jiangsu Chinagreen Biotechnology Co., Ltd., Suqian 223700, Jiangsu, China. The remaining authors declare that this research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
References
- Fan, L.; Ashok, P.; Soccol, C.R. Production of Flammulina velutipes on coffee husk and coffee spent-ground. Braz. Arch. Biol. Technol. 2001, 44, 205–212. [Google Scholar]
- Yi, C.; Fu, M.; Cao, X.; Tong, S.; Zheng, Q.; Firempong, C.K.; Jiang, X.; Xu, X.; Yu, J. Enhanced oral bioavailability and tissue distribution of a new potential anticancer agent, Flammulina velutipes sterols, through liposomal encapsulation. J. Agric. Food Chem. 2013, 61, 5961–5971. [Google Scholar] [CrossRef]
- Shah, S.R.; Ukaegbu, C.I.; Hamid, H.A.; Alara, O.R. Evaluation of antioxidant and antibacterial activities of the stems of Flammulina velutipes and Hypsizygus tessellatus (white and brown var.) extracted with different solvents. J. Food Meas. Charact. 2018, 12, 1947–1961. [Google Scholar] [CrossRef]
- Zhao, S.; Li, B.; Chen, G.; Hu, Q.; Zhao, L. Preparation, characterization, and anti-inflammatory effect of the chelate of Flammulina velutipes polysaccharide with Zn. Food Agric. Immunol. 2017, 28, 162–177. [Google Scholar] [CrossRef]
- Ma, Z.; Cui, F.; Gao, X.; Zhang, J.; Zheng, L.; Jia, L. Purification, characterization, antioxidant activity and anti-aging of exopolysaccharides by Flammulina velutipes SF-06. Antonie Van Leeuwenhoek 2015, 107, 73–82. [Google Scholar] [CrossRef]
- Hu, Y.N.; Sung, T.J.; Chou, C.H.; Liu, K.L.; Hsieh, L.P.; Hsieh, C.W. Characterization and antioxidant activities of yellow strain Flammulina velutipes (Jinhua Mushroom) polysaccharides and their effects on ROS content in L929 cell. Antioxidants 2019, 8, 298. [Google Scholar] [CrossRef] [PubMed]
- Yeh, M.Y.; Ko, W.C.; Lin, L.Y. Hypolipidemic and antioxidant activity of enoki mushrooms (Flammulina velutipes). BioMed Res. Int. 2014, 2014, 352385. [Google Scholar] [CrossRef]
- Feng, T.; Jia, W.; Wang, W.H.; Lin, C.C.; Fan, H.; Zhang, J.S.; Bao, H.Y. Structural Characterization and Immunological Activities of a Novel Water-Soluble Polysaccharide from the Fruiting Bodies of Culinary-Medicinal Winter Mushroom, Flammulina velutipes (Agaricomycetes). Int. J. Med. Mushrooms 2016, 18, 807–819. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Hoo, P.C.; Tan, L.T.; Pusparajah, P.; Khan, T.M.; Lee, L.H.; Goh, B.H.; Chan, K.G. Golden Needle Mushroom: A Culinary Medicine with Evidenced-Based Biological Activities and Health Promoting Properties. Front. Pharmacol. 2016, 7, 474. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Feng, J.; Zhang, J.S.; Lin, C.C.; Wang, W.H.; Chen, H.G. Structural Characteristics of the Novel Polysaccharide FVPA1 from Winter Culinary-Medicinal Mushroom, Flammulina velutipes (Agaricomycetes), Capable of Enhancing Natural Killer Cell Activity against K562 Tumor Cells. Int. J. Med. Mushrooms 2017, 19, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Castro-Alves, V.C.; do Nascimento, J.R.O. Speeding up the Extraction of Mushroom Polysaccharides. Food Analy Method 2016, 9, 2429–2433. [Google Scholar] [CrossRef]
- Zhu, M.; Nie, P.; Liang, Y.; Wang, B. Optimizing conditions of polysaccharide extraction from Shiitake mushroom using response surface methodology and its regulating lipid metabolism. Carbohydr. Polym. 2013, 95, 644–648. [Google Scholar] [CrossRef]
- Guo, H.; Yuan, Q.; Fu, Y.; Liu, W.; Su, Y.H.; Liu, H.; Wu, C.Y.; Zhao, L.; Zhang, Q.; Lin, D.R.; et al. Extraction Optimization and Effects of Extraction Methods on the Chemical Structures and Antioxidant Activities of Polysaccharides from Snow Chrysanthemum (Coreopsis tinctoria). Polymers 2019, 11, 215. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.Y.; Dong, F.; Liu, X.; Lv, Q.; YingYang; Liu, F.; Chen, L.; Wang, T.; Wang, Z.; Zhang, Y. Effects of extraction methods on the yield, chemical structure and anti-tumor activity of polysaccharides from Cordyceps gunnii mycelia. Carbohydr. Polym. 2016, 140, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Fang, Y.; Liang, J.; Hu, Q. Optimization of ultrasonic extraction of Flammulina velutipes polysaccharides and evaluation of its acetylcholinesterase inhibitory activity. Food Res. Int. 2011, 44, 1269–1275. [Google Scholar] [CrossRef]
- Alfonsi, K.; Colberg, J.; Dunn, P.J.; Fevig, T.; Stefaniak, M.J.G.C. Green chemistry tools to influence a medicinal chemistry and research chemistry based organisation. Green Chem. 2008, 10, 31–36. [Google Scholar] [CrossRef]
- Harde, S.M.; Singhal, R.S. Extraction of forskolin from Coleus forskohlii roots using three phase partitioning. Sep. Purif. Technol. 2012, 96, 20–25. [Google Scholar] [CrossRef]
- Mulchandani, K.; Kar, J.R.; Singhal, R.S. Extraction of Lipids from Chlorella saccharophila Using High-Pressure Homogenization Followed by Three Phase Partitioning. Appl. Biochem. Biotechnol. 2015, 176, 1613–1626. [Google Scholar] [CrossRef]
- Yan, J.K.; Wang, Y.Y.; Qiu, W.Y.; Wang, Z.B.; Ma, H. Ultrasound synergized with three-phase partitioning for extraction and separation of Corbicula fluminea polysaccharides and possible relevant mechanisms. Ultrason. Sonochem. 2018, 40, 128–134. [Google Scholar] [CrossRef]
- Boucherba, N.; Gagaoua, M.; Bouanane-Darenfed, A.; Bouiche, C.; Bouacem, K.; Kerbous, M.Y.; Maafa, Y.; Benallaoua, S. Biochemical properties of a new thermo- and solvent-stable xylanase recovered using three phase partitioning from the extract of Bacillus oceanisediminis strain SJ3. Bioresour. Bioprocess. 2017, 4, 29. [Google Scholar] [CrossRef]
- Tan, Z.J.; Wang, C.Y.; Yi, Y.J.; Wang, H.Y.; Zhou, W.L.; Tan, S.Y.; Li, F.F. Three phase partitioning for simultaneous purification of aloe polysaccharide and protein using a single-step extraction. Process. Biochem. 2015, 50, 482–486. [Google Scholar] [CrossRef]
- Palvska, W.; Chmielewski, A.G. Solvent extraction and emulsion separation in magnetic fields. Sep. Sci. Technol. 1993, 28, 127–138. [Google Scholar] [CrossRef]
- Zaguła, G.; Bajcar, M.; Saletnik, B.; Czernicka, M.; Puchalski, C.; Kapusta, I.; Oszmiański, J. Comparison of the Effectiveness of Water-Based Extraction of Substances from Dry Tea Leaves with the Use of Magnetic Field Assisted Extraction Techniques. Molecules 2017, 22, 1656. [Google Scholar] [CrossRef]
- Zhang, T.; Ye, J.; Xue, C.; Wang, Y.; Liao, W.; Mao, L.; Yuan, M.; Lian, S. Structural characteristics and bioactive properties of a novel polysaccharide from Flammulina velutipes. Carbohydr. Polym. 2018, 197, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.Q.; Zhang, J.S.; Tang, Q.J.; Jia, W.; Yang, Y.; Liu, Y.F.; Fan, J.M.; Pan, Y.J. Structural elucidation of a novel fucogalactan that contains 3-O-methyl rhamnose isolated from the fruiting bodies of the fungus, Hericium erinaceus. Carbohydr. Res. 2006, 341, 645–649. [Google Scholar] [CrossRef] [PubMed]
- Paramonov, N.; Bailey, D.; Rangarajan, M.; Hashim, A.; Kelly, G.; Curtis, M.A.; Hounsell, E.F. Structural analysis of the polysaccharide from the lipopolysaccharide of Porphyromonas gingivalis strain W50. Eur. J. Biochem. 2001, 268, 4698–4707. [Google Scholar] [CrossRef]
- Wang, W.H.; Zhang, J.S.; Feng, T.; Deng, J.; Lin, C.C.; Hua, F.; Yu, W.J.; Wang, W.H.; Bao, H.Y.; Jia, W. Structural elucidation of a polysaccharide from Flammulina velutipes and its immunomodulation activities on mouse b lymphocytes. Sci. Rep. 2018, 8, 3120. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Yue, S.; Zhang, S.; Lu, L. Lipid-Lowering Effect of the Pleurotus eryngii (King Oyster Mushroom) Polysaccharide from Solid-State Fermentation on Both Macrophage-Derived Foam Cells and Zebrafish Models. Polymers 2018, 10, 492. [Google Scholar] [CrossRef]
- O’Rourke, E.J.; Soukas, A.A.; Carr, C.E.; Ruvkun, G. C. elegans major fats are stored in vesicles distinct from lysosome-related organelles. Cell Metab. 2009, 10, 430–435. [Google Scholar] [CrossRef]
- Ge, Q.; Zhang, A.Q.; Sun, P.L. Structural investigation of a novel water-soluble heteropolysaccharide from the fruiting bodies of Phellinus baumii Pilát. Food Chem. 2009, 114, 391–395. [Google Scholar] [CrossRef]
- Jansson, P.E.; Kenne, L.; Widmalm, G. Computer-assisted structural analysis of polysaccharides with an extended version of CASPER using 1H- and 13C-n.m.r. data. Carbohydr. Res. 1989, 188, 169–191. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Zhang, J.; Ye, X.; Tang, Q.; Liu, Y.; Gong, C.; Du, X.; Pan, Y. Structural elucidation of the polysaccharide moiety of a glycopeptide (GLPCW-II) from Ganoderma lucidum fruiting bodies. Carbohydr. Res. 2008, 343, 746–752. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Xu, Y.Q.; Guo, S.Y.; Li, C.Q. Rapid analysis of hypolipidemic drugs in a live zebrafish assay. J. Pharmacol. Toxicol. Methods 2015, 72, 47–452. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xue, Y.; Pang, L.; Len, B.; Lin, Z.; Huang, J.; ShangGuan, Z.; Pan, Y. Agaricus bisporus-derived β-glucan prevents obesity through PPAR γ downregulation and autophagy induction in zebrafish fed by chicken egg yolk. Int. J. Biol. Macromol. 2019, 125, 820–828. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).