Poly(butylene adipate-co-terephthalate)/Polylactic Acid/Tetrapod-Zinc Oxide Whisker Composite Films with Antibacterial Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Surface Modification and Morphology of T-ZnO Whiskers
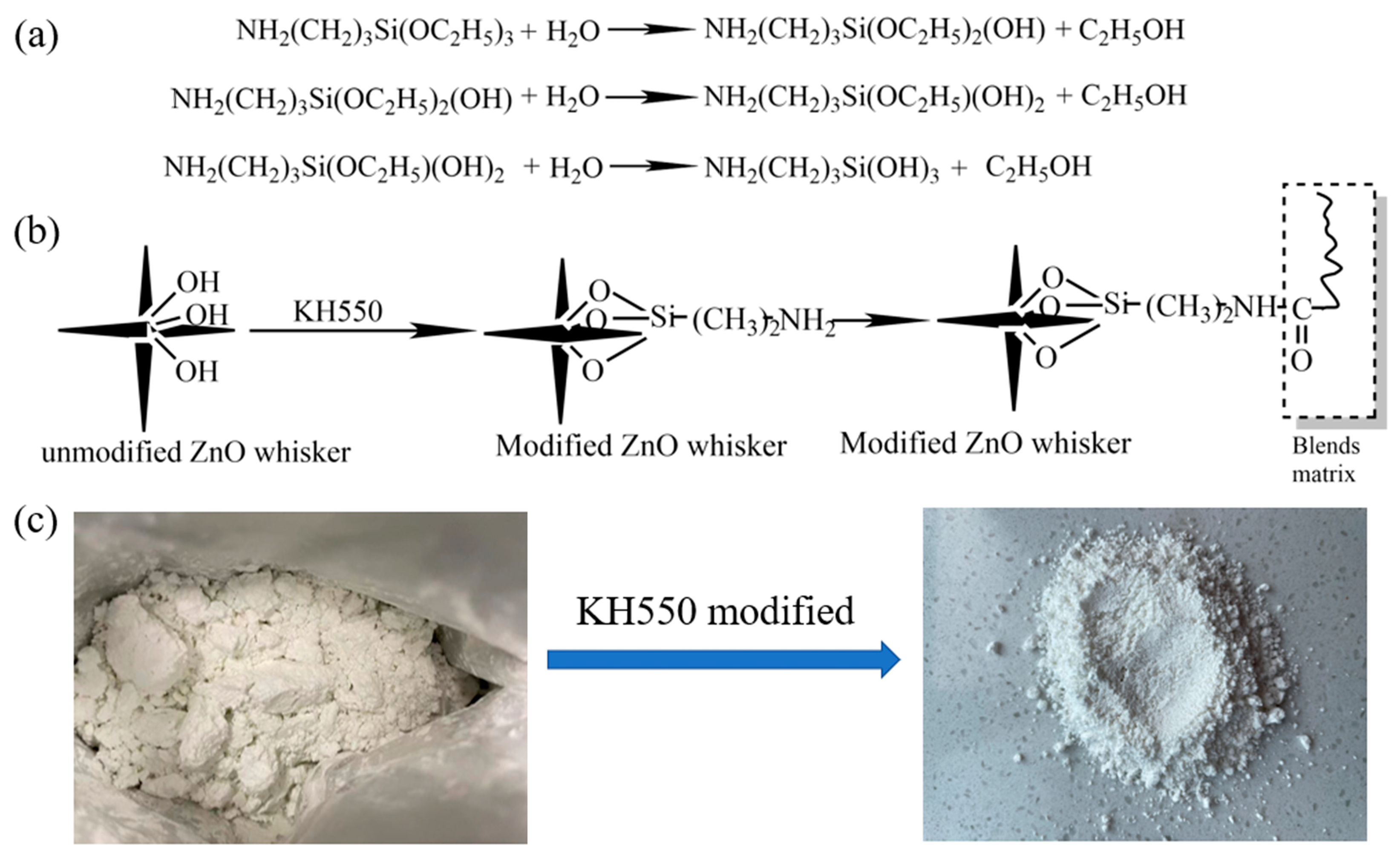
2.2.1. Surface Modification of T-ZnO Whisker
2.2.2. Morphology of Unmodified and Modified T-ZnO Whiskers
2.3. Preparation of PBAT/PLA/T-ZnO Whisker Composite Blends and Film Samples
2.3.1. Preparation of Composite Blends
2.3.2. Preparation of Composite Films
2.4. Characterization of PBAT/PLA/T-ZnO Whisker Composite Films
2.4.1. Optical Properties
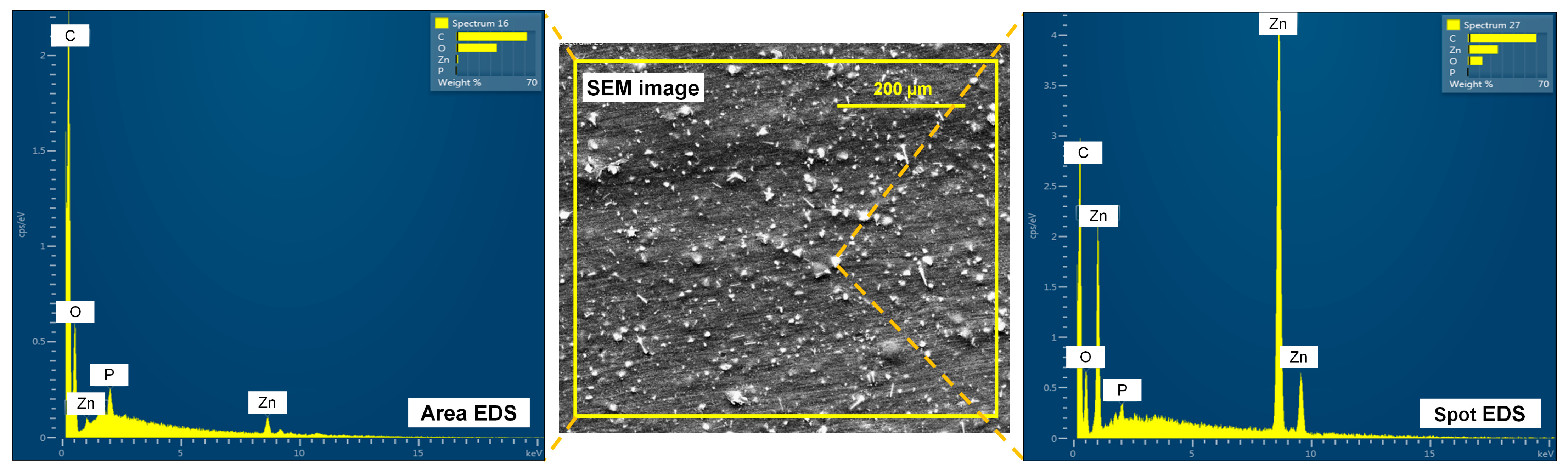
2.4.2. Morphology and Elemental Composition
2.4.3. Functional Groups
2.4.4. Mechanical Properties
2.4.5. Thermal Properties
2.4.6. Crystallinity and Intrinsic Structure
2.4.7. Rheological Behavior
2.4.8. Film Barrier Properties
2.4.9. Antibacterial Properties
3. Results and Discussion
3.1. Morphology of T-ZnO Whisker
3.2. Surface Chemistry and Morphology of Modified T-ZnO Whisker
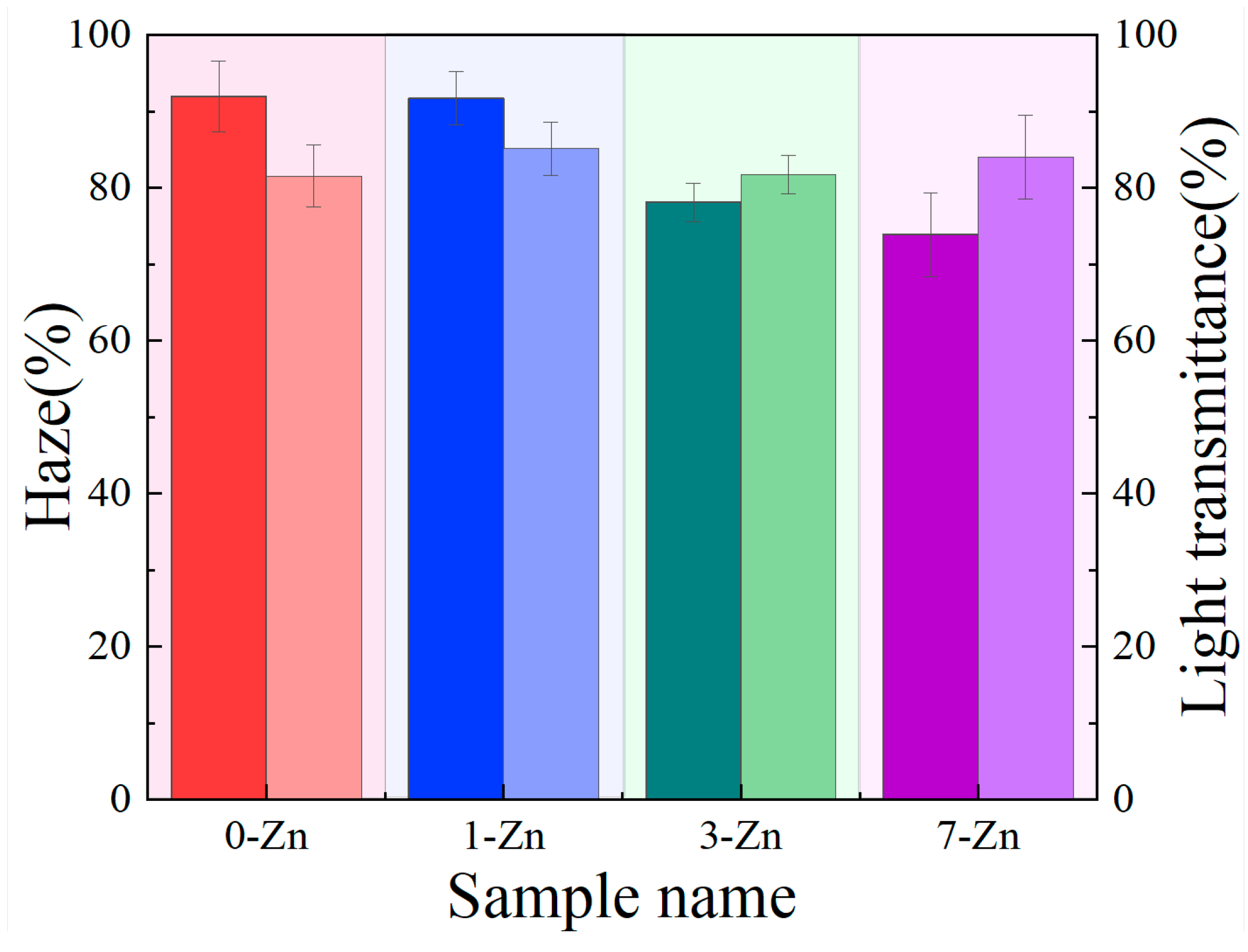
3.3. Appearance and Optical Properties of Films
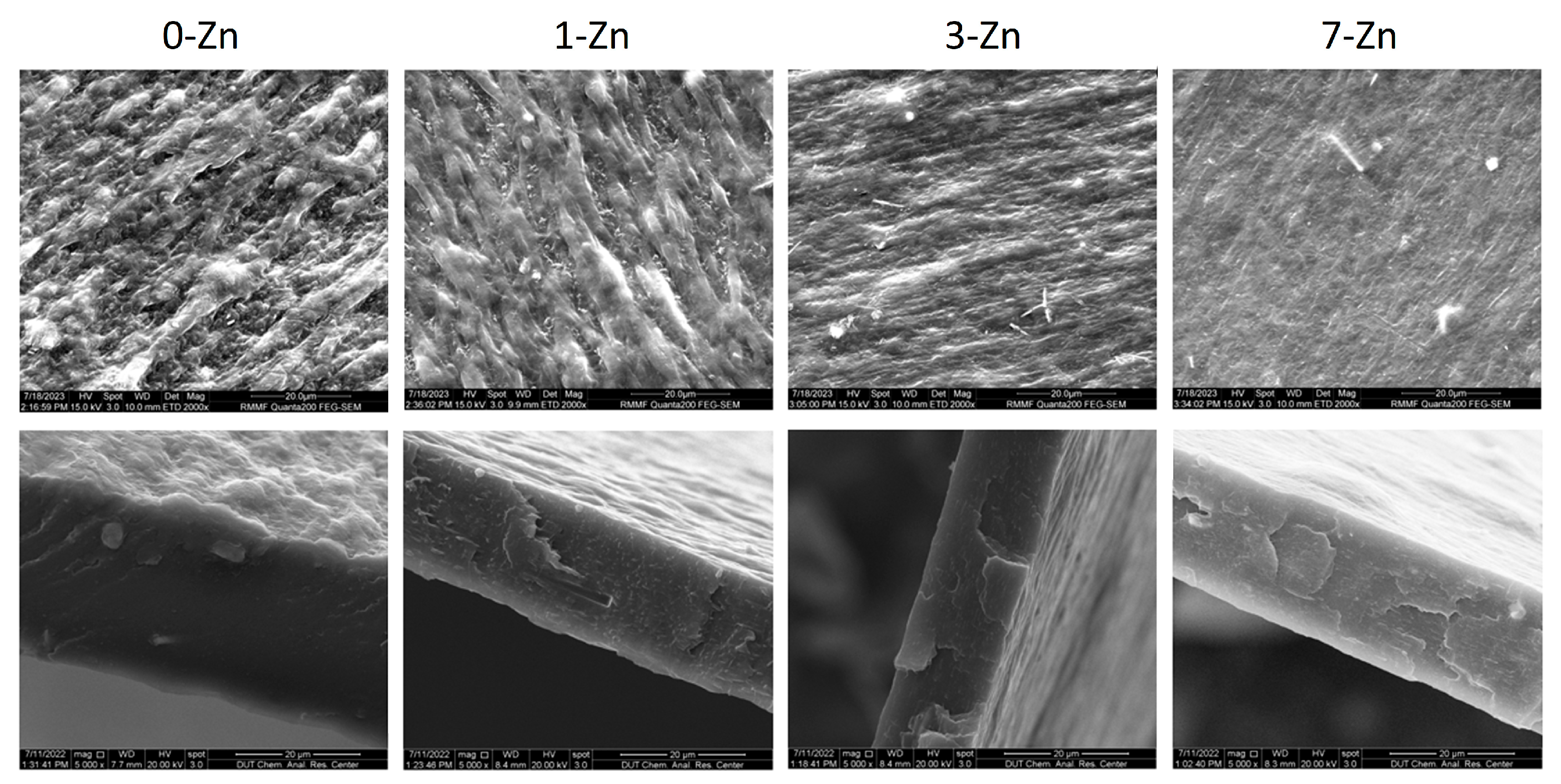
3.4. Surface and Cross-Sectional Morphology of Films
3.5. Functional Groups of Fabricated Films
3.6. Mechanical Properties of Fabricated Films
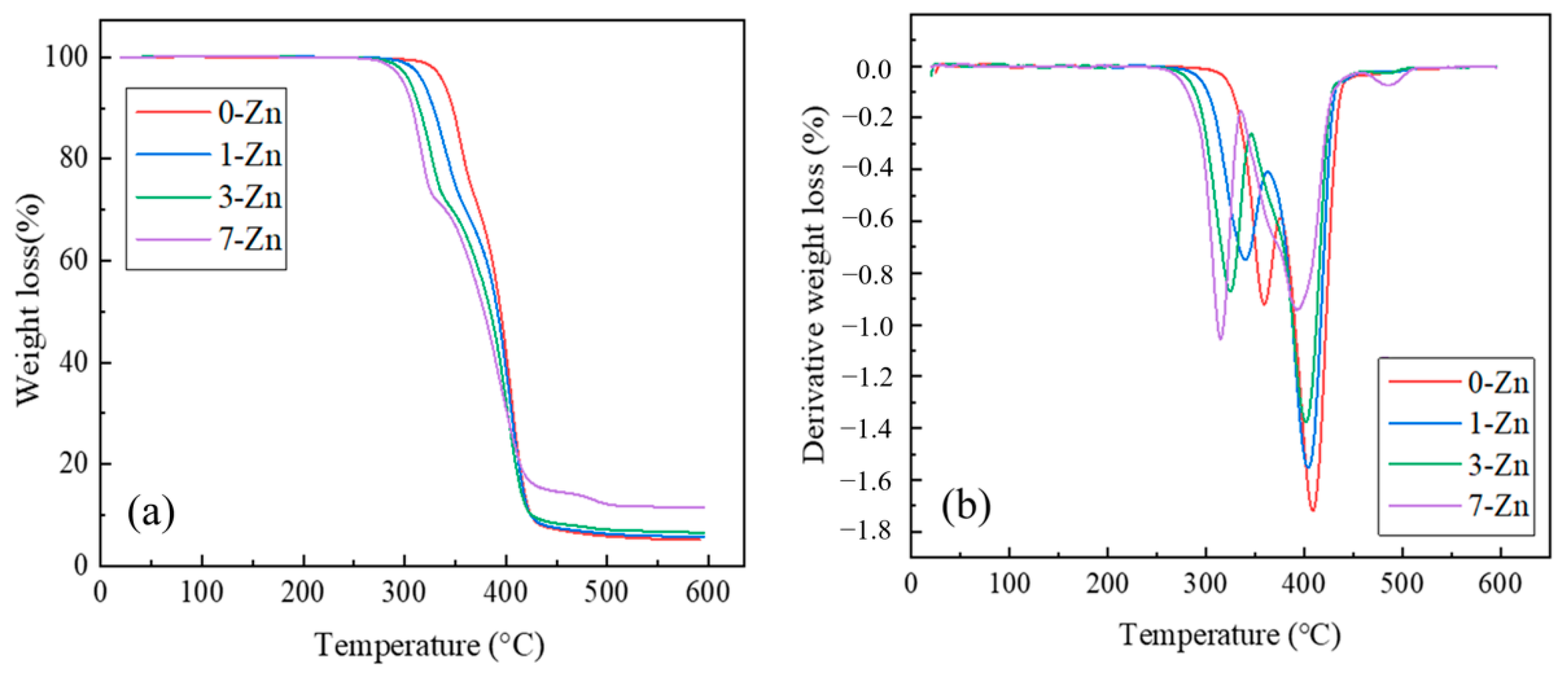
3.7. Thermal Properties of Fabricated Films
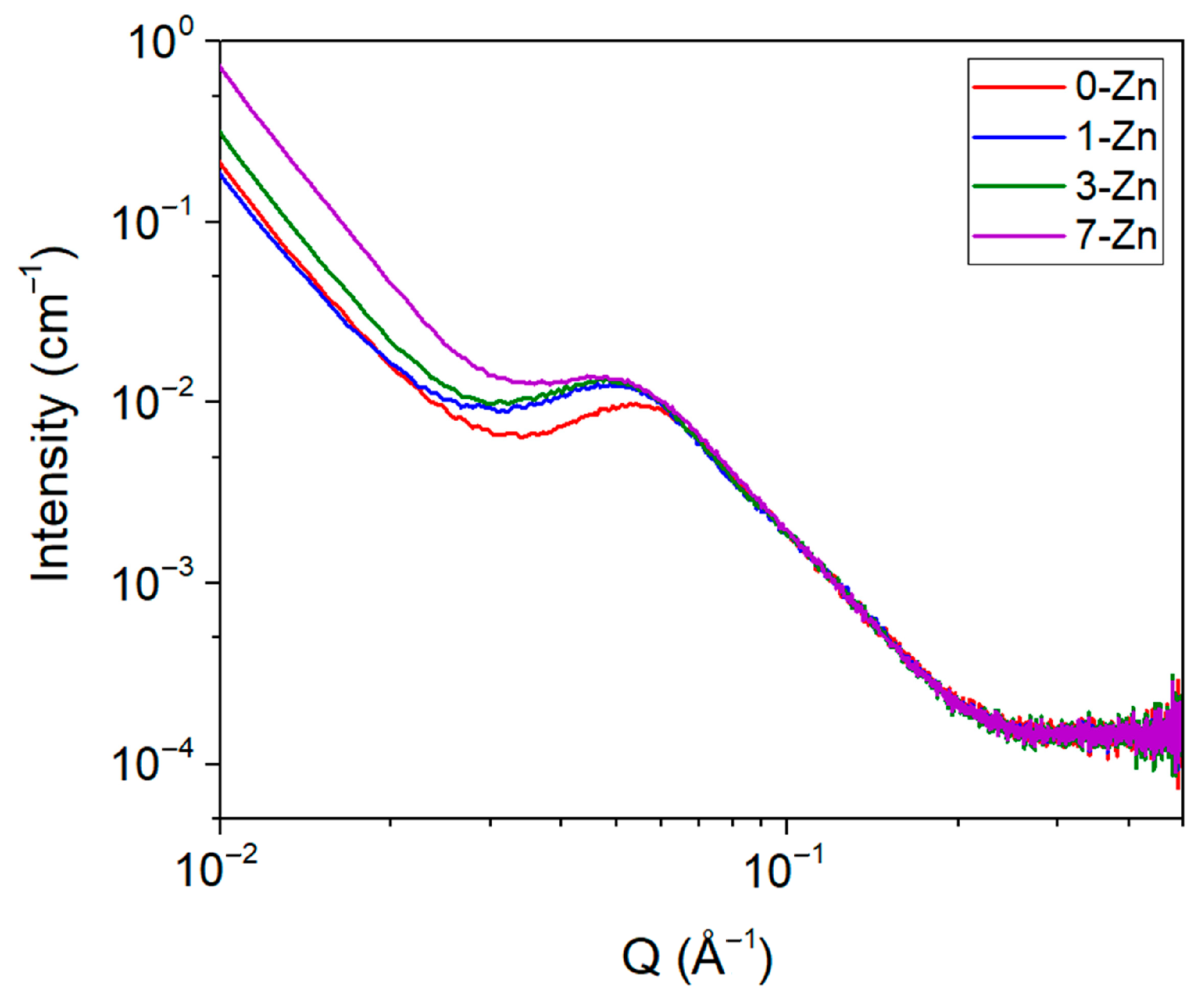
3.8. Crystallinity and Intrinsic Structure of Fabricated Films
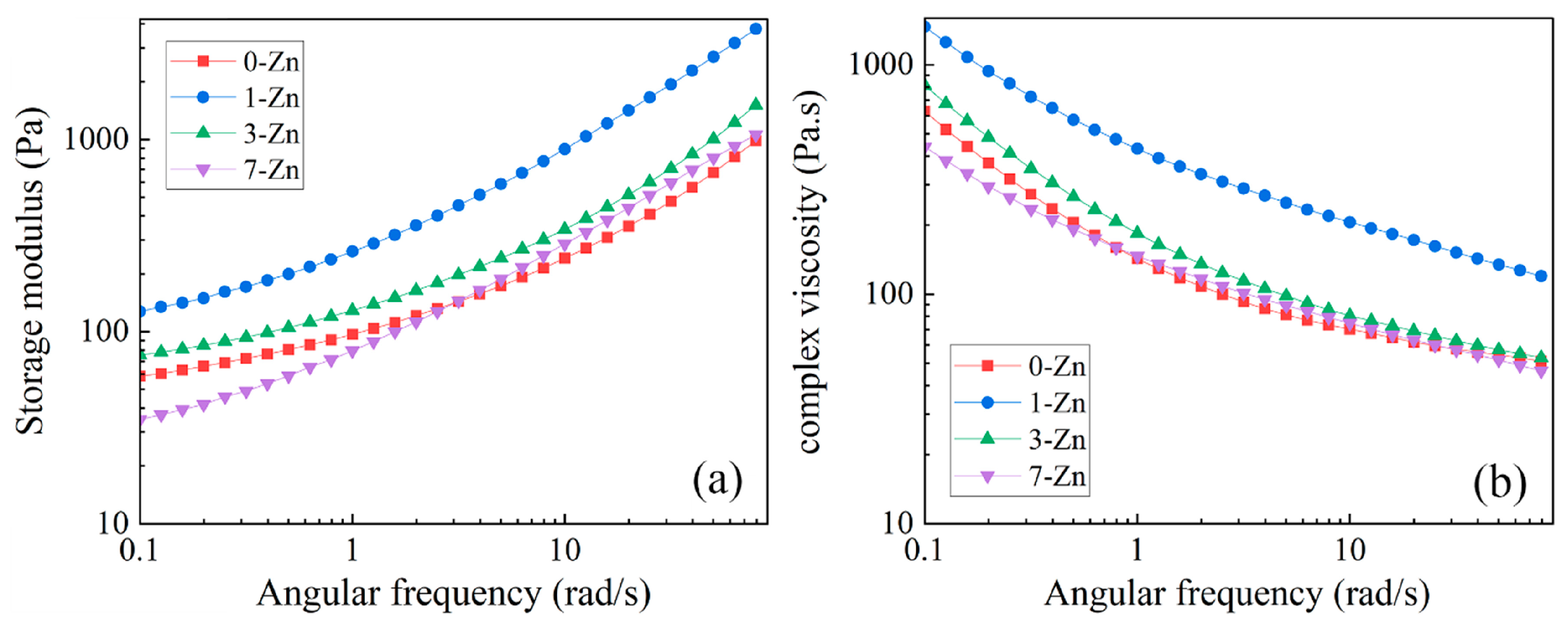
3.9. Rheological Properties of Fabricated Films
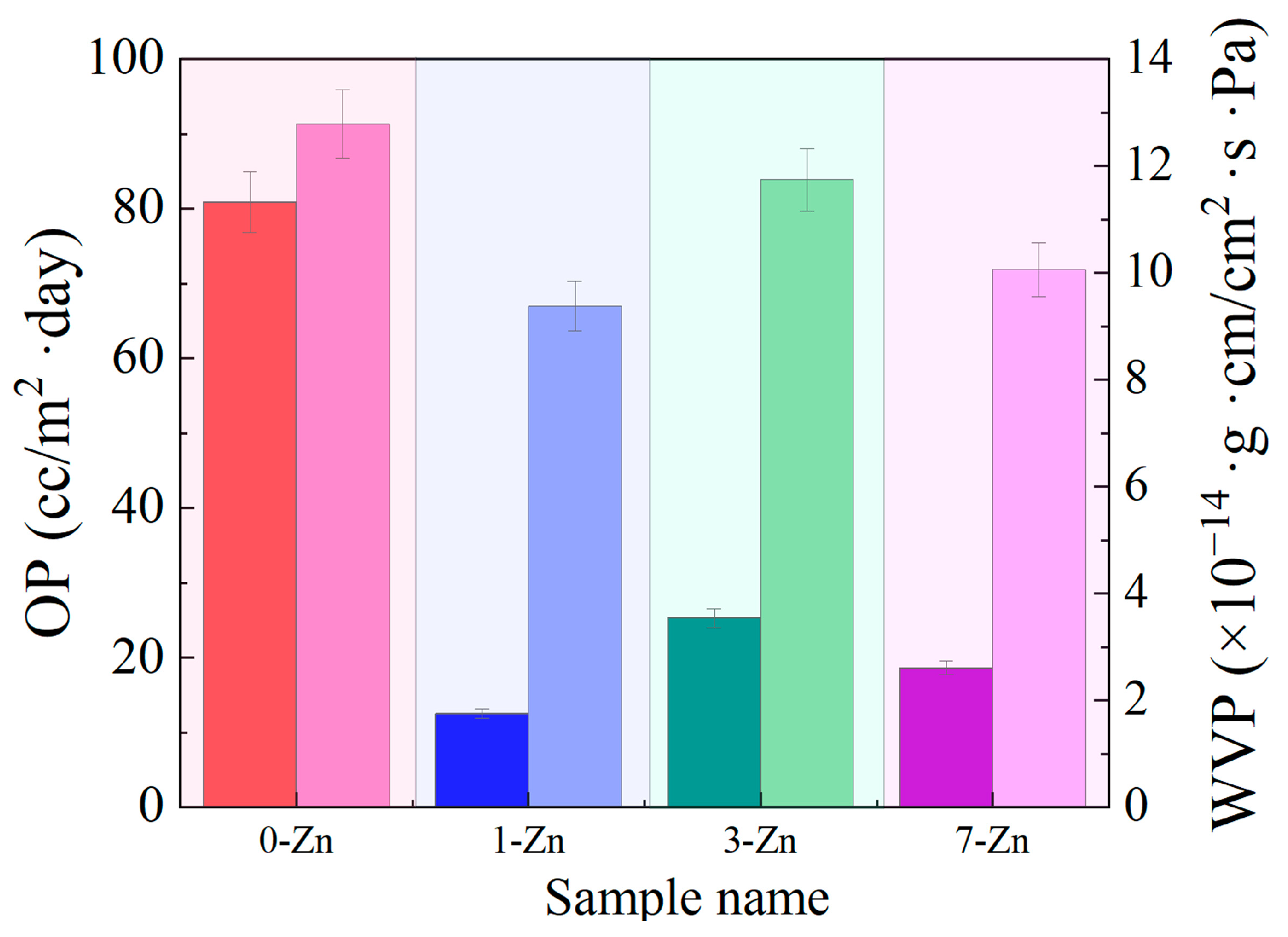
3.10. Film Barrier Properties of Fabricated Films
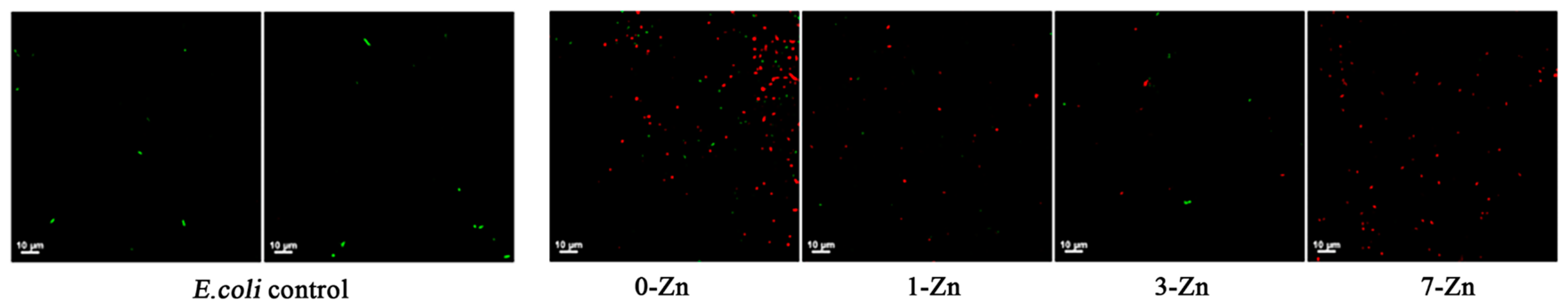
3.11. Antibacterial Properties of Fabricated Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dybka-Stępień, K.; Antolak, H.; Kmiotek, M.; Piechota, D.; Koziróg, A. Disposable Food Packaging and Serving Materials–Trends and Biodegradability. Polymers 2021, 13, 3606. [Google Scholar] [CrossRef] [PubMed]
- Mansoor, Z.; Tchuenbou-Magaia, F.; Kowalczuk, M.; Adamus, G.; Manning, G.; Parati, M.; Radecka, I.; Khan, H. Polymers Use as Mulch Films in Agriculture–A Review of History, Problems and Current Trends. Polymers 2022, 14, 5062. [Google Scholar] [CrossRef] [PubMed]
- Hameed, M.; Bhat, R.A.; Singh, D.V.; Mehmood, M.A. White Pollution: A Hazard to Environment and Sustainable Approach to Its Management. In Innovative Waste Management Technologies for Sustainable Development; Bhat, R.A., Qadri, H., Wani, K.A., Dar, G.H., Mehmood, M.A., Eds.; IGI Global: Hershey, PA, USA, 2020; pp. 52–81. [Google Scholar]
- Ziani, K.; Ioniță-Mîndrican, C.-B.; Mititelu, M.; Neacșu, S.M.; Negrei, C.; Moroșan, E.; Drăgănescu, D.; Preda, O.-T. Microplastics: A Real Global Threat for Environment and Food Safety: A State-of-the-Art Review. Nutrients 2023, 15, 617. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Gao, X.; Wu, J.; Zhou, T.; Nguyen, T.T.; Wang, Y. Biodegradable Polylactic Acid and Its Composites: Characteristics, Processing, and Sustainable Applications in Sports. Polymers 2023, 15, 3096. [Google Scholar] [CrossRef]
- Jian, J.; Xiangbin, Z.; Xianbo, H. An overview on synthesis, properties and applications of poly(butylene-adipate-co-terephthalate)–PBAT. Adv. Ind. Eng. Polym. Res. 2020, 3, 19–26. [Google Scholar] [CrossRef]
- Li, X.; Meng, L.; Zhang, Y.; Qin, Z.; Meng, L.; Li, C.; Liu, M. Research and Application of Polypropylene Carbonate Composite Materials: A Review. Polymers 2022, 14, 2159. [Google Scholar] [CrossRef]
- Huang, Y.; Brünig, H.; Müller, M.T.; Wießner, S. Melt spinning of PLA/PCL blends modified with electron induced reactive processing. J. Appl. Polym. Sci. 2022, 139, 51902. [Google Scholar] [CrossRef]
- Li, C.; Wang, B.; Shang, Z.; Yu, L.; Wei, C.; Wei, Z.; Sang, L.; Li, Y. High-Barrier Poly(butylene succinate-co-terephthalate) Blend with Poly(lactic acid) as Biodegradable Food Packaging Films. Ind. Eng. Chem. Res. 2023, 62, 7250–7261. [Google Scholar] [CrossRef]
- Teamsinsungvon, A.; Jarapanyacheep, R.; Ruksakulpiwat, Y.; Jarukumjorn, K. Melt processing of maleic anhydride grafted poly(lactic acid) and its compatibilizing effect on poly(lactic acid)/poly(butylene adipate-co-terephthalate) blend and their composite. Polym. Sci. Ser. A 2017, 59, 384–396. [Google Scholar] [CrossRef]
- Arruda, L.C.; Magaton, M.; Bretas, R.E.S.; Ueki, M.M. Influence of chain extender on mechanical, thermal and morphological properties of blown films of PLA/PBAT blends. Polym. Test. 2015, 43, 27–37. [Google Scholar] [CrossRef]
- Wu, F.; Misra, M.; Mohanty, A.K. Challenges and new opportunities on barrier performance of biodegradable polymers for sustainable packaging. Prog. Polym. Sci. 2021, 117, 101395. [Google Scholar] [CrossRef]
- Ma, F.; Wang, B.; Leng, X.; Wang, Y.; Sun, Z.; Wang, P.; Sang, L.; Wei, Z. Biodegradable PBAT/PLA/CaCO3 Blowing Films with Enhanced Mechanical and Barrier Properties: Investigation of Size and Content of CaCO3 Particles. Macromol. Mater. Eng. 2022, 307, 2200135. [Google Scholar] [CrossRef]
- Ma, P.; Jiang, L.; Yu, M.; Dong, W.; Chen, M. Green Antibacterial Nanocomposites from Poly(lactide)/Poly(butylene adipate-co-terephthalate)/Nanocrystal Cellulose–Silver Nanohybrids. ACS Sustain. Chem. Eng. 2016, 4, 6417–6426. [Google Scholar] [CrossRef]
- Kumar, C.R.; Betageri, V.S.; Nagaraju, G.; Pujar, G.H.; Onkarappa, H.S.; Latha, M.S. One-pot green synthesis of ZnO–CuO nanocomposite and their enhanced photocatalytic and antibacterial activity. Adv. Nat. Sci. Nanosci. Nanotechnol. 2020, 11, 015009. [Google Scholar] [CrossRef]
- Brindha, R.; Kandeeban, R.; Swarna Kamal, K.; Manojkumar, K.; Nithya, V.; Saminathan, K. Andrographis paniculata absorbed ZnO nanofibers as a potential antimicrobial agent for biomedical applications. Adv. Nat. Sci. Nanosci. Nanotechnol. 2021, 12, 045002. [Google Scholar] [CrossRef]
- Imran, H.J.; Aadim, K.A.; Hubeatir, K.A. Two–step pulsed laser ablation for preparation NiO@ZnO core-shell nanostructure and evaluation of their antibacterial activity. Adv. Nat. Sci. Nanosci. Nanotechnol. 2023, 14, 045003. [Google Scholar] [CrossRef]
- Kitano, M.; Hamabe, T.; Maeda, S. Growth of large tetrapod-like ZnO crystals: I. Experimental considerations on kinetics of growth. J. Cryst. Growth 1990, 102, 965–973. [Google Scholar] [CrossRef]
- Niu, L.N.; Fang, M.; Jiao, K.; Tang, L.H.; Xiao, Y.H.; Shen, L.J.; Chen, J.H. Tetrapod-like zinc oxide whisker enhancement of resin composite. J. Dent. Res. 2010, 89, 746–750. [Google Scholar] [CrossRef]
- Shi, J.; Wang, Y.; Liu, L.; Bai, H.; Wu, J.; Jiang, C.; Zhou, Z. Tensile fracture behaviors of T-ZnOw/polyamide 6 composites. Mater. Sci. Eng. A 2009, 512, 109–116. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, J.; Han, L.; Xiang, F. Crystallization and mechanical properties of T-ZnOw/HDPE composites. Mater. Sci. Eng. A 2009, 501, 220–228. [Google Scholar] [CrossRef]
- Rocha, D.B.; Souza de Carvalho, J.; de Oliveira, S.A.; dos Santos Rosa, D. A new approach for flexible PBAT/PLA/CaCO3 films into agriculture. J. Appl. Polym. Sci. 2018, 135, 46660. [Google Scholar] [CrossRef]
- Pan, H.; Li, Z.; Yang, J.; Li, X.; Ai, X.; Hao, Y.; Zhang, H.; Dong, L. The effect of MDI on the structure and mechanical properties of poly(lactic acid) and poly(butylene adipate-co-butylene terephthalate) blends. RSC Adv. 2018, 8, 4610–4623. [Google Scholar] [CrossRef] [PubMed]
- Zeng, A.; Zheng, Y.; Guo, Y.; Qiu, S.; Cheng, L. Effect of tetra-needle-shaped zinc oxide whisker (T-ZnOw) on mechanical properties and crystallization behavior of isotactic polypropylene. Mater. Des. 2012, 34, 691–698. [Google Scholar] [CrossRef]
- Sang, L.; Zhao, M.; Liang, Q.; Wei, Z. Silane-Treated Basalt Fiber(-)Reinforced Poly(butylene succinate) Biocomposites: Interfacial Crystallization and Tensile Properties. Polymers 2017, 9, 351. [Google Scholar] [CrossRef]
- Sang, L.; Wang, Y.; Chen, G.; Liang, J.; Wei, Z. A comparative study of the crystalline structure and mechanical properties of carbon fiber/polyamide 6 composites enhanced with/without silane treatment. RSC Adv. 2016, 6, 107739–107747. [Google Scholar] [CrossRef]
- Andreassen, E.; Larsen, Å.; Nord-Varhaug, K.; Skar, M.; Øysaed, H. Haze of polyethylene films-effects of material parameters and clarifying agents. Polym. Eng. Sci. 2002, 42, 1082–1097. [Google Scholar] [CrossRef]
- Agrawal, M.; Gupta, S.; Zafeiropoulos, N.E.; Oertel, U.; Häßler, R.; Stamm, M. Nano-Level Mixing of ZnO into Poly(methyl methacrylate). Macromol. Chem. Phys. 2010, 211, 1925–1932. [Google Scholar] [CrossRef]
- Zhang, J.; Cao, C.; Zheng, S.; Li, W.; Li, B.; Xie, X. Poly (butylene adipate-co-terephthalate)/magnesium oxide/silver ternary composite biofilms for food packaging application. Food Packag. Shelf Life 2020, 24, 100487. [Google Scholar] [CrossRef]
- Kim, I.; Viswanathan, K.; Kasi, G.; Sadeghi, K.; Thanakkasaranee, S.; Seo, J. Poly(Lactic Acid)/Zno Bionanocomposite Films with Positively Charged Zno as Potential Antimicrobial Food Packaging Materials. Polymers 2019, 11, 1427. [Google Scholar] [CrossRef]
- Guimarães, M.; Botaro, V.R.; Novack, K.M.; Teixeira, F.G.; Tonoli, G.H.D. High moisture strength of cassava starch/polyvinyl alcohol-compatible blends for the packaging and agricultural sectors. J. Polym. Res. 2015, 22, 192. [Google Scholar] [CrossRef]
- Xiang, S.; Feng, L.; Bian, X.; Li, G.; Chen, X. Evaluation of PLA content in PLA/PBAT blends using TGA. Polym. Test. 2020, 81, 106211. [Google Scholar] [CrossRef]
- Yu, F.; Fei, X.; He, Y.; Li, H. Poly(lactic acid)-based composite film reinforced with acetylated cellulose nanocrystals and ZnO nanoparticles for active food packaging. Int. J. Biol. Macromol. 2021, 186, 770–779. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.P.; Zheng, C.H. Optimized Growth of Tetra-Needlelike ZnO Whiskers in the Atmosphere for Field-Emission Digital Tube Application. IEEE Electron. Device Lett. 2016, 37, 504–507. [Google Scholar] [CrossRef]
- Echeverría, C.; Limón, I.; Muñoz-Bonilla, A.; Fernández-García, M.; López, D. Development of Highly Crystalline Polylactic Acid with β-Crystalline Phase from the Induced Alignment of Electrospun Fibers. Polymers 2021, 13, 2860. [Google Scholar] [CrossRef] [PubMed]
- Al-Itry, R.; Lamnawar, K.; Maazouz, A. Rheological, morphological, and interfacial properties of compatibilized PLA/PBAT blends. Rheol. Acta 2014, 53, 501–517. [Google Scholar] [CrossRef]
- Phothisarattana, D.; Wongphan, P.; Promhuad, K.; Promsorn, J.; Harnkarnsujarit, N. Blown film extrusion of PBAT/TPS/ZnO nanocomposites for shelf-life extension of meat packaging. Colloids Surf. B 2022, 214, 112472. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xu, J.; Zhang, M.; Zheng, H.; Li, L. Preservation of soy protein-based meat analogues by using PLA/PBAT antimicrobial packaging film. Food Chem. 2022, 380, 132022. [Google Scholar] [CrossRef]
- Buysschaert, B.; Byloos, B.; Leys, N.; Van Houdt, R.; Boon, N. Reevaluating multicolor flow cytometry to assess microbial viability. Appl. Microbiol. Biotechnol. 2016, 100, 9037–9051. [Google Scholar] [CrossRef] [PubMed]
- Rutherford, D.; Jíra, J.; Kolářová, K.; Remeš, Z.; Rezek, B. Bactericidal effect of zinc oxide nanoparticles on Grampositive and Gram-negative strains in reverse spin bioreactor. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1050, 012013. [Google Scholar] [CrossRef]
- Kumar, R.; Umar, A.; Kumar, G.; Nalwa, H.S. Antimicrobial properties of ZnO nanomaterials: A review. Ceram. Int. 2017, 43, 3940–3961. [Google Scholar] [CrossRef]
- Gangadoo, S.; Xu, C.; Cozzolino, D.; Latham, K.; Della Gaspera, E.; Chapman, J.; Truong, V.K. Probing Nanoscale Interactions of Antimicrobial Zinc Oxide Quantum Dots on Bacterial and Fungal Cell Surfaces. Adv. Mater. Interfaces 2021, 9, 2101484. [Google Scholar] [CrossRef]








| Sample Name | PLA (wt.%) | PBAT (wt.%) | T-ZnO Whisker (phr) | Joncryl ADR-4380 (phr) |
|---|---|---|---|---|
| 0-Zn | 30 | 70 | 0 | 0.5 |
| 1-Zn | 30 | 70 | 1 | 0.5 |
| 3-Zn | 30 | 70 | 3 | 0.5 |
| 7-Zn | 30 | 70 | 7 | 0.5 |
| Sample Name | Degradation Onset Temperature (°C) | Derivative Peak of PLA (°C) | Derivative Peak of PBAT (°C) | Residue at 600 °C (wt.%) |
|---|---|---|---|---|
| 0-Zn | 338.6 | 359.2 | 408.7 | 4.8 |
| 1-Zn | 317.8 | 340.0 | 404.2 | 5.7 |
| 3-Zn | 305.7 | 324.8 | 401.7 | 7.2 |
| 7-Zn | 303.7 | 315.0 | 393.0 | 11.1 |
| Sample Name | Tg of PLA (°C) | Tg of PBAT (°C) | Tm of PLA (°C) | Tm of PBAT (°C) | ΔHm of PLA (J/g) | ΔHcc of PLA (J/g) | ΔHm of PBAT (J/g) | χc of PLA (%) | χc of PBAT (%) | χc of Blends (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| 0-Zn | 60.28 | −32.19 | 168.52 | 130.11 | 8.262 | 6.955 | 7.252 | 4.65 | 9.09 | 7.76 |
| 1-Zn | 60.41 | −30.13 | 167.89 | 129.19 | 2.171 | 0.283 | 4.924 | 6.72 | 6.17 | 6.34 |
| 3-Zn | 59.96 | −30.62 | 168.15 | 129.24 | 9.085 | 5.470 | 9.685 | 12.87 | 12.14 | 12.36 |
| 7-Zn | 61.33 | −28.10 | 169.51 | 133.23 | 8.389 | 4.634 | 2.861 | 13.37 | 3.59 | 6.52 |
| Sample Name | Qmax (Å) | dac (Å) | χc (%) | dc (Å) | da (Å) |
|---|---|---|---|---|---|
| 0-Zn | 0.05416 | 116.01 | 7.76 | 9.00 | 107.00 |
| 1-Zn | 0.04853 | 129.47 | 6.34 | 8.20 | 121.26 |
| 3-Zn | 0.04845 | 129.69 | 12.36 | 16.03 | 113.66 |
| 7-Zn | 0.04838 | 129.86 | 6.52 | 8.46 | 121.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Z.; Balu, R.; Gangadoo, S.; Duta, N.K.; Choudhury, N.R. Poly(butylene adipate-co-terephthalate)/Polylactic Acid/Tetrapod-Zinc Oxide Whisker Composite Films with Antibacterial Properties. Polymers 2024, 16, 1039. https://doi.org/10.3390/polym16081039
Zhao Z, Balu R, Gangadoo S, Duta NK, Choudhury NR. Poly(butylene adipate-co-terephthalate)/Polylactic Acid/Tetrapod-Zinc Oxide Whisker Composite Films with Antibacterial Properties. Polymers. 2024; 16(8):1039. https://doi.org/10.3390/polym16081039
Chicago/Turabian StyleZhao, Zhibo, Rajkamal Balu, Sheeana Gangadoo, Naba Kumar Duta, and Namita Roy Choudhury. 2024. "Poly(butylene adipate-co-terephthalate)/Polylactic Acid/Tetrapod-Zinc Oxide Whisker Composite Films with Antibacterial Properties" Polymers 16, no. 8: 1039. https://doi.org/10.3390/polym16081039
APA StyleZhao, Z., Balu, R., Gangadoo, S., Duta, N. K., & Choudhury, N. R. (2024). Poly(butylene adipate-co-terephthalate)/Polylactic Acid/Tetrapod-Zinc Oxide Whisker Composite Films with Antibacterial Properties. Polymers, 16(8), 1039. https://doi.org/10.3390/polym16081039







