Enhancing Polyvinyl Alcohol Nanocomposites with Carboxy-Functionalized Graphene: An In-Depth Analysis of Mechanical, Barrier, Electrical, Antibacterial, and Chemical Properties
Abstract
1. Introduction
2. Experimental Methods
2.1. Materials
2.2. Preparation of Nanocomposite CFG/PVA Films
2.3. Mechanical Properties
2.4. X-ray Diffraction (XRD) Analysis
2.5. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
2.6. XPS Analysis
2.7. TGA Analysis
2.8. Evaluation of Water Vapor Barrier Properties
2.9. Oxygen Barrier Properties
2.10. Morphology Examination via Scanning Electron Microscopy
2.11. Morphology Examinatio via Transmission Electron Microscopy
2.12. Hydrophilicity
2.13. Electrical Conductivity
2.14. Antibacterial Test
3. Results and Discussion
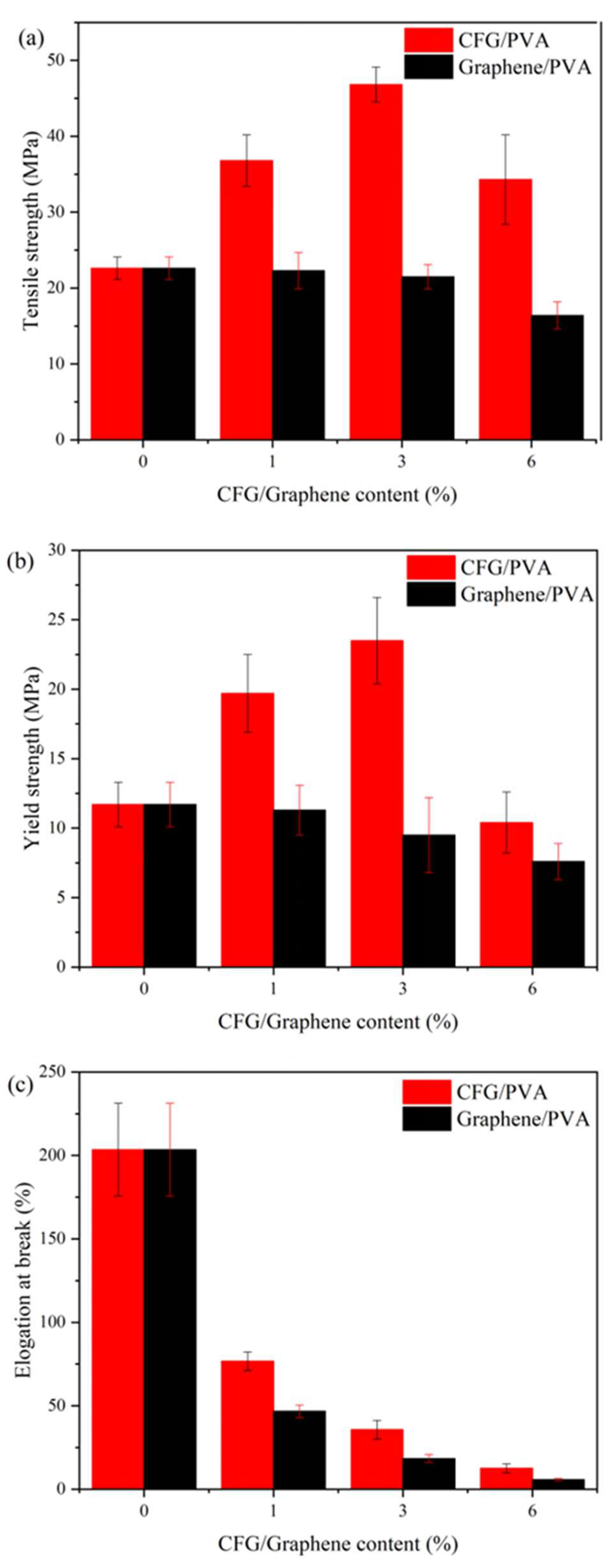
3.1. Mechanical Properties
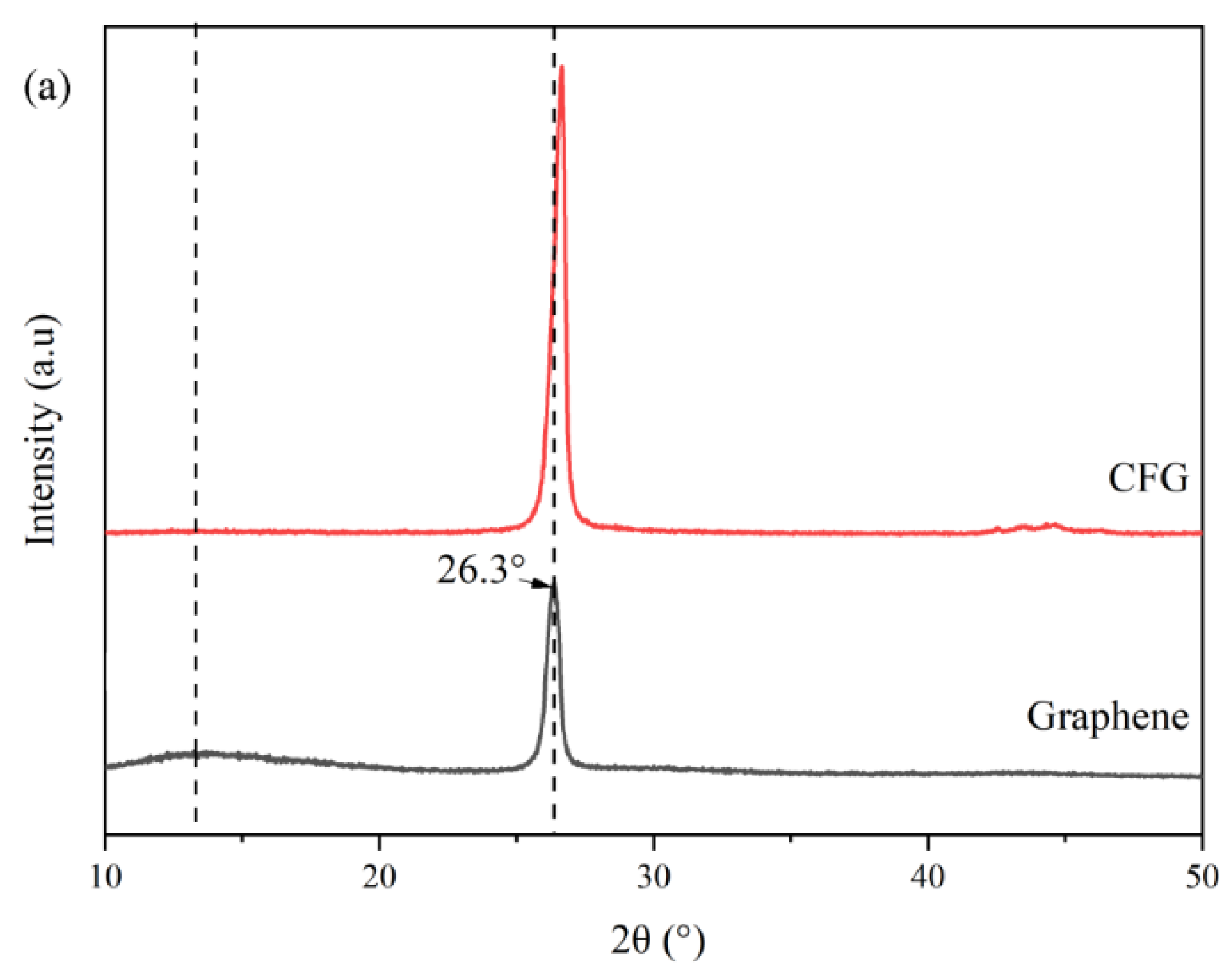
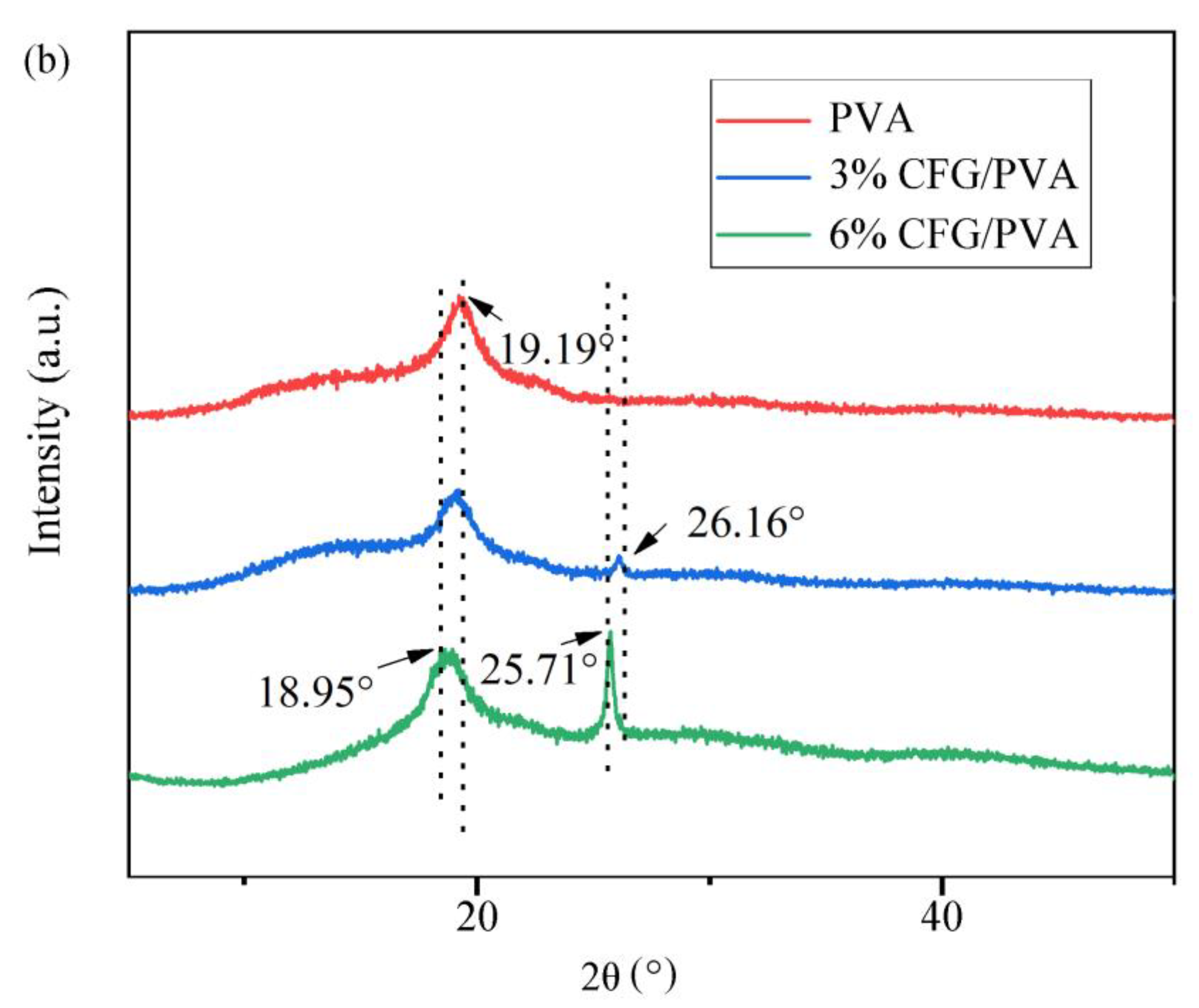
3.2. X-ray Diffraction (XRD) Analysis
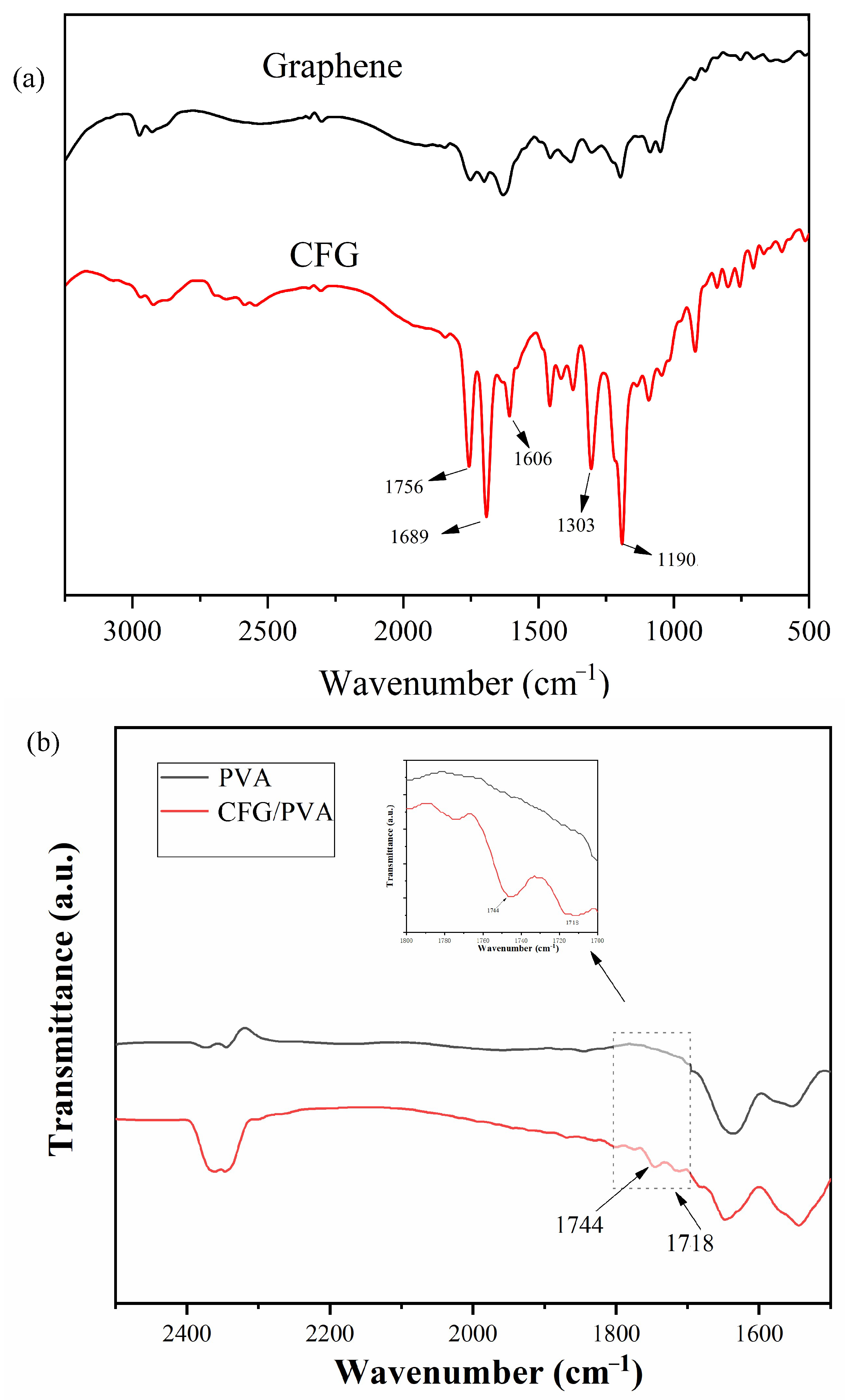
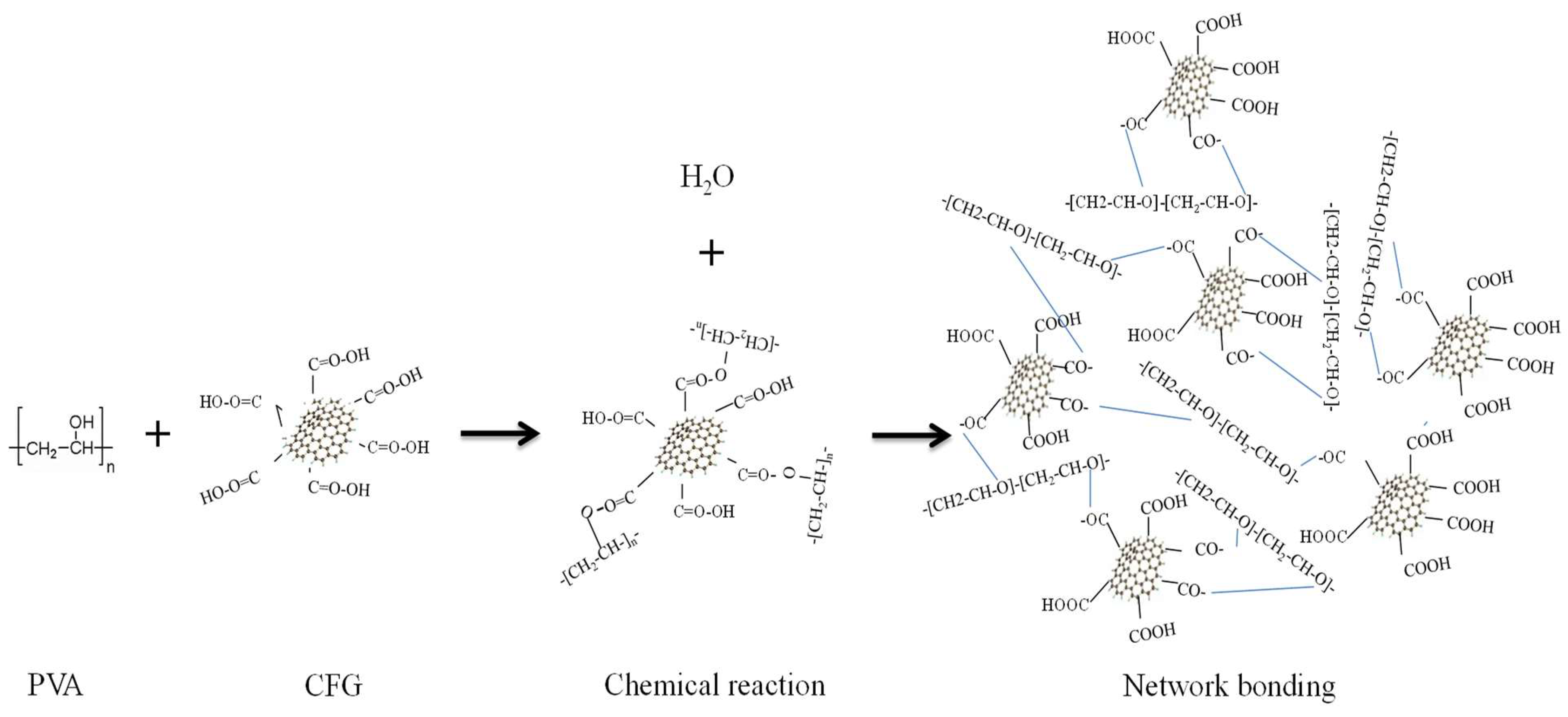
3.3. FTIR
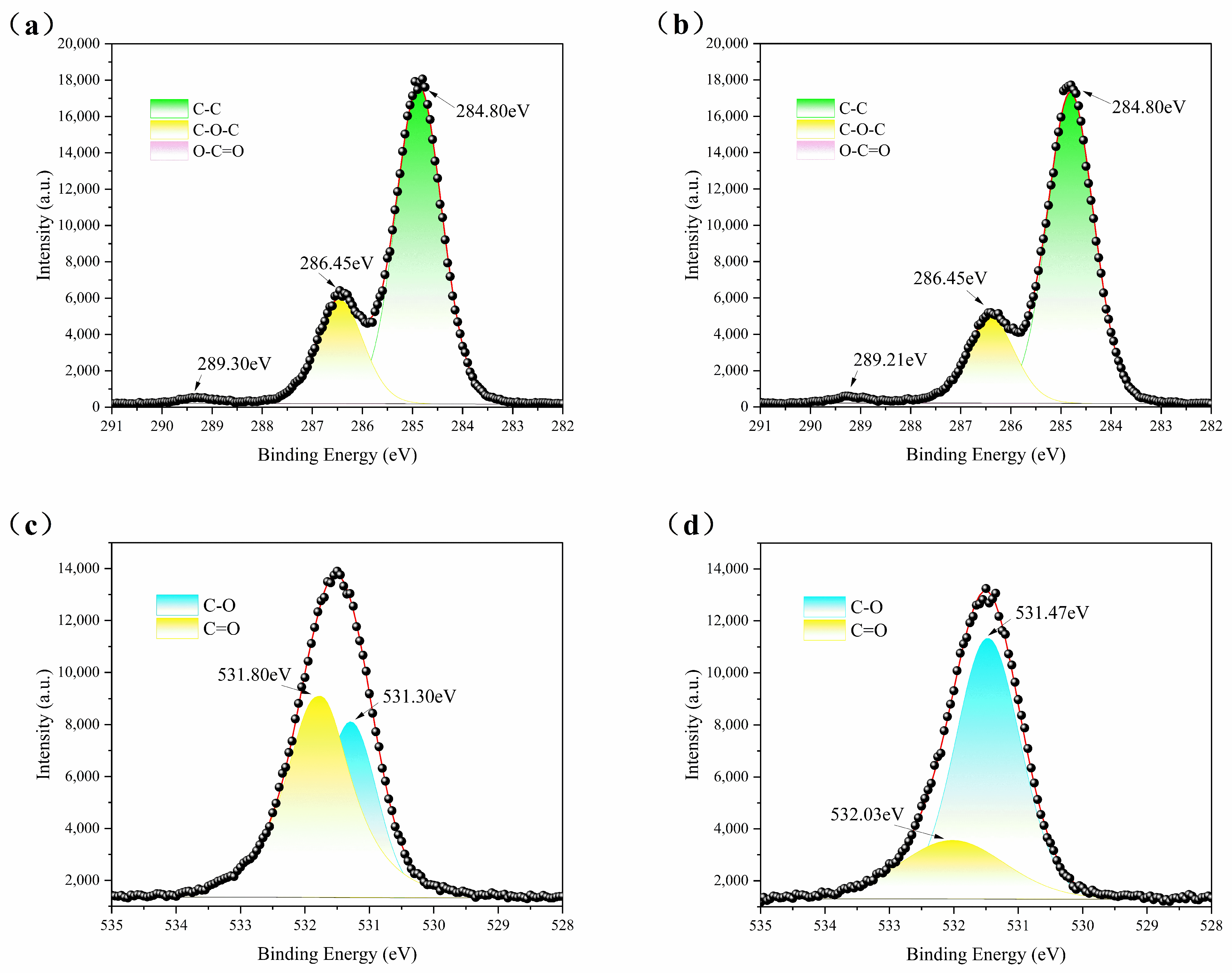
3.4. X-ray Photoelectron Spectroscopy (XPS)
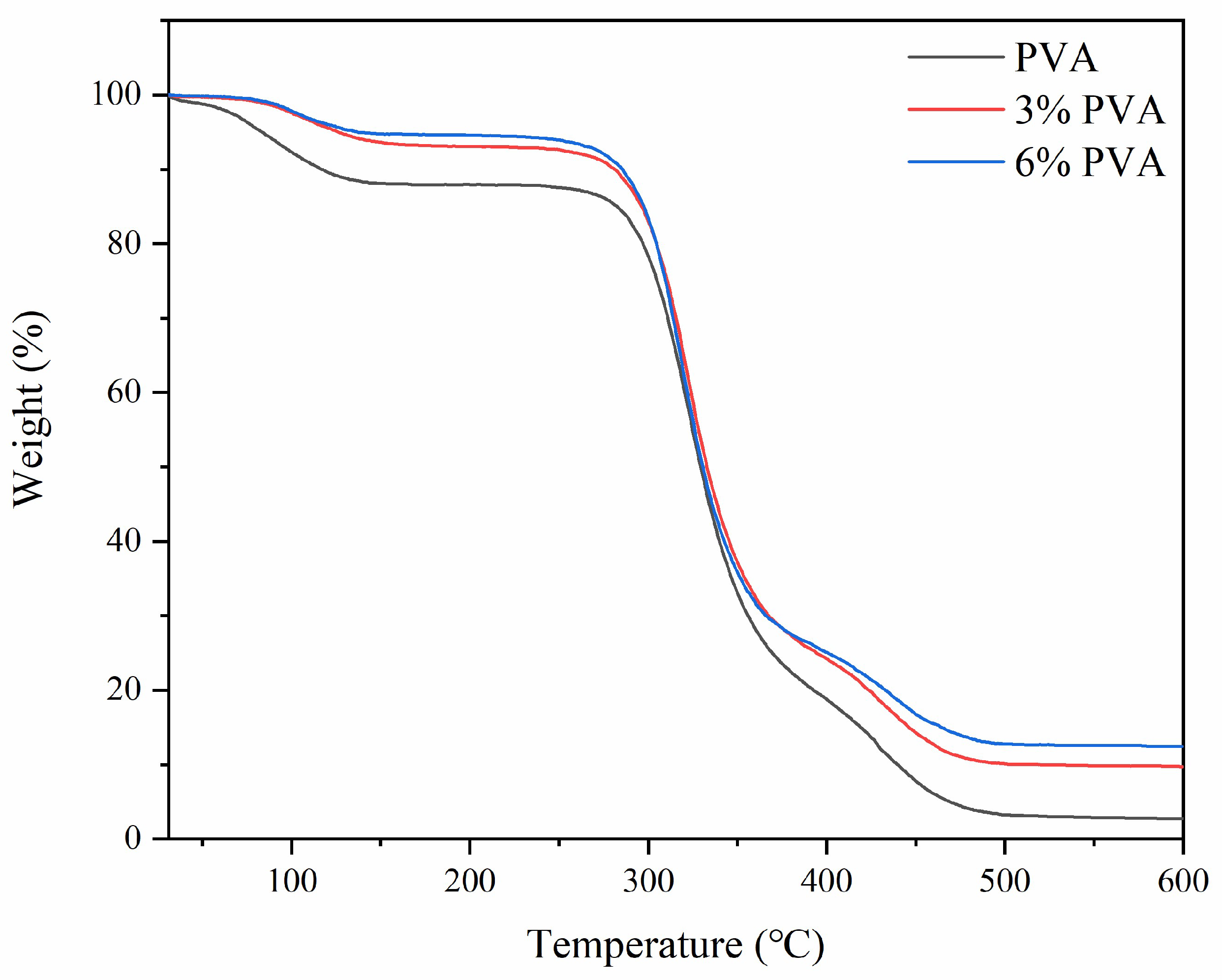
3.5. TGA
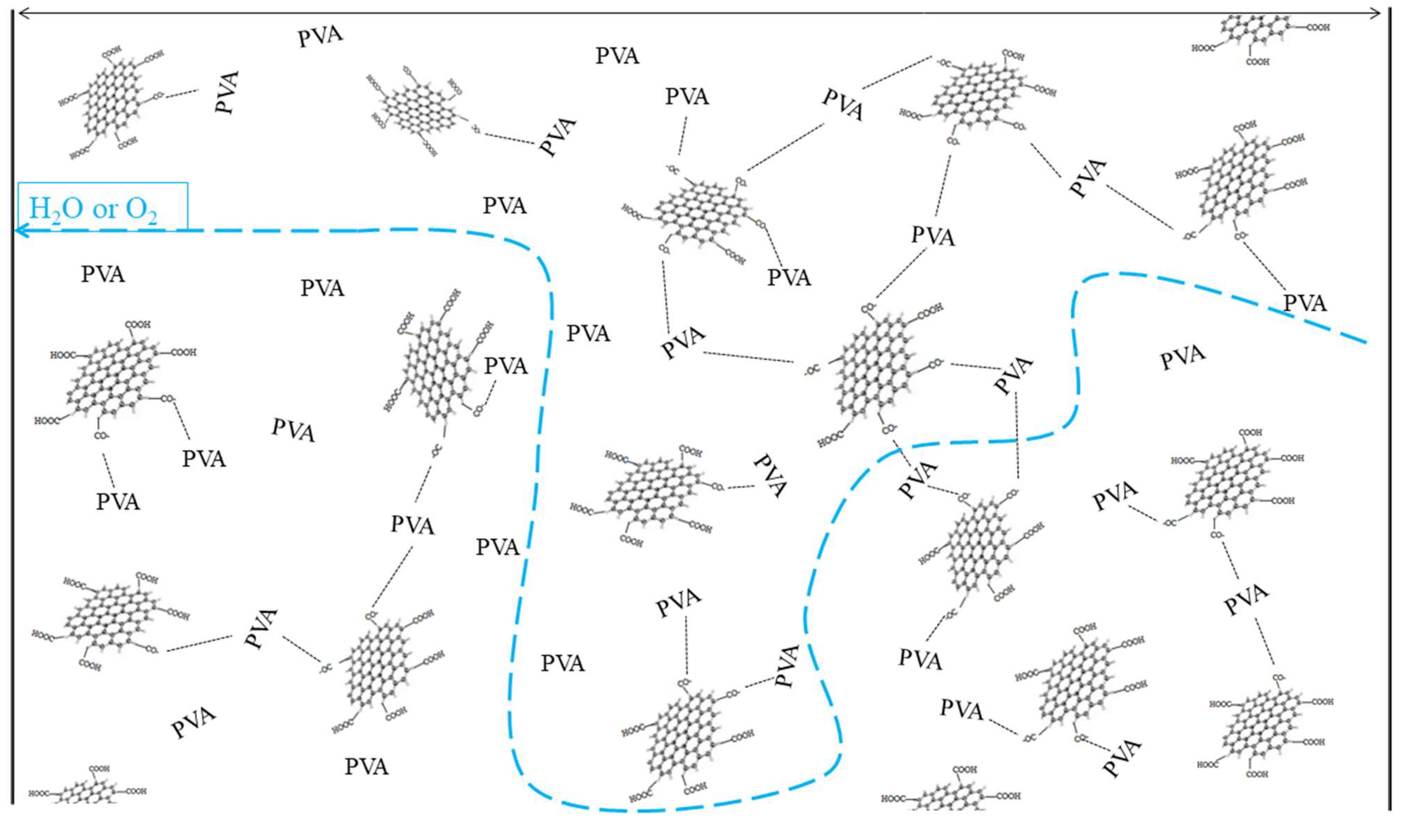
3.6. Water Vapor Barrier Properties
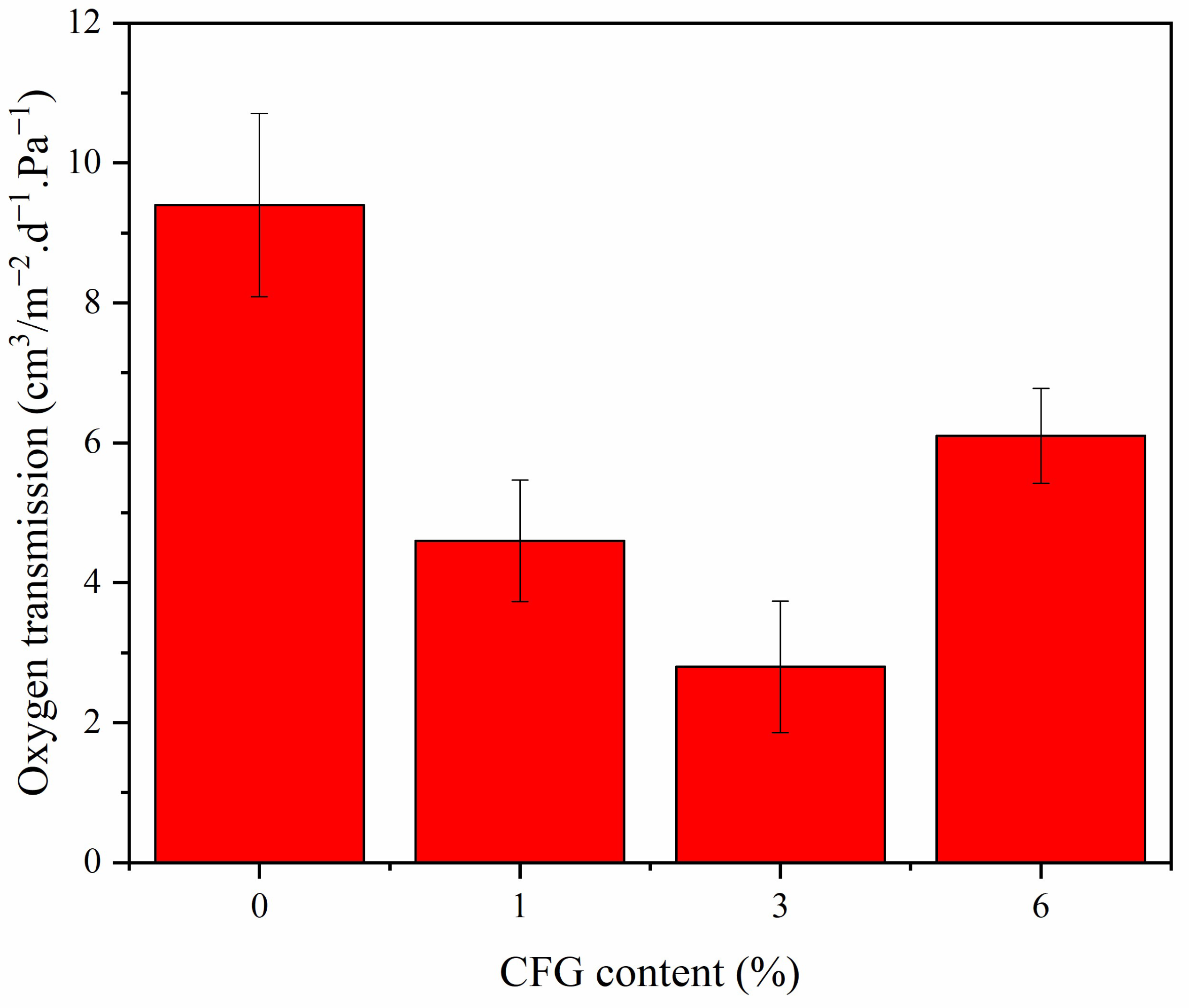
3.7. Oxygen Barrier Properties
| Sample | Oxygen Barrier Performance (%) | Water Vapor Barrier Performance (%) | Reference | ||
|---|---|---|---|---|---|
| Oxygen Transmission (cm3/m−2·d−1·Pa−1) | Permeability Reduction Factor (Times) | Water Vapor Transmission (g/m2/day) | Permeability Reduction Factor (Times) | ||
| PVA/CFG | 2.8 | 3.36 | 226.8 | 2.2 | This work |
| PVA/GO | 4 | 2.225 | n/a | n/a | [71] |
| PVA/GO | 107.14 | 1.07 | n/a | n/a | [72] |
| PVA/GO/CuSO4 5H2O | 4.2 | 2.476 | n/a | n/a | [73] |
| PVA/rGO | 2.98 | 2.48 | n/a | n/a | [74] |
| PVA/CNF/GO | 3 | 1.65 | n/a | n/a | [75] |
3.8. Scanning Electron Microscope (SEM) Analysis
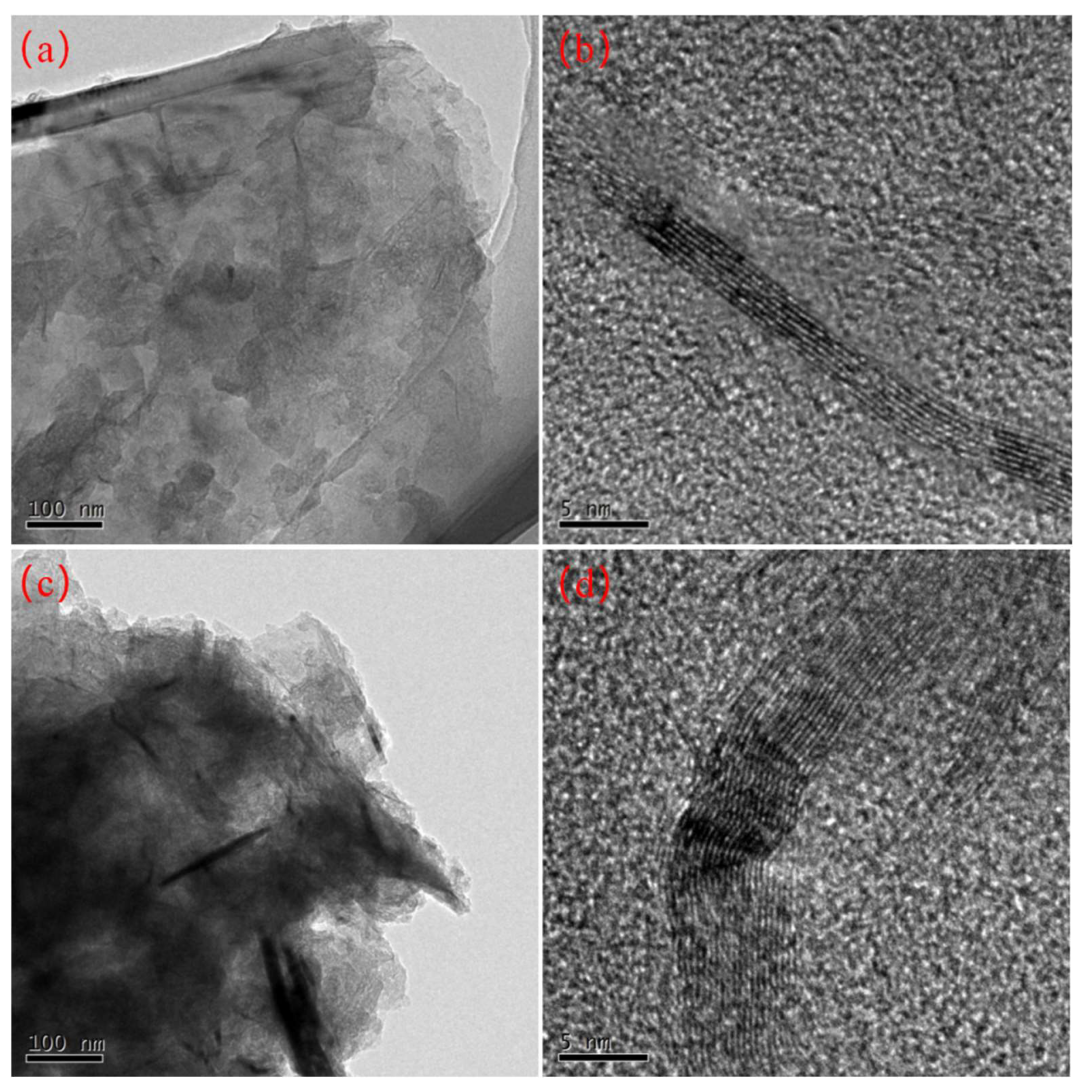
3.9. Transmission Electron Microscope (TEM) Analysis
3.10. Hydrophilicity
3.11. Conductivity
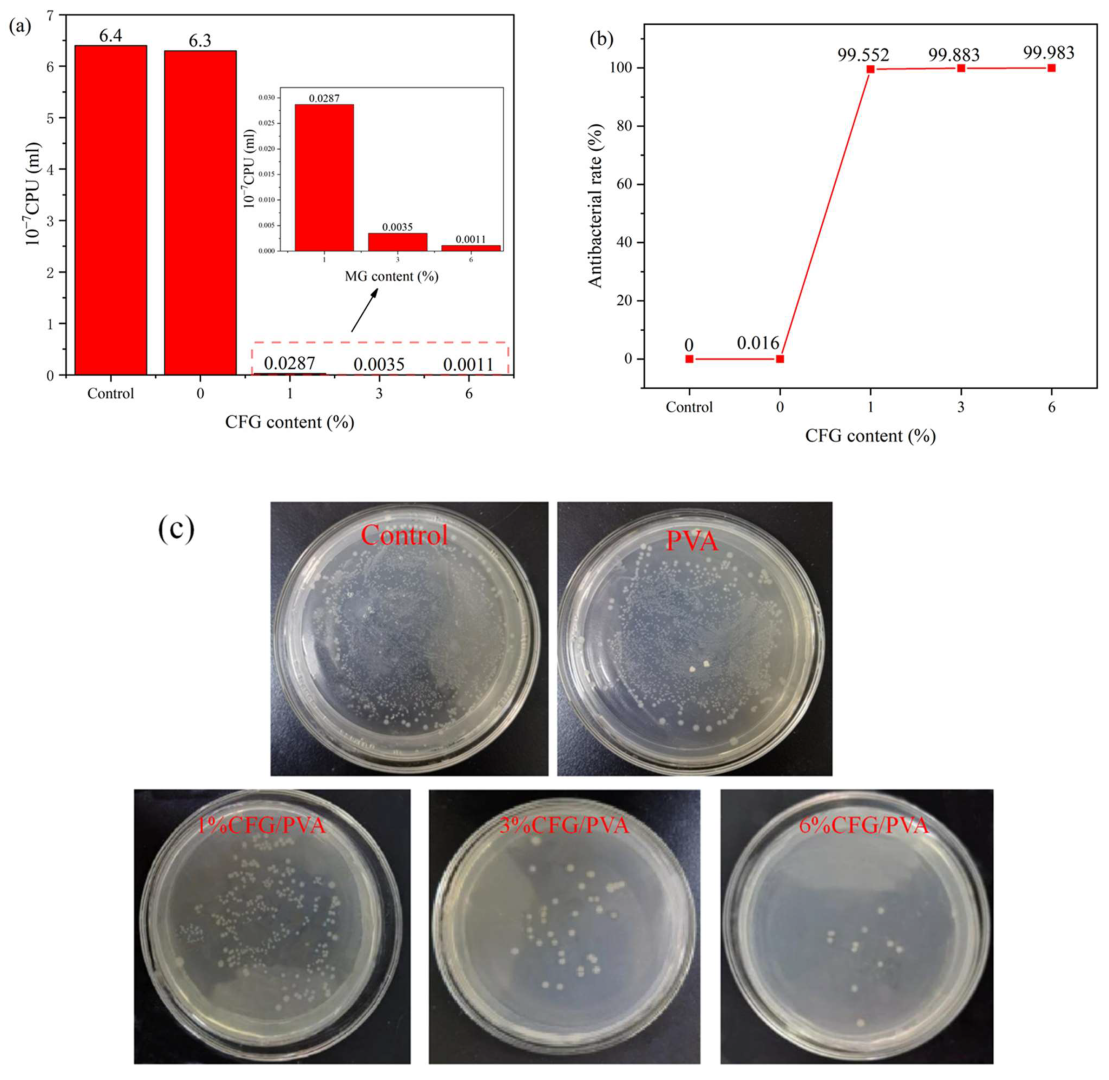
3.12. Antibacterial Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tsou, C.H.; Wu, C.S.; Hung, W.S.; De Guzman, M.R.; Gao, C.; Wang, R.Y.; Suen, M.C. Rendering polypropylene biocomposites antibacterial through modification with oyster shell powder. Polymer 2019, 160, 265–271. [Google Scholar] [CrossRef]
- Luo, X.L.; Xu, J.J.; Wang, J.L.; Chen, H.Y. Electrochemically deposited nanocomposite of chitosan and carbon nanotubes for biosensor application. Chem. Commun. 2005, 16, 2169–2171. [Google Scholar] [CrossRef] [PubMed]
- Tsou, C.H.; Yao, W.H.; Lu, Y.C.; Tsou, C.Y.; Wu, C.S.; Chen, J.; Wang, R.; Su, C.C.; Hung, W.S.; De Guzman, M.R.; et al. Antibacterial property and cytotoxicity of a poly (lactic acid)/nanosilver-doped multiwall carbon nanotube nanocomposite. Polymers 2019, 9, 100. [Google Scholar] [CrossRef]
- Lee, S.H.; Choi, S.H.; Kim, S.Y.; Choi, J.I.; Lee, J.R.; Youn, J.R. Degradation and Dynamic Properties of Poly(amide-co-imide)/Carbon Nanotube Composite Films. Polym. Polym. Compos. 2010, 18, 381–390. [Google Scholar] [CrossRef]
- Kuan, C.F.; Chiang, C.L.; Lin, S.H.; Huang, W.G.; Hsieh, W.Y.; Shen, M.Y. Characterization and properties of graphene nanoplatelets/XNBR nanocomposites. Polym. Polym. Compos. 2018, 26, 59–68. [Google Scholar] [CrossRef]
- Anwer, T.; Mohammad, F. Thermal stability of electrical properties and amine vapour sensitivity of in-situ prepared polyaniline/graphene nanocomposites assisted by sodium dodecyl sulfate micelles. Polym. Polym. Compos. 2015, 23, 261–270. [Google Scholar] [CrossRef]
- Bian, J.; Lin, H.L.; Wang, G.; Zhou, Q.; Wang, Z.J.; Zhou, X.; Lu, Y.; Zhao, X.W. Morphological, mechanical and thermal properties of chemically bonded graphene oxide nanocomposites with biodegradable poly (3-hydroxybutyrate) by solution intercalation. Polym. Polym. Compos. 2016, 24, 133–141. [Google Scholar] [CrossRef]
- Xu, B.; Tanaka, S.I. Formation of a new electric material: Fullerene/metal polycrystalline film. MRS Online Proc. Libr. 1997, 472, 179. [Google Scholar] [CrossRef]
- Yu, Z.; Meng, J.; Li, Y.; Li, Y. Efficient photocatalytic hydrogen production from water over a CuO and carbon fiber comodified TiO2 nanocomposite photocatalyst. Int. J. Hydrogen Energy 2013, 38, 16649–16655. [Google Scholar] [CrossRef]
- Qureshi, A.; Mergen, A.; Eroğlu, M.S.; Singh, N.L.; Güllüoğlu, A. Dielectric properties of polymer composites filled with different metals. J. Macromol. Sci. Part. A 2008, 45, 462–469. [Google Scholar] [CrossRef]
- Akhavan, A.; Khoylou, F.; Ataeivarjovi, E. Preparation and characterization of gamma irradiated Starch/PVA/ZnO nanocomposite films. Radiat. Phys. Chem. 2017, 138, 49–53. [Google Scholar] [CrossRef]
- Rusu, M.; Sofian, N.; Ibanescu, C.; Rusu, D. Mechanical and thermal properties of copper-powder-filled high density polyethylene composites. Polym. Polym. Compos. 2000, 8, 427–435. [Google Scholar]
- Lee, J.S.; Choi, Y.J.; Park, H.H.; Chul Pyun, J. Electrochromic properties of poly (3,4-ethylenedioxythiophene) nanocomposite film containing SiO2 nanoparticles. J. Appl. Polym. Sci. 2011, 122, 3080–3085. [Google Scholar] [CrossRef]
- Ou, C.F.; Shen, P.H. Thermal and gas barrier properties of COC/SiO2 nanocomposites. Polym. Polym. Compos. 2012, 20, 537–544. [Google Scholar] [CrossRef]
- Poisson, C.; Guerengomba, J.; Lacrampe, M.F.; Krawczak, P.; Gupta, B.; Miri, V.; Lefebvre, J.M. Mechanical, optical and barrier properties of PA6/nanoclay-based single-and multilayer blown films. Polym. Polym. Compos. 2008, 16, 349–358. [Google Scholar] [CrossRef]
- Zhang, J.; Ji, Q.; Shen, X.; Xia, Y.; Tan, L.; Wang, F.; Kong, Q. Flame Retardancy and Non-isothermal Crystallization Behaviour of PET/TiO2 Nanocomposites. Polym. Polym. Compos. 2012, 20, 399–406. [Google Scholar] [CrossRef]
- Ramesan, M.T. Poly (Ethylene-Co-Vinyl Acetate)/Magnetite Nanocomposites: Interaction of Some Liquid Fuels, Thermal and Oil Resistance Studies. Polym. Polym. Compos. 2015, 23, 85–92. [Google Scholar] [CrossRef]
- Farahmandjou, M.; Golabiyan, N. Synthesis and characterisation of Al2O3 nanoparticles as catalyst prepared by polymer co-precipitation method. Mater. Eng. Res. 2019, 1, 40–44. [Google Scholar] [CrossRef]
- Chen, Y.H.; Yao, X.Y.; Pan, Z.J.; Gu, Q. Preparation and isothermal crystallization behavior of poly (lactic acid)/graphene nanocomposites. Adv. Mater. Res. 2011, 284, 246–252. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, L.-j.; Wang, Y.-x.; Tang, J.-g.; Wang, Y.; Cheng, M.-m.; Du, Y.-c.; Yang, K.; Kipper, M.J.; Hedayati, M. The preparation and study of ethylene glycol-modified graphene oxide membranes for water purification. Polymers 2019, 11, 188. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Kishi, N.; Sugita, R.; Fukaya, A.; Khatri, I.; Liang, J.; Jimbo, T. Graphene synthesis by thermal chemical vapor deposition using solid precursor. J. Mater. Sci. Mater. Electron. 2013, 24, 2151–2155. [Google Scholar] [CrossRef]
- Fan, Y.C.; Wang, L.J.; Jiang, W. Graphene based oxide ceramic composites with high mechanical and functional performance: From preparation to property. J. Inorg. Mater. 2018, 33, 138. [Google Scholar] [CrossRef]
- Al-Tikrity, E.T.B.; Waheed, I.F.; Ali, S.M. Study of electrical properties of a reduced graphene-oxadiazole-2-thiol (rGS) PVA polymer composite. Polym. Polym. Compos. 2019, 27, 11–19. [Google Scholar] [CrossRef]
- Alizadeh, T.; Ahmadian, F. Thiourea-treated graphene aerogel as a highly selective gas sensor for sensing of trace level of ammonia. Anal. Chim. Acta 2015, 897, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, H. Doped graphene as a superconductor. Phys. Lett. A 2010, 29, 2953–2956. [Google Scholar] [CrossRef]
- Tan, L.; Zhang, Y.C.; Wang, B.; Luo, H.M.; Feng, H.X. Reduced Graphene Oxide as a Metal-Free Carbocatalyst for Polymerization of 1-Naphthylamine. ChemPlusChem 2014, 79, 929–935. [Google Scholar] [CrossRef]
- Song, E.; Han, W.; Li, C.; Cheng, D.; Li, L.; Liu, L.; Zhu, G.; Song, Y.; Tan, W. Hyaluronic acid-decorated graphene oxide nanohybrids as nanocarriers for targeted and pH-responsive anticancer drug delivery. ACS Appl. Mater. Interfaces 2014, 6, 11882–11890. [Google Scholar] [CrossRef] [PubMed]
- Zamharir, M.J.; Asl, M.S.; Kakroudi, M.G.; Vafa, N.P.; Zamharir, M.J. Significance of hot pressing parameters and reinforcement size on sinterability and mechanical properties of ZrB2–25 vol% SiC UHTCs. Ceram. Int. 2015, 41, 9628–9636. [Google Scholar] [CrossRef]
- Tsou, C.H.; Yao, W.H.; Hung, W.S.; Suen, M.C.; De Guzman, M.R.; Chen, J.; Tsou, C.Y.; Wang, R.Y.; Chen, J.C.; Wu, C.S. Innovative plasma process of grafting methyl diallyl ammonium salt onto polypropylene to impart antibacterial and hydrophilic surface properties. Ind. Eng. Chem. Res. 2018, 57, 2537–2545. [Google Scholar] [CrossRef]
- Ahmad, J.; Burduhos-Nergis, D.D.; Arbili, M.M.; Alogla, S.M.; Majdi, A.; Deifalla, A.F. A review on failure modes and cracking behaviors of polypropylene fibers reinforced concrete. Buildings 2022, 12, 1951. [Google Scholar] [CrossRef]
- De Albuquerque, T.L.; Júnior, J.E.M.; de Queiroz, L.P.; Ricardo, A.D.S.; Rocha, M.V.P. Polylactic acid production from biotechnological routes: A review. Int. J. Biol. Macromol. 2021, 186, 933–951. [Google Scholar] [CrossRef] [PubMed]
- Ilyas, R.A.; Zuhri, M.Y.M.; Aisyah, H.A.; Asyraf, M.R.M.; Hassan, S.A.; Zainudin, E.S.; Sari, N.H. Natural fiber-reinforced polylactic acid, polylactic acid blends and their composites for advanced applications. Polymers 2022, 14, 202. [Google Scholar] [CrossRef] [PubMed]
- Tsou, C.Y.; Wu, C.L.; Tsou, C.H.; Chiu, S.H.; Suen, M.C.; Hung, W.S. Biodegradable composition of poly (lactic acid) from renewable wood flour. Polym. Sci. Ser. B 2015, 57, 473–480. [Google Scholar] [CrossRef]
- Tsou, C.H.; Kao, B.J.; Suen, M.C.; Yang, M.C.; Wu, T.Y.; Tsou, C.Y.; Lai, J.Y. Crystallisation behaviour and biocompatibility of poly (butylene succinate)/poly (lactic acid) composites. Mater. Res. Innov. 2014, 18 (Suppl. S2), 372–376. [Google Scholar] [CrossRef]
- Mittal, A.; Garg, S.; Premi, A.; Giri, A.S. Synthesis of polyvinyl alcohol/modified starch-based biodegradable nanocomposite films reinforced with starch nanocrystals for packaging applications. Polym. Polym. Compos. 2021, 29, 405–416. [Google Scholar] [CrossRef]
- Al-Muntaser, A.A.; Pashameah, R.A.; Sharma, K.; Alzahrani, E.; Farea, M.O.; Morsi, M.A. α-MoO3 nanobelts/CMC-PVA nanocomposites: Hybrid materials for optoelectronic and dielectric applications. J. Polym. Res. 2022, 29, 274. [Google Scholar] [CrossRef]
- Afzal, H.M.; Iqbal MItu, S.S.; Al-Harthi, M.A. Microwave radiations effect on electrical and mechanical properties of poly (vinyl alcohol) and PVA/graphene nanocomposites. Surf. Interfaces 2018, 13, 65. [Google Scholar] [CrossRef]
- Chen, W.; Wu, K.; Liu, Q.; Lu, M. Functionalization of graphite via Diels-Alder reaction to fabricate poly (vinyl alcohol) composite with enhanced thermal conductivity. Polymer 2020, 186, 122075. [Google Scholar] [CrossRef]
- Mahendia, S.; Chahal, R.P.; Munjal, N.; Kumar, S. Graphene-Polyvinyl Alcohol (PVA) Composites with Enhanced Conductivity: A Comparative Study of Various Synthesis Approaches. Adv. Electrochem. 2014, 2, 50–54. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, K.; Luo, F.; Lu, M. Significantly enhanced thermal conductivity in polyvinyl alcohol composites enabled by dopamine modified graphene nanoplatelets. Compos. Part. A Appl. Sci. Manuf. 2019, 117, 134–143. [Google Scholar] [CrossRef]
- Morimune, S.; Nishino, T.; Goto, T. Poly (vinyl alcohol)/graphene oxide nanocomposites prepared by a simple eco-process. Polym. J. 2012, 44, 1056–1063. [Google Scholar] [CrossRef]
- Li, K.M.; Dong, H.; Lin, H.; Zhang, J.K. Study on the thermal conductivity of graphene oxide/polyvinyl alcohol composites. J. Eng. Therm. Energy Power 2017, 32, 14. Available online: https://www.cqvip.com/qk/94195x/201709/673311959.html (accessed on 25 May 2023).
- Chen, Z.C.; Chang, T.L.; Pan, T.C.; Chiang, D.; Tseng, S.F. A facile approach to fabrication and characterization of conductive conjugated polyvinyl alcohol/graphene composite nanofibers. Mater. Lett. 2018, 233, 130–133. [Google Scholar] [CrossRef]
- Peng, Y.Y.; Hsieh, T.E.; Hsu, C.H. White-light emitting ZnO–SiO2 nanocomposite thin films prepared by the target-attached sputtering method. Nanotechnology 2005, 17, 174. [Google Scholar] [CrossRef]
- Tamulevičius, T.; Peckus, D.; Tamulevičiene, A.; Vasiliauskas, A.; Čiegis, A.; Meškinis, Š.; Tamulevičius, S. Dynamic optical properties of amorphous diamond-like carbon nanocomposite films doped with Cu and Ag nanoparticles. In Proceedings of the Plasmonics: Metallic Nanostructures and Their Optical Properties XII, San Diego, CA, USA, 17 August 2014; Volume 9163, pp. 254–258. [Google Scholar] [CrossRef]
- Zahid, M.; Ali, S.; Saleem, S.; Salman, M.; Khan, M. Carbon nanoparticles/polyvinyl alcohol composites with enhanced optical, thermal, mechanical, and flame-retardant properties. J. Appl. Polym. Sci. 2020, 137, 49261. [Google Scholar] [CrossRef]
- Dragoman, M.; Modreanu, M.; Povey, I.M.; Dinescu, A.; Dragoman, D. Reconfigurable horizontal–vertical carrier transport in graphene/HfZrO field-effect transistors. Nanotechnology 2019, 31, 025203. [Google Scholar] [CrossRef]
- Kim, S.; Shimazu, J.; Fukaminato, T.; Ogata, T.; Kurihara, S. Thermal conductivity of graphene oxide-enhanced polyvinyl alcohol composites depending on molecular interaction. Polymer 2017, 129, 201–206. [Google Scholar] [CrossRef]
- Park, G.T.; Chang, J.H. Comparison of Properties of PVA Nanocomposites Containing Reduced Graphene Oxide and Functionalized Graphene. Polymers 2019, 11, 450. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, X.; Xu, C.; Zhang, M.; Shang, X. Preparation of graphene/poly (vinyl alcohol) nanocomposites with enhanced mechanical properties and water resistance. Polym. Int. 2011, 60, 816–822. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, Q.; Chen, D.J.; Lu, P. Enhanced Mechanical Properties of Graphene-Based Poly(vinyl alcohol) Composites. Macromolecules 2010, 43, 2357–2363. [Google Scholar] [CrossRef]
- Tao, C.A.; Zhang, H.; Huang, J.; Zou, X.; Zhu, H.; Wang, J. Reduction versus cross-linking: How to improve the tensile strength of graphene oxide/polyvinyl alcohol composite film. Mater. Res. Express 2017, 4, 085601. [Google Scholar] [CrossRef]
- Park, J.H.; Kim, I.K.; Choi, J.Y.; Karim, M.R.; Cheong, I.W.; Oh, W.; Yeum, J.H. Electrospinning Fabrication of polyvinyl alcohol)/waterborne polyurethane/silver composite nanofibre mats in aqueous solution for anti-bacterial exploits. Polym. Polym. Compos. 2011, 19, 753–762. [Google Scholar] [CrossRef]
- Hu, H.; Chen, G. Electrochemically modified graphite nanosheets and their nanocomposite films with poly (vinyl alcohol). Polym. Compos. 2010, 31, 1770–1775. [Google Scholar] [CrossRef]
- Pope, C.G. X-ray diffraction and the Bragg equation. J. Chem. Educ. 1997, 74, 129. [Google Scholar] [CrossRef]
- Jung, S.Y.; Paik, K.W. Effects of alignment of graphene flakes on water permeability of graphene-epoxy composite film. In Proceedings of the 2014 IEEE 64th Electronic Components and Technology Conference (ECTC), Orlando, FL, USA, 27–30 May 2014; pp. 2255–2259. Available online: https://ieeexplore.ieee.org/document/6897618 (accessed on 10 July 2020).
- Mehta, J.; Vinayak, P.; Tuteja, S.K.; Chhabra, V.A.; Bhardwaj, N.; Paul, A.K.; Deep, A. Graphene modified screen printed immunosensor for highly sensitive detection of parathion. Biosens. Bioelectron. 2016, 83, 339–346. [Google Scholar] [CrossRef]
- Pfaffeneder-Kmen, M.; Casas, I.F.; Naghilou, A.; Trettenhahn, G.; Kautek, W. A Multivariate Curve Resolution evaluation of an in-situ ATR-FTIR spectroscopy investigation of the electrochemical reduction of graphene oxide. Electrochim. Acta 2017, 255, 160–167. [Google Scholar] [CrossRef]
- Mawhinney, D.B.; Yates, J.T., Jr. FTIR study of the oxidation of amorphous carbon by ozone at 300 K—Direct COOH formation. Carbon 2001, 39, 1167–1173. [Google Scholar] [CrossRef]
- Vineeth, S.K.; Gadhave, R.V.; Gadekar, P.T. Polyvinyl alcohol–cellulose blend wood adhesive modified by citric acid and its effect on physical, thermal, mechanical and performance properties. Polym. Bull. 2023, 80, 8013–8030. [Google Scholar] [CrossRef]
- Bian, Y.; Colin, X.; Aressy, M. Thermal aging of high tenacity polyvinyl alcohol yarns. Polym. Degrad. Stab. 2020, 179, 109277. [Google Scholar] [CrossRef]
- Greczynski, G.; Hultman, L. The same chemical state of carbon gives rise to two peaks in X-ray photoelectron spectroscopy. Sci. Rep. 2021, 11, 11195. [Google Scholar] [CrossRef]
- Isaacs, M.A.; Davies-Jones, J.; Davies, P.R.; Guan, S.; Lee, R.; Morgan, D.J.; Palgrave, R. Advanced XPS characterization: XPS-based multi-technique analyses for comprehensive understanding of functional materials. Mater. Chem. Front. 2021, 5, 7931–7963. [Google Scholar] [CrossRef]
- Cortez-Lemus, N.A.; Salgado-Rodríguez, R.; Licea-Claveríe, A. Preparation of α, ω-telechelic hexyl acrylate polymers with —OH, —COOH, and —NH2 functional groups by RAFT. J. Polym. Sci. Part. A Polym. Chem. 2010, 48, 3033–3051. [Google Scholar] [CrossRef]
- Mirabedini, S.M.; Zareanshahraki, F.; Mannari, V. Enhancing thermoplastic road-marking paints performance using sustainable rosin ester. Prog. Org. Coat. 2020, 139, 105454. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, J.; Zheng, Y.; Wei, H.; Su, Z. Graphene-based hybrid aerogels for energy and environmental applications. Chem. Eng. J. 2021, 420, 129700. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Jiang, H.; Xu, L.; Cheng, Y.; Wang, P.G.; Wang, F. Preparation, antiangiogenic and antitumoral activities of the chemically sulfated glucan from Phellinus ribis. Carbohydr. Polym. 2014, 106, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Vitale, A.; Merlo, S.; Rizza, G.; Melilli, G.; Sangermano, M. UV Curing of Perfluoropolyether Oligomers Containing Graphene Nanosheets to Enhance Water-Vapor Barrier Properties. Macromol. Chem. Phys. 2014, 215, 1588–1592. [Google Scholar] [CrossRef]
- Yoo, B.M.; Shin, H.J.; Yoon, H.W.; Park, H.B. Graphene and graphene oxide and their uses in barrier polymers. J. Appl. Polym. Sci. 2014, 131, 39628. [Google Scholar] [CrossRef]
- Tsou, C.H.; Ge, F.F.; Lin, L.; Yuan, S.; De Guzman, M.R.; Potiyaraj, P. Barrier and Biodegradable Properties of Poly (butylene adipate-co-terephthalate) Reinforced with ZnO-Decorated Graphene Rendering it Antibacterial. ACS Appl. Polym. Mater. 2023, 5, 1681–1695. [Google Scholar] [CrossRef]
- Bian, Q.; Tian, H.; Wang, Y.; Liu, Q.; Ge, X.; Rajulu, A.V. Effect of graphene oxide on the structure and properties of poly (vinyl alcohol) composite films. Polym. Sci. Ser. A 2015, 57, 836–844. [Google Scholar] [CrossRef]
- Liu, D.; Bian, Q.; Li, Y.; Wang, Y.; Tian, H. Effect of oxidation degrees of graphene oxide on the structure and properties of poly (vinyl alcohol) composite films. Compos. Sci. Technol. 2016, 129, 146–152. [Google Scholar] [CrossRef]
- Liu, Q.; Ge, X.; Tian, H. Effect of copper sulfate pentahydrate on the structure and properties of poly (vinyl alcohol)/graphene oxide composite films. J. Appl. Polym. Sci. 2016, 133, 44135. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Chen, Y.; Guo, L.; Wang, Q.; Xu, C. Preparation and properties of functional graphene/polyvinyl alcohol composite films. J. For. Eng. 2018, 3, 109–115. [Google Scholar]
- Genorio, B.; Harrison, K.L.; Connell, J.G.; Dražić, G.; Zavadil, K.R.; Markovic, N.M.; Strmcnik, D. Tuning the selectivity and activity of electrochemical interfaces with defective graphene oxide and reduced graphene oxide. ACS Appl. Mater. Interfaces 2019, 11, 34517–34525. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.V.; Bee, S.T.; Tin Sin, L.; Ratnam, C.T.; Abdul Hamid, Z.A. A review on the synthesis, properties, and utilities of functionalized carbon nanoparticles for polymer nanocomposites. Polymers 2021, 13, 3547. [Google Scholar] [CrossRef] [PubMed]
- Dorigato, A.; Dzenis, Y.; Pegoretti, A. Filler aggregation as a reinforcement mechanism in polymer nanocomposites. Mech. Mater. 2013, 61, 79–90. [Google Scholar] [CrossRef]
- Wen, Y.H.; Tsou, C.H.; de Guzman, M.R.; Huang, D.; Yu, Y.Q.; Gao, C.; Wang, Z.H. Antibacterial nanocomposite films of poly (vinyl alcohol) modified with zinc oxide-doped multiwalled carbon nanotubes as food packaging. Polym. Bull. 2022, 79, 3847–3866. [Google Scholar] [CrossRef]
- Salavagione, H.J.; Martínez, G.; Gómez, R.; Segura, J.L. Synthesis of water-soluble perylenediimide-functionalized polymer through esterification with poly (vinyl alcohol). J. Polym. Sci. Part. A Polym. Chem. 2010, 48, 3613–3622. [Google Scholar] [CrossRef]
- Kapourani, A.; Chachlioutaki, K.; Andriotis, E.G.; Fatouros, D.G.; Barmpalexis, P. Evaluating PAA/PVA thermal crosslinking process during the preparation of in-situ high-drug loading amorphous solid dispersions. J. Drug Deliv. Sci. Technol. 2023, 79, 104030. [Google Scholar] [CrossRef]
- Van der Schueren, B.; El Marouazi, H.; Mohanty, A.; Lévêque, P.; Sutter, C.; Romero, T.; Janowska, I. Polyvinyl alcohol-few layer graphene composite films prepared from aqueous colloids. Investigations of mechanical, conductive and gas barrier properties. Nanomaterials 2020, 10, 858. [Google Scholar] [CrossRef] [PubMed]
- Hwang, E.H.; Sarma, S.D. Screening-induced temperature-dependent transport in two-dimensional graphene. Phys. Rev. B 2009, 79, 165404. [Google Scholar] [CrossRef]
- Li, J.; Wang, G.; Zhu, H.; Zhang, M.; Zheng, X.; Di, Z.; Wang, X. Antibacterial activity of large-area monolayer graphene film manipulated by charge transfer. Sci. Rep. 2014, 4, 4359. [Google Scholar] [CrossRef]
- Bao, Y.; Wei, C.; Su, E.; Zhang, Q.; Bai, Y. Study on the synthesis and antibacterial properties of silver-chitosan composite. Polym. Polym. Compos. 2013, 21, 519–524. [Google Scholar] [CrossRef]
- Mallakpour, S.; Abdolmaleki, A.; Khalesi, Z. Fabrication and physicochemical features study of crosslinked PVA/FGO nanocomposite films. Polym. Bull. 2018, 75, 1473–1486. [Google Scholar] [CrossRef]
- Wu, H.; Drzal, L.T. Graphene nanoplatelet paper as a light-weight composite with excellent electrical and thermal conductivity and good gas barrier properties. Carbon 2012, 50, 1135–1145. [Google Scholar] [CrossRef]
- Yao, Y.L.; De Guzman, M.R.; Duan, H.; Gao, C.; Lin, X.; Wen, Y.H.; Tsou, C.H. Infusing high-density polyethylene with graphene-zinc oxide to produce antibacterial nanocomposites with improved properties. Chin. J. Polym. Sci. 2020, 38, 898–907. [Google Scholar] [CrossRef]
- Ma, Z.L.; Tsou, C.H.; Yao, Y.L.; De Guzman, M.R.; Wu, C.S.; Gao, C.; Yang, T.; Chen, Z.J.; Zeng, R.; Li, Y.; et al. Thermal properties and barrier performance of antibacterial high-density polyethylene reinforced with carboxyl graphene-grafted modified high-density polyethylene. Ind. Eng. Chem. Res. 2021, 60, 12911–12922. [Google Scholar] [CrossRef]
- Liu, S.; Zeng, T.H.; Hofmann, M.; Burcombe, E.; Wei, J.; Jiang, R.; Chen, Y. Antibacterial activity of graphite, graphite oxide, graphene oxide, and reduced graphene oxide: Membrane and oxidative stress. ACS Nano 2011, 5, 6971–6980. [Google Scholar] [CrossRef] [PubMed]
- Akhavan, O.; Ghaderi, E. Enhancement of antibacterial properties of graphene sheets decorated with silver nanoparticles. J. Mater. Chem. 2010, 20, 8671–8676. [Google Scholar]





| Sample | PVA (%) | CFG (%) | Graphene (%) |
|---|---|---|---|
| PVA | 100 | 0 | 0 |
| 1% CFG/PVA | 99 | 1 | 0 |
| 3% CFG/PVA | 97 | 3 | 0 |
| 6% CFG/PVA | 94 | 6 | 0 |
| 1% graphene/PVA | 99 | 0 | 1 |
| 3% graphene/PVA | 97 | 0 | 3 |
| 6% graphene/PVA | 94 | 0 | 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shui, Y.-J.; Yao, W.-H.; Lin, J.-H.; Zhang, Y.; Yu, Y.; Wu, C.-S.; Zhang, X.; Tsou, C.-H. Enhancing Polyvinyl Alcohol Nanocomposites with Carboxy-Functionalized Graphene: An In-Depth Analysis of Mechanical, Barrier, Electrical, Antibacterial, and Chemical Properties. Polymers 2024, 16, 1070. https://doi.org/10.3390/polym16081070
Shui Y-J, Yao W-H, Lin J-H, Zhang Y, Yu Y, Wu C-S, Zhang X, Tsou C-H. Enhancing Polyvinyl Alcohol Nanocomposites with Carboxy-Functionalized Graphene: An In-Depth Analysis of Mechanical, Barrier, Electrical, Antibacterial, and Chemical Properties. Polymers. 2024; 16(8):1070. https://doi.org/10.3390/polym16081070
Chicago/Turabian StyleShui, Yu-Jie, Wei-Hua Yao, Jarrn-Horng Lin, Yingjun Zhang, Yongqi Yu, Chin-San Wu, Xuemei Zhang, and Chi-Hui Tsou. 2024. "Enhancing Polyvinyl Alcohol Nanocomposites with Carboxy-Functionalized Graphene: An In-Depth Analysis of Mechanical, Barrier, Electrical, Antibacterial, and Chemical Properties" Polymers 16, no. 8: 1070. https://doi.org/10.3390/polym16081070
APA StyleShui, Y.-J., Yao, W.-H., Lin, J.-H., Zhang, Y., Yu, Y., Wu, C.-S., Zhang, X., & Tsou, C.-H. (2024). Enhancing Polyvinyl Alcohol Nanocomposites with Carboxy-Functionalized Graphene: An In-Depth Analysis of Mechanical, Barrier, Electrical, Antibacterial, and Chemical Properties. Polymers, 16(8), 1070. https://doi.org/10.3390/polym16081070









