Adsorption of Aqueous Nickel Ion by Biomass Carboxymethyl Cellulose-Doped Boron Nitride Composites and Its Subsequent Energy Storage
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of Carboxymethyl Cellulose Boron Nitride Hydrogels
2.3. Adsorption Experiments
2.4. Heat Treatment
2.5. Electrochemical Testing
3. Result and Discussion
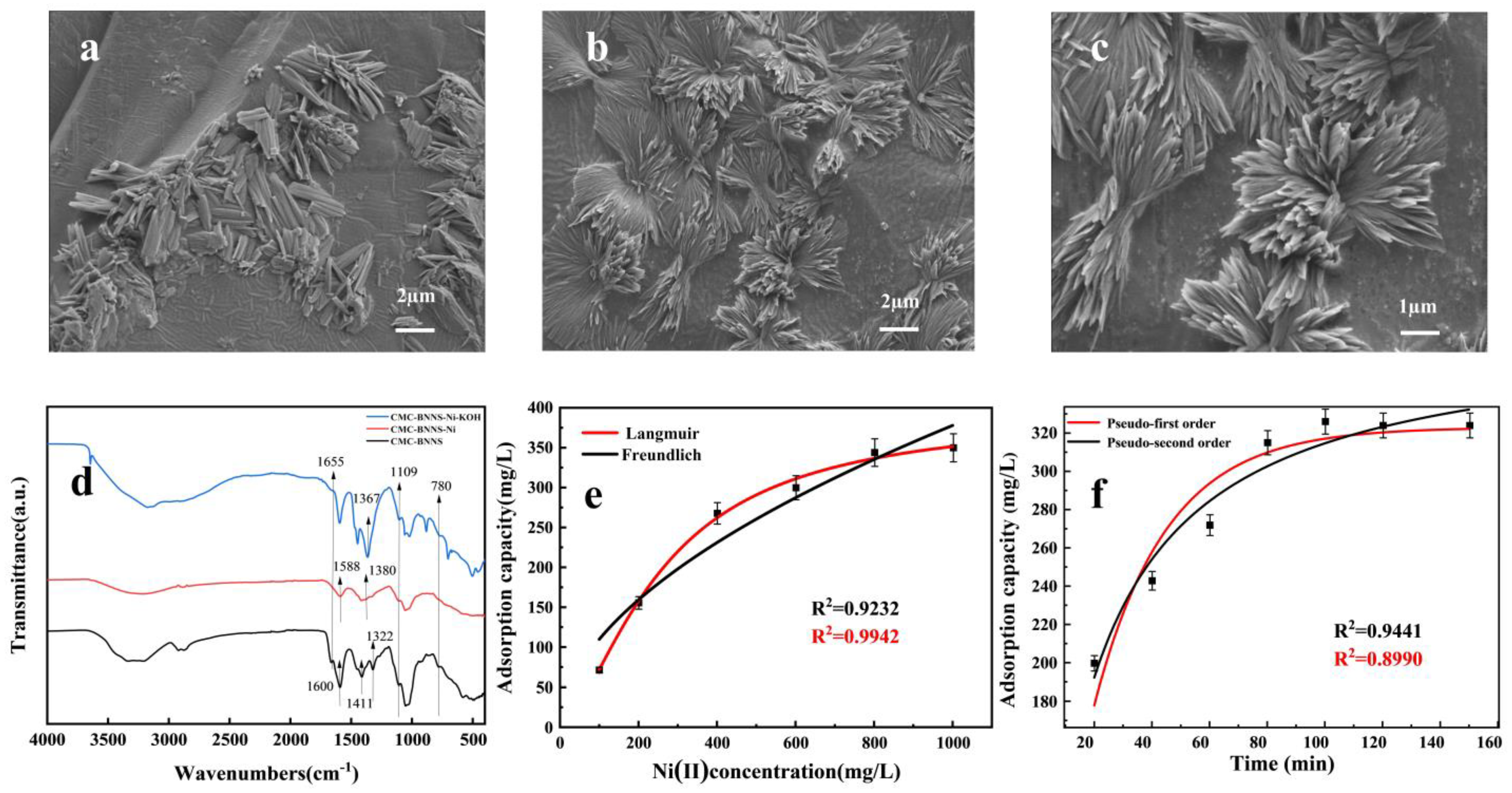
3.1. Design and Characterization of Carboxymethyl Cellulose Hydrogels
3.2. Adsorption Mechanism of CMC-BNNS
| Sample | Ni2+ (mg/g) | Author |
|---|---|---|
| GAMAAX | 80.75 | [38] |
| Cell-g-P(AN-co-Sty) | 139 | [39] |
| PHG | 158.68 | [40] |
| F-IIP | 69.93 | [41] |
| Biosorption | 3.008 | [42] |
| CMC-BNNS | 344 | This work |
| Metals | Parameters | Pseudo−First−Order Model | Pseudo−Second−Order Model | ||
|---|---|---|---|---|---|
| Ni2+ | R2 | 0.8018 | 0.9932 | ||
| Constants | k1 | 0.01113 min−1 | k1 | 0.00015 g/(mg·min) | |
| qe | 181.241 mg/g | qe | 370.37 mg/g | ||
3.3. Transformational Utilization of CMC-BNNS Hydrogels
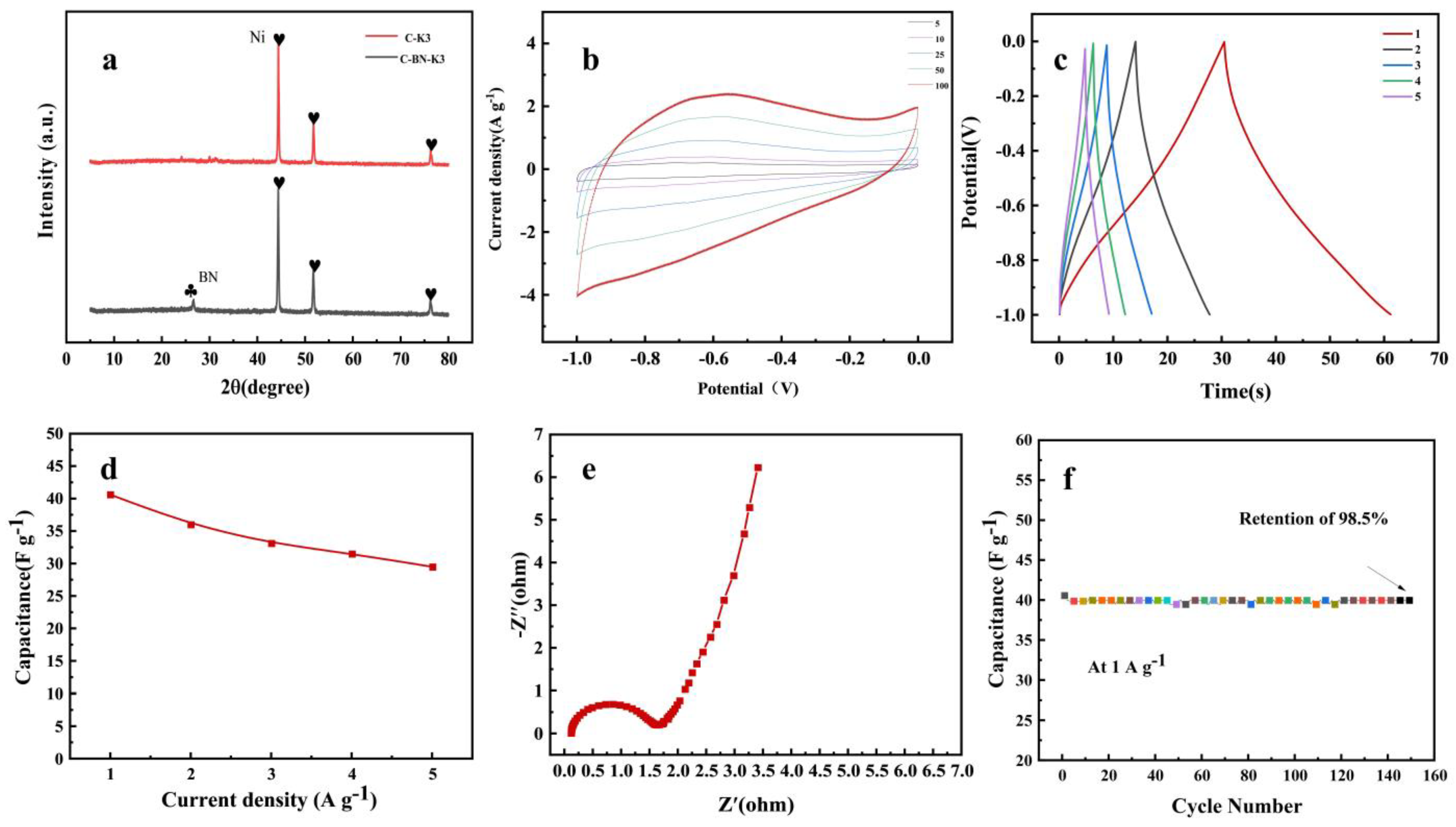
3.4. Study of the Electrical Properties of C-BN-K3
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Fei, Y.; Hu, Y.H. Design, synthesis, and performance of adsorbents for heavy metal removal from wastewater: A review. J. Mater. Chem. A 2022, 10, 1047–1085. [Google Scholar] [CrossRef]
- Zamora-Ledezma, C.; Negrete-Bolagay, D.; Figueroa, F.; Zamora-Ledezma, E.; Ni, M.; Alexis, F.; Guerrero, V.H. Heavy metal water pollution: A fresh look about hazards, novel and conventional remediation methods. Environ. Technol. Innov. 2021, 22, 101504. [Google Scholar] [CrossRef]
- Mamyrbayeva, K.K.; Kuandykova, A.N.; Chepushtanova, T.A.; Merkibayev, Y.S. Review of technology for hydrometallurgical processing of lateritic nickel ores over the past 20 years in the world. Non-Ferr. Met. 2024, 13–21. [Google Scholar] [CrossRef]
- Dilshara, P.; Abeysinghe, B.; Premasiri, R.; Dushyantha, N.; Ratnayake, N.; Senarath, S.; Sandaruwan Ratnayake, A.; Batapola, N. The role of nickel (Ni) as a critical metal in clean energy transition: Applications, global distribution and occurrences, production-demand and phytomining. J. Asian Earth Sci. 2024, 259, 105912. [Google Scholar] [CrossRef]
- Zhang, P.; Yang, M.; Lan, J.; Huang, Y.; Zhang, J.; Huang, S.; Yang, Y.; Ru, J. Water Quality Degradation Due to Heavy Metal Contamination: Health Impacts and Eco-Friendly Approaches for Heavy Metal Remediation. Toxics 2023, 11, 828. [Google Scholar] [CrossRef]
- Qasem, N.A.A.; Mohammed, R.H.; Lawal, D.U. Removal of heavy metal ions from wastewater: A comprehensive and critical review. npj Clean Water 2021, 4, 36. [Google Scholar] [CrossRef]
- Benalia, M.C.; Youcef, L.; Bouaziz, M.G.; Achour, S.; Menasra, H. Removal of Heavy Metals from Industrial Wastewater by Chemical Precipitation: Mechanisms and Sludge Characterization. Arab. J. Sci. Eng. 2021, 47, 5587–5599. [Google Scholar] [CrossRef]
- Sharma, M.; Singh, J.; Hazra, S.; Basu, S. Adsorption of heavy metal ions by mesoporous ZnO and TiO2@ZnO monoliths: Adsorption and kinetic studies. Microchem. J. 2019, 145, 105–112. [Google Scholar] [CrossRef]
- Yang, C.; Yang, H.-R.; Li, S.-S.; An, Q.-D.; Zhai, S.-R.; Xiao, Z.-Y. Rationally designed carboxymethylcellulose-based sorbents crosslinked by targeted ions for static and dynamic capture of heavy metals: Easy recovery and affinity mechanism. J. Colloid Interface Sci. 2022, 625, 651–663. [Google Scholar] [CrossRef] [PubMed]
- Zeng, R.; Zheng, J.; Zuo, Y.; Xiao, C.; Zhu, Y. Synergistic and simultaneous removal of heavy metal ions over waste bamboo shoot particles encapsulated carboxymethyl cellulose/gelatin composite hydrogel. Int. J. Biol. Macromol. 2024, 283, 137578. [Google Scholar] [CrossRef]
- Krishna Kumar, A.S.; Warchol, J.; Matusik, J.; Tseng, W.-L.; Rajesh, N.; Bajda, T. Heavy metal and organic dye removal via a hybrid porous hexagonal boron nitride-based magnetic aerogel. NPJ Clean Water 2022, 5, 24. [Google Scholar] [CrossRef]
- Li, P.-H.; Wu, W.-J. Co-doping mechanism of biomass-derived nitrogen-boron porous carbon and its applications in energy storage and environmental purification. J. Alloys Compd. 2024, 1002, 175098. [Google Scholar] [CrossRef]
- Peng, D.; Jiang, W.; Li, F.-F.; Zhang, L.; Liang, R.-P.; Qiu, J.-D. One-Pot Synthesis of Boron Carbon Nitride Nanosheets for Facile and Efficient Heavy Metal Ions Removal. ACS Sustain. Chem. Eng. 2018, 6, 11685–11694. [Google Scholar] [CrossRef]
- Baskar, A.V.; Bolan, N.; Hoang, S.A.; Sooriyakumar, P.; Kumar, M.; Singh, L.; Jasemizad, T.; Padhye, L.P.; Singh, G.; Vinu, A.; et al. Recovery, regeneration and sustainable management of spent adsorbents from wastewater treatment streams: A review. Sci. Total Environ. 2022, 822, 153555. [Google Scholar] [CrossRef] [PubMed]
- Jalalah, M.; Han, H.; Nayak, A.K.; Harraz, F.A. High-performance supercapacitor based on self-heteroatom-doped porous carbon electrodes fabricated from Mikania micrantha. Adv. Compos. Hybrid Mater. 2024, 7, 20. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, H.; Wang, S.; Wu, Q.; Xia, N.; Kong, F. Electroless decoration of cellulose paper with nickel nanoparticles: A hybrid carbon fiber for supercapacitors. Mater. Chem. Phys. 2018, 215, 157–162. [Google Scholar] [CrossRef]
- Kim, S.-I.; Kang, J.-H.; Kim, S.-W.; Jang, J.-H. A new approach to high-performance flexible supercapacitors: Mesoporous three-dimensional Ni-electrodes. Nano Energy 2017, 39, 639–646. [Google Scholar] [CrossRef]
- Li, W.; Zhang, W.; Xu, Y.; Wang, G.; Sui, W.; Xu, T.; Yuan, Z.; Si, C. Heteroatom-doped lignin derived carbon materials with improved electrochemical performance for advanced supercapacitors. Chem. Eng. J. 2024, 497, 154829. [Google Scholar] [CrossRef]
- Yang, J.; Peng, J.; Lei, Y.; Tang, Y.; Liu, P.; Zeng, J.; Yi, C.; Shen, Y.; Zheng, L.; Wang, X. Activated Carbon Derived from the Agricultural Waste Camellia Seed Shell for High-Performance Supercapacitors. ACS Appl. Energy Mater. 2024, 7, 469–478. [Google Scholar] [CrossRef]
- Liu, L.; Xie, Z.; Du, X.; Yu, D.; Yang, B.; Li, B.; Liu, X. Large-scale mechanical preparation of graphene containing nickel, nitrogen and oxygen dopants as supercapacitor electrode material. Chem. Eng. J. 2022, 430, 132815. [Google Scholar] [CrossRef]
- Thomas, S.A.; Cherusseri, J. Boron carbon nitride (BCN): An emerging two-dimensional nanomaterial for supercapacitors. J. Mater. Chem. A 2023, 11, 23148–23187. [Google Scholar] [CrossRef]
- Chaney, L.E.; Hyun, W.J.; Khalaj, M.; Hui, J.; Hersam, M.C. Fully Printed, High-Temperature Micro-Supercapacitor Arrays Enabled by a Hexagonal Boron Nitride Ionogel Electrolyte. Adv. Mater. 2023, 36, 2305161. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, J.; Zhang, B.; Dong, S.; Guo, X.; Mu, X.; Fei, B. A novel hierarchical porous nitrogen-doped carbon derived from bamboo shoot for high performance supercapacitor. Sci. Rep. 2017, 7, 7362. [Google Scholar] [CrossRef]
- Wang, B.; Zhong, Y.; Liu, Z.; Zhang, W.; Hu, Z.; Chen, L.; Wang, X.; Zhang, X. Fluorescent carbon dots crosslinked Salix psammophila microcrystalline cellulose interpenetrating hydrogel system for sensitive detection and efficient adsorption of Fe3+. Microchem. J. 2024, 200, 110410. [Google Scholar] [CrossRef]
- Sun, L.; Gong, P.; Sun, Y.; Qin, Q.; Song, K.; Ye, J.; Zhang, H.; Zhou, B.; Xue, Y. Modified chicken manure biochar enhanced the adsorption for Cd2+ in aqueous and immobilization of Cd in contaminated agricultural soil. Sci. Total Environ. 2022, 851, 158252. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wei, X.; Kong, H.; Zheng, X.; Guo, H. Hydrothermal Ammonia Carbonization of Rice Straw for Hydrochar to Separate Cd(II) and Zn(II) Ions from Aqueous Solution. Polymers 2023, 15, 4548. [Google Scholar] [CrossRef]
- Balu, R.; Panneerselvam, A.; Rajabathar, J.R.; Devendrapandi, G.; Subburaj, S.; Anand, S.; Veerasamy, U.S.; Palani, S. Synergistic effect of Echinops flower-like Copper sulfide@Cadmium sulfide heterostructure for high-performance all-solid-state asymmetric supercapacitor. J. Energy Storage 2023, 72, 108447. [Google Scholar] [CrossRef]
- Chen, K.; Peng, L.; Fang, Z.; Lin, X.; Sun, C.; Qiu, X. Dispersing boron nitride nanosheets with carboxymethylated cellulose nanofibrils for strong and thermally conductive nanocomposite films with improved water-resistance. Carbohydr. Polym. 2023, 321, 121250. [Google Scholar] [CrossRef]
- Chen, J.; Huang, X.; Zhu, Y.; Jiang, P. Cellulose Nanofiber Supported 3D Interconnected BN Nanosheets for Epoxy Nanocomposites with Ultrahigh Thermal Management Capability. Adv. Funct. Mater. 2016, 27, 1604754. [Google Scholar] [CrossRef]
- Guo, Y.; Zhao, C.; Yan, C.; Cui, L. Construction of cellulose/carboxymethyl chitosan hydrogels for potential wound dressing application. Cellulose 2021, 28, 10013–10023. [Google Scholar] [CrossRef]
- Marchesini, S.; McGilvery, C.M.; Bailey, J.; Petit, C. Template-Free Synthesis of Highly Porous Boron Nitride: Insights into Pore Network Design and Impact on Gas Sorption. ACS Nano 2017, 11, 10003–10011. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.K.; Pandey, L.M.; Uppaluri, R.V.S. Synthesized carboxymethyl-chitosan variant composites for cyclic adsorption-desorption based removal of Fe, Pb, and Cu. Chemosphere 2023, 340, 139780. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Huang, C.; Li, Y.; Liu, Y.; Bian, H.; Xiang, Z.; Wang, H.; Wang, H.; Xiao, H. Freeze-casting production of thermal insulating and fire-retardant lightweight aerogels based on nanocellulose and boron nitride. Int. J. Biol. Macromol. 2023, 252, 126370. [Google Scholar] [CrossRef]
- Chen, D.; Wang, X.; Wang, X.; Feng, K.; Su, J.; Dong, J. The mechanism of cadmium sorption by sulphur-modified wheat straw biochar and its application cadmium-contaminated soil. Sci. Total Environ. 2020, 714, 136550. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, H.; Chen, Z.; Yang, Z.; Zhang, X.; Wang, X. In Situ Construction of CdS/g-C3N4 Heterojunctions in Spent Thiolation@Wood-Aerogel for Efficient Excitation Peroxymonosulfate to Degradation Tetracycline. ACS Appl. Mater. Interfaces 2024, 16, 28353–28366. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.H.; Kareem, A.B.; Al-Rawi, U.A.; Khalid, U.; Zhang, S.; Zafar, F.; Papracanin, E.; Hatshan, M.R.; Sher, F. Kinetic and equilibrium study of graphene and copper oxides modified nanocomposites for metal ions adsorption from binary metal aqueous solution. Front. Chem. 2023, 11, 1279948. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Lin, J.; Gao, X.; Yu, C.; Tang, C.; Huang, Y. Modification of boron nitride aerogels with APTES for enhanced iodine capture performance. Ceram. Int. 2024, 50, 27111–27119. [Google Scholar] [CrossRef]
- Jafarigol, E.; Afshar Ghotli, R.; Hajipour, A.; Pahlevani, H.; Baghban Salehi, M. Tough dual-network GAMAAX hydrogel for the efficient removal of cadmium and nickle ions in wastewater treatment applications. J. Ind. Eng. Chem. 2021, 94, 352–360. [Google Scholar] [CrossRef]
- El-Khouly, A.S.; Takahashi, Y. Synthesis, Characterization, and Evaluation of the Adsorption Behavior of Cellulose-Graft-Poly(Acrylonitrile-co-Acrylic Acid) and Cellulose-Graft-Poly(Acrylonitrile-co-Styrene) towards Ni(II) and Cu(II) Heavy Metals. Polymers 2024, 16, 445. [Google Scholar] [CrossRef] [PubMed]
- Lv, A.; Lv, X. Porous chitosan quaternary ammonium salt hydrogel embedded with Cu, Ni and Pd nanoparticles for efficient coupled adsorption-catalytic reduction of methylene blue and 4-nitrophenol. Int. J. Biol. Macromol. 2024, 282, 136842. [Google Scholar] [CrossRef] [PubMed]
- Guo, N.; Su, S.-J.; Liao, B.; Ding, S.-L.; Sun, W.-Y. Preparation and properties of a novel macro porous Ni 2+ -imprinted chitosan foam adsorbents for adsorption of nickel ions from aqueous solution. Carbohydr. Polym. 2017, 165, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, R.M.; Dhanyashree, J.K.; Varma, E.; Sirivibha, S.P. Batch and continuous packed bed column studies on biosorption of nickel (II) by sugarcane bagasse. Results Chem. 2022, 4, 100328. [Google Scholar] [CrossRef]
- Yu, M.; Li, J.; Wang, L. KOH-activated carbon aerogels derived from sodium carboxymethyl cellulose for high-performance supercapacitors and dye adsorption. Chem. Eng. J. 2017, 310, 300–306. [Google Scholar] [CrossRef]
- Samage, A.; Halakarni, M.; Yoon, H.; Sanna Kotrappanavar, N. Sustainable conversion of agricultural biomass waste into electrode materials with enhanced energy density for aqueous zinc-ion hybrid capacitors. Carbon 2024, 219, 118774. [Google Scholar] [CrossRef]
- Li, Y.; Mei, J.; Wu, L.; Xu, Q.; Li, Z. Ultrahigh surface area hierarchical porous carbon derived from biomass via a new KOH activation strategy for high-performance supercapacitor. Int. J. Hydrogen Energy 2024, 49, 67–80. [Google Scholar] [CrossRef]
- Nanaji, K.; Sarada, B.V.; Varadaraju, U.V.; N Rao, T.; Anandan, S. A novel approach to synthesize porous graphene sheets by exploring KOH as pore inducing agent as well as a catalyst for supercapacitors with ultra-fast rate capability. Renew. Energy 2021, 172, 502–513. [Google Scholar] [CrossRef]
- Li, D.; Zhao, L.; Cao, X.; Xiao, Z.; Nan, H.; Qiu, H. Nickel-catalyzed formation of mesoporous carbon structure promoted capacitive performance of exhausted biochar. Chem. Eng. J. 2021, 406, 126856. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, Y.; Xie, N.; Guo, R.; Wang, Y.; Hu, Z.-N.; Xu, W.; Ai, Y.; Gao, J.; Wang, J.; et al. Ternary NiFeMnOx compounds for adsorption of antimony and subsequent application in energy storage to avoid secondary pollution. Sep. Purif. Technol. 2021, 276, 119237. [Google Scholar] [CrossRef]




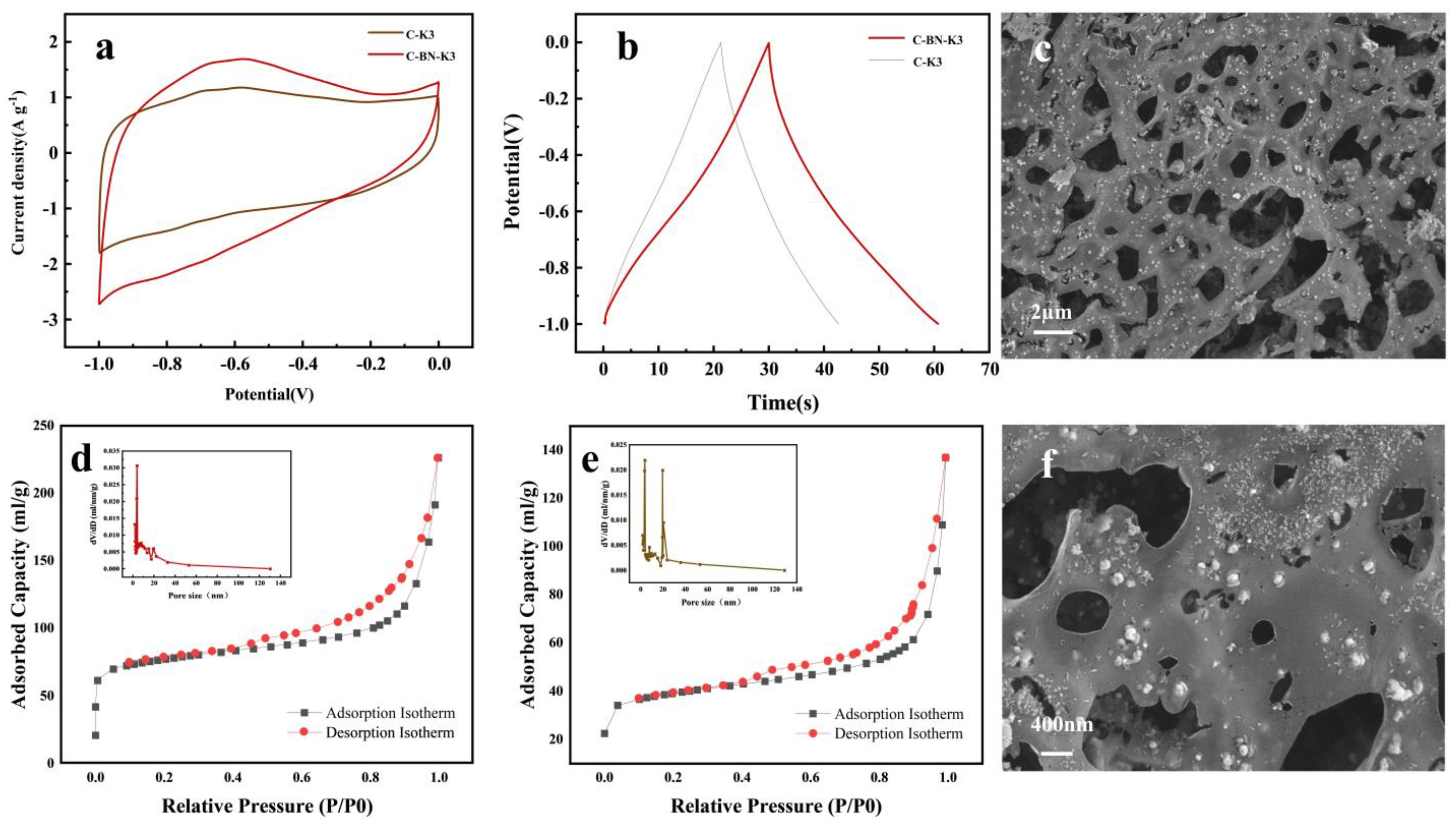
| BET Surface Area (m2/g) | Micropore Volume (cm2/g) | Micropore Area (m2/g) | Average Pore Size (nm) | |
|---|---|---|---|---|
| C-K3 | 125.5 ± 2.5 | 85.4 ± 4.3 | 0.046 ± 0.002 | 6.53 ± 0.33 |
| C-BN-K3 | 241.8 ± 4.8 | 187.5 ± 9.4 | 0.098 ± 0.005 | 5.03 ± 0.25 |
| C-BN-K5 | 96.4 ± 1.9 | 54.8 ± 2.7 | 0.029 ± 0.001 | 9.21 ± 0.46 |
| C-BN | 215.7 ± 4.3 | 179.2 ± 9.0 | 0.096 ± 0.005 | 6.28 ± 0.31 |
| C-BN-K3-600 | 238.3 ± 4.8 | 182.2 ± 9.1 | 0.094 ± 0.005 | 5.04 ± 0.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Wang, B.; Zhang, W.; Zhang, X.; Wang, X. Adsorption of Aqueous Nickel Ion by Biomass Carboxymethyl Cellulose-Doped Boron Nitride Composites and Its Subsequent Energy Storage. Polymers 2025, 17, 567. https://doi.org/10.3390/polym17050567
Li X, Wang B, Zhang W, Zhang X, Wang X. Adsorption of Aqueous Nickel Ion by Biomass Carboxymethyl Cellulose-Doped Boron Nitride Composites and Its Subsequent Energy Storage. Polymers. 2025; 17(5):567. https://doi.org/10.3390/polym17050567
Chicago/Turabian StyleLi, Xinran, Boyun Wang, Wanqi Zhang, Xiaotao Zhang, and Ximing Wang. 2025. "Adsorption of Aqueous Nickel Ion by Biomass Carboxymethyl Cellulose-Doped Boron Nitride Composites and Its Subsequent Energy Storage" Polymers 17, no. 5: 567. https://doi.org/10.3390/polym17050567
APA StyleLi, X., Wang, B., Zhang, W., Zhang, X., & Wang, X. (2025). Adsorption of Aqueous Nickel Ion by Biomass Carboxymethyl Cellulose-Doped Boron Nitride Composites and Its Subsequent Energy Storage. Polymers, 17(5), 567. https://doi.org/10.3390/polym17050567






