The Effect of Polysaccharide Colloids on the Thermal Stability of Water-in-Oil Emulsions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Emulsions
2.3. General Appearance
2.4. Optical Microscopy and Droplet Size
2.5. Encapsulation Efficiency (EE)
2.6. Turbiscan Stability Index (TSI)
2.7. Viscosity
2.8. Friction Coefficient
2.9. Rheological Measurements
2.9.1. Frequency Sweep
2.9.2. Temperature Scanning
2.10. Statistical Analysis
3. Results and Discussion
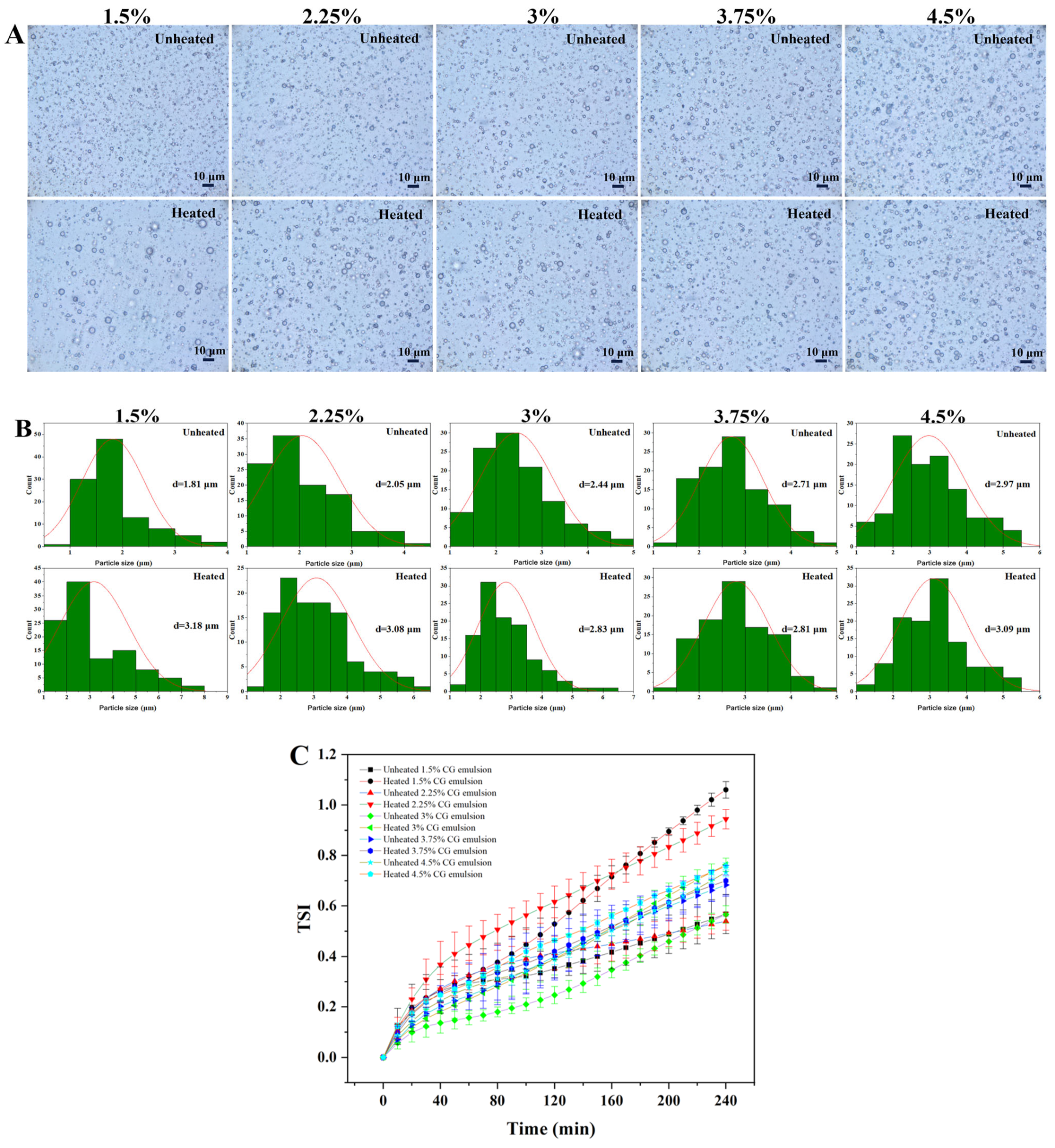
3.1. Effect of CG Concentration on the Thermal Stability of Emulsions
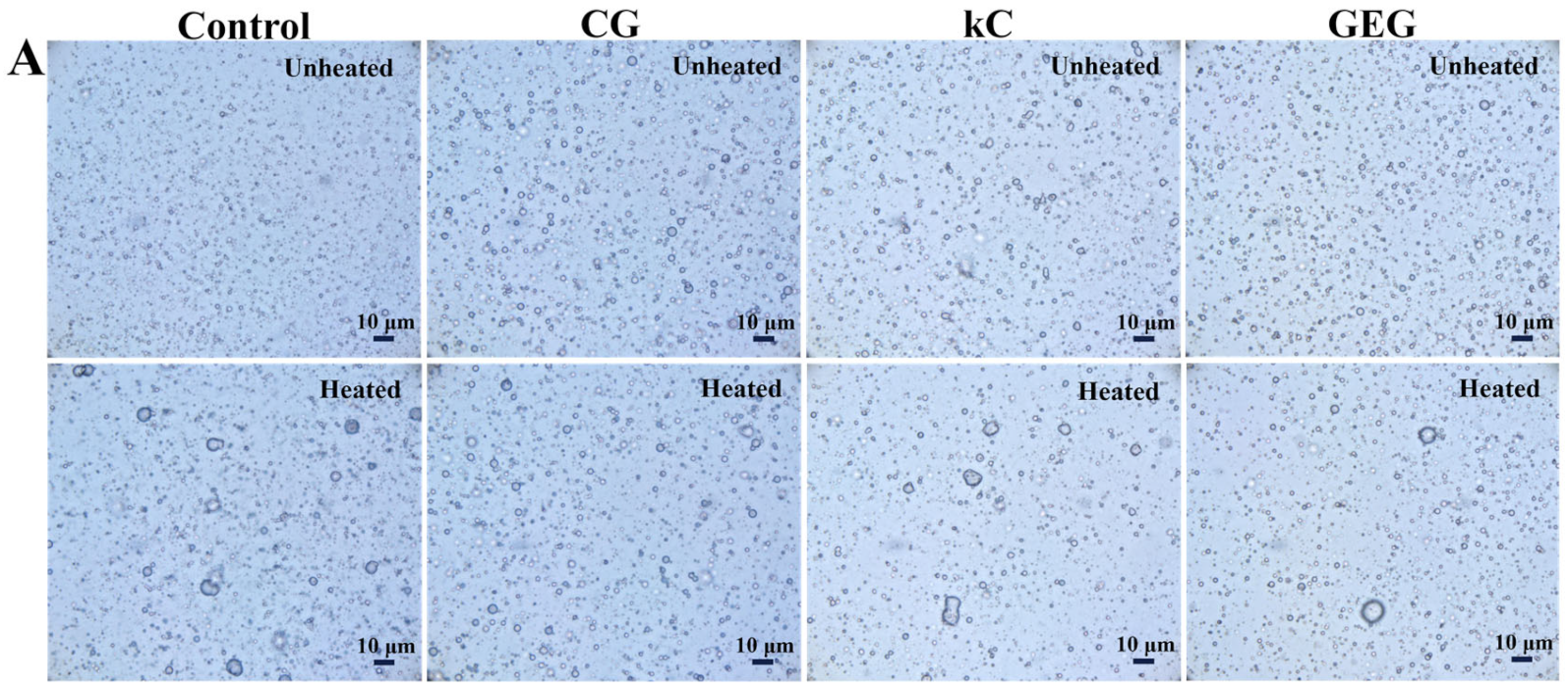
3.2. Appearance Observation of Emulsions
3.3. Analysis of Optical Microscopy and Particle Size
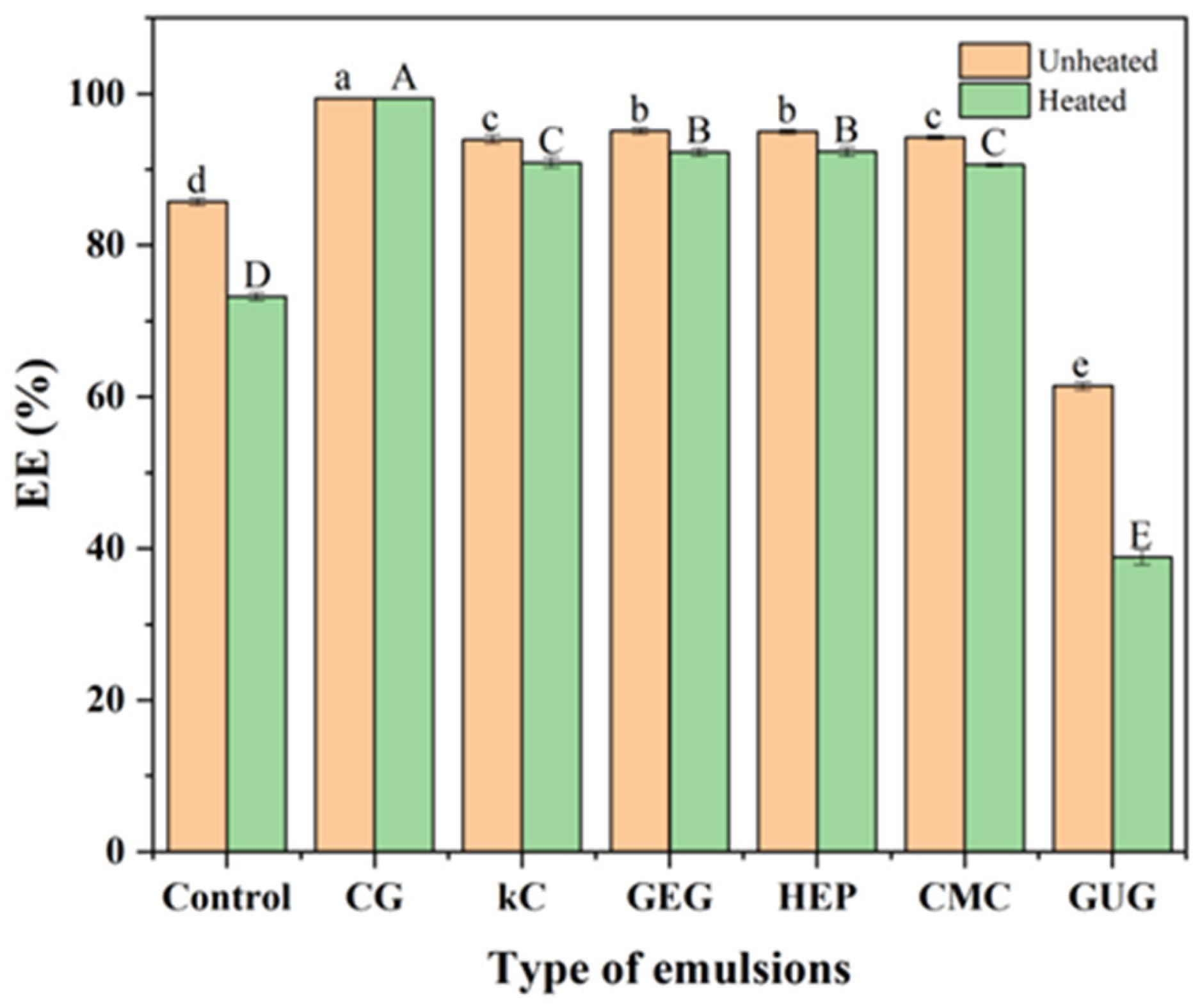
3.4. Analysis of Encapsulation Efficiency
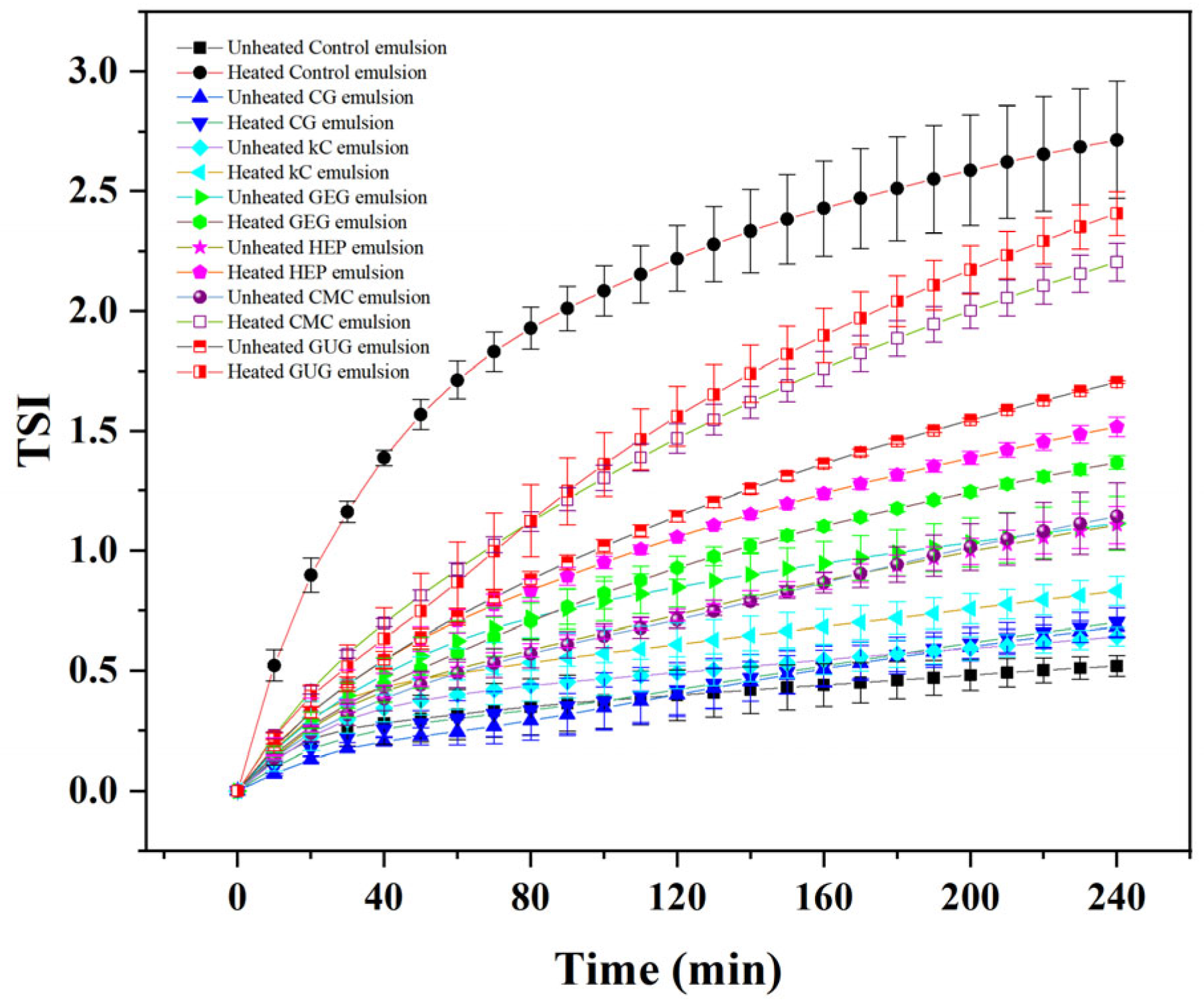
3.5. Analysis of Turbiscan Stability Index
3.6. Analysis of Viscosity
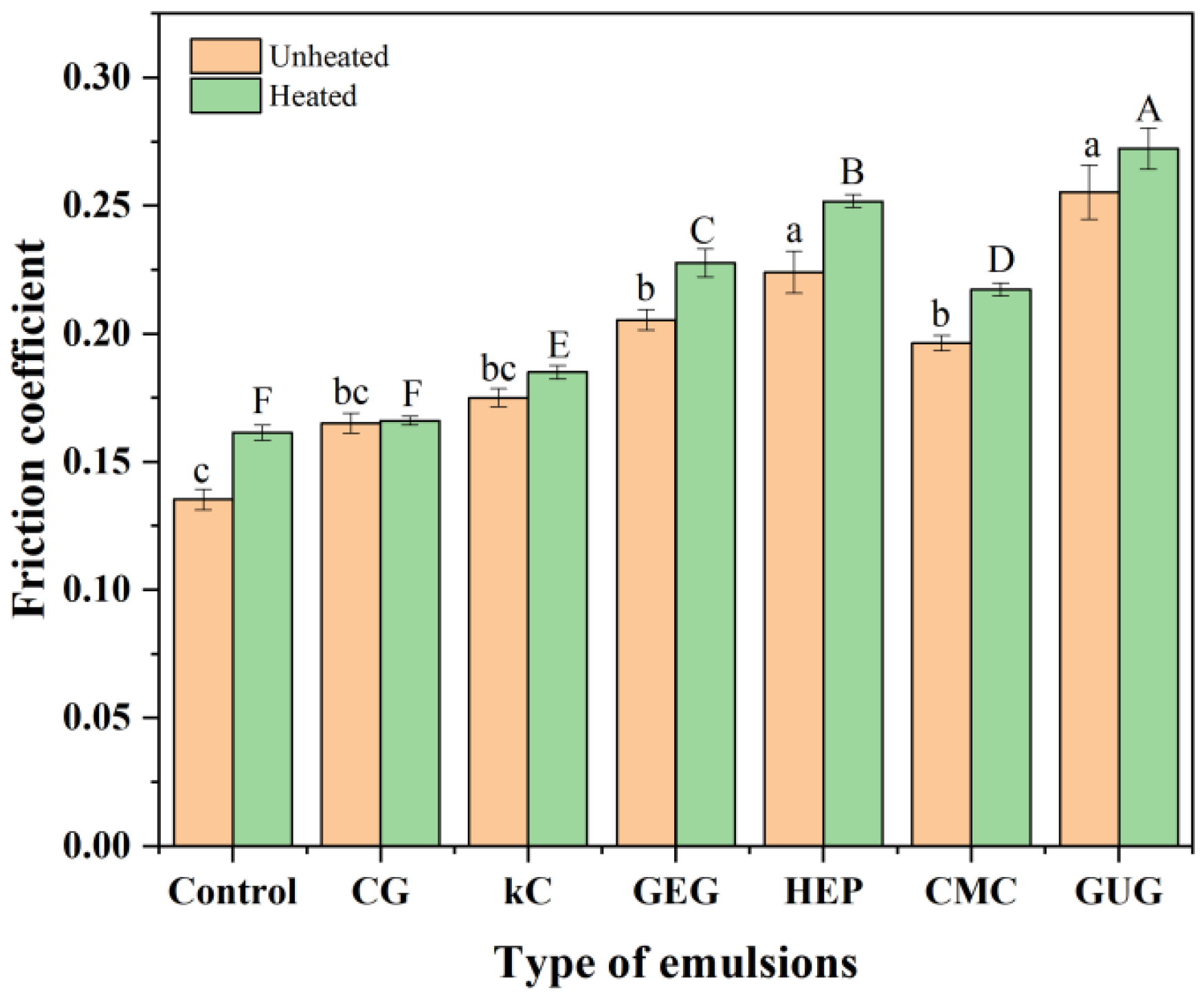
3.7. Analysis of Friction Coefficient
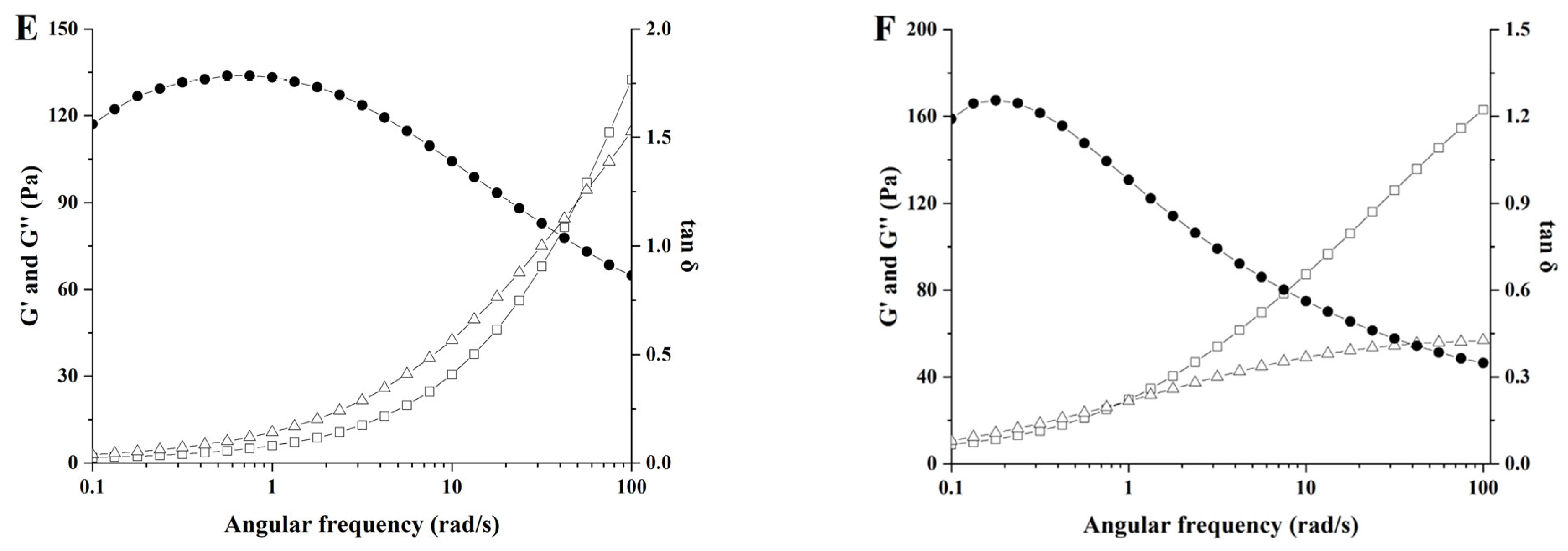
3.8. Analysis of Frequency Sweep
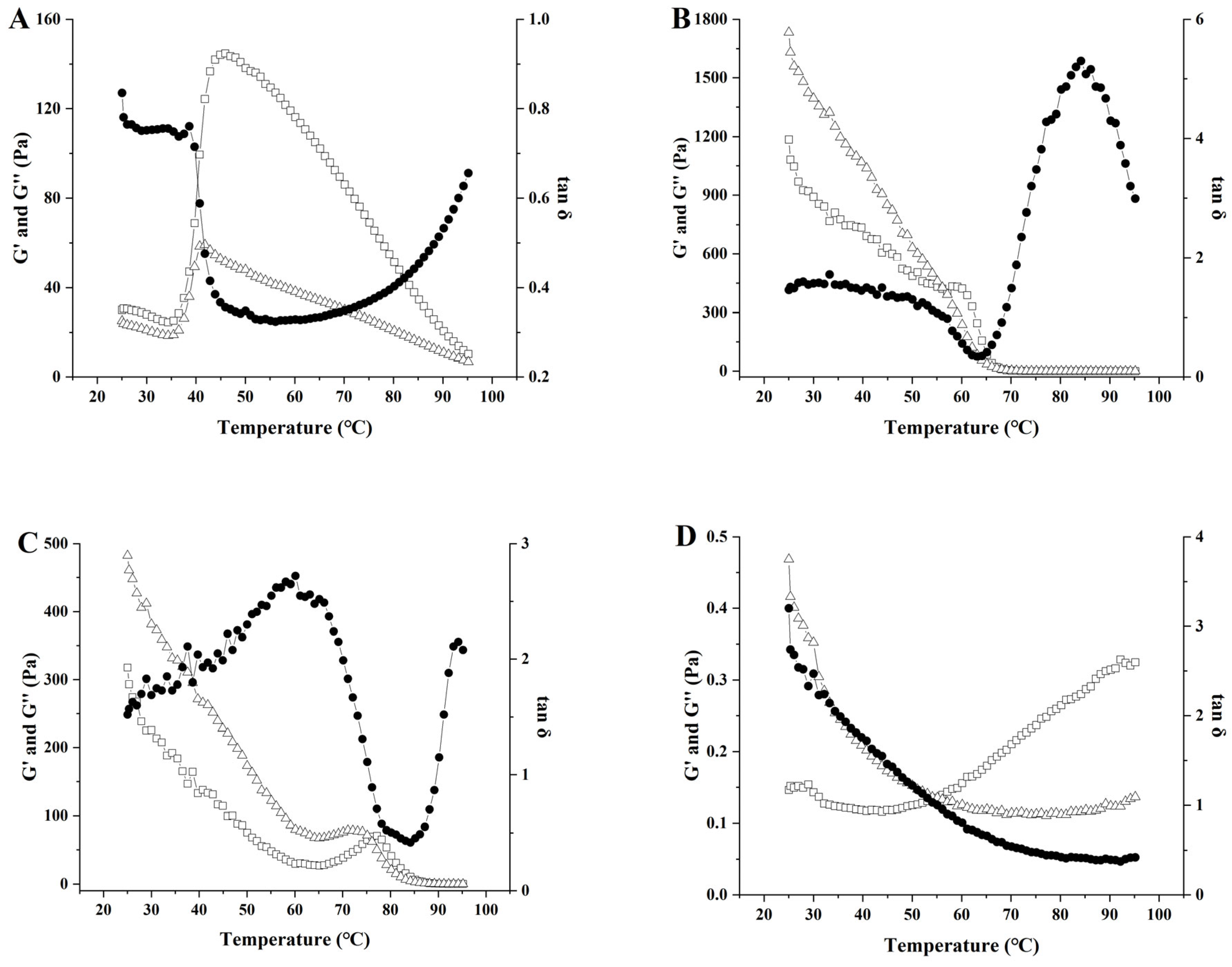
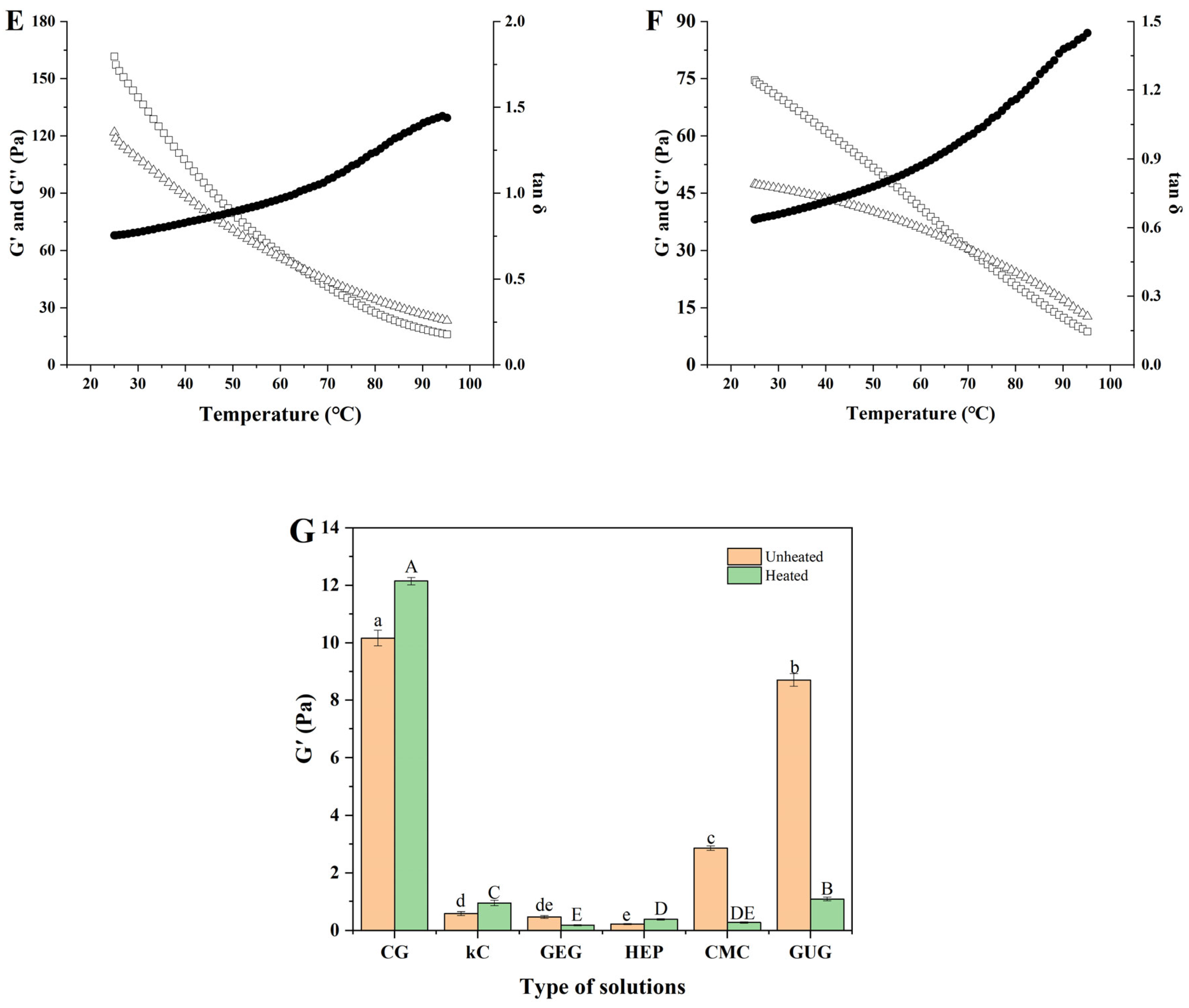
3.9. Analysis of Temperature Scanning
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CG | curdlan gum |
| kC | kappa-carrageenan |
| GEG | gellam gum |
| HEP | high-ester pectin |
| CMC | carboxymethyl cellulose |
| GUG | guar gum |
| TSI | Turbiscan stability index |
| EE | encapsulation efficiency |
| G′ | storage modulus |
| G″ | loss modulus |
| tan δ | phase angle |
References
- Delaš, I. Benefits and hazards of fat-free diets. Trends Food Sci. Technol. 2011, 22, 576–582. [Google Scholar] [CrossRef]
- Zhao, Y.; Khalesi, H.; He, J.; Fang, Y. Application of different hydrocolloids as fat replacer in low-fat dairy products: Ice cream, yogurt and cheese. Food Hydrocoll. 2023, 138, 108493. [Google Scholar] [CrossRef]
- Wang, F.; Tong, Q.; Luo, J.; Xu, Y.; Ren, F. Effect of Carrageenan on Physicochemical and Functional Properties of Low-Fat Colby Cheese. J. Food Sci. 2016, 81, E1949–E1955. [Google Scholar] [CrossRef] [PubMed]
- Kondyli, E.; Pappa, E.C.; Kremmyda, A.; Arapoglou, D.; Metafa, M.; Eliopoulos, C.; Israilides, C. Manufacture of Reduced Fat White-Brined Cheese with the Addition of β-Glucans Biobased Polysaccharides as Textural Properties Improvements. Polymers 2020, 12, 2647. [Google Scholar] [CrossRef] [PubMed]
- Stankey, J.A.; Lu, Y.; Abdalla, A.; Govindasamy-Lucey, S.; Jaeggi, J.J.; Ø Mikkelsen, B.; Pedersen, K.T.; Andersen, C.B. Low-fat Cheddar cheese made using microparticulated whey proteins: Effect on yield and cheese quality. Int. J. Dairy Technol. 2017, 70, 481–491. [Google Scholar] [CrossRef]
- Kumar, S.S.; Balasubramanian, S.; Biswas, A.K.; Chatli, M.K.; Devatkal, S.K.; Sahoo, J. Efficacy of soy protein isolate as a fat replacer on physico-chemical and sensory characteristics of low-fat paneer. J. Food Sci. Technol. 2010, 48, 498–501. [Google Scholar] [CrossRef]
- Pérez, M.P.; Wagner, J.R.; Márquez, A.L. Influence of Different Factors on the Particle Size Distribution and Solid Fat Content of Water-in-Oil Emulsions. J. Am. Oil Chem. Soc. 2016, 93, 793–801. [Google Scholar] [CrossRef]
- Zhu, Q.; Pan, Y.; Jia, X.; Li, J.; Zhang, M.; Yin, L. Review on the Stability Mechanism and Application of Water-in-Oil Emulsions Encapsulating Various Additives. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1660–1675. [Google Scholar] [CrossRef]
- Tian, H.; Xiang, D.; Wang, B.; Zhang, W.; Li, C. Using hydrogels in dispersed phase of Water-in-oil emulsion for encapsulating tea polyphenols to sustain their release. Colloids Surf. A Physicochem. Eng. Asp. 2021, 612, 125999. [Google Scholar] [CrossRef]
- Wang, F.; Guo, L.; Liu, H.; Tao, H.; Yu, B.; Zhao, H.; Li, J.; Tao, H.; Cui, B.; Wang, Y. Water-in-oil oleogel with biphasic stabilization for fabrication of low-fat salad dressing. Food Hydrocoll. 2023, 142, 108805. [Google Scholar] [CrossRef]
- Iqbal, S.; Xu, Z.; Huang, H.; Chen, X.D. Structuring of water-in-oil emulsions using controlled aggregation of polysaccharide in aqueous phases. J. Food Eng. 2019, 258, 34–44. [Google Scholar] [CrossRef]
- Cheng, C.; Gao, H.; McClements, D.J.; Zeng, H.; Ma, L.; Zou, L.; Miao, J.; Wu, X.; Tan, J.; Liang, R.; et al. Impact of polysaccharide mixtures on the formation, stability and EGCG loading of water-in-oil high internal phase emulsions. Food Chem. 2021, 372, 131225. [Google Scholar] [CrossRef] [PubMed]
- Cheung, Y.; Yin, J.; Wu, J. Effect of polysaccharide chain conformation on ultrasonic degradation of curdlan in alkaline solution. Carbohydr. Polym. 2018, 195, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Liang, C.; Chang, Y. Effect of starch source on gel properties of kappa-carrageenan-starch dispersions. Food Hydrocoll. 2016, 60, 509–515. [Google Scholar] [CrossRef]
- Ikeda, S.; Henry, K. Effects of Partial Replacement of Gelatin in High Sugar Gels with Gellan on their Textural, Rhelogical, and Thermal Properties. Food Biophys. 2016, 11, 400–409. [Google Scholar] [CrossRef]
- Lv, R.; Kong, Q.; Mou, H.; Fu, X. Effect of guar gum on stability and physical properties of orange juice. Int. J. Biol. Macromol. 2017, 98, 565–574. [Google Scholar] [CrossRef]
- Xie, F.; Gu, B.; Saunders, S.R.; Ganjyal, G.M. High methoxyl pectin enhances the expansion characteristics of the cornstarch relative to the low methoxyl pectin. Food Hydrocoll. 2021, 110, 106131. [Google Scholar] [CrossRef]
- Horinaka, J.; Chen, K.; Takigawa, T. Entanglement properties of carboxymethyl cellulose and related polysaccharides. Rheol. Acta 2017, 57, 51–56. [Google Scholar] [CrossRef]
- Pu, X.; Yu, S.; Cui, Y.; Tong, Z.; Wang, C.; Wang, L.; Han, J.; Zhu, H.; Wang, S. Stability of electrostatically stabilized emulsions and its encapsulation of astaxanthin against environmental stresses: Effect of sodium caseinate-sugar beet pectin addition order. Curr. Res. Food Sci. 2024, 9, 100821. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Zhang, S.; Li, P.; Jiang, J. Influence of pH-shifting and transglutaminase on the freeze-thaw stability and thermal gel properties of mung bean protein-based liquid egg substitute prepared with two different oil phases. Food Hydrocoll. 2023, 146, 109128. [Google Scholar] [CrossRef]
- Li, Y.; Wu, C.; Liu, J.; Zhu, Y.; Zhang, X.; Jiang, L.; Qi, B.; Zhang, X.; Wang, Z.; Teng, F. Soy Protein Isolate-Phosphatidylcholine Nanoemulsions Prepared Using High-Pressure Homogenization. Nanomaterials 2018, 8, 307. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Zheng, B.; Miao, S. Improved emulsion stability and modified nutrient release by structuring O/W emulsions using konjac glucomannan. Food Hydrocoll. 2018, 81, 120–128. [Google Scholar] [CrossRef]
- Kapanyk, U. Experimental determination of the frictional characteristics of fabrics made of natural silk. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1188, 012007. [Google Scholar] [CrossRef]
- Zhu, Y.; McClements, D.J.; Zhou, W.; Peng, S.; Zhou, L.; Zou, L.; Liu, W. Influence of ionic strength and thermal pretreatment on the freeze-thaw stability of Pickering emulsion gels. Food Chem. 2019, 303, 125401. [Google Scholar] [CrossRef]
- Liu, S.; Lei, H.; Li, L.; Liu, F.; Li, L.; Yan, J. Effects of direct addition of curdlan on the gelling characteristics of thermally induced soy protein isolate gels. Int. J. Biol. Macromol. 2023, 253, 127092. [Google Scholar] [CrossRef]
- Jiang, Q.; Sun, N.; Kumar, P.; Li, Q.; Liu, B.; Li, A.; Wang, W.; Gao, Z. Real-Time Analysis of the Stability of Oil-in-Water Pickering Emulsion by Electrochemical Impedance Spectroscopy. Molecules 2020, 25, 2904. [Google Scholar] [CrossRef]
- Queiroz, D.G.; da Silva, C.M.F.; Minale, M.; Merino, D.; Lucas, E.F. The effect of monoethylene glycol on the stability of water-in-oil emulsions. Can. J. Chem. Eng. 2021, 100, 44–53. [Google Scholar] [CrossRef]
- Zeng, C.; Wang, Y.; Liu, Y.; Su, S.; Lu, Y.; Qin, S.; Shi, M. Self-constructed water-in-oil Pickering emulsions as a tool for increasing bioaccessibility of betulin. Food Chem. X 2023, 21, 101056. [Google Scholar] [CrossRef]
- Su, Y.; Sun, Y.; McClements, D.J.; Chang, C.; Li, J.; Xiong, W.; Sun, Y.; Cai, Y.; Gu, L.; Yang, Y. Encapsulation of amino acids in water-in-oil-in-water emulsions stabilized by gum arabic and xanthan gum. Int. J. Biol. Macromol. 2022, 220, 1493–1500. [Google Scholar] [CrossRef]
- Tepsongkroh, B.; Harnsilawat, T.; Maisuthisakul, P.; Chantrapornchai, W. Influence of Polyglycerol Polyricinoleate and Biopolymers on Physical Properties and Encapsulation Efficiency of Water-in-Oil-in-Water Emulsions Containing Mango Seed Kernel Extract. J. Dispers. Sci. Technol. 2014, 36, 1126–1133. [Google Scholar] [CrossRef]
- Jiang, J.; Jiang, Y.; Li, H.; Zhu, D.; He, Y.; Yang, L.; Wang, S.; Liu, J.; Zhang, Y.; Liu, H. Application of soybean isolate protein (SPI) and soy hull polysaccharide (SHP) complex in fermentation products. Int. J. Biol. Macromol. 2023, 258, 128806. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Fan, L.; Liu, Y.; Li, J. A mechanistic investigation of the effect of dispersion phase protein type on the physicochemical stability of water–in–oil emulsions. Food Res. Int. 2022, 157, 111293. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhao, Y.; Wang, X.; Tang, H.; Wu, N.; Wu, F.; Yu, D.; Elfalleh, W. Effects o-f (+)-catechin on a rice bran protein oil-in-water emulsion: Droplet size, zeta-potential, emulsifying properties, and rheological behavior. Food Hydrocoll. 2020, 98, 105306. [Google Scholar] [CrossRef]
- Le Priol, L.; Dagmey, A.; Morandat, S.; Saleh, K.; El Kirat, K.; Nesterenko, A. Comparative study of plant protein extracts as wall materials for the improvement of the oxidative stability of sunflower oil by microencapsulation. Food Hydrocoll. 2019, 95, 105–115. [Google Scholar] [CrossRef]
- Castel, V.; Rubiolo, A.C.; Carrara, C.R. Droplet size distribution, rheological behavior and stability of corn oil emulsions stabilized by a novel hydrocolloid (Brea gum) com-pared with gum arabic. Food Hydrocoll. 2017, 63, 170–177. [Google Scholar] [CrossRef]
- Zhang, R.; Yu, J.; Liu, N.; Gao, Y.; Mao, L. W/O emulsions featuring ethylcellulose structuring in the water phase, interface and oil phase for multiple delivery. Carbohydr. Polym. 2022, 283, 119158. [Google Scholar] [CrossRef]
- Zhang, L.; Yu, Y. Improving the Stability of Water-in-Oil Emulsions with Medium Internal Phase by the Introduction of Gelatin. Foods 2023, 12, 2863. [Google Scholar] [CrossRef]
- Brenner, T.; Tuvikene, R.; Parker, A.; Matsukawa, S.; Nishinari, K. Rheology and structure of mixed kappa-carrageen-an/iota-carrageenan gels. Food Hydrocoll. 2014, 39, 272–279. [Google Scholar] [CrossRef]
- Yan, J.; Cai, W.; Wang, C.; Yu, Y.; Zhang, H.; Yang, Y.; Wang, W. Macromolecular behavior, structural characteristics and rheological properties of alkali-neutralization curdlan at different concentrations. Food Hydrocoll. 2020, 105, 105785. [Google Scholar] [CrossRef]
- Rudge, R.E.D.; van de Sande, J.P.M.; Dijksman, J.A.; Scholten, E. Uncovering friction dynamics using hydrogel particles as soft ball bearings. Soft Matter 2020, 16, 3821–3831. [Google Scholar] [CrossRef]
- Guo, Y.; Li, J.; Zhou, X.; Tang, Y.; Zeng, X. Formulation of lyotropic liquid crystal emulsion based on natural sucrose ester and its tribological behavior as novel lubricant. Friction 2022, 10, 1879–1892. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, Y.; Yu, J.; Gao, Y.; Mao, L. Rheology and Tribology of Ethylcellulose-Based Oleogels and W/O Emulsions as Fat Substitutes: Role of Glycerol Monostearate. Foods 2022, 11, 2364. [Google Scholar] [CrossRef] [PubMed]
- Hao, Z.; Chen, Z.; Chang, M.; Meng, J.; Liu, J.; Feng, C. Rheological properties and gel characteristics of polysaccharides from fruit-bodies of Sparassis crispa. Int. J. Food Prop. 2018, 21, 2283–2295. [Google Scholar] [CrossRef]
- de Morais, S.C.; Cardoso, O.R.; de Carvalho Balaban, R. Thermal stability of water-soluble polymers in solution. J. Mol. Liq. 2018, 265, 818–823. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, C.; Chen, X.; Li, P.; Ma, F.; Lu, Q. Contribution of Three Ionic Types of Polysaccharides to the Thermal Gelling Properties of Chicken Breast Myosin. J. Agric. Food Chem. 2014, 62, 2655–2662. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, S.; Wang, R.; Xu, Y.; Wang, C.; Xu, J.; Wang, P.; Fu, Y.; Su, J.; Chai, H.; He, J.; et al. The Effect of Polysaccharide Colloids on the Thermal Stability of Water-in-Oil Emulsions. Polymers 2025, 17, 809. https://doi.org/10.3390/polym17060809
Zhao S, Wang R, Xu Y, Wang C, Xu J, Wang P, Fu Y, Su J, Chai H, He J, et al. The Effect of Polysaccharide Colloids on the Thermal Stability of Water-in-Oil Emulsions. Polymers. 2025; 17(6):809. https://doi.org/10.3390/polym17060809
Chicago/Turabian StyleZhao, Shunfa, Ran Wang, Ying Xu, Caiyun Wang, Jun Xu, Pengjie Wang, Yonggang Fu, Jiaqi Su, Hanyu Chai, Jian He, and et al. 2025. "The Effect of Polysaccharide Colloids on the Thermal Stability of Water-in-Oil Emulsions" Polymers 17, no. 6: 809. https://doi.org/10.3390/polym17060809
APA StyleZhao, S., Wang, R., Xu, Y., Wang, C., Xu, J., Wang, P., Fu, Y., Su, J., Chai, H., He, J., & Chen, H. (2025). The Effect of Polysaccharide Colloids on the Thermal Stability of Water-in-Oil Emulsions. Polymers, 17(6), 809. https://doi.org/10.3390/polym17060809






