Synthesis of Polymer Sodium Alginate–Red Mud Adsorbent and Its Application in the Removal of Low-Concentration Fluoride
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of Adsorbents
2.2.1. Modification and Activation of Red Mud
2.2.2. Preparation of SA@RM
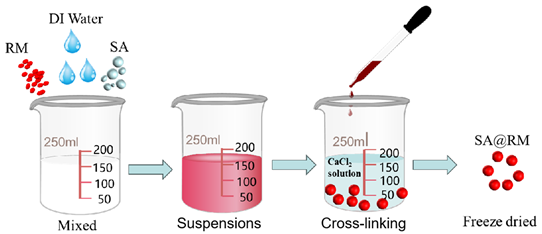
2.3. Characterization of Materials
2.4. Experimental Methods
3. Results and Discussion
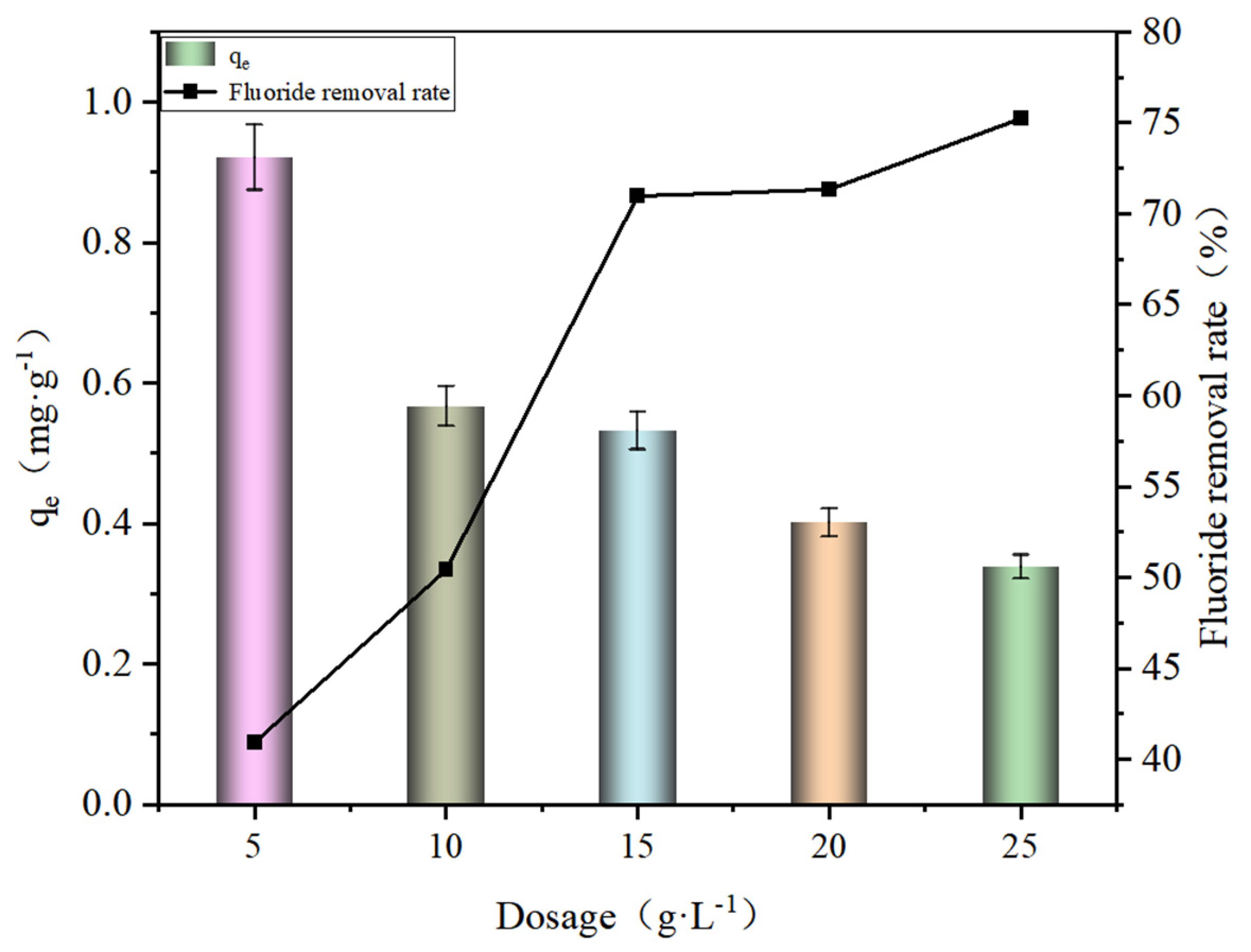
3.1. Effect of Dosage on Fluoride Removal
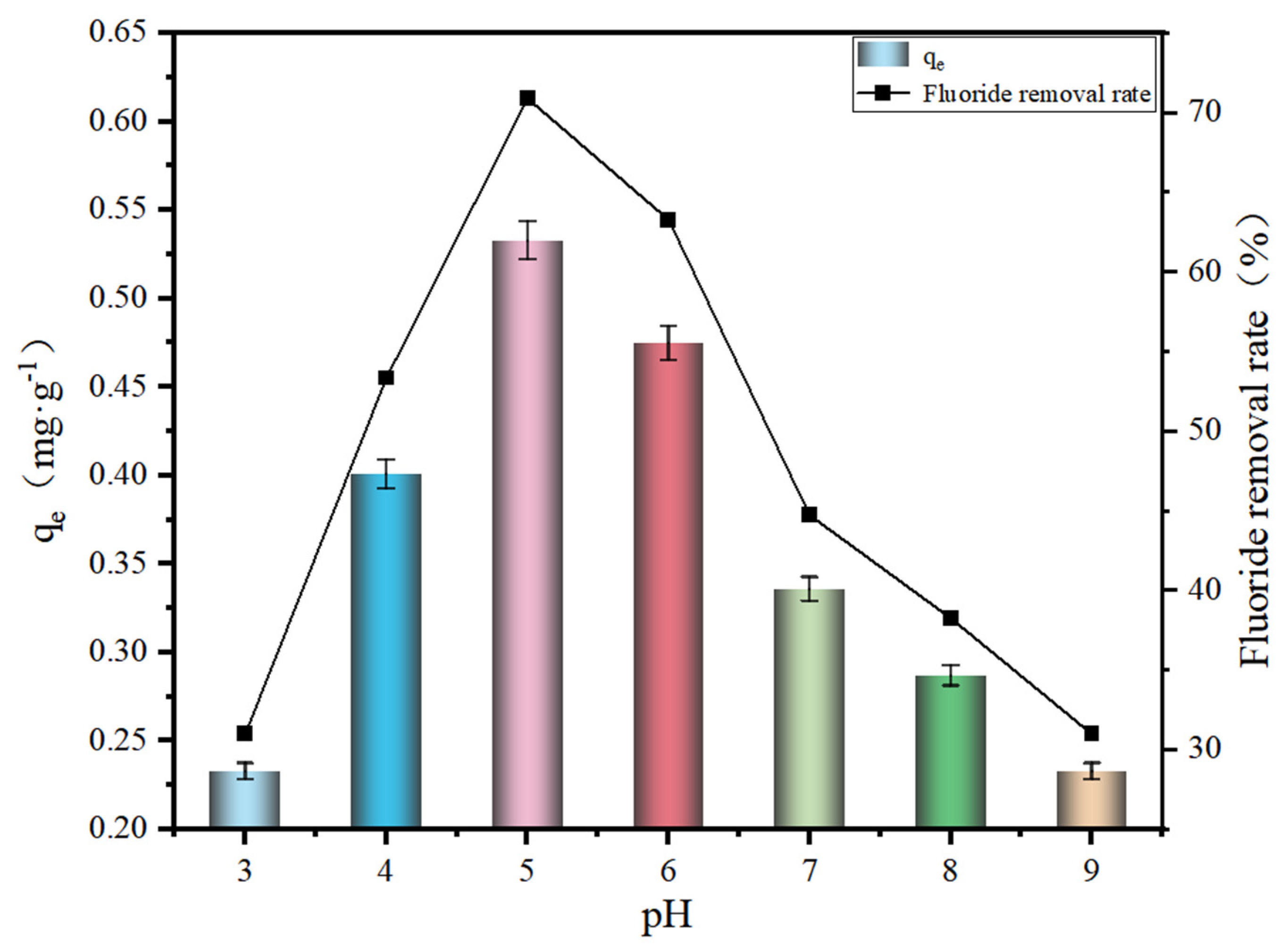
3.2. Effect of pH on Adsorption Effect
3.3. Adsorption Kinetics and Isotherms
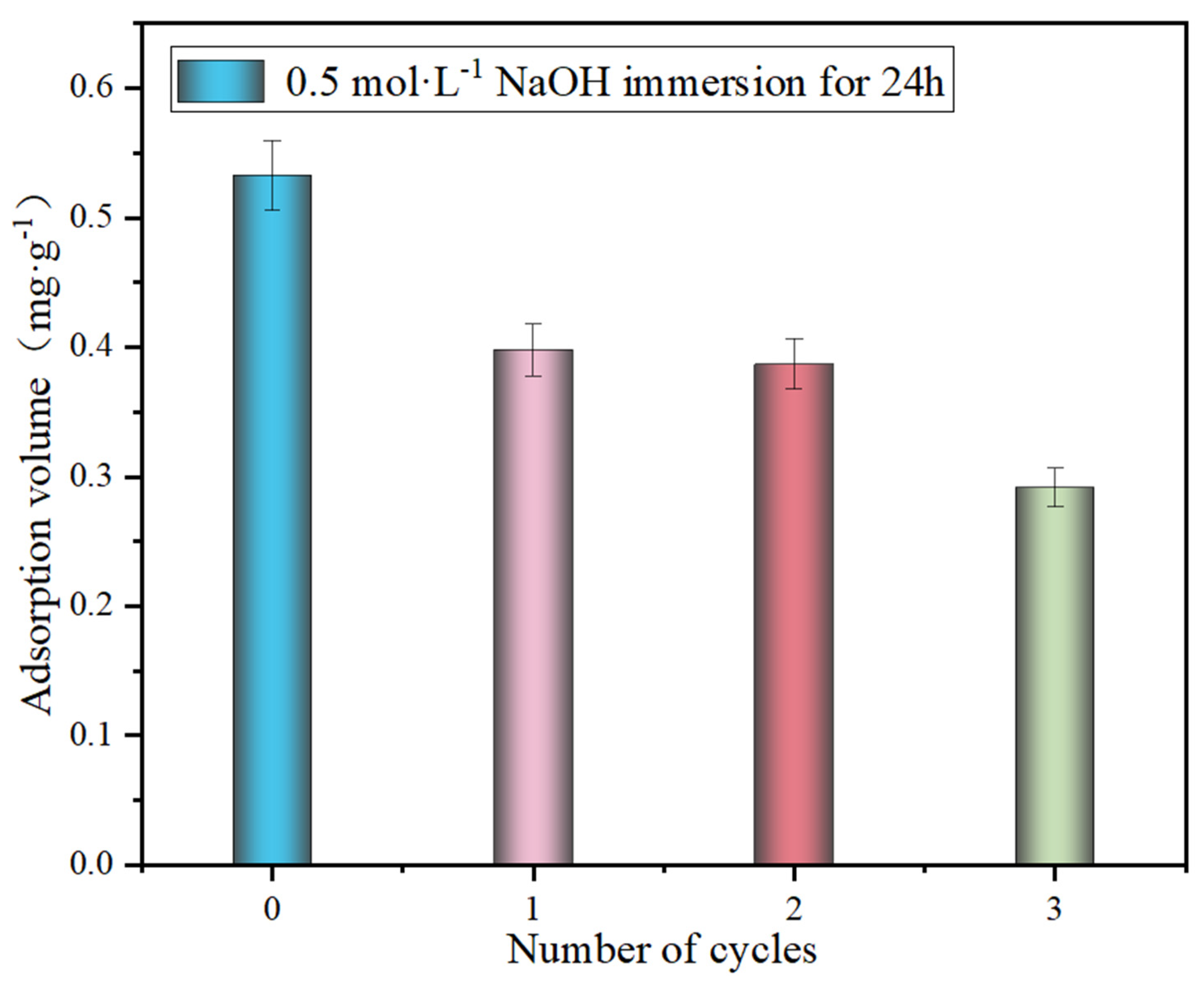
3.4. Regeneration of Adsorbents
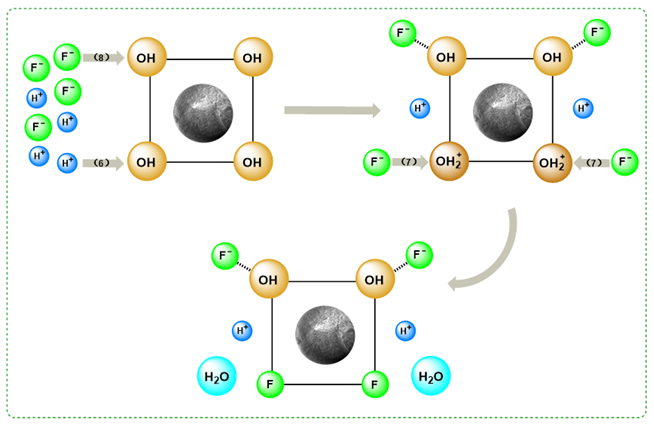
3.5. Exploration of the Adsorption Mechanism
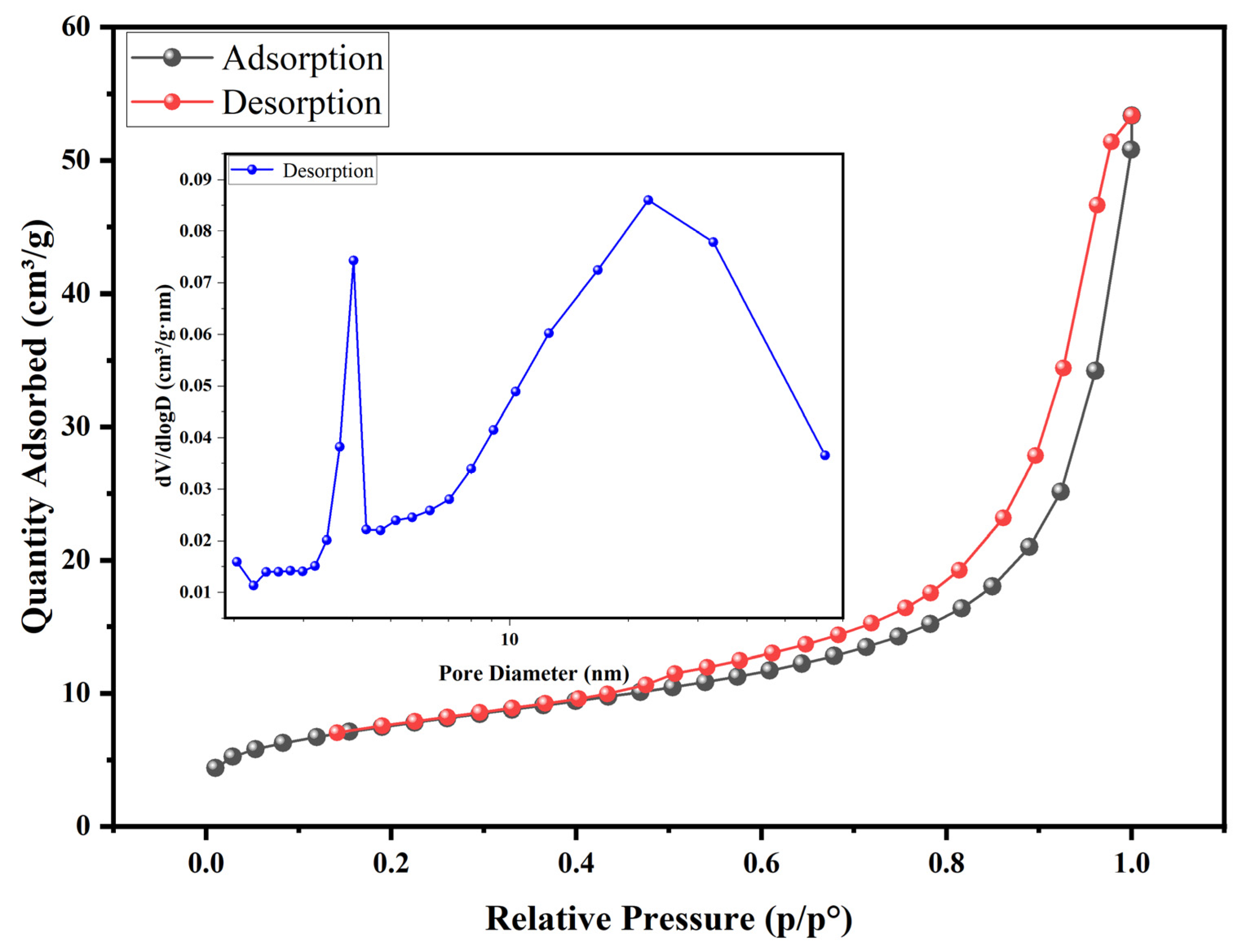
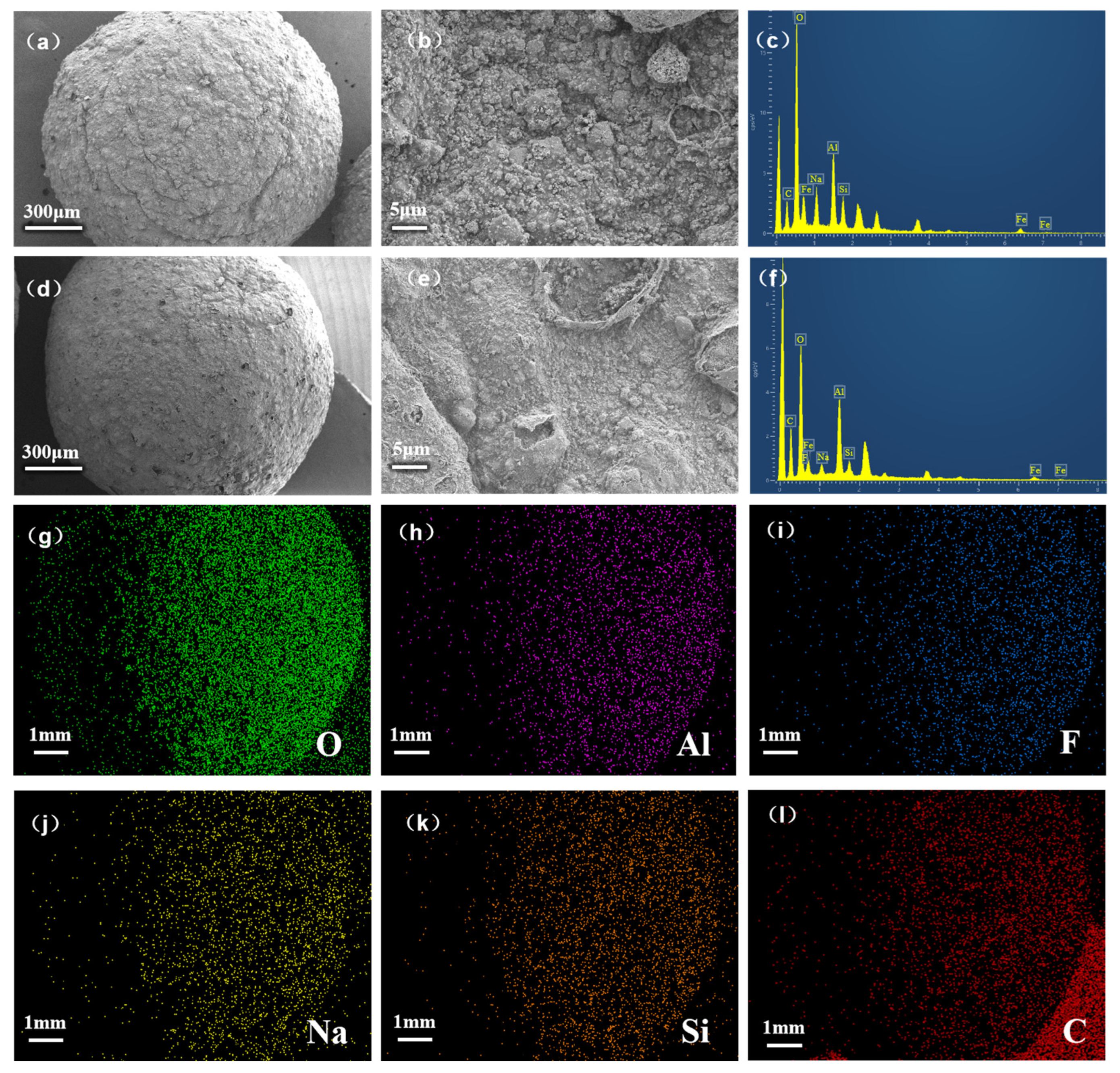
3.5.1. BET and SEM-EDS Analysis
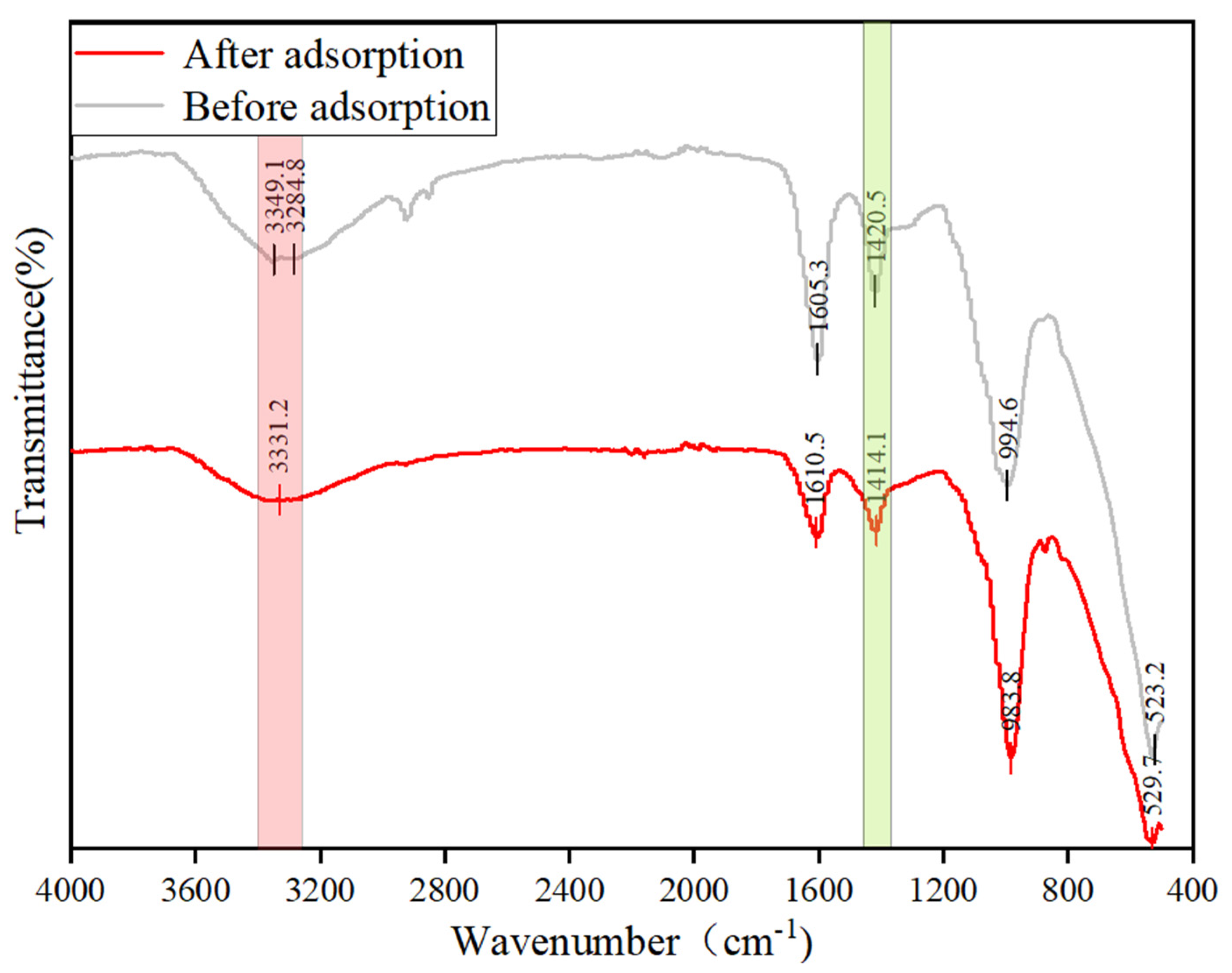
3.5.2. FTIR and XPS Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Li, L.; Zhu, Q.; Man, K.; Xing, Z. Fluoride Removal from Liquid Phase by Fe-Al-La Trimetal Hydroxides Adsorbent Prepared by Iron and Aluminum Leaching from Red Mud. J. Mol. Liq. 2017, 237, 164–172. [Google Scholar] [CrossRef]
- Chen, M.; Li, Z.; Huang, P.; Li, X.; Qu, J.; Yuan, W.; Zhang, Q. Mechanochemical Transformation of Apatite to Phosphoric Slow-Release Fertilizer and Soluble Phosphate. Process Saf. Environ. Prot. 2018, 114, 91–96. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, J.; Wu, H.; Pan, Y.; Xia, Y.; Pan, Z.; Wang, D. Synthesis and Characterization of Heterogeneous Catalyst EDTMPA-Cu-LDH and Study of the Mechanism of Visible-Light Photocatalytic Degradation of Rhodamine B. Desalination Water Treat. 2020, 196, 177–188. [Google Scholar] [CrossRef]
- Ghorai, S.; Pant, K.K. Equilibrium, Kinetics and Breakthrough Studies for Adsorption of Fluoride on Activated Alumina. Sep. Purif. Technol. 2005, 42, 265–271. [Google Scholar] [CrossRef]
- Kaygusuz, H.; Çoşkunırmak, M.H.; Kahya, N.; Erim, F.B. Aluminum Alginate–Montmorillonite Composite Beads for Defluoridation of Water. Water Air Soil Pollut 2015, 226, 2257. [Google Scholar] [CrossRef]
- Silva, D.; Pinto, L.F.V.; Bozukova, D.; Santos, L.F.; Serro, A.P.; Saramago, B. Chitosan/Alginate Based Multilayers to Control Drug Release from Ophthalmic Lens. Colloids Surf. B Biointerfaces 2016, 147, 81–89. [Google Scholar] [CrossRef]
- He, Y.; Huang, L.; Song, B.; Wu, B.; Yan, L.; Deng, H.; Yang, Z.; Yang, W.; Wang, H.; Liang, Z.; et al. Defluorination by Ion Exchange of SO42- on Alumina Surface: Adsorption Mechanism and Kinetics. 2021, 273, 129678. Chemosphere. [CrossRef]
- Dash, S.; Gutti, P.; Behera, B.; Mishra, D. Emergence of Anionic Counterparts of Divalent Metal Salts as the Fine-Tuners of Alginate Hydrogel Properties for Tissue Engineering and Drug Delivery Applications. ChemRxiv 2023. [Google Scholar] [CrossRef]
- Pepper, R.A.; Couperthwaite, S.J.; Millar, G.J. Value Adding Red Mud Waste: Impact of Red Mud Composition upon Fluoride Removal Performance of Synthesised Akaganeite Sorbents. J. Environ. Chem. Eng. 2018, 6, 2063–2074. [Google Scholar] [CrossRef]
- Gao, X.; Guo, C.; Hao, J.; Zhao, Z.; Long, H.; Li, M. Adsorption of Heavy Metal Ions by Sodium Alginate Based Adsorbent-a Review and New Perspectives. Int. J. Biol. Macromol. 2020, 164, 4423–4434. [Google Scholar] [CrossRef]
- Wang, Y.; Yao, W.; Wang, Q.; Yang, Z.; Liang, L.; Chai, L. Synthesis of Phosphate-Embedded Calcium Alginate Beads for Pb(II) and Cd(II) Sorption and Immobilization in Aqueous Solutions. Trans. Nonferrous Met. Soc. China 2016, 26, 2230–2237. [Google Scholar] [CrossRef]
- Zhang, S.; Han, D.; Ding, Z.; Wang, X.; Zhao, D.; Hu, Y.; Xiao, X. Fabrication and Characterization of One Interpenetrating Network Hydrogel Based on Sodium Alginate and Polyvinyl Alcohol. J. Wuhan Univ. Technol.-Mat. Sci. Edit. 2019, 34, 744–751. [Google Scholar] [CrossRef]
- Li, D.; He, B.; Qin, M.; Huang, X. Research on activation of red mud and the performance of its defluorination agent. J. Env. Pollution. 2017, 39, 1117–1121. [Google Scholar] [CrossRef]
- Wei, N.; Luan, Z.; Wang, J.; Shi, L.; Zhao, Y.; Wu, J. Preparation of Modified Red Mud with Aluminum and Its Adsorption Characteristics on Fluoride Removal. J. Inorg. Chem. 2009, 25, 849–854. [Google Scholar] [CrossRef]
- Li, C.; Chen, N.; Zhao, Y.; Li, R.; Feng, C. Polypyrrole-Grafted Peanut Shell Biological Carbon as a Potential Sorbent for Fluoride Removal: Sorption Capability and Mechanism. Chemosphere 2016, 163, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, Z.; Zhang, X.; Zhang, Y.; Long, T.; Wang, X.; Zhang, J.; Liu, J. Tailored Design of a Novel Composite Foam of Sodium Alginate Used for Fluoride Ion Removal. Water Sci. Technol. 2022, 86, 643–655. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Chen, Y.; Zhang, Y.; Tan, X.; Xie, T.; Shao, B.; Huang, X. Novel Reusable Sulfate-Type Zirconium Alginate Ion-Exchanger for Fluoride Removal. Chin. Chem. Lett. 2021, 32, 3410–3415. [Google Scholar] [CrossRef]
- Bian, X.; Qiu, Z.; Yang, J.; Yan, R.; Lv, S. Study on fluoride removal by modified Y zeolite particles with sodium alginate-lanthanum hydroxide J. Inorg. Salt Ind. 2020, 52, 86–91. [Google Scholar] [CrossRef]
- Zhang, L.; Cui, C.; Zhu, C.; Gong, L.; Li, M.; Xiang, S.; Yu, B. High-Efficiency Fluoride Removal Using Hierarchical Flower-like Magnesium Oxide: Adsorption Characteristics and Mechanistic Insights. New J. Chem. 2024, 48, 17268–17276. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, L.; Zhou, B.; Wang, S.; Fan, L.; Hu, S.; Wu, Y. Adsorption Performance and Mechanism of Synthetic Schwertmannite to Remove Low-Concentration Fluorine in Water. Bull Env. Contam Toxicol 2021, 107, 1191–1201. [Google Scholar] [CrossRef]
- Wang, A.; Zhou, K.; Liu, X.; Liu, F.; Chen, Q. Development of Mg–Al–La Tri-Metal Mixed Oxide Entrapped in Alginate for Removal of Fluoride from Wastewater. RSC Adv. 2017, 7, 31221–31229. [Google Scholar] [CrossRef]
- Mahdavinia, G.R. Magnetic Hydrogel Beads Based on PVA/Sodium Alginate/Laponite RD and Studying Their BSA Adsorption. Carbohydr. Polym. 2016, 147, 379–391. [Google Scholar] [CrossRef]
- Nie, Y.X.; Dai, J.F.; Hou, Y.D.; Zhu, Y.; Wang, C.; He, D.; Mei, Y. An efficient and environmentally friendly process for the reduction of SO2 by using waste phosphate mine tailings as adsorbent. J. Hazard. Mater. 2020, 388, 121748. [Google Scholar] [CrossRef]
- Qiao, W.; Bai, H.; Tang, T.; Miao, J.; Yang, Q. Recovery and utilization of phosphorus in wastewater by magnetic Fe3O4/Zn-Al-FeLa layered double hydroxides(LDHs). Colloids Surf. A 2019, 577, 118–128. [Google Scholar] [CrossRef]
- Hobbs, C.E. Recent Advances in Bio-Based Flame Retardant Additives for Synthetic Polymeric Materials. Polymers 2019, 11, 224. [Google Scholar] [CrossRef] [PubMed]
- Fuseini, M.; Zaghloul, M.M.Y.; Eikady, M.F.; EI-Shazly, A.H. Evaluation of synthesized polyaniline nanofibres as corrosion protection film coating on copper substrate by electrophoretic deposition. J. Mater. Sci. 2022, 57, 6085–6101. [Google Scholar] [CrossRef]
- Zhu, Z.M.; Wang, L.X.; Lin, X.B.; Dong, L.P. Synthesis of a novel phosphorus-nitrogen flame retardant and its application in epoxy resin. Polym. Degrad. Stabil. 2019, 169, 108981. [Google Scholar] [CrossRef]
- Mirzaei, S.; Javanbakht, V. Dye Removal from Aqueous Solution by a Novel Dual Cross-Linked Biocomposite Obtained from Mucilage of Plantago Psyllium and Eggshell Membrane. Int. J. Biol. Macromol. 2019, 134, 1187–1204. [Google Scholar] [CrossRef]
- Zare, K.; Banihashemi, A.; Javanbakht, V.; Mohammadifard, H. Fluoride Removal from Aqueous Solutions Using Alginate Beads Modified with Functionalized Silica Particles. J. Mol. Struct. 2022, 1252, 132217. [Google Scholar] [CrossRef]
- Bekeshev, A.; Mostovoy, A.; Kadykova, Y.; Akhmetova, M.; Tastanova, L.; Lopukhova, M. Development and Analysis of the Physicochemical and Mechanical Properties of Diorite-Reinforced Epoxy Composites. Polymers 2021, 13, 2421. [Google Scholar] [CrossRef]
- Gao, D.Q.; Zhang, J.; Yang, G.J.; Zhang, J.; Shi, Z.; Qi, J.; Zhang, Z.; Xue, D. Ferromagnetism in ZnO nanoparticles induced by doping of a nonmagnetic element: Al. J. Phys. Chem. C 2010, 114, 13477–13481. [Google Scholar] [CrossRef]
- Kim, Y.J.; Park, C.R. Analysis of problematic complexing behavior of ferric chloride with N,N-dimethylformamide using combined techniques of FT-IR, XPS, and TGA/DTG. Inorg. Chem. 2002, 41, 6211–6216. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.M.; Lin, P.L.; Wang, H.; Wang, L.X.; Yu, B.; Yang, F.H. A facile one-step synthesis of highly efficient melamine salt reactive flame retardant for epoxy resin. J. Mater. Sci. 2020, 55, 12836–12847. [Google Scholar] [CrossRef]




| SiO2 | Fe2O3 | Al2O3 | CaO and MgO | Na2O | K2O | TiO2 | Ignition Loss |
|---|---|---|---|---|---|---|---|
| 16.3% | 30.1% | 26.25% | 1.81% | 16.2% | 0.128% | 3.619% | 13.48% |
| pseudo-first-order kinetic model | qe (mg·g−1) | k1 (min−1) | R2 |
| ) | 0.4649 | 0.0624 | 0.9423 |
| pseudo-second-order kinetic model | qe (mg·g−1) | k2 (g·mg−1·min−1) | R2 |
| qt = k2qe2t/(1 + kqet) | 0.5341 | 0.1523 | 0.9745 |
| Langmuir adsorption isothermal model | qmax (mg·g−1) | KL (L·mg−1) | R2 |
| qe = KLqmaxCe/(1 + Ce) | 0.73 | 0.3843 | 0.9942 |
| Freundlich adsorption isothermal model | KF (L·mg−1) | n | R2 |
| qe = KFCe(-n) | 0.2574 | −0.3640 | 0.9258 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Zhang, H.; Wang, N.; Mo, H.; Yang, Z.; Dong, Y.; Liu, Q.; Huang, X.; Han, B. Synthesis of Polymer Sodium Alginate–Red Mud Adsorbent and Its Application in the Removal of Low-Concentration Fluoride. Polymers 2025, 17, 826. https://doi.org/10.3390/polym17060826
Wang J, Zhang H, Wang N, Mo H, Yang Z, Dong Y, Liu Q, Huang X, Han B. Synthesis of Polymer Sodium Alginate–Red Mud Adsorbent and Its Application in the Removal of Low-Concentration Fluoride. Polymers. 2025; 17(6):826. https://doi.org/10.3390/polym17060826
Chicago/Turabian StyleWang, Jiahao, Huali Zhang, Nenghao Wang, Han Mo, Zhen Yang, Yangyang Dong, Qingwei Liu, Xiao Huang, and Baoyi Han. 2025. "Synthesis of Polymer Sodium Alginate–Red Mud Adsorbent and Its Application in the Removal of Low-Concentration Fluoride" Polymers 17, no. 6: 826. https://doi.org/10.3390/polym17060826
APA StyleWang, J., Zhang, H., Wang, N., Mo, H., Yang, Z., Dong, Y., Liu, Q., Huang, X., & Han, B. (2025). Synthesis of Polymer Sodium Alginate–Red Mud Adsorbent and Its Application in the Removal of Low-Concentration Fluoride. Polymers, 17(6), 826. https://doi.org/10.3390/polym17060826






