Current Status and Future Trends for Modification Technology of Flame Retardant Nylon 66
Abstract
:1. Introduction
2. Flame Retardant Mechanism of Flame Retardants
2.1. The Combustion Mechanism of PA66
2.2. The Flame Retardant Mechanism of PA66
2.2.1. Gas Phase Flame Retardant Mechanism
2.2.2. Condensed Phase Flame Retardant Mechanism
2.2.3. Synergistic Flame Retardant Mechanism
2.2.4. Interrupting Heat Exchange Flame Retardant Mechanism
3. The Development of Flame Retardant PA66
3.1. Blending Flame Retardant Modification
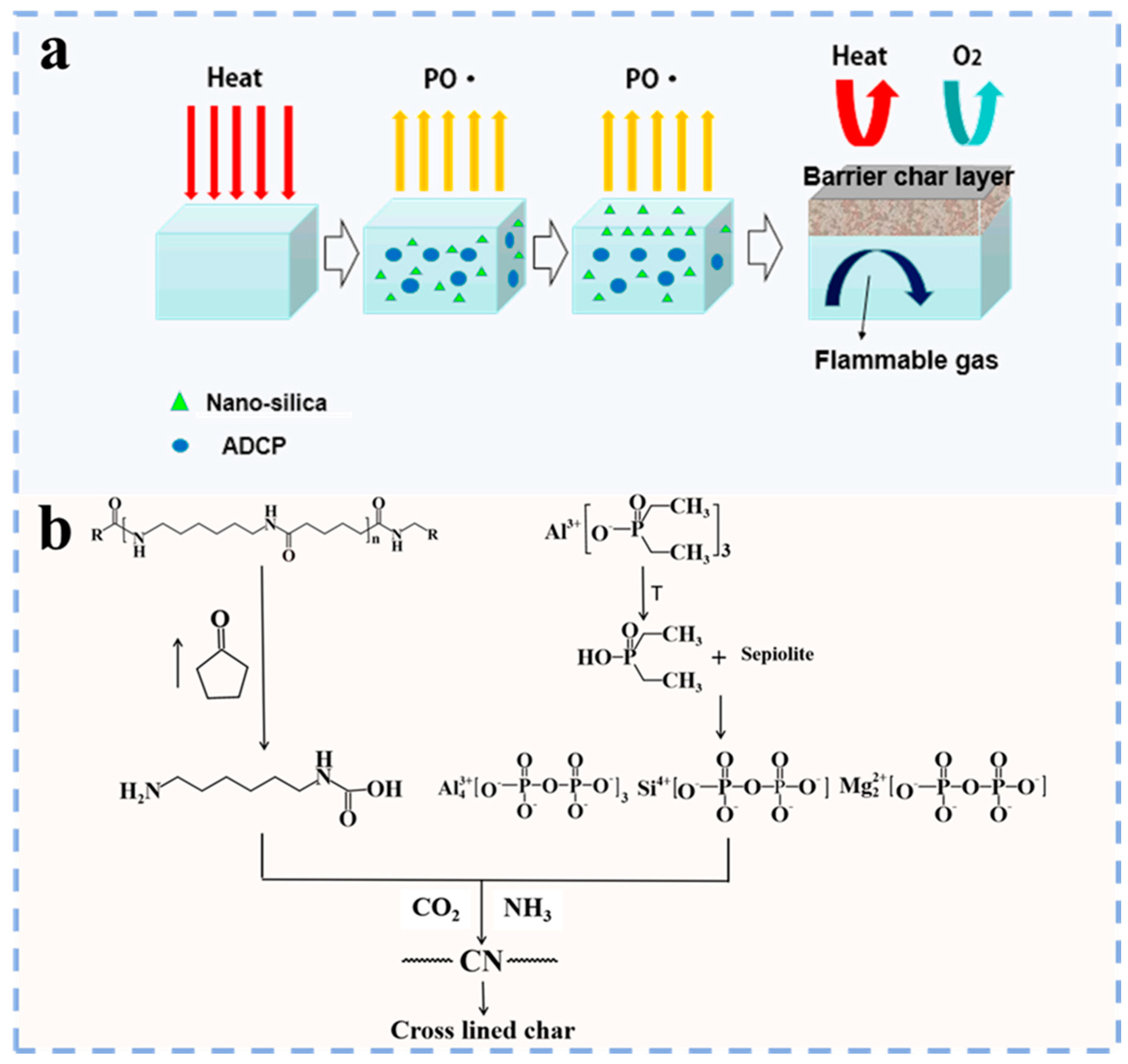
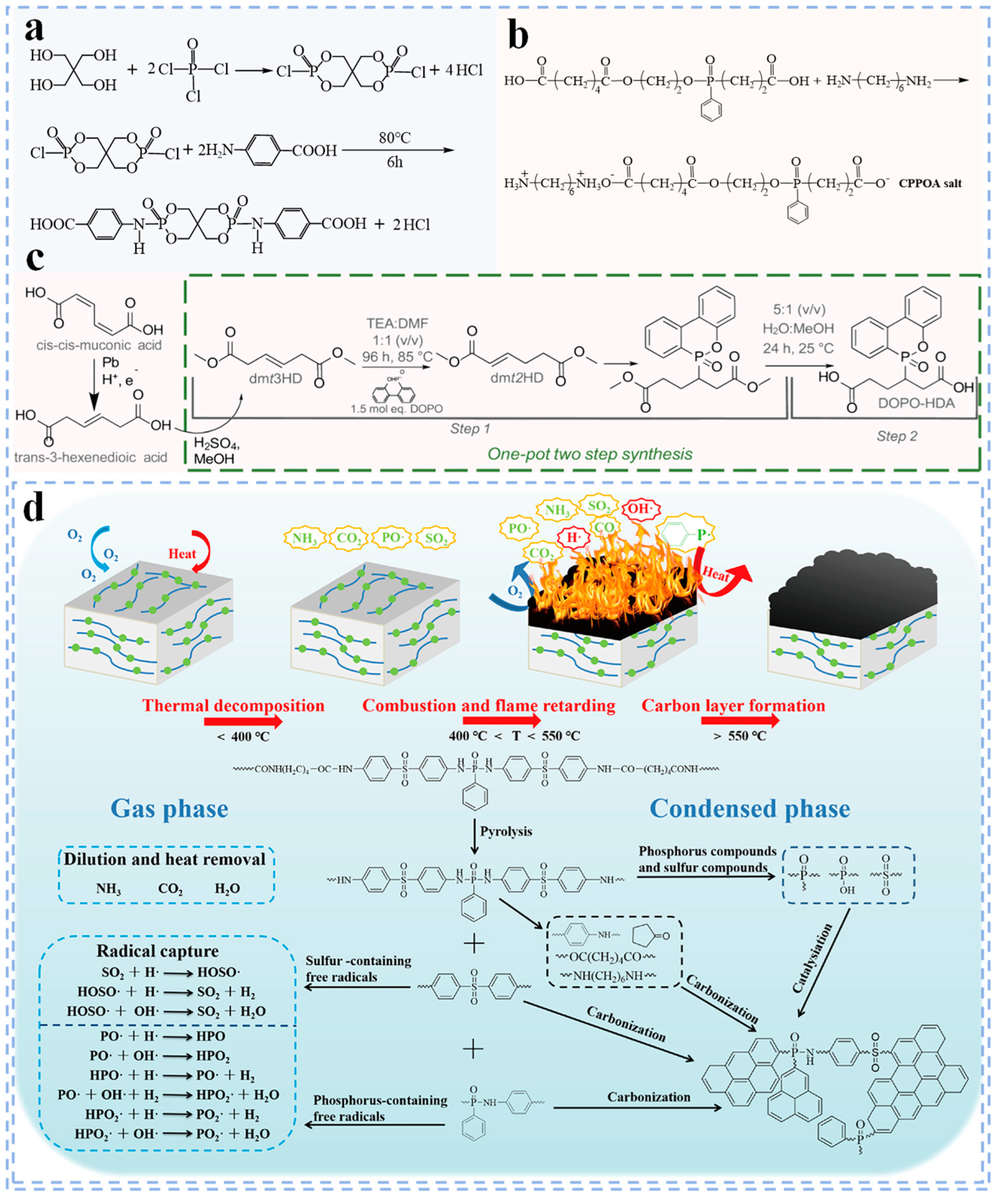
3.1.1. Phosphorus Flame Retardants
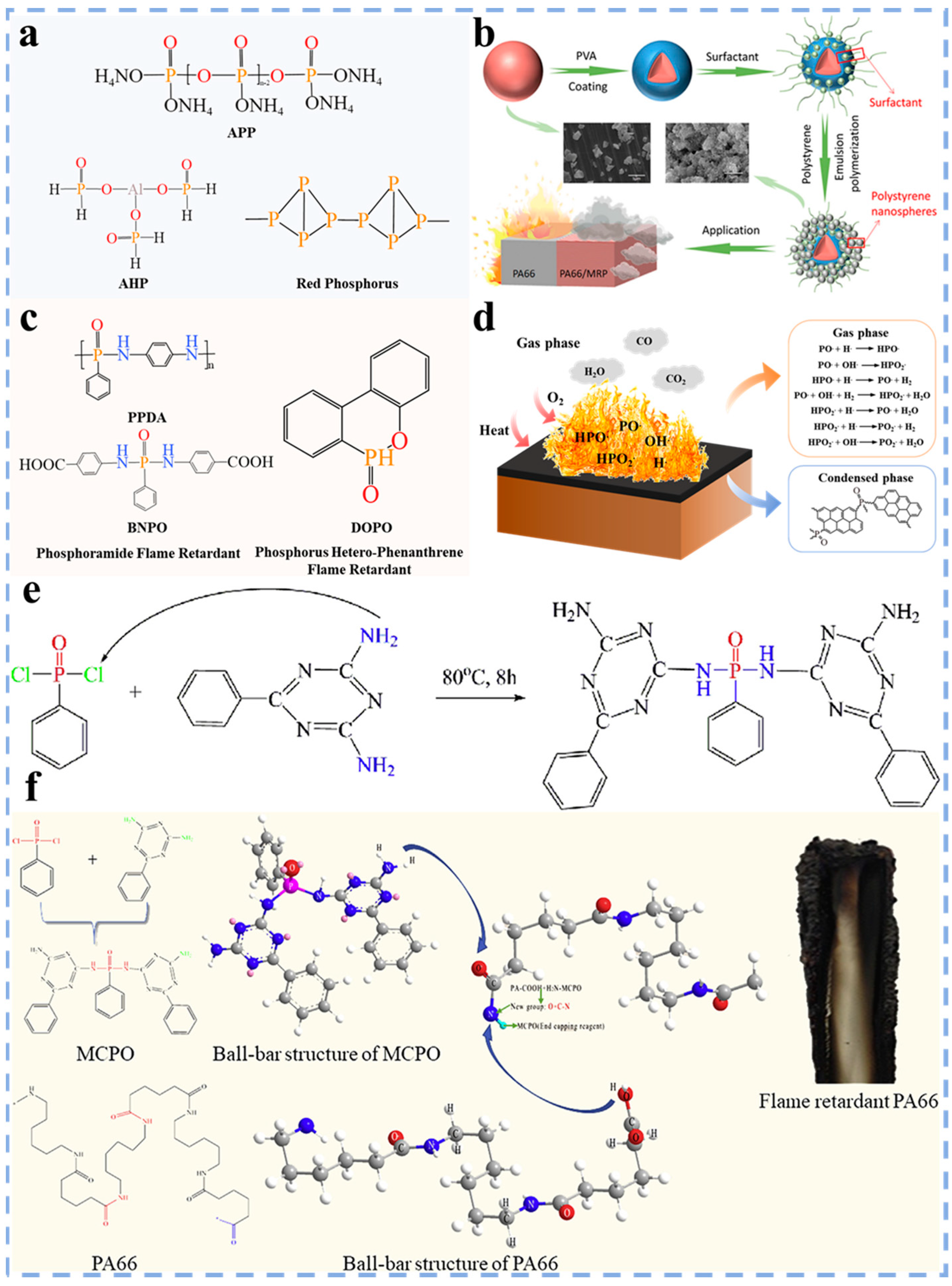
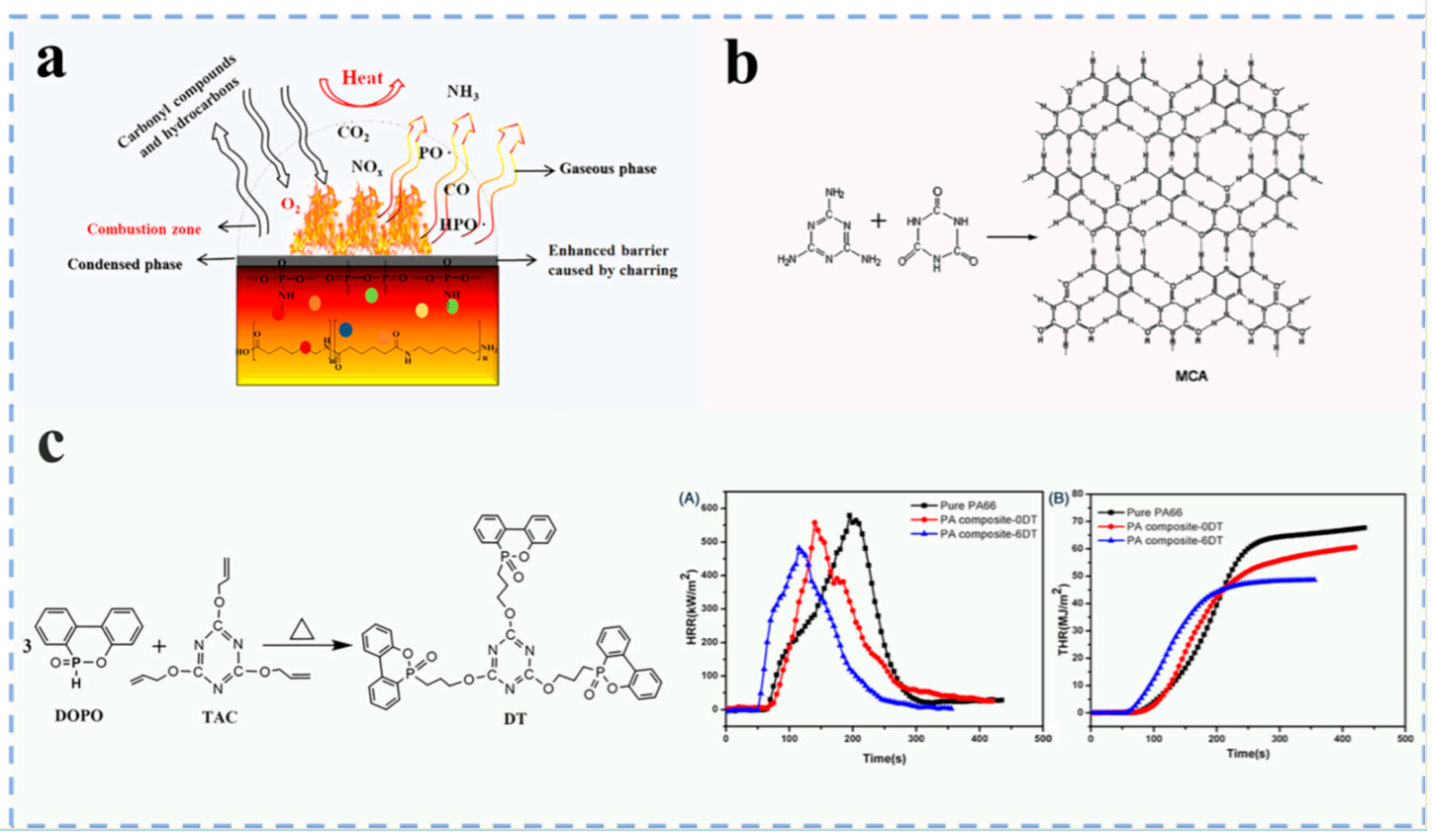
3.1.2. Nitrogen Flame Retardants
3.1.3. Phosphorus–Nitrogen Flame Retardants
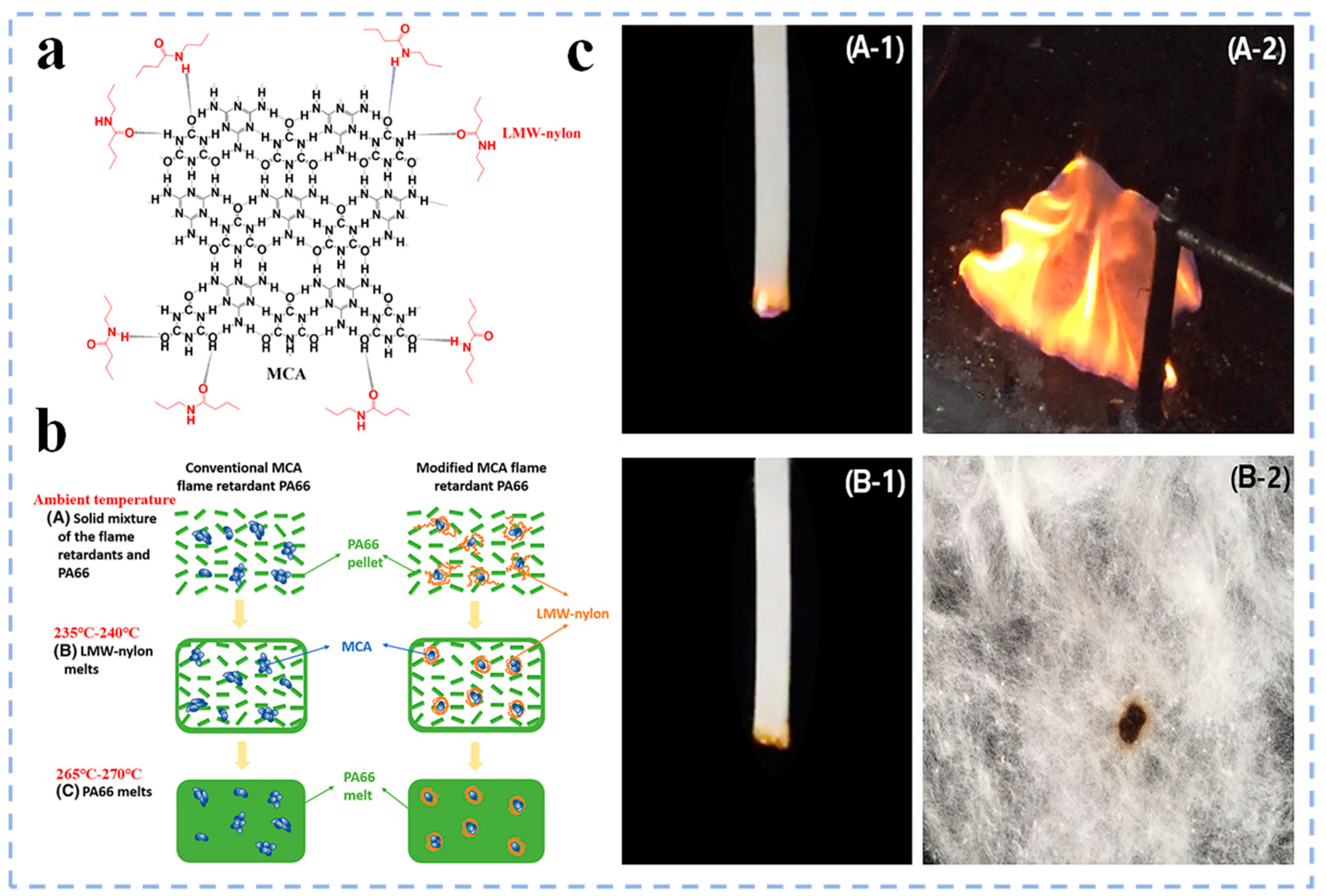
3.1.4. Organic–Inorganic Flame Retardants
3.2. Copolymerization Flame Retardant Modification
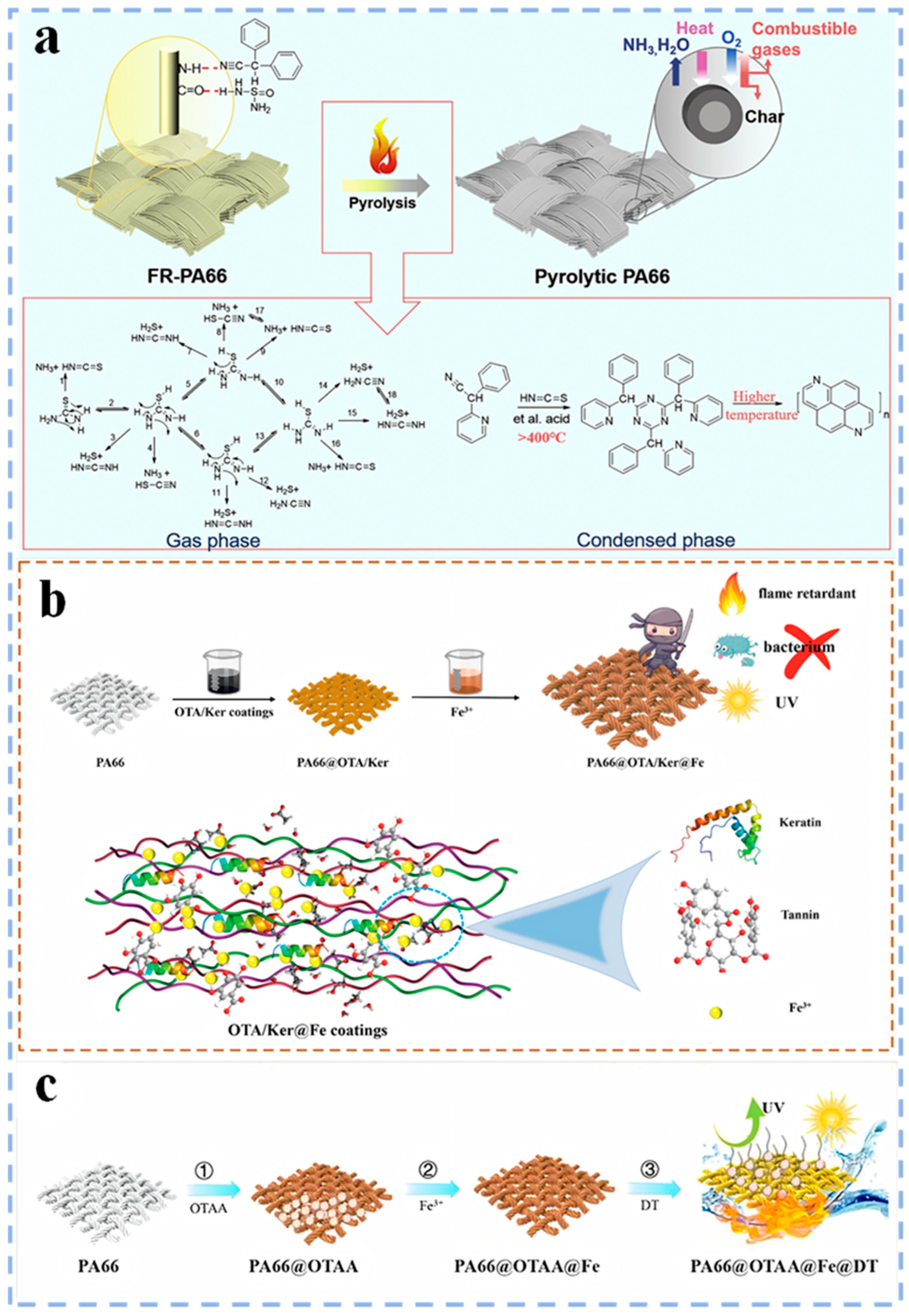
3.3. Post–Finishing Flame Retardant Modification
| Flame Retardants | Modification Method | References |
|---|---|---|
| Vinyltrimethoxysilane (VTMS) | Microwave grafting | [68] |
| Chitosan (CS), phytic acid (PA) and oxidized sodium alginate (OSA) | Deposit | [69] |
| Bio–based polyelectrolytes, CS, PA, (3–aminopropyl) triethoxysilane (APTES) and boron–doped APTES | Deposit and sol–gel treatment | [70] |
| Graphene oxide functionalized bio–macromolecule | One–pot deposition | [71] |
| CS and PA | Layer–by–layer assembly | [72] |
| CS, phosphorylated chitosan (PCS), and poly–acrylate sodium (PAS) | One–pot and layer–by–layer assembly | [73] |
| PA and Al3+ | Layer–by–layer deposition technology | [74] |
| CS and phytic acid ammonia (PAA) | Pad–dry–cure technique | [75] |
4. Conclusions and Prospect
- (1)
- High efficiency: With the continuous progress and enrichment of coating, microencapsulation, masterbatch and other technical means, the development of efficient flame retardants not only reduces the adverse impact on the original properties of PA66 but also significantly decreases the amount of flame retardants added, thereby leading to lower material costs. However, achieving high efficiency of flame retardants still requires continuous optimization and improvement of related technologies, such as further enhancing the performance and competitiveness of flame retardants through finer process control and innovation.
- (2)
- Compound: For PA66 with high flame retardant requirements, a single flame retardant is usually difficult to meet strict flame retardant standards. In contrast, compound flame retardants can not only significantly improve the flame retardant efficiency through the synergistic effect of a variety of flame retardant components but also reduce the total amount of addition; this approach better preserves the comprehensive performance of the material. In recent years, some emerging flame retardant strategies (such as intumescent systems and layer–by–layer assemblies) have shown outstanding application potential by promoting the formation of char layers and strengthening synergies. Therefore, the development of high–performance flame retardant PA66 based on compounding technology will become an important direction of future research.
- (3)
- Green and environmentally friendly: Green and environmentally friendly have become an important trend in the development of flame retardant materials. With the advancement of industry technology and the increasing public awareness of health and environmental protection, halogen flame retardants are gradually facing elimination. In the future, the investigation and application of halogen–free flame retardants and environmentally friendly flame retardants will become the development focus of flame retardant PA66. To meet the growing market demand for safety, environmental protection and sustainable development, the use of these flame retardants is expected to continue increasing.
- (4)
- Multifunctionalization: The multifunctionalization of flame retardant PA66 will become an important direction of future research. With the increasing complexity of industrial application scenarios, it is difficult for materials with single properties to meet diverse needs. For this reason, PA66 is evolving from simple flame retardant to multifunctionalization, such as combining flame retardancy with antibacterial, antistatic, UV–resistant or mechanically enhanced properties. Relevant applications include smart textiles, automotive wiring requiring durability and fire resistance, and enclosures that demand both thermal stability and EMI shielding. Therefore, the development of multifunctional flame retardant PA66 to meet the urgent needs of modern industry for high–performance, multipurpose materials will become a key research direction in the future.
- (5)
- Additional emerging trends: In the future, the development trend of flame retardant PA66 will pay more attention to recyclability and circular economy integration, reducing the environmental burden by designing flame retardant systems that are compatible with recycling. At the same time, the widespread application of advanced characterization and simulation technologies (e.g., AI, modeling and high–throughput screening) will accelerate the prediction of flame behavior and material optimization. In addition, the rise in smart flame retardants, such as thermally triggered char formation and other stimuli–responsive systems that can dynamically enhance flame retardancy in the event of a fire, further promotes the development of efficient and adaptive flame retardant PA66. These innovative directions reflect the evolution of flame retardant technology toward sustainability, precision and functionality.
Author Contributions
Funding
Conflicts of Interest
References
- Duan, X.; Wu, Y.; Chen, Z.; Yang, T.; Cheng, Y.; Yu, H.; Huang, T. In–situ polymerization of high–molecular weight nylon 66 modified clay nanocomposites with low apparent viscosity. Polymers 2019, 11, 510. [Google Scholar] [CrossRef] [PubMed]
- Al–Shawabkeh, A.F. Optoelectronic investigation and spectroscopic characteristics of polyamide–66 polymer. e–Polym 2022, 22, 858–869. [Google Scholar]
- Tseng, C.H.; Tsai, P.S. The isothermal and nonisothermal crystallization kinetics and morphology of solvent–precipitated nylon 66. Polymers 2022, 14, 442. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Wu, Y.; Li, J.; Peng, X.; Yin, D.; Jin, H.; Wang, S.; Wang, J.; Wang, X.; Jin, M.; et al. A review of the recent developments in flame–retardant nylon composites. Compos. Part C Open Access. 2022, 9, 100297. [Google Scholar]
- Liu, Y.; Wang, Q. Melamine cyanurate–microencapsulated red phosphorus flame retardant unreinforced and glass fiber reinforced polyamide 66. Polym. Degrad. Stab. 2006, 91, 3103–3109. [Google Scholar]
- Huang, X.; Li, B.; Shi, B.; Li, L. Investigation on interfacial interaction of flame retarded and glass fiber reinforced PA66 composites by IGC/DSC/SEM. Polymer 2008, 49, 1049–1055. [Google Scholar]
- Li, Y.; Liu, K.; Zhang, J.; Xiao, R. Preparation and characterizations of inherent flame retarded polyamide 66 containing the phosphorus linking pendent group. Polym. Adv. Technol. 2018, 29, 951–960. [Google Scholar] [CrossRef]
- Liu, W.; Shi, R.; Ge, X.; Huang, H.; Chen, X.; Mu, M. A bio–based flame retardant coating used for polyamide 66 fabric. Prog. Org. Coat. 2021, 156, 106271. [Google Scholar]
- Jiang, L.; Liu, Y.; Zuo, C.; Zhao, J.; Tan, W.; Ren, Y.; Liu, X. Improving the flame retardant and anti–dripping performance of polyamide 66 inspired by vegetable tanning. Polym. Degrad. Stab. 2023, 217, 110517. [Google Scholar]
- Kim, D.K.; Lee, A.S.; Baek, B.K.; Song, K.H.; Hong, S.M.; Koo, C.M. PPE/nylon 66 blends with high mechanical toughness and flame retardancy. Macromol. Res. 2020, 28, 103–109. [Google Scholar]
- Jou, W.S.; Chen, K.N.; Chao, D.Y.; Lin, C.Y.; Yeh, J.T. Flame retardant and dielectric properties of glass fibre reinforced nylon-66 filled with red phosphorous. Polym. Degrad. Stab. 2001, 74, 239–245. [Google Scholar] [CrossRef]
- Fu, H.; Cui, Y.; Lv, J. Fire retardant mechanism of PA66 modified by a “trinity” reactive flame retardant. J. Appl. Polym. Sci. 2020, 137, 47488. [Google Scholar]
- Ma, X.; Chen, X.; Wang, X.; Yang, X.; Yao, Z.; Yu, H.; Zhang, Y. Enhancing flame retardancy and heat insulation performances of polyamide 66 composite film by adding CNC/Al2O3 nanohybrids. Int. J. Biol. Macromol. 2024, 278, 134702. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Yang, T.; Cheng, Y.; Huang, T.; Yu, B.; Wu, Q.; Zhu, M.; Yu, H. Synthesis phosphorus–sulfur reactive flame retardant for polyamide 66 with high flame retardant efficiency and low smoke. Polym. Degrad. Stab. 2023, 214, 110378. [Google Scholar] [CrossRef]
- Zhang, J.; Lian, S.; He, Y.; Cao, X.; Shang, J.; Liu, Q.; Ye, G.; Zheng, K.; Ma, Y. Intrinsically flame–retardant polyamide 66 with high flame retardancy and mechanical properties. RSC. Adv. 2021, 11, 433–441. [Google Scholar] [CrossRef]
- Carter, P.; Meyer, P.M.; Lee, T.H.; Dileep, D.; Chalgren, N.L.; Noreen, S.; Forrester, M.J.; Shanks, B.H.; Tessonnier, J.P.; Cochran, E.W. Leveraging the bio–enabled muconic acid platform via phospha–Michael–addition: Intrinsically flame–retardant nylon-66/DOPO copolymers. RSC Sustain. 2024, 2, 2968–2978. [Google Scholar] [CrossRef]
- Tamura, K.; Ohyama, S.; Umeyama, K.; Kitazawa, T.; Yamagishi, A. Preparation and properties of halogen–free flame–retardant layered silicate–polyamide 66 nanocomposites. Appl. Clay Sci. 2016, 126, 107–112. [Google Scholar] [CrossRef]
- Liu, W.; Shi, R.; Zhang, Z.; Ge, X.; Li, P.; Chen, X. Facile Strategy to Fabricate the Flame Retardant Polyamide 66 Fabric Modified with an Inorganic–Organic Hybrid Structure. ACS Appl. Mater. Interfaces 2021, 13, 9122–9133. [Google Scholar] [CrossRef]
- Li, Y.Y.; Liu, K.; Xiao, R. Preparation and characterizations of flame retardant polyamide 66 fiber. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2017. [Google Scholar]
- Wu, Y.; Yang, T.; Cheng, Y.; Huang, T.; Yu, B.; Wu, Q.; Zhu, M.; Yu, H. Flame Retardancy and Mechanical Properties of Melt-Spun PA66 Fibers Prepared by End–Group Blocking Technology. Polymers 2023, 15, 1183. [Google Scholar] [CrossRef]
- Purser, D.A. Toxicity assessment of combustion products. In SFPE Handbook of Fire Protection Engineering; DiNenno, P.J., Ed.; Springer: Berlin/Heidelberg, Germany, 2002; pp. 298–386. [Google Scholar]
- Behzadfar, E.; Kim, K.; Lee, B.; Jordan, A.M.; Lhost, O.; Jaffer, S.A.; Bates, F.S.; Macosko, C.W. Effects of a layered morphology on drip suppression in burning polymers. ACS Appl. Polym. Mater. 2021, 3, 1664–1674. [Google Scholar] [CrossRef]
- Kishore, K.; Nagarajan, R. Polymer combustion: A review. J. Polym. Eng. 1987, 7, 319–347. [Google Scholar]
- Liu, B.W.; Zhao, H.B.; Wang, Y.Z. Advanced flame–retardant methods for polymeric materials. Adv. Mater. 2022, 34, 2107905. [Google Scholar]
- Shinohara, Y.; Ballantine, D. Free radicals in irradiated nylon. J. Chem. Phys. 1962, 36, 3042–3049. [Google Scholar]
- Morgan, A.B.; Gilman, J.W. An overview of flame retardancy of polymeric materials: Application, technology, and future directions. Fire Mater. 2013, 37, 259–279. [Google Scholar]
- Lv, W.; Lv, J.; Zhu, C.; Zhang, Y.; Cheng, Y.; Zeng, L.; Wang, L.; Liao, C. Thermal Stabilities and Flame Retardancy of Polyamide 66 Prepared by In Situ Loading of Amino–Functionalized Polyphosphazene Microspheres. Polymers 2022, 15, 218. [Google Scholar] [CrossRef]
- Hörold, S. Phosphorus–based and intumescent flame retardants. In Polymer Green Flame Retardants; Papaspyrides, C., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 221–254. [Google Scholar]
- Kundu, C.K.; Yu, B.; Gangireddy, C.S.R.; Mu, X.; Wang, B.; Wang, X.; Song, L.; Hu, Y. UV grafting of a DOPO–based phosphoramidate monomer onto polyamide 66 fabrics for flame retardant treatment. Ind. Eng. Chem. Res. 2017, 56, 1376–1384. [Google Scholar]
- Lyu, W.; Cui, Y.; Zhang, X.; Yuan, J.; Zhang, W. Thermal stability, flame retardance, and mechanical properties of polyamide 66 modified by a nitrogen–phosphorous reacting flame retardant. J. Appl. Polym. Sci. 2016, 133, 43538. [Google Scholar]
- Zhan, Z.; Xu, M.; Li, B. Synergistic effects of sepiolite on the flame retardant properties and thermal degradation behaviors of polyamide 66/aluminum diethylphosphinate composites. Polym. Degrad. Stab. 2015, 117, 66–74. [Google Scholar]
- Chen, Y.; Wang, Q.; Yan, W.; Tang, H. Preparation of flame retardant polyamide 6 composite with melamine cyanurate nanoparticles in situ formed in extrusion process. Polym. Degrad. Stab. 2006, 91, 2632–2643. [Google Scholar]
- Xie, M.; Zhang, S.; Ding, Y.; Wang, F.; Liu, P.; Tang, H.; Wang, Y.; Yang, M. Synthesis of a heat–resistant DOPO derivative and its application as flame–retardant in engineering plastics. J. Appl. Polym. Sci. 2017, 134, 44892. [Google Scholar]
- Zhang, Z.; Cai, K.; Guo, Y.; Liu, X.; He, S.; Liu, H.; Huang, M.; Yang, M.; Liu, W. Halogen Free Flame–Retardant Glass Fiber Reinforced PA66/PPO Alloys. Res. Sq. 2021. [Preprint]. [Google Scholar] [CrossRef]
- Lyu, W.; Chen, X.; Li, Y.; Cao, S.; Han, Y. Thermal stability and heat release effect of flame retarded PA66 prepared by end-pieces capping technology. Compos. Part B. 2019, 167, 34–43. [Google Scholar]
- Schartel, B.; Kunze, R.; Neubert, D. Red phosphorus–controlled decomposition for fire retardant PA66. J. Appl. Polym. Sci. 2002, 83, 2060–2071. [Google Scholar]
- Ding, Y.; McCoy, C.G.; Stoliarov, S.I.; Hu, H. Prediction of mass loss and heat release rates measured in cone calorimeter experiments performed on glass fiber reinforced nylon 66 blended with red phosphorus. Int. J. Therm. Sci. 2023, 190, 108320. [Google Scholar]
- Chen, X.; Lan, W.; Dou, W. Polystyrene nanospheres coated red phosphorus flame retardant for polyamide 66. J. Appl. Polym. Sci. 2022, 139, e52772. [Google Scholar]
- Lyu, W.; Cui, Y.; Zhang, X.; Yuan, J.; Zhang, W. Synthesis, thermal stability, and flame retardancy of PA66, treated with dichlorophenylphsophine derivatives. Des. Monomers Polym. 2016, 19, 420–428. [Google Scholar]
- Guo, S.; Xu, J.; Ni, X. Synthesis, structures, and properties of a new pentaerythritol–derived flame retardant used in polyamide 66. ACS Omega 2021, 6, 12887–12897. [Google Scholar]
- Camino, G.; Costa, L.; Trossarelli, L. Study of the mechanism of intumescence in fire retardant polymers: Part I–Thermal degradation of ammonium polyphosphate–pentaerythritol mixtures. Polym. Degrad. Stab. 1984, 6, 243–252. [Google Scholar]
- Howell, B.A. Thermal degradation of organophosphorus flame retardants. Polymers 2022, 14, 4929. [Google Scholar] [CrossRef]
- Huo, S.; Song, P.; Yu, B.; Ran, S.; Chevali, V.S.; Liu, L.; Fang, Z.; Wang, H. Phosphorus–containing flame retardant epoxy thermosets: Recent advances and future perspectives. Prog. Polym. Sci. 2021, 114, 101366. [Google Scholar]
- Luo, D.; Duan, W.; Liu, Y.; Chen, N.; Wang, Q. Melamine cyanurate surface treated by nylon of low molecular weight to prepare flame–retardant polyamide 66 with high flowability. Fire Mater. 2019, 43, 323–331. [Google Scholar]
- Wang, W.; Wang, F.; Li, H.; Liu, Y. Synthesis of phosphorus–nitrogen hybrid flame retardant and investigation of its efficient flame–retardant behavior in PA6/PA66. J. Appl. Polym. Sci. 2023, 140, e53536. [Google Scholar]
- Chen, W.; Liu, P.; Liu, Y.; Liu, Y.; Wang, Q. Synergistic flame–retardant effect and mechanism of nitrogen–phosphorus–containing compounds for glass fiber–reinforced polyamide 66. Polym. Plast. Technol. Eng. 2017, 56, 1118–1127. [Google Scholar]
- Guo, S.; Bao, M.; Ni, X. The synthesis of meltable and highly thermostable triazine–DOPO flame retardant and its application in PA66. Polym. Adv. Technol. 2021, 32, 815–828. [Google Scholar]
- Sheng, F.; Tang, X.Z.; Zhang, S.; Ding, X.; Yu, Z.Z.; Qiu, Z. Flame retardancy of polyamide 66 nanocomposites with thermally stable organoclay. Polym. Adv. Technol. 2012, 23, 137–142. [Google Scholar]
- Qin, H.; Su, Q.; Zhang, S.; Zhao, B.; Yang, M. Thermal stability and flammability of polyamide 66/montmorillonite nanocomposites. Polymer 2003, 44, 7533–7538. [Google Scholar]
- Liu, Y.; Wang, Q. The investigation of melamine cyanurate–encapsulating magnesium hydroxide flame retarded polyamide–66. Polym. Polym. Compos. 2010, 18, 315–320. [Google Scholar]
- Zhang, H.; Lu, J.; Yang, H.; Yang, H.; Lang, J.; Zhang, Q. Synergistic flame–retardant mechanism of dicyclohexenyl aluminum hypophosphite and nano–silica. Polymers 2019, 11, 1211. [Google Scholar] [CrossRef]
- Zhan, Z.; Li, B.; Xu, M.; Guo, Z. Synergistic effects of nano–silica on aluminum diethylphosphinate/polyamide 66 system for fire retardancy. High Perform. Polym. 2016, 28, 140–146. [Google Scholar]
- Zhan, Z.; Shi, J.; Zhang, Y.; Zhang, Y.; Zhang, B.; Liu, W. The study on flame retardancy synergetic mechanism of magnesium oxide for PA66/AlPi composite. Mater. Res. Express. 2019, 6, 115317. [Google Scholar]
- Bourbigot, S.; Duquesne, S. Fire retardant polymers: Recent developments and opportunities. J. Mater. Chem. 2007, 17, 2283–2300. [Google Scholar] [CrossRef]
- Wazarkar, K.; Kathalewar, M.; Sabnis, A. Reactive modification of thermoplastic and thermoset polymers using flame retardants: An overview. Polym. Plast. Technol. Eng. 2016, 55, 71–91. [Google Scholar] [CrossRef]
- Ridgway, J.S. Nylon 6, 6 copolyamides of bis (2–carboxyethyl) methylphosphine oxide. J. Appl. Polym. Sci. 1988, 35, 215–227. [Google Scholar] [CrossRef]
- Yang, X.; Li, Q.; Chen, Z.; Han, H. Fabrication and thermal stability studies of polyamide 66 containing triaryl phosphine oxide. Bull. Mater. Sci. 2009, 32, 375–380. [Google Scholar] [CrossRef]
- Feng, Y.X.; Ling, L.Q.; Ping, C.Z.; Feng, Y.Y.; Lei, Z. Study on crystallization behaviour of co–polyamide 66 containing triaryl phosphine oxide. Bull. Mater. Sci. 2012, 35, 233–242. [Google Scholar] [CrossRef]
- Lyu, W.; Cui, Y.; Zhang, X.; Yuan, J.; Zhang, W. Fire and thermal properties of PA66 resin treated with poly–N–aniline–phenyl phosphamide as a flame retardant. Fire Mater. 2017, 41, 349–361. [Google Scholar] [CrossRef]
- Chen, X.; Xu, D.; Zhang, H.; Feng, X.; Deng, J.; Pan, K. In situ polymerization of flame retardant modification polyamide 6, 6 with 2–carboxy ethyl (phenyl) phosphinic acid. J. Appl. Polym. Sci. 2020, 137, 48687. [Google Scholar] [CrossRef]
- Zheng, Y.L.; Chen, S.; Wang, J.H.; Xiao, R. Synthesis and Characterization of Phosphorus–Containing Flame Retardant Polyamide 66. In Materials Science Forum; Trans Tech Publications Ltd.: Wollerau, Switzerland, 2020. [Google Scholar]
- Cao, Z.; Zhang, L.; Zhao, S.; Li, X.; Zhao, B.; Zhou, Y.; Gong, S.; Chen, X.; Feng, X.; Pan, K. Study on polymerization kinetics of copolymerized flame–retardant polyamide 66: Apparent rate constant and reaction activation energy. Polymer 2024, 299, 126936. [Google Scholar] [CrossRef]
- Weil, E.D.; Levchik, S.V. Flame Retardants for Plastics and Textiles: Practical Applications; Carl Hanser Verlag GmbH Co KG: Munich, Germany, 2015. [Google Scholar]
- Meng, D.; Guo, J.; Wang, A.; Gu, X.; Wang, Z.; Jiang, S.; Zhang, S. The fire performance of polyamide66 fabric coated with soybean protein isolation. Prog. Org. Coat. 2020, 148, 105835. [Google Scholar] [CrossRef]
- Kundu, C.K.; Song, L.; Hu, Y. Sol–gel coatings from DOPO–alkoxysilanes: Efficacy in fire protection of polyamide 66 textiles. Eur. Polym. J. 2020, 125, 109483. [Google Scholar] [CrossRef]
- Jiang, L.; Zhao, J.; Zuo, C.; Tan, W.; Ren, Y.; Liu, X. Protein–tannin interactions towards fabricating flame–retardant, UV–resistance, antibacterial and mechanical–reinforced PA66 fabric. Prog. Org. Coat. 2024, 197, 108798. [Google Scholar] [CrossRef]
- Jiang, L.; Zhao, J.; Zuo, C.; Tan, W.; Ren, Y.; Liu, X. A green approach to endow PA66 with superhydrophobic, flame retardant and ultra UV resistance inspired by mussel chemistry. Appl. Surf. Sci. 2024, 681, 161589. [Google Scholar] [CrossRef]
- Wang, A.; Duan, Y.; Gu, X.; Zhang, S.; Zang, W. Surface modification of polyamide66 fabric by grafting with vinyltrimethoxysilane. Chem. Res. Chin. Univ. 2017, 33, 492–498. [Google Scholar] [CrossRef]
- Kundu, C.K.; Wang, W.; Zhou, S.; Wang, X.; Sheng, H.; Pan, Y.; Song, L.; Hu, Y. A green approach to constructing multilayered nanocoating for flame retardant treatment of polyamide 66 fabric from chitosan and sodium alginate. Carbohydr. Polym. 2017, 166, 131–138. [Google Scholar] [CrossRef]
- Kundu, C.K.; Wang, X.; Liu, L.; Song, L.; Hu, Y. Few layer deposition and sol–gel finishing of organic–inorganic compounds for improved flame retardant and hydrophilic properties of polyamide 66 textiles: A hybrid approach. Prog. Org. Coat. 2019, 129, 318–326. [Google Scholar] [CrossRef]
- Kundu, C.K.; Li, Z.; Li, X.; Zhang, Z.; Hu, Y. Graphene oxide functionalized biomolecules for improved flame retardancy of Polyamide 66 fabrics with intact physical properties. Int. J. Biol. Macromol. 2020, 156, 362–371. [Google Scholar] [CrossRef]
- Kundu, C.K.; Wang, X.; Song, L.; Hu, Y. Borate cross–linked layer–by–layer assembly of green polyelectrolytes on polyamide 66 fabrics for flame–retardant treatment. Prog. Org. Coat. 2018, 121, 173–181. [Google Scholar] [CrossRef]
- Kundu, C.K.; Wang, X.; Song, L.; Hu, Y. Chitosan–based flame retardant coatings for polyamide 66 textiles: One–pot deposition versus layer–by–layer assembly. Int. J. Biol. Macromol. 2020, 143, 1–10. [Google Scholar] [CrossRef]
- Liu, W.; Shi, R.; Zhang, Z.; Yan, M.; Chen, X.; Chen, Y. Coordination driven layer–by–layer deposition technology used for fabrication of flame retardant polyamide 66 fabric. Polym. Adv. Technol. 2021, 32, 3232–3241. [Google Scholar] [CrossRef]
- Rahman, M.Z.; Wang, X.; Song, L.; Hu, Y. A novel green phosphorus–containing flame retardant finishing on polysaccharide-modified polyamide 66 fabric for improving hydrophilicity and durability. Int. J. Biol. Macromol. 2023, 239, 124252. [Google Scholar] [CrossRef]


| Reactive Flame Retardants | Molecular Structures | References |
|---|---|---|
| Bis (4–carboxyphenyl) phenyl phosphine oxide (BCPPO) | 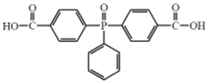 | [56,57,58] |
| N–benzoic acid (ethyl–N–benzoic acid formamide) phosphamide (NENP) | 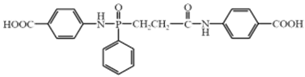 | [30] |
| Poly–N–aniline–phenyl phosphamide (PDPPD) | 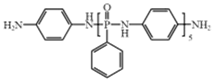 | [59] |
| 2–carboxy ethyl (phenyl) phosphinic acid (CEPPA) | 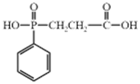 | [60] |
| Reactive phosphorus–containing flame retardant (FR–B) | – | [61] |
| Reactive flame retardant (DPDA) | 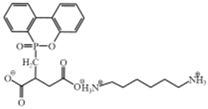 | [62] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, B.; Yu, S.; Xiang, H.; Li, L.; Zhu, M. Current Status and Future Trends for Modification Technology of Flame Retardant Nylon 66. Polymers 2025, 17, 1074. https://doi.org/10.3390/polym17081074
Feng B, Yu S, Xiang H, Li L, Zhu M. Current Status and Future Trends for Modification Technology of Flame Retardant Nylon 66. Polymers. 2025; 17(8):1074. https://doi.org/10.3390/polym17081074
Chicago/Turabian StyleFeng, Bingtao, Senlong Yu, Hengxue Xiang, Lili Li, and Meifang Zhu. 2025. "Current Status and Future Trends for Modification Technology of Flame Retardant Nylon 66" Polymers 17, no. 8: 1074. https://doi.org/10.3390/polym17081074
APA StyleFeng, B., Yu, S., Xiang, H., Li, L., & Zhu, M. (2025). Current Status and Future Trends for Modification Technology of Flame Retardant Nylon 66. Polymers, 17(8), 1074. https://doi.org/10.3390/polym17081074







