High-Energy Milling as a Pre-Treatment Alternative for Lignocellulosic Fibers Derived from Brewer’s Spent Grain
Abstract
1. Introduction
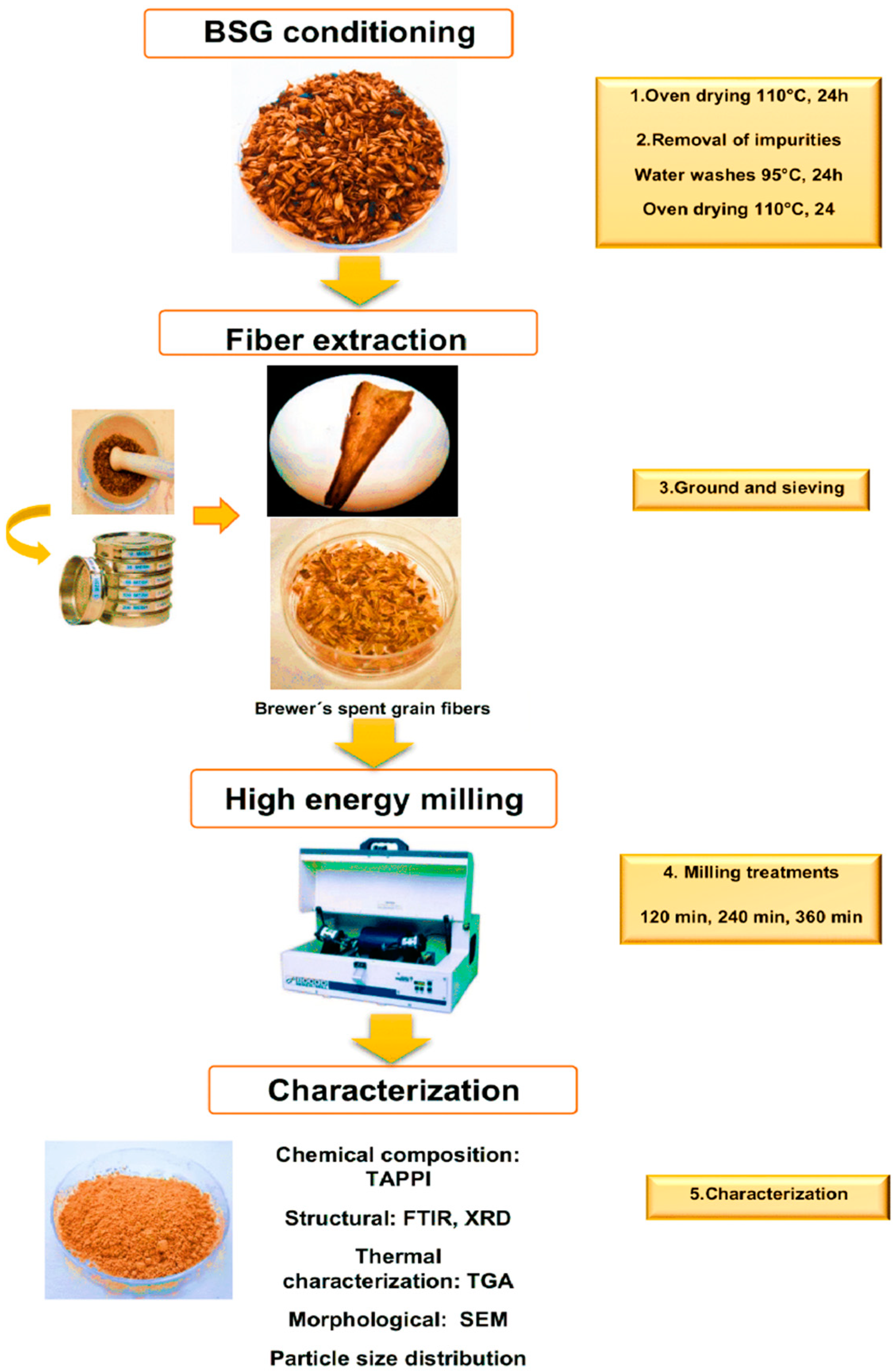
General Workflow
2. Materials and Methods
2.1. Extraction of Fibers from Brewer’s Spent Grain
2.2. Effect of Milling Conditions on Composition Particle Size Distribution, and Morphology of Lignocellulosic Material
2.3. Chemical Composition
2.3.1. Determination of Soluble Extractible Compounds
2.3.2. Determination of Lignin
2.3.3. Determination of Cellulose
2.3.4. Determination of Holocellulose
2.4. Particle Size Analysis
2.5. Structural Characterization of Brewer’s Spent Grain Fibers (BSGFs)
2.5.1. Fourier-Transform Infrared Spectroscopy (FTIR)
2.5.2. X-Ray Diffraction
2.6. Thermal Characterization
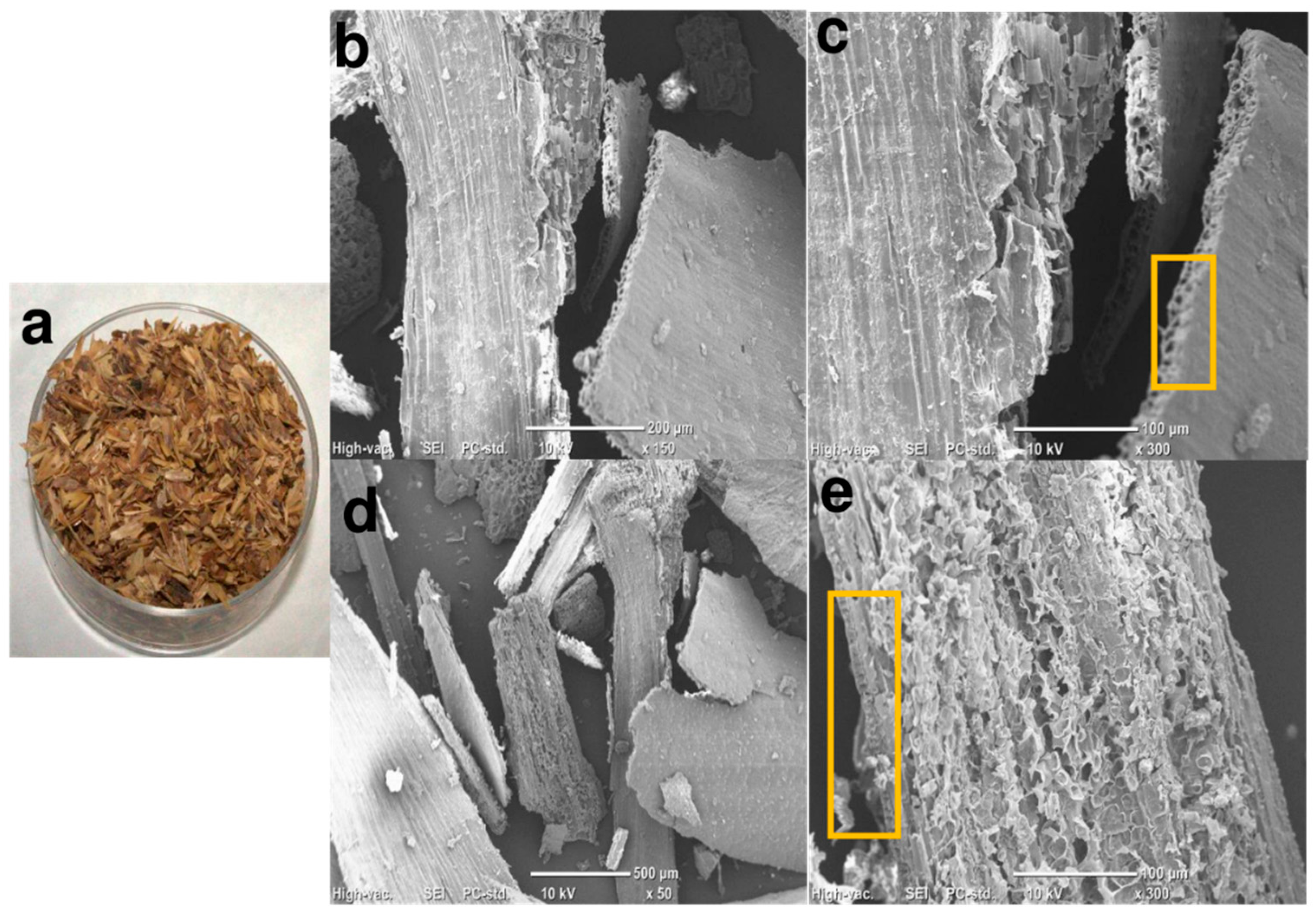
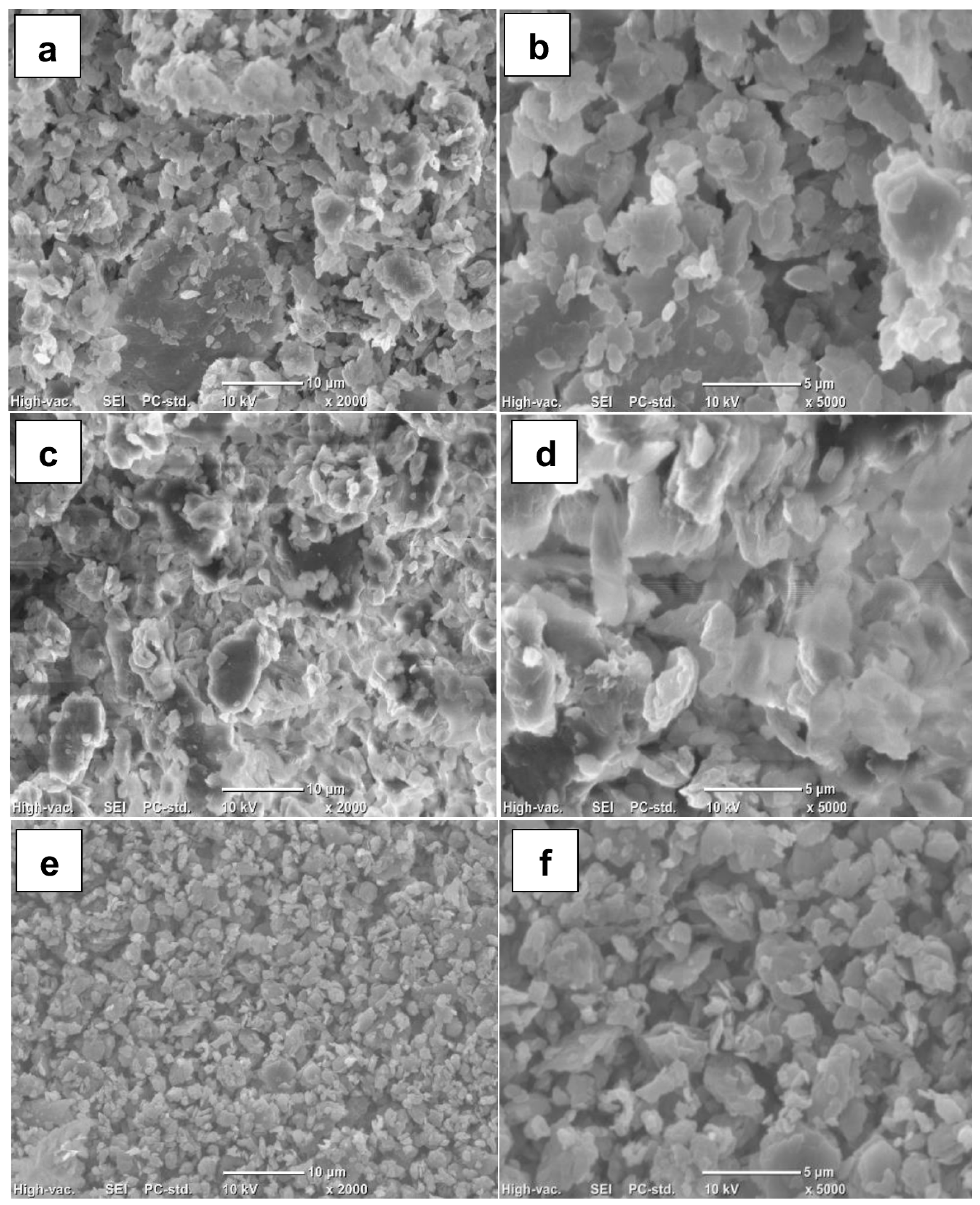
2.7. Morphological Study
3. Results and Discussion
3.1. Chemical Composition of Brewer’s Spent Grain Fibers and Treatments
3.2. Morphological Study
3.3. Infrared Spectroscopy (FTIR)
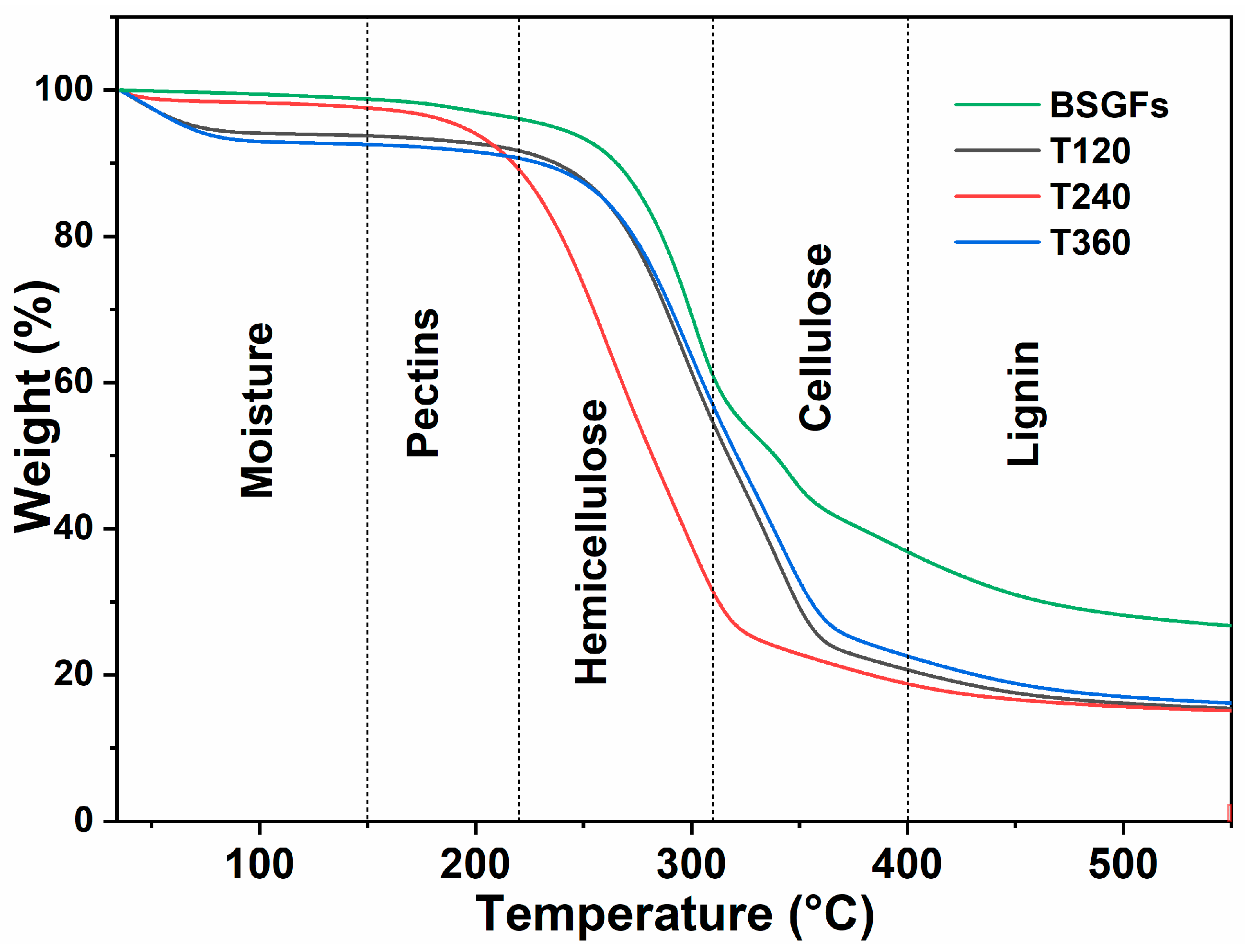
3.4. Thermogravimetric Analysis (TGA)
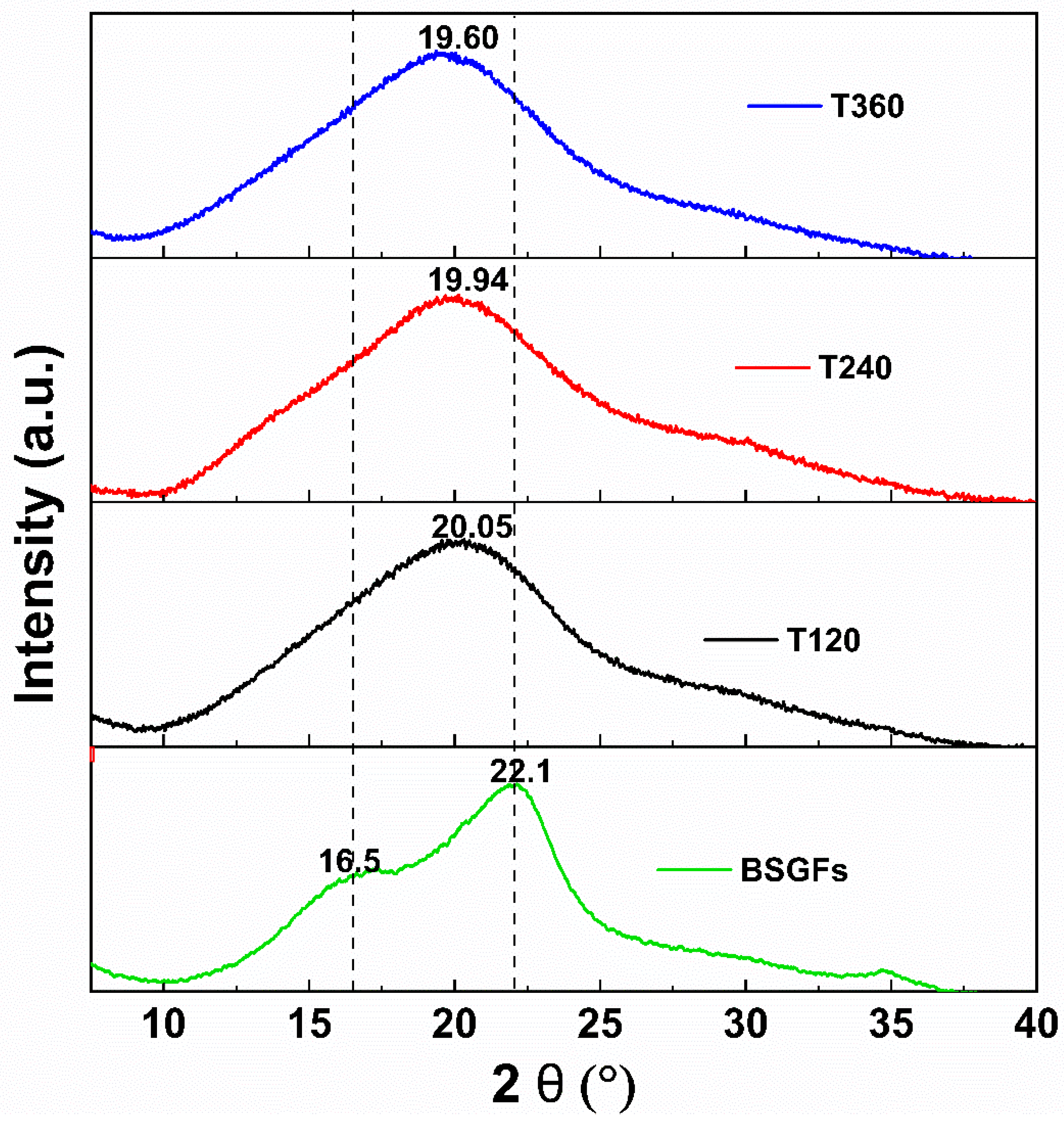
3.5. X-Ray Diffraction
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hejna, A.; Marć, M.; Kowalkowska-Zedler, D.; Pladzyk, A.; Barczewski, M. Insights into the Thermo-Mechanical Treatment of Brewers’ Spent Grain as a Potential Filler for Polymer Composites. Polymers 2021, 13, 879. [Google Scholar] [CrossRef] [PubMed]
- Chetrariu, A.; Dabija, A. Brewer’s Spent Grains: Possibilities of Valorization, a Review. Appl. Sci. 2020, 10, 5619. [Google Scholar] [CrossRef]
- Mitri, S.; Salameh, S.J.; Khelfa, A.; Leonard, E.; Maroun, R.G.; Louka, N.; Koubaa, M. Valorization of Brewers’ Spent Grains: Pretreatments and Fermentation, a Review. Fermentation 2022, 8, 50. [Google Scholar] [CrossRef]
- Kumla, J.; Suwannarach, N.; Sujarit, K.; Penkhrue, W.; Kakumyan, P.; Jatuwong, K.; Vadthanarat, S.; Lumyong, S. Cultivation of Mushrooms and Their Lignocellulolytic Enzyme Production through the Utilization of Agro-Industrial Waste. Molecules 2020, 25, 2811. [Google Scholar] [CrossRef]
- Ravindran, R.; Jaiswal, A.K. A Comprehensive Review on Pre-Treatment Strategy for Lignocellulosic Food Industry Waste: Challenges and Opportunities. Bioresour. Technol. 2016, 199, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Mussatto, S.I. Brewer’s Spent Grain: A Valuable Feedstock for Industrial Applications. J. Sci. Food Agric. 2014, 94, 1264–1275. [Google Scholar] [CrossRef]
- Bonifácio-Lopes, T.; Teixeira, J.A.; Pintado, M. Current Extraction Techniques towards Bioactive Compounds from Brewer’s Spent Grain–A Review. Crit. Rev. Food Sci. Nutr. 2019, 60, 2730–2741. [Google Scholar] [CrossRef]
- Ravindran, R.; Jaiswal, S.; Abu-Ghannam, N.; Jaiswal, A.K. A Comparative Analysis of Pretreatment Strategies on the Properties and Hydrolysis of Brewers’ Spent Grain. Bioresour. Technol. 2018, 248, 272–279. [Google Scholar] [CrossRef]
- Pérez-Merchán, A.M.; Rodríguez-Carballo, G.; Torres-Olea, B.; García-Sancho, C.; Maireles-Torres, P.J.; Mérida-Robles, J.; Moreno-Tost, R. Recent Advances in Mechanochemical Pretreatment of Lignocellulosic Biomass. Energies 2022, 15, 5948. [Google Scholar] [CrossRef]
- Liu, C.; Ullah, A.; Gao, X.; Shi, J. Synergistic Ball Milling–Enzymatic Pretreatment of Brewer’s Spent Grains to Improve Volatile Fatty Acid Production through Thermophilic Anaerobic Fermentation. Processes 2023, 11, 1648. [Google Scholar] [CrossRef]
- Khan, A.S.; Man, Z.; Bustam, M.A.; Kait, C.F.; Khan, M.I.; Muhammad, N.; Nasrullah, A.; Ullah, Z.; Ahmad, P. Impact of Ball-Milling Pretreatment on Pyrolysis Behavior and Kinetics of Crystalline Cellulose. Waste Biomass Valorization 2016, 7, 571–581. [Google Scholar] [CrossRef]
- Zhang, Z.; Tahir, N.; Li, Y.; Zhang, T.; Zhu, S.; Zhang, Q. Tailoring of Structural and Optical Parameters of Corncobs through Ball Milling Pretreatment. Renew. Energy 2019, 141, 298–304. [Google Scholar] [CrossRef]
- Ji, G.; Han, L.; Gao, C.; Xiao, W.; Zhang, Y.; Cao, Y. Quantitative approaches for illustrating correlations among the Mechanical Fragmentation Scales, Crystallinity and Enzymatic Hydrolysis Glucose Yield of Rice Straw. Bioresour. Technol. 2017, 241, 262–268. [Google Scholar] [CrossRef]
- Liu, H.; Chen, X.; Ji, G.; Yu, H.; Gao, C.; Han, L.; Xiao, W. Mechanochemical Deconstruction of Lignocellulosic Cell Wall Polymers with Ball-Milling. Bioresour. Technol. 2019, 286, 121364. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Wang, J.; Zhang, Y.; Qi, C.; Wolcott, M.P.; Yu, Z. A Co-Production of Sugars, Lignosulfonates, Cellulose, and Cellulose Nanocrystals from Ball-Milled Woods. Bioresour. Technol. 2017, 238, 254–262. [Google Scholar] [CrossRef]
- Wise, L.E.; Murphy, M.; Adieco, A.A.D. A chlorite holocel lulose, its fractionation and bearing on summative wood analysis and studies on the hemicellulsoes. Paper Trade J. 1946, 122, 35–42. [Google Scholar]
- Segal, L.; Creely, J.J.; Martin, A.E.; Conrad, C.M. An empirical method for estimating the degree of crystallinity of native cellulose using the X-Ray diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Coronado, M.A.; Montero, G.; Montes, D.G.; Valdez-Salas, B.; Ayala, J.R.; García, C.; Carrillo, M.; León, J.A.; Moreno, A. Physicochemical Characterization and SEM-EDX Analysis of Brewer’s Spent Grain from the Craft Brewery Industry. Sustainability 2020, 12, 7744. [Google Scholar] [CrossRef]
- Klímek, P.; Wimmer, R.; Kumar Mishra, P.; Kúdela, J. Utilizing Brewer’s-Spent-Grain in Wood-Based Particleboard Manufacturing. J. Clean. Prod. 2017, 141, 812–817. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Y.X.; Hou, T.; Chen, X.; Gao, C.; Han, L.; Xiao, W. Mechanical Deconstruction of Corn Stover as an Entry Process to Facilitate the Microwave-Assisted Production of Ethyl Levulinate. Fuel Process. Technol. 2018, 174, 53–60. [Google Scholar] [CrossRef]
- Neitzel, N.; Eder, M.; Hosseinpourpia, R.; Walther, T.; Adamopoulos, S. Chemical Composition, Particle Geometry, and Micro-Mechanical Strength of Barley Husks, Oat Husks, and Wheat Bran as Alternative Raw Materials for Particleboards. Mater. Today Commun. 2023, 36, 106602. [Google Scholar] [CrossRef]
- Ji, G.; Gao, C.; Xiao, W.; Han, L. Mechanical Fragmentation of Corncob at Different Plant Scales: Impact and Mechanism on Microstructure Features and Enzymatic Hydrolysis. Bioresour. Technol. 2016, 205, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Xiao, W.; Ji, G.; Zhang, Y.; Cao, Y.; Han, L. Regularity and Mechanism of Wheat Straw Properties Change in Ball Milling Process at Cellular Scale. Bioresour. Technol. 2017, 241, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Baheti, V.K.; Abbasi, R.; Militky, J. Ball Milling of Jute Fibre Wastes to Prepare Nanocellulose. World J. Eng. 2012, 9, 45–50. [Google Scholar] [CrossRef]
- Barakat, A.; Mayer-Laigle, C.; Solhy, A.; Arancon, R.A.D.; De Vries, H.; Luque, R. Mechanical Pretreatments of Lignocellulosic Biomass: Towards Facile and Environmentally Sound Technologies for Biofuels Production. RSC Adv. 2014, 4, 48109–48127. [Google Scholar] [CrossRef]
- Nagarajaganesh, B.; Ganeshan, P.; Ramshankar, P.; Raja, K. Industrial Crops & Products Assessment of Natural Cellulosic Fibers Derived from Senna Auriculata for Making Light Weight Industrial Biocomposites. Ind. Crops. Prod. 2019, 139, 111546. [Google Scholar] [CrossRef]
- Narayanasamy, P.; Balasundar, P.; Senthil, S.; Sanjay, M.R.; Siengchin, S.; Khan, A.; Asiri, A.M. International Journal of Biological Macromolecules Characterization of a Novel Natural Cellulosic Fi Ber from Calotropis Gigantea Fruit Bunch for Ecofriendly Polymer Composites. Int. J. Biol. Macromol. 2020, 150, 793–801. [Google Scholar] [CrossRef]
- Hyness, N.R.J.; Vignesh, N.J.; Senthamaraikannan, P.; Saravanakumar, S.S.; Sanjay, M.R. Characterization of New Natural Cellulosic Fiber from Heteropogon Contortus Plant. J. Nat. Fibers 2018, 15, 146–153. [Google Scholar] [CrossRef]
- Outeiriño, D.; Costa-Trigo, I.; Paz, A.; Deive, F.J.; Rodríguez, A.; Domínguez, J.M. Biorefining Brewery Spent Grain Polysaccharides through Biotuning of Ionic Liquids. Carbohydr. Polym. 2019, 203, 265–274. [Google Scholar] [CrossRef]
- Indran, S.; Edwin Raj, R.; Sreenivasan, V.S. Characterization of New Natural Cellulosic Fiber from Cissus Quadrangularis Root. Carbohydr. Polym. 2014, 110, 423–429. [Google Scholar] [CrossRef]
- Bergamasco, S.; Zikeli, F.; Vinciguerra, V.; Sobolev, A.P.; Scarnati, L.; Tofani, G.; Scarascia Mugnozza, G.; Romagnoli, M. Extraction and Characterization of Acidolysis Lignin from Turkey Oak (Quercus cerris L.) and Eucalypt (Eucalyptus camaldulensis Dehnh.) Wood from Population Stands in Italy. Polymers 2023, 15, 3591. [Google Scholar] [CrossRef] [PubMed]
- Maache, M.; Bezazi, A.; Amroune, S.; Scarpa, F.; Dufresne, A. Characterization of a Novel Natural Cellulosic Fiber from Juncus effusus L. Carbohydr. Polym. 2017, 171, 163–172. [Google Scholar] [CrossRef]
- Reddy, K.O.; Maheswari, C.U.; Muzenda, E.; Shukla, M.; Rajulu, A.V.; Reddy, K.O.; Maheswari, C.U.; Muzenda, E.; Shukla, M.; Rajulu, A.V. Extraction and Characterization of Cellulose from Pretreated Ficus (Peepal Tree) Leaf Fibers. J. Nat. Fibers 2016, 13, 54–64. [Google Scholar] [CrossRef]
- Qu, T.; Zhang, X.; Gu, X.; Han, L.; Ji, G.; Chen, X.; Xiao, W. Ball Milling for Biomass Fractionation and Pretreatment with Aqueous Hydroxide Solutions. ACS Sustain. Chem. Eng. 2017, 5, 7733–7742. [Google Scholar] [CrossRef]
- Lim, C.J.; Arumugam, M.; Lim, C.K. Mercerizing Extraction and Physicochemical Characterizations of Lignocellulosic Fiber from the Leaf Waste of Mikania micrantha Kunth ex H.B.K. J. Nat. Fibers 2018, 17, 726–737. [Google Scholar] [CrossRef]
- Lu, Q.; Lin, W.; Tang, L.; Wang, S.; Chen, X.; Huang, B. A Cellulose Mechanochemical Approach to Manufacturing Bamboo Nanocrystals. J. Mater. Sci. 2014, 50, 611–619. [Google Scholar] [CrossRef]
- Sitotaw, Y.W.; Habtu, N.G.; Gebreyohannes, A.Y.; Nunes, S.P.; Van Gerven, T. Ball Milling as an Important Pretreatment Technique in Lignocellulose Biorefineries: A Review. Biomass Convers. Biorefinery 2023, 13, 15593–15616. [Google Scholar] [CrossRef]
- Licari, A.; Monlau, F.; Solhy, A.; Buche, P.; Barakat, A. Comparison of Various Milling Modes Combined to the Enzymatic Hydrolysis of Lignocellulosic Biomass for Bioenergy Production: Glucose Yield and Energy Efficiency. Energy 2016, 102, 335–342. [Google Scholar] [CrossRef]
- Ribeiro, L.S.; Órfão, J.J.M.; Pereira, M.F.R. Enhanced Direct Production of Sorbitol by Cellulose Ball-Milling. Green Chem. 2015, 17, 2973–2980. [Google Scholar] [CrossRef]


| Fiber | % Cellulose | Hemicellulose Content | % Lignin | % Soluble Extractible Compounds | Reference |
|---|---|---|---|---|---|
| Brewer’s spent grain | 27.61 | 32.62 | 28.89 | 8.6 | Current Study |
| Brewer’s spent grain | 26.80 | 37.17 | 17.13 | - | [18] |
| Brewer’s spent grain | 24.5 | 23.8 | 15.8 | - | [19] |
| Brewer’s spent grain fibers (BSGFs) | 36.78 | 41.82 | 27.82 | 15.58 | Current Study |
| Microfiber (T120) | 41.97 | 23.38 | 30.98 | 14.32 | Current Study |
| Microfiber (T240) | 34.93 | 41.26 | 29.70 | 12.13 | Current Study |
| Microfiber (T360) | 45.17 | 27.86 | 26.83 | 13.96 | Current Study |
| Milling Time (min) | |||
|---|---|---|---|
| Particle Size (µm) | T120 | T240 | T360 |
| Mean | 19.4 ± 0.74 | 26.9 ± 1.68 | 23.3 ± 0.81 |
| Median | 14.9 ± 0.35 | 21.5 ± 1.36 | 17.6 ± 0.71 |
| Mode | 19.2 ± 1.01 | 27.9 ± 1.47 | 25.4 ± 1.34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomez-Hernandez, E.; Hernández-Hernández, E.; Castro-Rosas, J.; Vázquez-García, R.A.; Cadena-Ramírez, A.; Jiménez-Villeda, B.E.; Gomez-Aldapa, C.A. High-Energy Milling as a Pre-Treatment Alternative for Lignocellulosic Fibers Derived from Brewer’s Spent Grain. Polymers 2025, 17, 1156. https://doi.org/10.3390/polym17091156
Gomez-Hernandez E, Hernández-Hernández E, Castro-Rosas J, Vázquez-García RA, Cadena-Ramírez A, Jiménez-Villeda BE, Gomez-Aldapa CA. High-Energy Milling as a Pre-Treatment Alternative for Lignocellulosic Fibers Derived from Brewer’s Spent Grain. Polymers. 2025; 17(9):1156. https://doi.org/10.3390/polym17091156
Chicago/Turabian StyleGomez-Hernandez, Erik, Ernesto Hernández-Hernández, Javier Castro-Rosas, Rosa A. Vázquez-García, Arturo Cadena-Ramírez, Brenda E. Jiménez-Villeda, and Carlos A. Gomez-Aldapa. 2025. "High-Energy Milling as a Pre-Treatment Alternative for Lignocellulosic Fibers Derived from Brewer’s Spent Grain" Polymers 17, no. 9: 1156. https://doi.org/10.3390/polym17091156
APA StyleGomez-Hernandez, E., Hernández-Hernández, E., Castro-Rosas, J., Vázquez-García, R. A., Cadena-Ramírez, A., Jiménez-Villeda, B. E., & Gomez-Aldapa, C. A. (2025). High-Energy Milling as a Pre-Treatment Alternative for Lignocellulosic Fibers Derived from Brewer’s Spent Grain. Polymers, 17(9), 1156. https://doi.org/10.3390/polym17091156







