Abstract
Sucrose is an important component of fruit flavor, but whether sucrose signaling affects the postharvest ripening process of kiwifruit is unclear. The aim of this article was to study the effect of sucrose application on postharvest kiwifruit ripening to clarify the effect of sucrose in this process. Our present study found that exogenous sucrose can promote ethylene synthesis, which increases the ethylene content during fruit ripening, thereby accelerating the ripening and softening of kiwifruit after harvest. A significantly higher expression of AcACS1 and AcACO2 was found in sucrose-treated fruits compared to that in mannitol-treated fruits. Blocking the ethylene signal significantly inhibited the sucrose-modulated expression of most selected ripening-related genes. Sucrose transport is essential for sucrose accumulation in fruits; therefore, we isolated the gene family related to sucrose transport in kiwifruit and analyzed the gene expression of its members. The results show that AcSUT1 and AcTST1 expression increased with fruit ripening and AcSUT4 expression decreased with ripening, indicating that they may have different roles in the regulation of fruit ripening. Additionally, many cis-elements associated with phytohormones and sugar responses were found in the promoter of the three genes, which suggests that they were transcriptionally regulated by sugar signal and phytohormones. This study demonstrates the effect of sucrose on postharvest ripening of kiwifruit, providing a good foundation for further research.
1. Introduction
When stored at room temperature, kiwifruit (Actinidia chinensis) is a typical climacteric fruit. The ripening of fruit after harvesting is a complicated process involving a series of physiological and biochemical processes which involve sugar, acid, hormones, and other changes in metabolism. With the continuous pursuit of fruit quality and the rapid development of molecular biology and modern biotechnology, research on the physiological and molecular basic of fruit ripening has made great progress over the years. To date, research on the regulation of postharvest ripening of kiwifruit has focused on changes in the content of endogenous hormones during this process, particularly the effects of ethylene and abscisic acid (ABA) [1,2,3]. The ripening and softening of kiwifruit were accelerated by ethylene and retarded by 1-MCP [4,5,6,7,8].
However, increasing attention has been paid to the effect of sucrose signaling on fruit ripening. Sugar is an important indicator used to judge fruit quality [9]. The sugar content in fleshy fruits is generally quite high. In sweet orange fruit, for example, sucrose accumulates rapidly before 180 day after flowering, and then slows down. Sucrose content accounts for more than 60% of the total soluble sugars during fruit ripening [10]. Studies have shown that ASR, a gene related to maturation, is found in grapes, and its expression is induced by sucrose. When sucrose and ABA exist simultaneously, sucrose can strongly induce the gene expression of ASR [11]. Soluble sugar accumulates gradually after pear post-harvest ripening [12]. Sucrose is an important signaling molecule which regulates ABA synthesis and participates in the ripening of strawberry fruit through the ABA-stress-ripening transcription factor [13,14]. Treatment with exogenous sucrose accelerates postharvest tomato fruit ripening by influencing its metabolism and enhancing ethylene biosynthesis and signaling [15].
The main source of accumulated sugar in fruit is the photosynthetic products of leaf assimilation, which are transported in the phloem. In the phloem of most plants, the carbon source is mainly sucrose, which is transported to the fruit where it is then used in physiological metabolism [16]. Sucrose is mainly transported in the phloem through the apoplast and symplast pathways [17,18]. As the apoplast pathway requires transmembrane transport of sucrose into the phloem, transport via this pathway requires a sucrose transporter. After sugar is transported to the fruit through the phloem, it is discharged through the phloem and enters the storage parenchyma cells for metabolism and accumulation. In fruits with high soluble sugar accumulation, sucrose unloading may involve the apoplast pathways, such as in apple [19,20], citrus [21], grape [22], tomato [23], and kiwifruit [24]. In most plants, sucrose is loaded directly into the phloem by two types of sugar transporters: the Sugar Will Eventually be Exported Transporters (SWEET) protein, which exports sucrose from the leaves and the sucrose transporter (SUT/SUC) protein, which mediates sucrose import into the phloem [25,26]. The vacuole is the main organelle for sugar accumulation in fruit. Sugar enters the vacuole, and then requires a transport protein to penetrate the plasma membrane and the tonoplast. Two sugar transporter gene families can mediate sucrose across the vacuole: the tonoplast monosaccharide transporter family (TMTs) [27,28], and members of the sucrose transporter (SUCs/SUTs) family [18,29,30,31].
Although many studies have shown that sucrose can affect fruit ripening, in kiwifruit, changes in sucrose accumulation are a sign of fruit ripening and are a key factor of fruit quality. However, few studies have assessed how sucrose signaling affects kiwifruit ripening and softening. In this study, the effect of sucrose application on postharvest kiwifruit ripening was investigated to clarify the effect of sucrose in this process. Then, the putative kiwifruit sucrose transporter gene family (SUT, SWEET, and TMT) was searched and a phylogenetic analysis with corresponding genes from Arabidopsis and other species was conducted. Finally, the gene expression patterns of putative kiwifruit sugar transporter family members during postharvest kiwifruit fruit ripening were profiled.
2. Materials and Methods
2.1. Plant Material and Treatments
Five-year-old ‘Hongyang’ kiwifruit (A. chinensis) vines grafted on ‘Jinkui’ kiwifruit (A. deliciosa) vines at the Institute of Kiwifruit Research in Fengxin county, Jiangxi Province, China (28.7° N, 115.38° E, and elevation 65 m). Orchard soil, fertilization regimes, training and pruning, thinning levels, and irrigation are all under unified management. The fruits were collected at the commercial mature stage (145 days after pollination, mean TSS of 6.4%) in October 2018. Fruits of uniform size free from visible defects were divided into two groups; the first group was treated with sucrose (50 mM, soak for 10 min, 20 °C), the second group was treated with mannitol (50 mM, soak for 10 min, 20 °C), mannitol is usually used as a reference for exogenous sugar treatment to exclude the influence of osmotic pressure, so it was used as a control in this study. Each treatment contained three biological replicates of approximately 200 fruit and then placed at 20 °C for storage. Pulp samples of treated fruits were separated at 0, 2, 4, 6, 8, 10, and 12 days, then rapidly frozen in liquid nitrogen and kept at −80 °C until use.
2.2. Experiments with Detached Fruits
To assess the transient effect of exogenous sucrose on the expression of the genes of interest in kiwifruit, we adopted a detached-fruit experimental system. Kiwifruit flesh cubes (1 cm thick) were prepared from pulp tissue. Immediately after cutting, the tissue cylinders were immersed in 200 mL of equilibration solution containing 50 mM MES (pH 5.5), 10 mM MgCl2, 10 mM EDTA, 5 mM CaCl2 (sucrose-treated: 50 mM Sucrose, mannitol-treated: 50mM mannitol). Freshly cut discs were washed by gently stirring for 0, 1, 3, 5, 7, and 9 h in equilibration solution. After washing, the discs were blotted on absorbent paper and then rapidly frozen in liquid nitrogen and kept at −80 °C until use. Sucrose-treated fruit samples received an ethylene signal inhibitor (1-MCP) at a concentration of 5 μL/L.
2.3. Fruit Firmness
Fruit firmness was measured on opposite sides of each fruit after the removal of peel (1 mm thick), using a fruit texture analyzer (FTA, model GS, Güss Manufacturing Ltd., Strand, South Africa) with a 10 mm probe. Data were recorded as Newton (N) and fruit firmness was expressed as the mean of 15–20 fruits.
2.4. Ethylene Production
Ethylene production was determined by incubating ten fruits per treatment in a 1 L container for 2 h, after which 1 mL of headspace gas was withdrawn and injected into a gas chromatograph (Model-GC4 CMPF, Shimadzu, Kyoto, Japan) equipped with a flame ionization detector and an activated alumina column. The GC conditions were as follows: Capillary column (0.53 mm), column temperature 50 °C, flow rate 1 mL/min, FID detector detection. Each treatment repeated three times and the average values are presented.
2.5. Quantification of Sucrose
The concentration of sucrose was analyzed by high-performance liquid chromatography (HPLC). Frozen samples (about 2 g) were ground in liquid nitrogen. Then, 5 mL of 80% ethanol was added and the sample was placed in a 70 °C water bath for 20 min. Next, the sample was centrifuged at 10,000 rpm for 15 min at 25 °C and the supernatant was taken. The extraction was repeated three times and the supernatant was combined to obtain a volume of 25 mL. Then, 1 mL of the extract was taken, filtered through a water filter with a pore size of 0.45 μm, and the filtrate was used for HPLC to determine the sucrose content. The HPLC conditions were as follows: Waters Spherisorb NH2 column (4.6 × 250 mm, 5 μm), column temperature 30 °C, mobile phase acetonitrile: water = 7.5:2.5, flow rate 1 mL/min, RID detector detection. Sucrose was used as a standard (Sigma Chemical Co, St. Louis, MO, USA). The sucrose concentration was determined by the retention time compared with external standards and quantified using standard calibration curves.
2.6. RNA Extraction and qRT-PCR
Total RNA was extracted using a plant RNA extraction kit (Huayueyang, Beijing, China). DNA contamination in the isolated RNA was digested before cDNA was synthesized by incubating with DNase I (Takara, Dalian, China) for 30 min at 37 °C; 1 μg RNA was used for cDNA synthesis using the Verso cDNA kit (Takara, Dalian, China). qRT-PCR was performed using a CFX96 Touch Real-time PCR instrument (Bio-RAD, USA) with 2 μL cDNA, 1X TB Green Master Mix (Takara, Dalian, China), and 1 μM each of two gene-specific primers (Table S2), in a final volume of 20 μL. The thermocycling protocol consisted of 30 s at 95 °C, 40 cycles of 5 s at 95 °C, and 34 s at 60 °C. Three biological replicates and three technical replicates were performed to verify the accuracy of the expression data.
2.7. Identification of Kiwifruit Sugar Transporter Genes and Phylogenetic Analyses
Candidate genes encoding sweet orange sugar transporters were retrieved by BLASTP searching against the kiwifruit genome database (http://bioinfo.bti.cornell.edu/cgi-bin/kiwi/home.cgi), using Arabidopsis sugar transporter proteins as queries. Phylogenetic analyses were conducted using ClustalW in MEGA 7.0 software and an unrooted phylogenetic tree of the sugar transporter gene families was constructed using the MEGA 7.0 software [32]. The evolutionary history was inferred using the neighbor-joining method with 1000 replicates.
2.8. Cis-Elements Analysis
To investigate cis-elements in the promoter regions of genes, sequences of the 2 kb regions upstream of the start codons (ATG) were extracted from the kiwifruit genome database. cis-elements in the promoter sequences were analyzed via the PlantCARE database (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/) [33].
2.9. Statistical Analyses
SigmaPlot 10.0 was used for plot and data analysis, and the data are expressed as mean ± standard deviation. SPSS 20.0 software was used for statistical analyses (p < 0.05).
3. Results
3.1. Exogenous Sucrose Modulates Kiwifruit Ripening
The exogenous sucrose content of kiwifruit was detected during storage after harvest. The results show that the sucrose content gradually increased during storage and reached its highest level at day 6 for the kiwifruit treated with sucrose and day 8 for the one with mannitol, then gradually decreased, and sucrose treatment increased sucrose content during postharvest fruit ripening (Figure 1A). As seen in Figure 1B, firmness decreased substantially with fruit ripening in both mannitol-treated and sucrose-treated fruits; however, significantly lower levels were observed in sucrose-treated fruits on days 4, 6, and 8 after treatment. Kiwifruit is a typical climacteric fruit at temperatures in the order of 20 °C, and its ripening process is accompanied by ethylene production. Therefore, ethylene production was also determined during storage following treatment with mannitol and sucrose. As shown in Figure 1C, ethylene production was higher during the first 8 days after sucrose-treated fruits than that in mannitol-treated fruits. Taken together, these results indicate that sucrose positively regulates kiwifruit ripening.
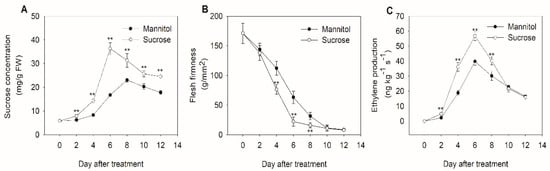
Figure 1.
Influence of sucrose treatment on the ripening process of kiwifruit during storage at 20 °C. (A) Concentration of sucrose during kiwifruit ripening. (B) Changes in flesh firmness following sucrose and mannitol (control) treatment. (C) Ethylene production under different treatments. The data are expressed as the mean ± standard deviation. The asterisks indicate a significant difference relative to mannitol treatment.
3.2. Changes in the Expression of Genes Involved in Ethylene Biosynthesis and Ripening
Ethylene plays an important role in kiwifruit ripening; therefore, the effect of sucrose treatment on ethylene biosynthesis was further investigated. AcACS1 and AcACO2 are key genes and their products are involved in the auto-catalytic system of ethylene biosynthesis. A high expression of AcACS1 and AcACO2 was observed during the first 6 days of treatment in both groups of fruits. Nevertheless, a significantly higher expression of AcACS1 was observed on days 2 and 4 in sucrose-treated fruits and AcACO2 was expressed at higher levels on days 2, 4, and 6 in sucrose-treated fruits compared to that in mannitol-treated fruits (Figure 2). We adopted a detached-fruit experimental system to observe the transient effects of exogenous sucrose on the expression of the genes of interest in kiwifruit and to investigate the association between sucrose-modulated gene expression and ethylene signaling. Following treatment with sucrose, gene expression increased significantly at 7 h, the expression of AcACS1 increased more than eight-fold at 9 h compared to that in the mannitol-treated group, and the expression of AcACO2 increased more than six-fold (Figure 3). These results suggest that sucrose plays an important role in the regulation of ethylene accumulation during kiwifruit ripening.
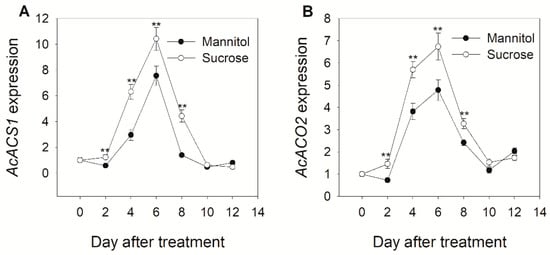
Figure 2.
Changes in the expression of genes encoding enzymes involved in ethylene biosynthesis after sucrose and mannitol (control) treatment in kiwifruit during storage at 20 °C. (A) AcACS1; (B) AcACO2. The data are expressed as the mean ± S.D. The asterisks indicate a significant difference relative to mannitol treatment according to the student’s t-test (** p < 0.01).
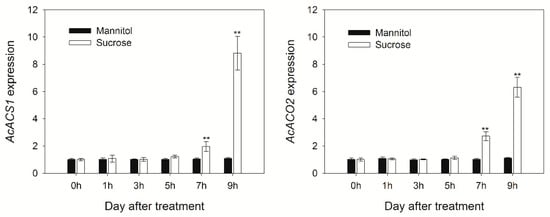
Figure 3.
The time course of sucrose induced the expression of AcACS1 and AcACO2 genes encoding enzymes involved in ethylene biosynthesis. Kiwifruit flesh cubes (1 cm thick) were prepared from pulp tissue. Immediately after cutting, the tissue cylinders were immersed in 200 mL of equilibration solution (sucrose-treated: 50 mM Sucrose, mannitol-treated (control): 50 mM mannitol). Freshly cut discs were washed by gently stirring for 0, 1, 3, 5, 7, and 9 h in equilibration solution, and gene expression was examined by qRT-PCR. The data are expressed as the mean ± S.D. The asterisks indicate a significant difference relative to mannitol treatment according to a student’s t-test (** p < 0.01).
Given that exogenous sucrose was demonstrated to modulate ethylene accumulation and fruit ripening, it was important to determine whether sucrose modulated fruit ripening upstream of ethylene signaling. To address this, we examined the effect of an ethylene signal inhibitor, 1-MCP, on the sucrose-modulation of some genes related to ripening, such as those controlling changes in fruit texture (polygalacturonase, PG; pectate lyase, PL; pectinmethylesterase, PME) and sugar metabolism (sucrose synthase, SS; sucrose-phosphate synthase, SPS). The results show that blocking the ethylene signal significantly inhibited the sucrose-modulated expression of most selected ripening-related genes, except for AcSS and AcSPS (Figure 4). This result indicates that sucrose modulated kiwifruit ripening upstream of the ethylene signal, and that this may be mediated by an ethylene-dependent pathway.
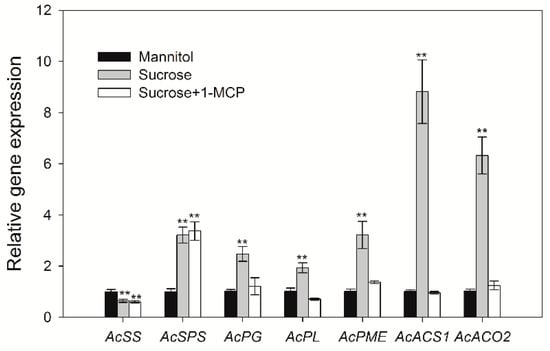
Figure 4.
Sucrose-modulated expression of genes involved in fruit ripening or ethylene biosynthesis. Detached kiwifruit were treated with 50 mM sucrose or mannitol for 9 h. To block ethylene signaling, samples were treated with 50 mM sucrose containing 10 mM l-MCP. The data are expressed as the mean ± S.D. The asterisks indicate a significant difference relative to mannitol treatment according to the student’s t-test (** p < 0.01).
3.3. Isolation and Phylogenetic Relationship of the Kiwifruit Sucrose Transporter Genes
To isolate putative genes encoding sucrose transporters, a BLASTP search was performed against the kiwifruit genome using Arabidopsis sugar transporter proteins as queries. In total, 22 genes encoding five SUTs, four TSTs, and 14 SWEETs were isolated (Table S1). Then, phylogenetic trees of the three transporter families were constructed with protein sequences using the MEGA 7.0 software. The SUT gene family in Arabidopsis is divided into three clades: SUT1, SUT3, and SUT4. The phylogenetic tree analysis showed that AcSUT1, AcSUT2, and AcSUT5 belonged to the SUT1 clade, AcSUT3 and AcSUT4 shared high homology with AtSUC3 and AtSUC4, which belonged to the SUT3 and SUT4 clade, respectively (Figure 5A). Of the four ortholog genes of TST identified in A. chinensis, AcTST3.1 and AcTST3.2 shared a high amino acid sequence similarity, which showed high homology with CmTST3 in Cucumis melo. AcTST1 and AcTST2 shared high homology with CmTST1 and CmTST2, respectively (Figure 5B). The SWEET gene family was divided into four clades. AcSWEET1a, AcSWEET1b, AcSWEET1c, AcSWEET2a, AcSWEET2b, AcSWEET2c, and AcSWEET3 belonged to clade I. AcSWEET7a, AcSWEET7b, and AcSWEET7c belonged to clade II. AcSWEET11 belonged to clade Ⅲ, and AcSWEET17a, AcSWEET17b, and AcSWEET17c belonged to clade IV (Figure 6).
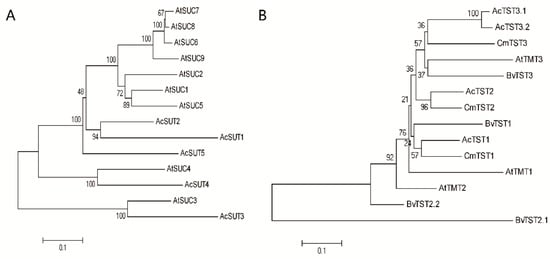
Figure 5.
Phylogenetic tree of amino acid sequences of SUTs and TSTs in Actinidia chinensis and other species. (A) SUTs; (B) TSTs. Trees were constructed by the neighbor-joining method based on amino acid differences (p-distance) using the MEGA 7.0 program with complete deletion and 1000 bootstrap replicates. Accession numbers were as follows: Arabidopsis, AtSUC1-9 (At1g71880, At1g22710, At2g02860, At1g09960, At1g71890, At5g43610, At1g66570, At2g14670, and At5g06170), AtTMT1-3 (At1g20840, At4g35300, and At3g51490); Beta vulgaris, BvTST1 (XP_010686712.1), BvTST2.1 (XP_010678631.1), BvTST2.2 (XP_010690557.1), and BvTST3 (XP_010680636.1); Cucumis melo, CmTST1 (XP_008464819.1), CmTST2 (XP_008448165.1), and CmTST3 (XP_016899284.1); A. chinensis (shown in Table S1).
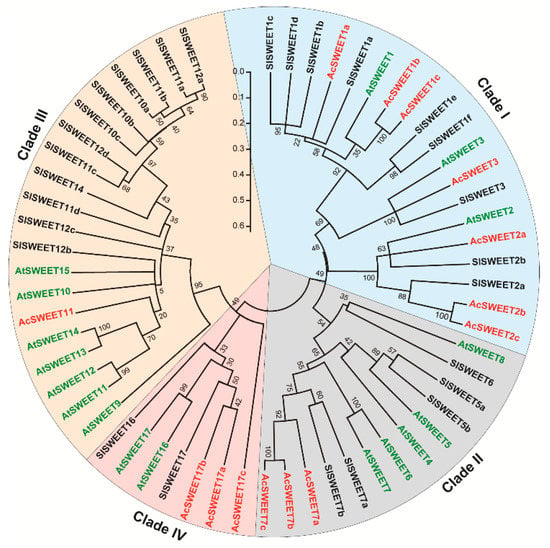
Figure 6.
Phylogenetic tree of amino acid sequences of SWEETs among A. chinensis and other species. The trees were constructed by the neighbor-joining method based on amino acid differences (p-distance) using the MEGA 7.0 program with complete deletion and 1000 bootstrap replicates. Accession numbers or gene ID (Table S1).
3.4. Expression of Genes during Postharvest Fruit Ripening
Sucrose transport is essential for the accumulation of soluble sugar in fruits. To determine the expression levels of genes in the sugar transporter family, qRT-PCR was used to analyze relative mRNA expression during postharvest kiwifruit ripening. The expression of AcSUT1 gradually increased with fruit maturity, peaking 8 days after harvest, after which it decreased slightly; this was consistent with the accumulation of sucrose during fruit ripening. The expression of AcSUT4 gradually increased in the early stage of fruit ripening but decreased in the later stage. Although AcSUT3 was highly expressed in fruit, its expression is not consistent, whereas AcSUT2 and AcSUT5 were only expression at low levels in fruits (Figure 7A). Regarding the AcTST gene family, AcTST3.1 and AcTST3.2 were expressed at low levels in fruits and the transcript abundance of AcTST2 decreased during fruit ripening. However, the expression of AcTST1 increased with fruit ripening, suggesting that it may play an important role in the process of kiwifruit ripening (Figure 7B). Regarding the SWEET gene family, the expression of most genes was low in fruit, except for AcSWEET1a, AcSWEET2a, AcSWEET2b, AcSWEET2c, and AcSWEET3. These genes were more highly expressed in the early stage of fruit ripening than in the later stage (Figure 8).
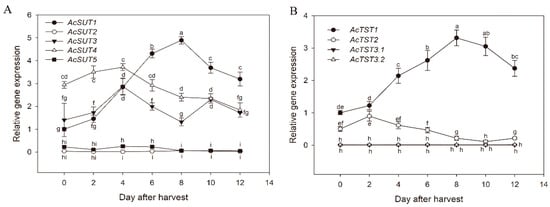
Figure 7.
Expression patterns of AcSUTs and AcTSTs during postharvest kiwifruit ripening. The stages were 0, 2, 4, 6, 8, 10, and 12 days after harvest (DAH). The error bars represent the means ± standard deviation from three independent biological replicates. The bars with the same letter are not significantly different at the 0.01 level according to the student’s t-test. For each family, the expression level of the first member in each figure at 0 DAH was set to ‘1’.
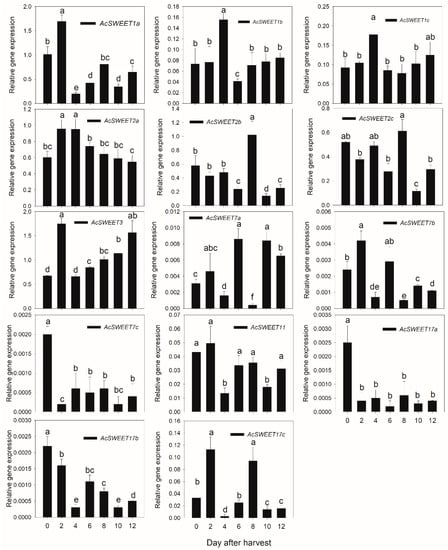
Figure 8.
Expression patterns of AcSWEETs during kiwifruit ripening. The stages were 0, 2, 4, 6, 8, 10, and 12 DAH. The error bars represent the means ± standard deviation from three independent biological replicates. The bars with the same letter are not significantly different at the 0.01 level according to the student’s t-test. The expression level of the first member in each figure at 0 DAH was set to ‘1’.
3.5. Cis-Elements in Sugar Transporter Gene Promoters in Kiwifruit
The gene expression analysis revealed that AcSUT1, AcSUT4, and AcTST1 are abundantly expressed in kiwifruit, suggesting that they may be closely related to sucrose accumulation in fruits. To understand the transcriptional control of these genes, sequences of the 2 kb regions upstream of the start codons (ATG) were determined. The cis-elements in the upstream sequences of the three genes were searched using the PlantCARE server. Notably, a large number of phytohormone-responsive cis-elements were identified in the promoter regions of AcSUT1, AcSUT4, and AcTST1, including abscisic acid-responsive elements (DPBFCOREDCDC3 and MYCCONSENSUSAT) [34,35], gibberellin-responsive elements (WRKY71OS) [19], and auxin-responsive elements (NTBBF1ARROLB) [36] (Table 1). Additionally, many sugar-responsive cis-elements were identified, including CGACGOSAMY3, AMYBOX1, WBOXHVISO1, and SUCROSE BOX 3 [37,38] (Table 1). These results indicate that the expression sugar transporter genes can be induced by phytohormones and sugar signals.

Table 1.
Putative cis-elements located in the promoter regions of the AcSUT1, AcSUT4, and AcTST1 genes.
4. Discussion
Sucrose is considered a simple component of fruit ripening and its content is closely related to the quality of fleshy fruit. With ripening, the sucrose content in fruit gradually increases. In our study, the sucrose content in kiwifruit also increased with ripening and decreased when the fruit was fully ripened, which is consistent with the previous report [39]. Because of the important role of sucrose in the development of fleshy fruit, researchers have started to consider its effect on the fruit development and ripening process. Sucrose has been reported to play an important role in fruit ripening. In the non-climacteric fruit strawberry, sucrose is an important signaling molecule which regulates ABA synthesis and participates in fruit ripening. Exogenous sucrose can accelerate the ripening of strawberry fruit [14]. In mutant sweet orange, which has a lower sucrose content, fruit ripening is delayed [40]. In the climacteric fruit tomato, exogenous sucrose treatment can promote postharvest fruit ripening by affecting its metabolism and enhancing ethylene synthesis and signal transduction [15]. Our results show that sucrose can accelerate the ripening and softening of kiwifruit, which can promote ethylene synthesis. Therefore, sucrose accumulation is important for regulation of fruit ripening.
Sucrose transport is essential for the accumulation of soluble sugar in fruits. As an irreversible reservoir organ, sucrose is mainly transported into storage organs by exoplasmic transport during fruit ripening; the sucrose transporter protein (SUC/SUT) plays an important role in this process. SUT1, SUT2, and SUT4 are subfamilies of sucrose transporters in dicotyledonous plants [41,42]. Studies have shown that sucrose transporters are involved in the regulation of sugar accumulation in fruits, thereby improving the yield and quality of fruits. There are four sucrose transporters in grape, all of which are expressed in the fruit and have the ability to transport sucrose [43]. The transcript of citrus CitSUT1 increases with fruit development and ripening, and its overexpression increases sucrose accumulation in transgenic citrus [10,44]. Seven sucrose transporter genes have been identified in strawberry. As the strawberry fruit ripens, expression of the FaSUT1 gene was found to increase significantly and sucrose accumulation in the fruit also increased. When FaSUT1 gene expression was inhibited, the ripening of strawberry fruit was significantly inhibited, indicating that FaSUT1 can regulate the accumulation of sucrose and affect ripening [14]. In Arabidopsis, AtSUC4 is located in the vacuolar membrane and facilitates the release of sucrose from the vacuole to the cytosol [30]. Similar results were obtained in peach, PpSUT1 is located in the tonoplast membrane, which is involved in sucrose loading in the leaves, while PpSUT4 is located in the tonoplast, which is involved in sucrose efflux from the vacuole [45]. The expression of genes in the SUT4 subfamily gradually decreases with the development of apple fruit [46]. In our study, the expression of AcSUT1 increased with the accumulation of sucrose and fruit ripening, suggesting that it may play an important role in this process. AcSUT4 belongs to the SUT4 subfamily and its expression gradually decreased during fruit ripening. To confirm the effect of these two genes on fruit postharvest ripening, further research is needed.
In addition to the SUT family, several sugar transporters have been identified, which can transport sucrose. Among the tonoplast monosaccharide transporter (TMT) family, BvTST2.1 is a sucrose-specific vacuolar transporter responsible for sucrose accumulation in sugar beet tap roots [27]. CmTST2 from melon can accumulate sucrose and facilitate fruit ripening when overexpressed in plant [47]. In sweet orange, CsTMT1 and CsTMT2 are expressed at relatively high levels in fruits, with transcripts most abundant in the early and late stages of fruit development, respectively [10]. Both MdTMT1 and MdTMT2 were expressed higher in fruit than in shoot tips and mature leaves, with the highest expression detected in mature fruit [46]. Abundant transcripts of AcTST1 have been observed during fruit ripening. Therefore, those findings suggest that AcTST1 can increase sucrose accumulation to promote fruit ripening. It is a good research object to investigate sucrose accumulation in vacuoles during the ripening process of kiwifruit. Regarding the SWEET family, clade III includes sucrose transporters, which are targeted to the plasma membrane [25], including AtSWEET9, 11, 12, 13, 15 and OsSWEET11 (Os8N3/Xa13) [25,48,49,50]. However, in our study, only AcSWEET11 belonged to clade III and its expression in fruits was very low, indicating that it may have no effect on fruit ripening.
5. Conclusions
In summary, our research shows that sucrose can promote the ripening of kiwifruit. We analyzed the expression of sucrose transport-related gene families in kiwifruit and found that AcSUT1, AcSUT4, and AcTST1 play important roles in sucrose accumulation during fruit ripening, indicating that they may be involved in the regulation of this process.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4395/10/2/245/s1. Table S1: Naming of SUT, TST and SWEET genes from A. chinensis, Arabidopsis and Salnum lycopersicum corresponding to gene ID; Table S2: Primers for qRT-PCR.
Author Contributions
Data curation, L.F.; Formal analysis, X.Y. and Z.G.; Funding acquisition, J.C.; Resources, Z.G.; Software, C.C., Y.F. and Z.G.; Supervision, J.C.; Validation, Z.G.; Writing—original draft, L.F.; Writing—review and editing, C.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Natural Science Foundation of China (NO.31760598), and Natural Science Foundation and Advantage Innovation Team Project of Jiangxi Province (NO. 20171BCB24006).
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| 1-MCP | 1-Methylcyclopropene |
| MES | 2-(N-Morpholino) ethanesulfonic acid |
| EDTA | Ethylenediaminetetraacetic acid |
| SUT | sucrose transporter |
| TST | tonoplast monosaccharide transporter |
| SWEET | Sugar Will Eventually be Exported Transporters |
| qRT-PCR | Quantitative real time polymerase chain reaction |
| DAH | days after harvest |
References
- Ampa, K.; Saito, T.; Okawa, K.; Ohara, H.; Kondo, S. Effects of ethephon and abscisic acid application on ripening-related genes in ‘kohi’ kiwifruit (Actinidia chinensis) on the vine. Hortic. Plant J. 2017, 3, 29–33. [Google Scholar] [CrossRef]
- Yin, X.; Allan, A.C.; Chen, K.; Ferguson, I.B. Kiwifruit EIL and ERF genes involved in regulating fruit ripening. Plant Physiol. 2010, 153, 1280–1292. [Google Scholar] [CrossRef]
- Yin, X.; Chen, K.; Allan, A.C.; Wu, R.; Zhang, B.; Lallu, N.; Ferguson, I.B. Ethylene-induced modulation of genes associated with the ethylene signalling pathway in ripening kiwifruit. J. Exp. Bot. 2008, 59, 2097–2108. [Google Scholar] [CrossRef] [PubMed]
- Gunaseelan, K.; McAtee, P.A.; Nardozza, S.; Pidakala, P.; Wang, R.L.; David, K.; Burdon, J.; Schaffer, R.J. Copy number variants in kiwifruit ETHYLENE RESPONSE FACTOR/APETALA2 (ERF/AP2)-like genes show divergence in fruit ripening associated cold and ethylene responses in C-REPEAT/DRE BINDING FACTOR-like genes. PLoS ONE 2019, 14, e0216120. [Google Scholar] [CrossRef] [PubMed]
- Asiche, W.O.; Mitalo, O.W.; Kasahara, Y.; Tosa, Y.; Mworia, E.G.; Owino, W.O.; Ushijima, K.; Nakano, R.; Yano, K.; Kubo, Y. Comparative transcriptome analysis reveals distinct ethylene-independent regulation of ripening in response to low temperature in kiwifruit. BMC Plant Biol. 2018, 18, 47. [Google Scholar] [CrossRef] [PubMed]
- Minas, I.S.; Tanou, G.; Karagiannis, E.; Belghazi, M.; Molassiotis, A. Coupling of Physiological and Proteomic Analysis to Understand the Ethylene- and Chilling-Induced Kiwifruit Ripening Syndrome. Front. Plant Sci. 2016, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.D.; Hu, X.; Kuang, S.; Ge, H.; Yin, X.R.; Chen, K.S. Isolation, classification and transcription profiles of the Ethylene Response Factors (ERFs) in ripening kiwifruit. Sci. Hortic. 2016, 199, 209–215. [Google Scholar] [CrossRef]
- Zhang, A.D.; Wang, W.Q.; Tong, Y.; Li, M.J.; Grierson, D.; Ferguson, I.; Chen, K.S.; Yin, X.R. Transcriptome Analysis Identifies a Zinc Finger Protein Regulating Starch Degradation in Kiwifruit. Plant Physiol. 2018, 178, 850–863. [Google Scholar] [CrossRef]
- Rai, M.K.; Shekhawat, N.S. Recent advances in genetic engineering for improvement of fruit crops. Plant Cell Tissue Organ Cult. 2014, 116, 1–15. [Google Scholar] [CrossRef]
- Zheng, Q.; Tang, Z.; Xu, Q.; Deng, X. Isolation, phylogenetic relationship and expression profiling of sugar transporter genes in sweet orange (Citrus sinensis). Plant Cell Tissue Organ Cult. 2014, 119, 609–624. [Google Scholar] [CrossRef]
- Cakir, B.; Agasse, A.; Gaillard, C.; Saumonneau, A.; Delrot, S.; Atanassova, R. A grape ASR protein involved in sugar and abscisic acid signaling. Plant Cell 2003, 15, 2165–2180. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chen, Y.; Wang, S.; Xue, H.; Su, Y.; Yang, J.; Li, X. Identification of candidate genes involved in the sugar metabolism and accumulation during pear fruit post-harvest ripening of ‘Red Clapp’s Favorite’ (Pyrus communis L.) by transcriptome analysis. Hereditas 2018, 155, 11. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Jiu, S.; Zhang, C.; Wang, C.; Tariq, P.; Liu, Z.; Wang, B.; Cui, L.; Fang, J. Abscisic acid and sucrose regulate tomato and strawberry fruit ripening through the abscisic acid-stress-ripening transcription factor. Plant Biotechnol. J. 2016, 14, 2045–2065. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Wang, Y.; Sun, M.; Li, B.; Han, Y.; Zhao, Y.; Li, X.; Ding, N.; Li, C.; Ji, W.; et al. Sucrose functions as a signal involved in the regulation of strawberry fruit development and ripening. New Phytol. 2013, 198, 453–465. [Google Scholar] [CrossRef]
- Li, D.; Mou, W.; Wang, Y.; Li, L.; Mao, L.; Ying, T.; Luo, Z. Exogenous sucrose treatment accelerates postharvest tomato fruit ripening through the influence on its metabolism and enhancing ethylene biosynthesis and signaling. Acta Physiol. Plant. 2016, 38, 225. [Google Scholar] [CrossRef]
- Orlich, G. Analysis of the driving forces of phloem transport in Ricinus seedlings: Sucrose export and volume flow are determined by the source. Planta 1998, 206, 266–271. [Google Scholar] [CrossRef]
- Berthier, A.; Desclos, M.; Amiard, V.; Morvan-Bertrand, A.; Demmig-Adams, B.; Adams, W.W., III; Turgeon, R.; Prud’homme, M.-P.; Noiraud-Romy, N. Activation of sucrose transport in defoliated lolium Perenne l.: An example of apoplastic phloem loading plasticity. Plant Cell Physiol. 2009, 50, 1329–1344. [Google Scholar] [CrossRef]
- Rennie, E.A.; Turgeon, R. A comprehensive picture of phloem loading strategies. Proc. Natl. Acad. Sci. USA 2009, 106, 14162–14167. [Google Scholar] [CrossRef]
- Zhang, Z.L.; Xie, Z.; Zou, X.L.; Casaretto, J.; Ho, T.H.D.; Shen, Q.X.J. A rice WRKY gene encodes a transcriptional repressor of the gibberellin signaling pathway in aleurone cells. Plant Physiol. 2004, 134, 1500–1513. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Peng, Y.B.; Pelleschi-Travier, S.; Fan, Y.; Lu, Y.F.; Lu, Y.M.; Gao, X.P.; Shen, Y.Y.; Delrot, S.; Zhang, D.P. Evidence for apoplasmic phloem unloading in developing apple fruit. Plant Physiol. 2004, 135, 574–586. [Google Scholar] [CrossRef]
- Koch, K.E.; Avigne, W.T. Postphloem, nonvascular transfer in citrus—Kinetics, metabolism, and sugar gradients. Plant Physiol. 1990, 93, 1405–1416. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, X.; Wang, X.; Xia, G.; Pan, Q.; Fan, R.; Wu, F.; Yu, X.; Zhang, D. A shift of phloem unloading from symplasmic to apoplasmic pathway is involved in developmental onset of ripening in grape berry. Plant Physiol. 2006, 142, 220–232. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Y.L.; Patrick, J.W. The cellular pathway of postphloem sugar-transport in developing tomato fruit. Planta 1995, 196, 434–444. [Google Scholar] [CrossRef]
- Chen, C.; Yuan, Y.; Zhang, C.; Li, H.; Ma, F.; Li, M. Sucrose phloem unloading follows an apoplastic pathway with high sucrose synthase in Actinidia fruit. Plant Sci. 2017, 255, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Qu, X.; Hou, B.; Sosso, D.; Osorio, S.; Fernie, A.R.; Frommer, W.B. Sucrose efflux mediated by SWEET proteins as a key step for phloem transport. Science 2012, 335, 207–211. [Google Scholar] [CrossRef]
- Sivitz, A.B.; Reinders, A.; Ward, J.M. Arabidopsis sucrose transporter AtSUC1 is important for pollen germination and sucrose-induced anthocyanin accumulation. Plant Physiol. 2008, 147, 92–100. [Google Scholar] [CrossRef]
- Jung, B.; Ludewig, F.; Schulz, A.; Meißner, G.; Wöstefeld, N.; Flügge, U.I.; Pommerrenig, B.; Wirsching, P.; Sauer, N.; Koch, W. Identification of the transporter responsible for sucrose accumulation in sugar beet taproots. Nat. Plants 2015, 1, 14001. [Google Scholar] [CrossRef]
- Wormit, A.; Trentmann, O.; Feifer, I.; Lohr, C.; Tjaden, J.; Meyer, S.; Schmidt, U.; Martinoia, E.; Neuhaus, H.E. Molecular identification and physiological characterization of a novel monosaccharide transporter from Arabidopsis involved in vacuolar sugar transport. Plant Cell 2006, 18, 3476–3490. [Google Scholar] [CrossRef]
- Schneider, S.; Hulpke, S.; Schulz, A.; Yaron, I.; Höll, J.; Imlau, A.; Schmitt, B.; Batz, S.; Wolf, S.; Hedrich, R. Vacuoles release sucrose via tonoplast-localised SUC4-type transporters. Plant Biol. 2012, 14, 325–336. [Google Scholar] [CrossRef]
- Schulz, A.; Beyhl, D.; Marten, I.; Wormit, A.; Neuhaus, E.; Poschet, G.; Büttner, M.; Schneider, S.; Sauer, N.; Hedrich, R. Proton-driven sucrose symport and antiport are provided by the vacuolar transporters SUC4 and TMT1/2. Plant J. 2011, 68, 129–136. [Google Scholar] [CrossRef]
- Reinders, A.; Sivitz, A.; Starker, C.; Gantt, J.; Ward, J. Functional analysis of LjSUT4, a vacuolar sucrose transporter from Lotus japonicus. Plant Mol. Biol. 2008, 68, 289–299. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870. [Google Scholar] [CrossRef]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van, P.Y.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Abe, H.; Urao, T.; Ito, T.; Seki, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Arabidopsis AtMYC2 (bHLH) and AtMYB2 (MYB) function as transcriptional activators in abscisic acid signaling. Plant Cell 2003, 15, 63–78. [Google Scholar] [CrossRef]
- Finkelstein, R.R.; Lynch, T.J. The Arabidopsis abscisic acid response gene ABI5 encodes a basic leucine zipper transcription factor. Plant Cell 2000, 12, 599–609. [Google Scholar] [CrossRef]
- Baumann, K.; Paolis, A.D.; Costantino, P.; Gualberti, G. The DNA binding site of the Dof protein NtBBF1 is essential for tissue-specific and auxin-regulated expression of the rolB oncogene in plants. Plant Cell 1999, 11, 323–333. [Google Scholar] [CrossRef]
- Grierson, C.; Du, J.S.; Zabala, M.D.; Beggs, K.; Smith, C.; Holdsworth, M.; Bevan, M. Separate cis sequences and trans factors direct metabolic and developmental regulation of a potato-tuber storage protein gene. Plant J. 1994, 5, 815–826. [Google Scholar] [CrossRef]
- Hwang, Y.S.; Karrer, E.E.; Thomas, B.R.; Chen, L.; Rodriguez, R.L. Three cis-elements required for rice alpha-amylase Amy3D expression during sugar starvation. Plant Mol. Biol. 1998, 36, 331–341. [Google Scholar] [CrossRef]
- Hu, X.; Kuang, S.; Zhang, A.D.; Zhang, W.S.; Chen, M.J.; Yin, X.R.; Chen, K.S. Characterization of Starch Degradation Related Genes in Postharvest Kiwifruit. Int. J. Mol. Sci. 2016, 17, 2112. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Wu, J.; Chen, S.; Chen, H.; Chai, L.; Yi, H. Comparative transcriptome analyses between a spontaneous late-ripening sweet orange mutant and its wild type suggest the functions of ABA, sucrose and ja during citrus fruit ripening. PLoS ONE 2014, 9, e116056. [Google Scholar] [CrossRef]
- Barker, L.; Kuhn, C.; Weise, A.; Schulz, A.; Gebhardt, C.; Hirner, B.; Hellmann, H.; Schulze, W.; Ward, J.M.; Frommer, W.B. SUT2, a putative sucrose sensor in sieve elements. Plant Cell 2000, 12, 1153–1164. [Google Scholar] [CrossRef]
- Weise, A.; Barker, L.; Kuhn, C.; Lalonde, S.; Buschmann, H.; Frommer, W.B.; Ward, J.M. A new subfamily of sucrose transporters, SUT4, with low affinity/high capacity localized in enucleate sieve elements of plants. Plant Cell 2000, 12, 1345–1355. [Google Scholar] [CrossRef]
- Manning, K.; Davies, C.; Bowen, H.C.; White, P.J. Functional characterization of two ripening-related sucrose transporters from grape berries. Ann. Bot. 2001, 87, 125–129. [Google Scholar] [CrossRef]
- Li, C.Y.; Shi, J.X.; Weiss, D.; Goldschmidt, E.E. Sugars regulate sucrose transporter gene expression in citrus. Biochem. Biophys. Res. Commun. 2003, 306, 402–407. [Google Scholar] [CrossRef]
- Zanon, L.; Falchi, R.; Hackel, A.; Kuehn, C.; Vizzotto, G. Expression of peach sucrose transporters in heterologous systems points out their different physiological role. Plant Sci. 2015, 238, 262–272. [Google Scholar] [CrossRef]
- Li, M.; Feng, F.; Cheng, L. Expression patterns of genes involved in sugar metabolism and accumulation during apple fruit development. PLoS ONE 2012, 7, e33055. [Google Scholar] [CrossRef]
- Cheng, J.; Wen, S.; Xiao, S.; Lu, B.; Ma, M.; Bie, Z. Overexpression of the tonoplast sugar transporter CmTST2 in melon fruit increases sugar accumulation. J. Exp. Bot. 2018, 69, 511–523. [Google Scholar] [CrossRef]
- Chen, L.; Lin, I.W.; Qu, X.; Sosso, D.; McFarlane, H.E.; Londono, A.; Samuels, A.L.; Frommer, W.B. A cascade of sequentially expressed sucrose transporters in the seed coat and endosperm provides nutrition for the Arabidopsis embryo. Plant Cell 2015, 27, 607–619. [Google Scholar] [CrossRef]
- Eom, J.S.; Chen, L.; Sosso, D.; Julius, B.T.; Lin, I.W.; Qu, X.; Braun, D.M.; Frommer, W.B. SWEETs, transporters for intracellular and intercellular sugar translocation. Curr. Opin. Plant Biol. 2015, 25, 53–62. [Google Scholar] [CrossRef]
- Lin, I.W.; Sosso, D.; Chen, L.; Gase, K.; Kim, S.; Kessler, D.; Klinkenberg, P.M.; Gorder, M.K.; Hou, B.; Qu, X.; et al. Nectar secretion requires sucrose phosphate synthases and the sugar transporter SWEET9. Nature 2014, 508, 546–549. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).








