Absence of Yield Reduction after Controlled Water Stress during Prehaverst Period in Table OliveTrees
Abstract
1. Introduction
2. Material and Methods
2.1. Site Description and Experimental Design
2.2. Meteorological Conditions throughout the Experiment
2.3. Measurements
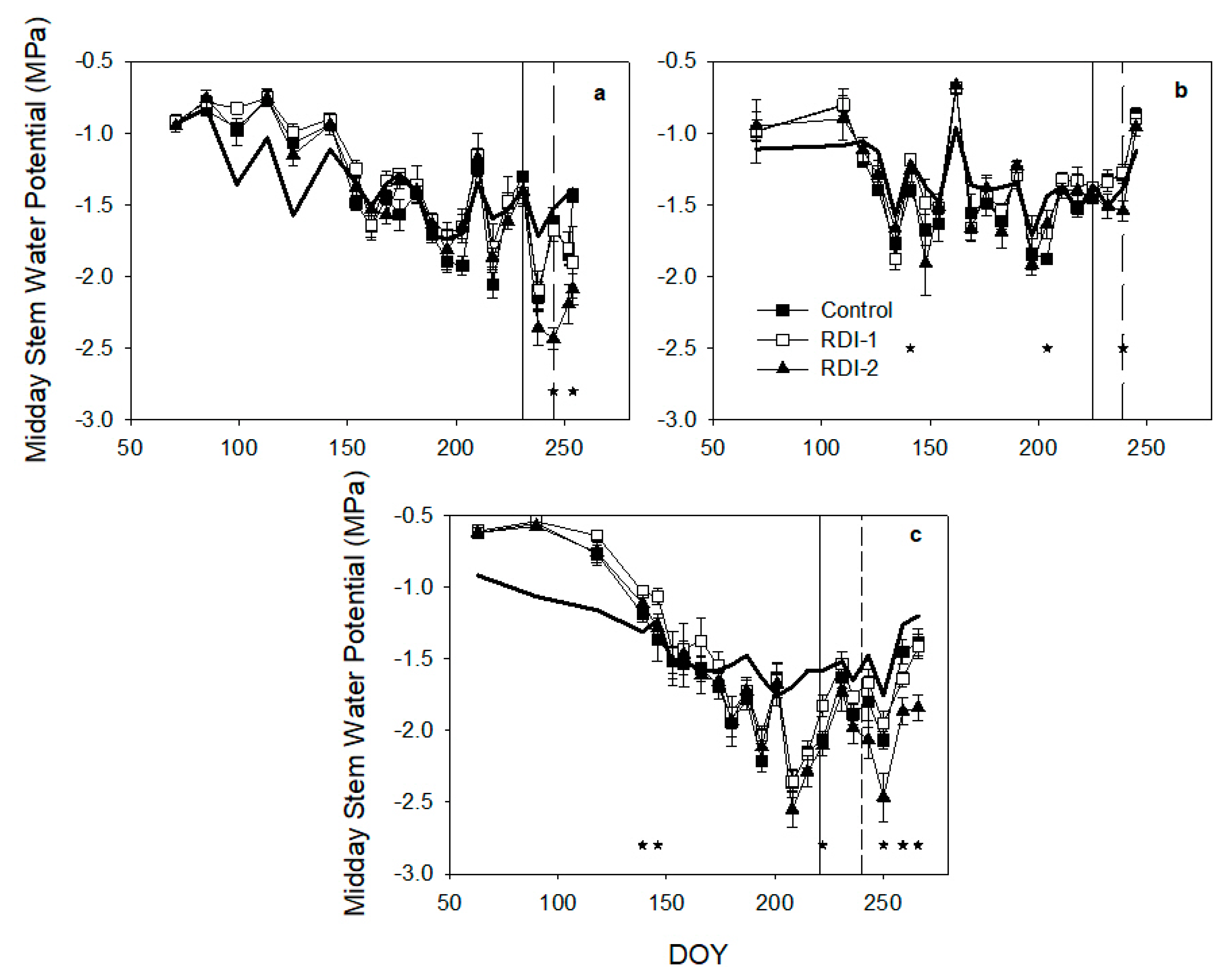
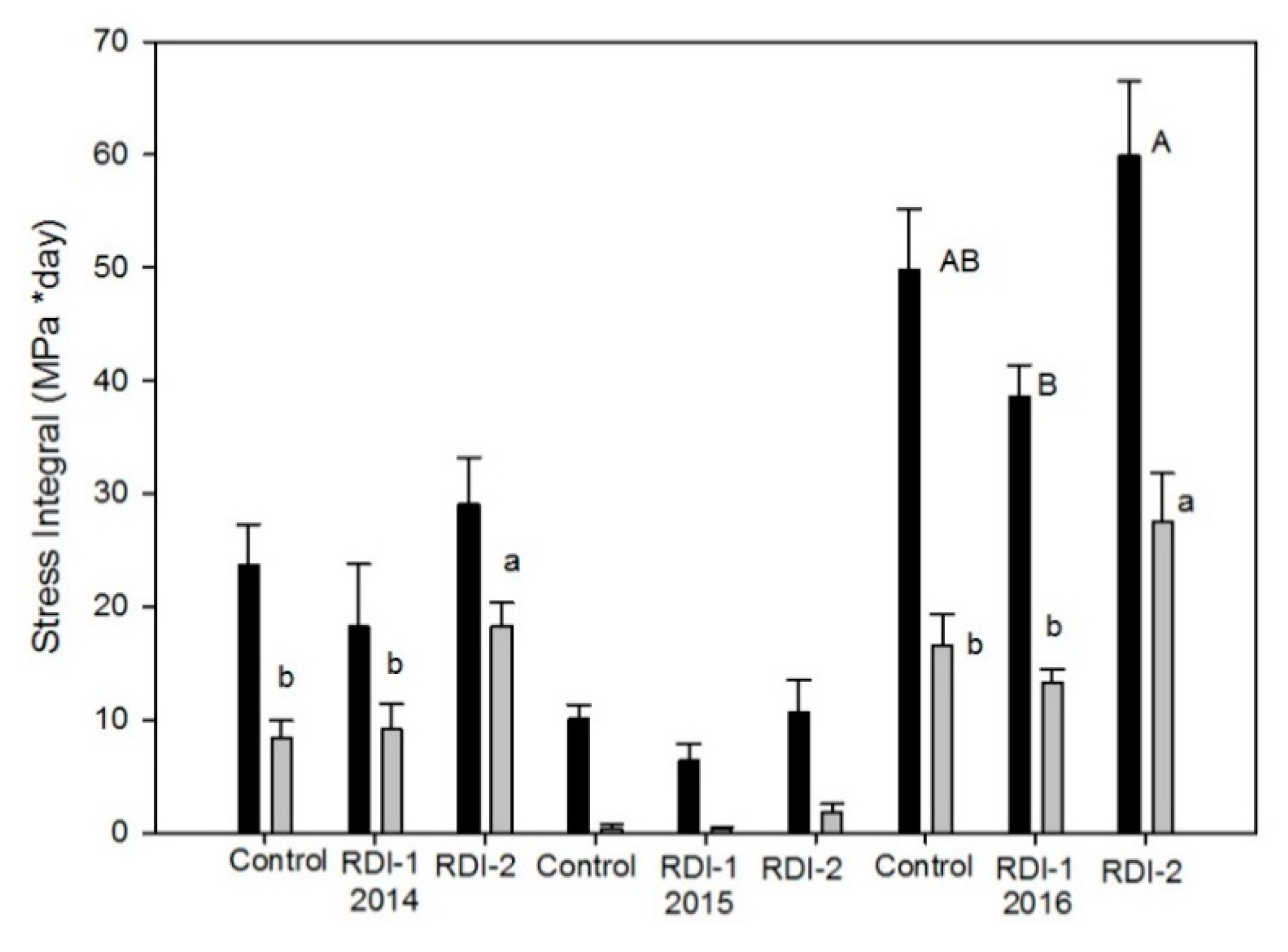
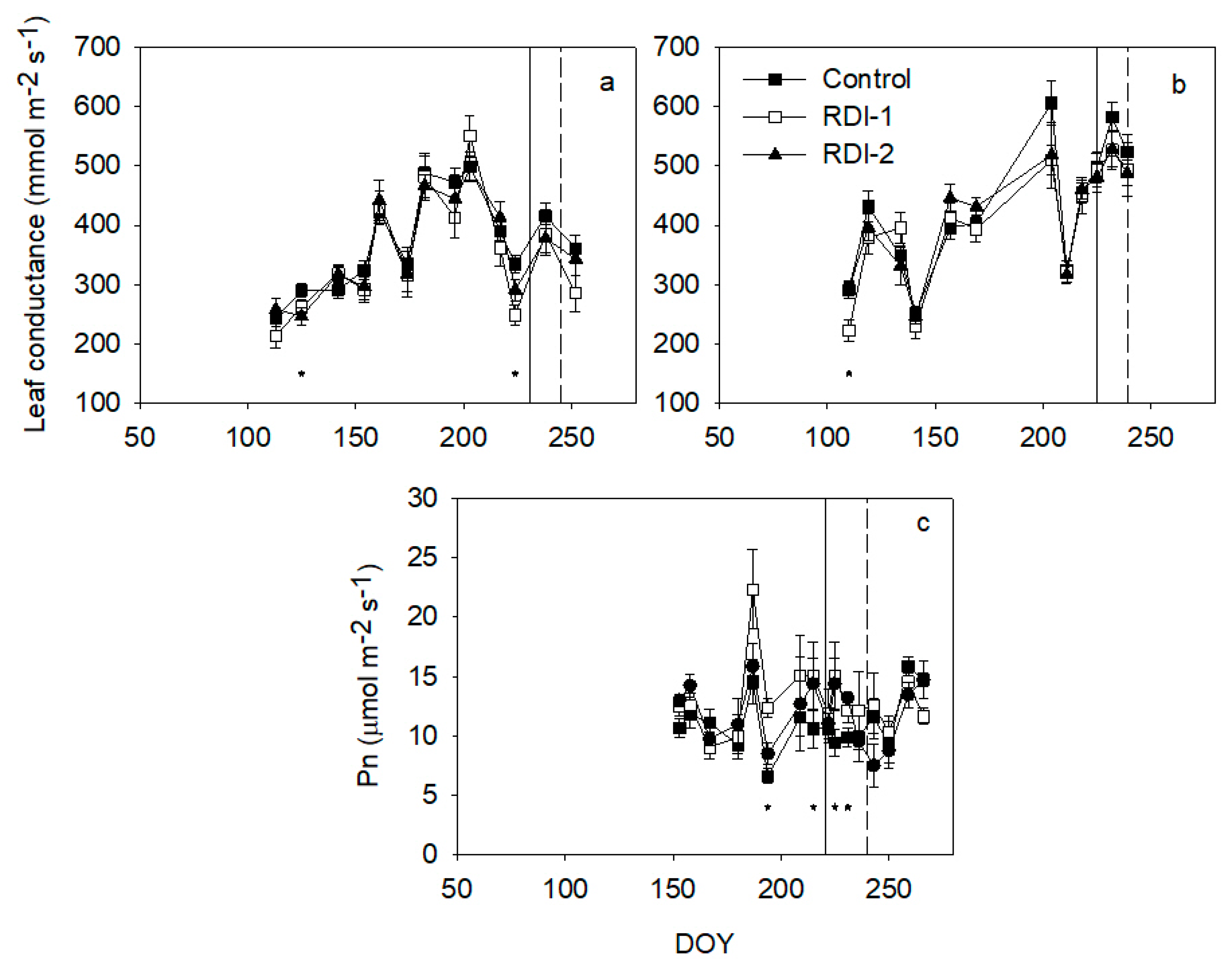
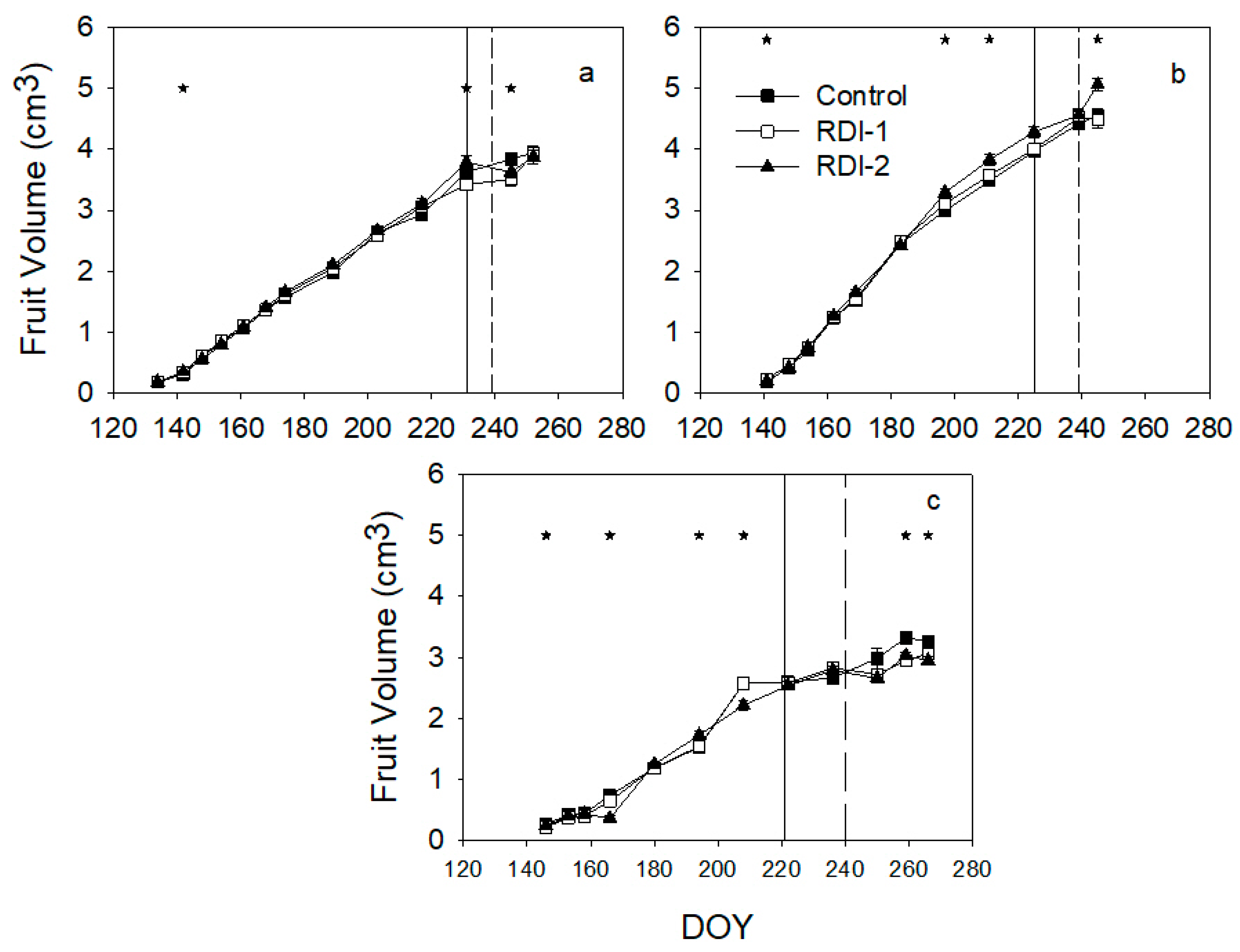
3. Results
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Hsiao, T.C. Measurements of plant water status. In Irrigation of Agricultural Crops; Stewart, B.A., Nielsen, D.R., Eds.; American Society of Agronomy: Madison, WI, USA, 1990; pp. 243–279. ISBN 0-89118-102-4. [Google Scholar]
- Díaz-Espejo, A.; Fernández, J.E.; Torres-Ruíz, J.M.; Rodríguez-Domínguez, C.M.; Pérez-Martín, A.; Hernández-Santana, V. The olive tree under water stress: Fitting the pieces of response mechanisms in the crop performance puzzle. In Water Scarcity and Sustainable Agriculture in Semiarid Environment; García-Tejero, I., Durán-Zuazo, V.H., Eds.; Academic Press: London, UK; Elsevier: Amsterdam, The Netherlands, 2018; pp. 439–480. ISBN 978-0-12-813164-0. [Google Scholar]
- Moriana, A.; Orgaz, F.; Fereres, E.; Pastor, M. Yield responses of a mature olive orchard to water deficits. J. Am. Soc. Hortic. Sci. 2003, 128, 425–431. [Google Scholar] [CrossRef]
- Lavee, S.; Wodner, M. Factors affecting the nature of oil acccumulation in fruit of olive (Olea europea L.) cultivars. J. Hortic. Sci. Biotechnol. 1991, 66, 583–591. [Google Scholar] [CrossRef]
- Goldhamer, D.A. Regulated deficit irrigation for California canning olives. Acta Hortic. 1999, 474, 369–372. [Google Scholar] [CrossRef]
- Chalmers, D.J.; Canterford, R.L.; Jerie, P.H.; Jones, T.R.; Ugalde, T.D. Photosynthesis in Relation to Growth and Distribution of Fruit in Peach Trees. Aust. J. Plant Physiol. 1975, 2, 635–645. [Google Scholar] [CrossRef]
- Johnstone, P.R.; Hartz, T.K.; LeStrange, M.; Nunez, J.L.; Miyao, E.M. Managing fruit soluble solids with late-season deficit irrigation in drip irrigated processing tomato production. HortScience 2005, 40, 1857–1861. [Google Scholar] [CrossRef]
- Besset, J.; Genard, M.; Girard, T.; Serra, V.; Bussi, C. Effect of water stress applied during the final stage of rapid growth on peach trees (cv. Big-Top). Sci. Hortic. 2001, 91, 289–303. [Google Scholar] [CrossRef]
- Girona, J.; Fereres, E.; Marsal, J.; Goldhamer, D.A.; Naor, A.; Soriano, M.A. Peach. In Crop Yield Response to Water, Irrigation and Drainage Paper No. 66; Steduto, P., Hsiao, T.C., Fereres, E., Raes, D., Eds.; FAO: Roma, Italy, 2012; pp. 392–409. ISBN 978-92-5-107274-5. [Google Scholar]
- Girona, J. Regulated deficit irrigation in Peach. A global analysis. Acta Hortic. 2002, 592, 335–342. [Google Scholar] [CrossRef]
- D’Amato, R.; Proietti, P.; Onofri, A.; Regni, L.; Esposto, S.; Servili, M.; Businelli, D.; Selvaggini, R. Biofortification (Se): Does it increase the content of phenolic compounds in virgin olive oil (VOO). PLoS ONE 2017. [Google Scholar] [CrossRef]
- Casanova, L.; Corell, M.; Suárez, M.P.; Rallo, P.; Martín-Palomo, M.J.; Jiménez, M.R. Bruising susceptibility of Manzanilla de Sevilla table olive cultivar under regulated deficit irrigation. Agric. Water Manag. 2017, 189, 1–4. [Google Scholar] [CrossRef]
- Lavee, S.; Hanoch, E.; Wodner, M.; Abramowitch, H. The effect of predetermined deficit irrigation on the performance of cv. Muhasan olives (Olea europaea L.) in the eastern coastal plain of Israel. Sci. Hortic. 2007, 1121, 156–163. [Google Scholar] [CrossRef]
- Girón, I.F.; Corell, M.; Martín-Palomo, M.J.; Galindo, A.; Torrecillas, A.; Moreno, F.; Moriana, A. Feasibility of trunk diameter fluctuations in the scheduling of regulated deficit irrigation for table olive trees without references trees. Agric. Water Manag. 2015, 161, 114–126. [Google Scholar] [CrossRef]
- Marino, G.; Caruso, T.; Ferguson, L.; Marra, F.P. Gas Exchange and stem water potential define stress thresholds for efficient irrigation management in olive (Olea europaea L.). Water 2018, 10, 342. [Google Scholar] [CrossRef]
- Moriana, A.; Pérez-López, D.; Prieto, M.H.; Ramírez-Santa-Pau, M.; Pérez-Rodríguez, J.M. Midday stem water potential as a useful tool for estimating irrigation requirements in olive trees. Agric. Water Manag. 2012, 112, 43–54. [Google Scholar] [CrossRef]
- Rapoport, H.F.; Pérez-López, D.; Hammami, S.B.M.; Aguera, J.; Moriana, A. Fruit pit hardening: Physical measurements during olive growth. Ann. Appl. Biol. 2013, 163, 200–208. [Google Scholar] [CrossRef]
- Orgaz, F.; Fereres, E. Riego. In El Cultivo del Olivo; Barranco, D., Fernández Escobar, R., Rallo, L., Eds.; Mundiprensa: Madrid, Spain, 1997; pp. 251–271. ISBN 84-7114-657-6. [Google Scholar]
- Meteorological Spanish Agency (AEMET). Available online: http://www.aemet.es/es/serviciosclimaticos/datosclimatologicos/valoresclimatologicos (accessed on 8 January 2020).
- Fernández, J.E.; Moreno, F.; Cabrera, F.; Arrue, J.L.; Martín-Aranda, J. Drip irrigation, soil characteristics and the root distribution and root activity of olive trees. Plant Soil 1991, 133, 239–251. [Google Scholar] [CrossRef]
- Xiloyannis, C.; Pezzarosa, B.; Jorba, J.; Angelini, P. Effects of soil water content on gas exchange in olive trees. Adv. Hortic. Sci. 1999, 2, 58–63. [Google Scholar]
- Moriana, A.; Villalobos, F.J.; Fereres, E. Stomatal and photosynthetic responses of olive (Olea europaea L.) leaves to water deficits. Plant Cell Environ. 2002, 25, 395–405. [Google Scholar] [CrossRef]
- Scholander, P.F.; Hammel, H.T.; Bradstreest, E.A.; Hemmingsen, E.A. Sap pressure in vascular plant. Science 1965, 148, 339–346. [Google Scholar] [CrossRef]
- Myers, B.J. Water stress integral a link between short term stress and long term growth. Tree Physiol. 1988, 4, 315–323. [Google Scholar] [CrossRef]
- Hermoso, M.; Uceda, M.; Frías, L.; Beltrán, G. Maduración. In El Cultivo del Olivo; Barranco, D., Fernández Escobar, R., Rallo, L., Eds.; Mundiprensa: Madrid, Spain, 1997; pp. 137–153. [Google Scholar]
- Szychowski, P.J.; Frutos, M.J.; Burlo, F.; Pérez-López, A.J.; Carbonell-Barrachina, A.A.; Hernández, F. Instrumental and sensory texture attributes of pomegranate arils and seeds as affected by cultivar. LWT Food Sci. Technol. 2015, 60, 656–663. [Google Scholar] [CrossRef]
- Molden, D.; Murray-Rust, H.; Sakthivadivel, R.; Makin, I. A water-productivity framework for understanding and action. In Water Productivity in Agriculture. Limits and Opportunities for Improvement; Kijne, J.W., Barker, R., Molden, D., Eds.; CABI Publishing: Oxford, UK, 2003; pp. 1–19. ISBN 0-85199-669-8. [Google Scholar]
- Fernández, J.E.; Diaz-Espejo, A.; Cuevas, M.V.; Hernandez-Santana, V. The crop water productivity, that false friend. In Proceedings of the XIV International Plant Water Relations Symposium, Madrid, Spain, 5 October 2018; pp. 119–123. [Google Scholar]
- Corell, M.; Pérez-López, D.; Martín-Palomo, M.J.; Centeno, A.; Girón, I.; Galindo, A.; Moreno, M.M.; Moreno, C.; Memmi, H.; Torrecillas, A.; et al. Comparison of the water potential baseline in different locations. Usefulness for irrigation scheduling of olive orchards. Agric. Water Manag. 2016, 177, 308–316. [Google Scholar] [CrossRef]
- Martín-Vertedor, A.; Pérez-Rodriguez, J.M.; Prieto, H.; Fereres, E. Interactive responses to water deficits and crop load in olive (Olea europaea L. cv. Morisca) I. Growth and water relations. Agric. Water Manag. 2011, 98, 941–949. [Google Scholar]
- Naor, A.; Schneider, D.; Ben-Gal, A.; Zipori, I.; Dag, A.; Kerem, Z.; Birger, R.; Peres, M.; Gal, Y. The effects of crop laod and irrigation rate in the oil accumulation stage on oil yield and water relations of “Koroneiki” olives. Irrig. Sci. 2013, 31, 781–791. [Google Scholar] [CrossRef]
- Shackel, K. A plant-based approach to deficit irrigation in trees and vines. HortScience 2011, 46, 173–177. [Google Scholar] [CrossRef]
- Steduto, P.; Hsiao, T.C.; Fereres, E.; Raes, D. Crop Yield Response to Water; FAO Irrigation and Drainage Paper no. 66; FAO: Roma, Italy, 2012. [Google Scholar]
- Rallo, L. Fructificación y producción. In El Cultivo del Olivo; Barranco, D., Fernández Escobar, R., Rallo, L., Eds.; Mundiprensa: Madrid, Spain, 1997; pp. 107–135. ISBN 84-7114-657-6. [Google Scholar]
- Fabbri, A.; Benelli, C. Flower bud induction and differentiation in olive. J. Hortic. Sci. Biotechnol. 2000, 75, 131–141. [Google Scholar] [CrossRef]
- Gómez-del-campo, M. Summer deficit-irrigation strategies in a hedgerow olive orchard cv. Arbequina: Effect on fruit characteristics and yield. Irrig. Sci. 2011. [Google Scholar] [CrossRef]
- Motilva, M.J.; Tovar, M.J.; Romero, M.P.; Alegre, S.; Girona, J. Influence of regultaed deficit irrigation strategies applied to olive trees (Arbequina cultivar) on oil yield and oil composition during fruit ripening period. J. Sci. Food Agric. 2000, 80, 2037–2043. [Google Scholar] [CrossRef]
- Inglese, P.; Barone, E.; Gullo, G. THe effect of complementary irrigation on fruit growth, ripening pattern and oil characteristics of olive (Olea europea L.) cv. Carolea. J. Hortic. Sci. Biotechnol. 1996, 71, 257–263. [Google Scholar] [CrossRef]
- Fereres, E. Variability in adaptive mechanisms to water deficits in annual and perennial crop plants. Bull. Soc. Bot. Fr. 1984, 131, 17–32. [Google Scholar] [CrossRef]
- Cuevas, M.V.; Martín-Palomo, M.J.; Díaz-Espejo, A.; Torres-Ruiz, J.M.; Rodriguez-Dominguez, C.M.; Pérez-Martín, A.; Pino-Mejias, R.; Fernandez, J.E. Assessing water stress in a hedgerow olive orchard from sap flow and trunk diameter measurements. Irrig. Sci. 2013, 31, 729–746. [Google Scholar] [CrossRef]
- Caruso, G.; Gucci, R.; Sifola, M.I.; Selvaggini, R.; Urbani, S.; Esposto, S.; Taticchi, A.; Servilli, M. Irrigation and fruit canopy position modify oil quantity of olive trees (cv. Frantoio). J. Sci. Food Agri. 2017, 97, 3530–3539. [Google Scholar] [CrossRef] [PubMed]
- Gucci, R.; Lodolini, E.; Rapoport, H.F. Productivity of olive trees with different water status and crop load. J. Hortic. Sci. Biotechnol. 2007, 82, 648–656. [Google Scholar] [CrossRef]
- Dell’Amico, J.; Moriana, A.; Corell, M.; Girón, I.F.; Morales, D.; Torrecillas, A.; Moreno, F. Low water stress conditions in table olive trees (Olea europaea L.) during pit hardening produced a different response of fruit and leaf water relations. Agric. Water Manag. 2012, 114, 11–17. [Google Scholar] [CrossRef]
- Girón, I.F.; Corell, M.; Galindo, A.; Torrecillas, E.; Morales, D.; Dell’Amico, J.; Torrecillas, A.; Moreno, F.; Moriana, A. Changes in the physiological response between leaves and fruits during a moderate water stress in table olive trees. Agric. Water Manag. 2015, 148, 280–286. [Google Scholar] [CrossRef]
- Hueso, A.; Trentacoste, E.R.; Junquera, P.; Gómez-Miguel, V.; Gómez-del-Campo, M. Differences in stem water potential during oil synthesis determine fruit characteristics and production but not vegetative growth or return blom in an olive hedgerow orchard (cv. Arbequina). Agric. Water Manag. 2019, 223, 105589. [Google Scholar] [CrossRef]
- Gómez-del-Campo, M.; Pérez-Exposito, M.A.; Hammami, S.B.M.; Centeno, A.; Rapoport, H.F. Effect of varied summer déficit irrigation on components of olive fruit growth and development. Agric. Water Manag. 2014, 137, 84–91. [Google Scholar] [CrossRef]





| % of Decrease from the Threshold | Amount of Irrigation (mm day−1) | % of Average Maximum ETc |
|---|---|---|
| Less than 15% | 1 mm | 25% |
| Between 15% and 30% | 2 mm | 50% |
| Greater than 30% | 4 mm | 100% |
| 2014 | ||||
| Control | RDI 1 | RDI 2 | ||
| Vegetative Growth (71–168) | Irr | 54 | 56 | 56 |
| ETo 4.7/R 78.6 | SWP | −1.12 | −1.04 | −1.11 |
| SI | 2.3 | 1.1 | 1.6 | |
| Pit Hardening (169–230) | Irr | 149 | 139 | 87 |
| ETo 5.7/R 5.9 | SWP | −1.62 | −1.49 | −1.54 |
| SI | 17.5 | 11.6 | 14.2 | |
| Deficit Period (231–258) | Irr | 75 | 47 | 0 |
| ETo 4.9/ R 1.9 | SWP | −1.76 | −1.87 | −2.27 |
| SI | 6.2 | 6.6 | 14.9 | |
| 2015 | ||||
| Control | RDI 1 | RDI 2 | ||
| Vegetative Growth (70–161) | Irr | 134 | 124 | 105 |
| ETo 4.7/R 55.9 | SWP | −1.28 | −1.22 | −1.25 |
| SI | 5.7 | 3.6 | 5.1 | |
| Pit hardening (162–225) | Irr | 244 | 252 | 228 |
| ETo 6.0/R 1.6 | SWP | −1.46 | −1.40 | −1.44 |
| SI | 9.9 | 6.2 | 9.5 | |
| Deficit Period (225–245) | Irr | 74 | 33 | 0 |
| ETo 4.9 /R 0.0 | SWP | −1.16 | −1.17 | −1.34 |
| SI | 3.2 | 1.8 | 10.7 | |
| 2016 | ||||
| Control | RDI 1 | RDI 2 | ||
| Vegetative Growth (63–166) | Irr | 62 | 63 | 73 |
| ETo 4.1/R 253.9 | SWP | −1.14 | −1.02 | −1.12 |
| SI | 3.3 | 0.9 | 2.0 | |
| Pit hardening (167–221) | Irr | 210 | 190 | 222 |
| ETo 6.3 /R 0.0 | SWP | −1.98 | −1.90 | −2.01 |
| SI | 36.5 | 29.0 | 37.0 | |
| Deficit period (222–263) | Irr | 185 | 67 | 0 |
| ETo 4.8/R 13.9 | SWP | −1.70 | −1.70 | −1.99 |
| SI | 16.5 | 13.3 | 27.5 |
| 2014 | |||
| Control | RDI 1 | RDI 2 | |
| Yield (T ha−1) | 14.7±1.6 | 12.2±2.4 | 12.0±2.4 |
| Size (Fruit kg−1) | 244±9 | 261±21 | 275±15 |
| FL (Fruit tree−1) | 12710±1656 | 11952±2953 | 11981±2922 |
| MI | 0.82±0.03 | 0.98±0.09 | 0.78±0.08 |
| Pulp Stone Fresh | 4.6±0.1 | 4.6±0.2 | 4.4±0.2 |
| Pulp Stone Dry | 2.1±0.1 | 2.2±0.1 | 2.2±0.1 |
| Fruit texture (N) | |||
| Applied Water (mm) | 278±22a | 242±54ab | 143±13b |
| CWP (kg m−3) | 5.1±0.3 | 5.0±1.0 | 8.3±1.5 |
| 2015 | |||
| Control | RDI 1 | RDI 2 | |
| Yield (T ha−1) | 0.2 ± 0.1 | 0.4 ± 0.1 | 0.5 ± 0.4 |
| Size (Fruit kg−1) | 208 ± 4 | 192 ± 6 | 194 ± 4 |
| FL (Fruit tree−1) | 144 ± 61 | 277 ± 110 | 348 ± 307 |
| MI | 0.87 ± 0.1b | 1.26 ± 0.1ab | 1.39 ± 0.1a |
| Pulp Stone Fresh | 5.8 ± 0.1 | 6.2 ± 0.1 | 5.7 ± 0.1 |
| Pulp Stone Dry | 2.5 ± 0.1b | 2.7 ± 0.1a | 2.5 ± 0.1ab |
| Fruit texture (N) | 5.9 ± 0.1a | 5.2 ± 0.2b | 5.2 ± 0.2b |
| Applied Water (mm) | 452 ± 28 | 409 ± 75 | 333 ± 33 |
| CWP (kg m−3) | 0.1 ± 0.0 | 0.1 ± 0.1 | 0.1 ± 0.1 |
| 2016 | |||
| Control | RDI 1 | RDI 2 | |
| Yield (T ha−1) | 17.6 ± 1.8 | 16.1 ± 2.3 | 17.0 ± 2.0 |
| Size (Fruit kg−1) | 309 ± 17 | 338 ± 10 | 331 ± 3 |
| FL (Fruit tree−1) | 20,083 ± 4198 | 19,183 ± 4095 | 19,622 ± 556 |
| MI | 0.85 ± 0.1 | 0.71 ± 0.0 | 0.87 ± 0.1 |
| Pulp Stone Fresh | 4.8 ± 0.3a | 4.0 ± 0.2b | 3.9 ± 0.1b |
| Pulp Stone Dry | 1.8 ± 0.1 | 1.8 ± 0.1 | 1.8 ± 0.1 |
| Fruit texture (N) | 6.2 ± 0.2a | 5.0 ± 0.1b | 5.0 ± 0.1b |
| Applied Water (mm) | 457 ± 30 | 320 ± 68 | 295 ± 15 |
| CWP (kg m−3) | 3.9 ± 0.8 | 5.5 ± 0.7 | 5.3 ± 0.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín-Palomo, M.J.; Corell, M.; Girón, I.; Andreu, L.; Galindo, A.; Centeno, A.; Pérez-López, D.; Moriana, A. Absence of Yield Reduction after Controlled Water Stress during Prehaverst Period in Table OliveTrees. Agronomy 2020, 10, 258. https://doi.org/10.3390/agronomy10020258
Martín-Palomo MJ, Corell M, Girón I, Andreu L, Galindo A, Centeno A, Pérez-López D, Moriana A. Absence of Yield Reduction after Controlled Water Stress during Prehaverst Period in Table OliveTrees. Agronomy. 2020; 10(2):258. https://doi.org/10.3390/agronomy10020258
Chicago/Turabian StyleMartín-Palomo, María José, Mireia Corell, Ignacio Girón, Luis Andreu, Alejandro Galindo, Ana Centeno, David Pérez-López, and Alfonso Moriana. 2020. "Absence of Yield Reduction after Controlled Water Stress during Prehaverst Period in Table OliveTrees" Agronomy 10, no. 2: 258. https://doi.org/10.3390/agronomy10020258
APA StyleMartín-Palomo, M. J., Corell, M., Girón, I., Andreu, L., Galindo, A., Centeno, A., Pérez-López, D., & Moriana, A. (2020). Absence of Yield Reduction after Controlled Water Stress during Prehaverst Period in Table OliveTrees. Agronomy, 10(2), 258. https://doi.org/10.3390/agronomy10020258







