Response of Rice Algal Assemblage to Fertilizer and Chemical Application: Implications for Early Algal Bloom Management
Abstract
1. Introduction
2. Materials and Methods
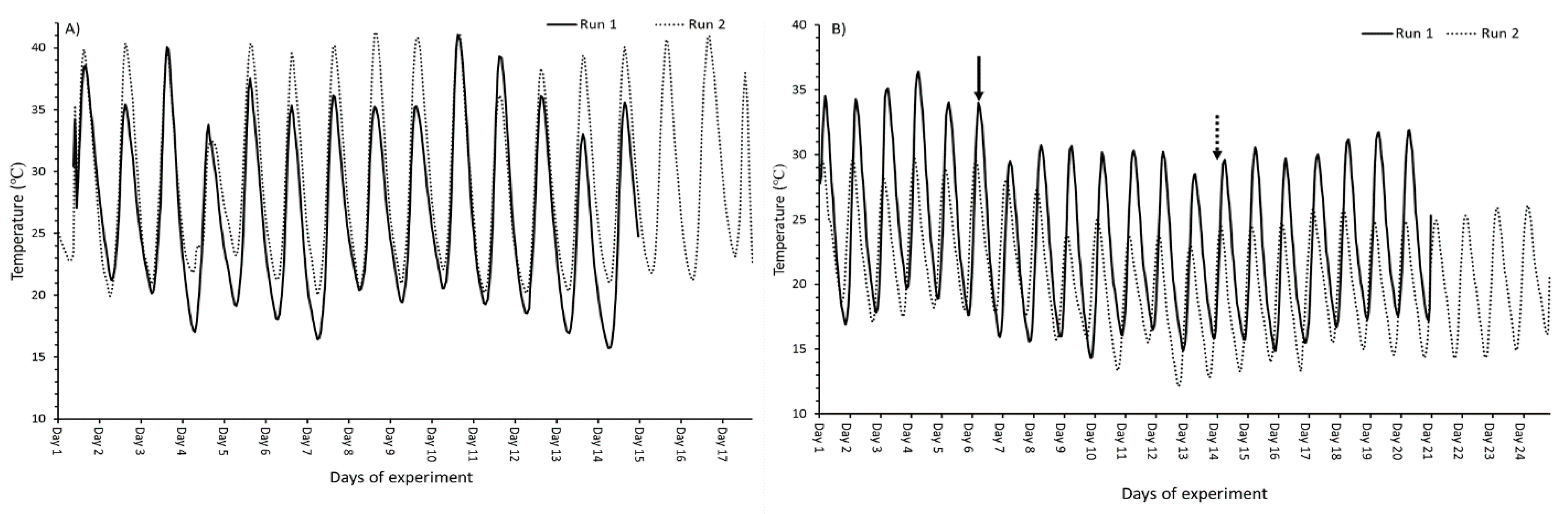
2.1. Nutrient Evaluation Study
2.2. Algaecide Evaluation Study
2.3. Data Analysis
3. Results and Discussion
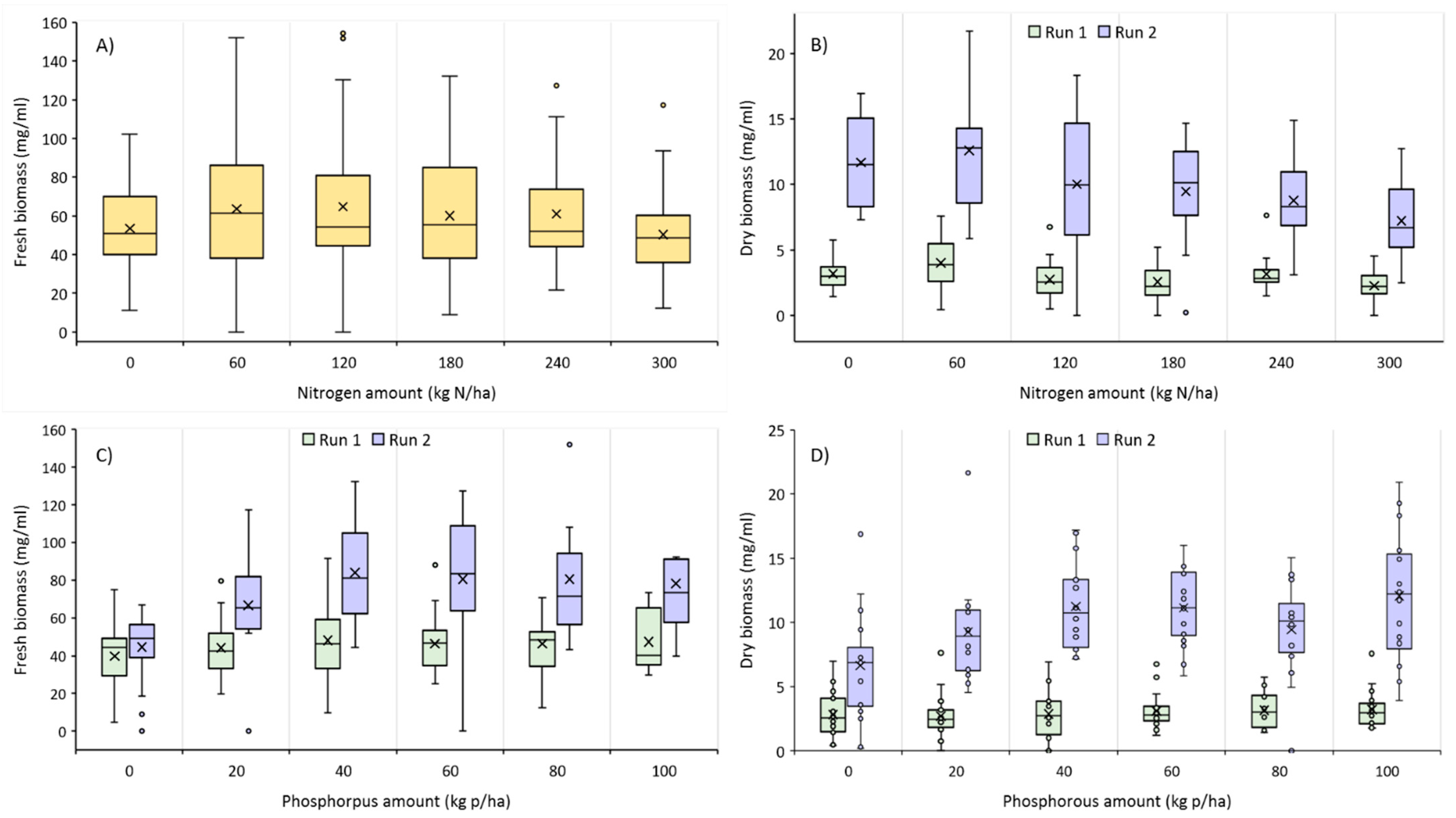
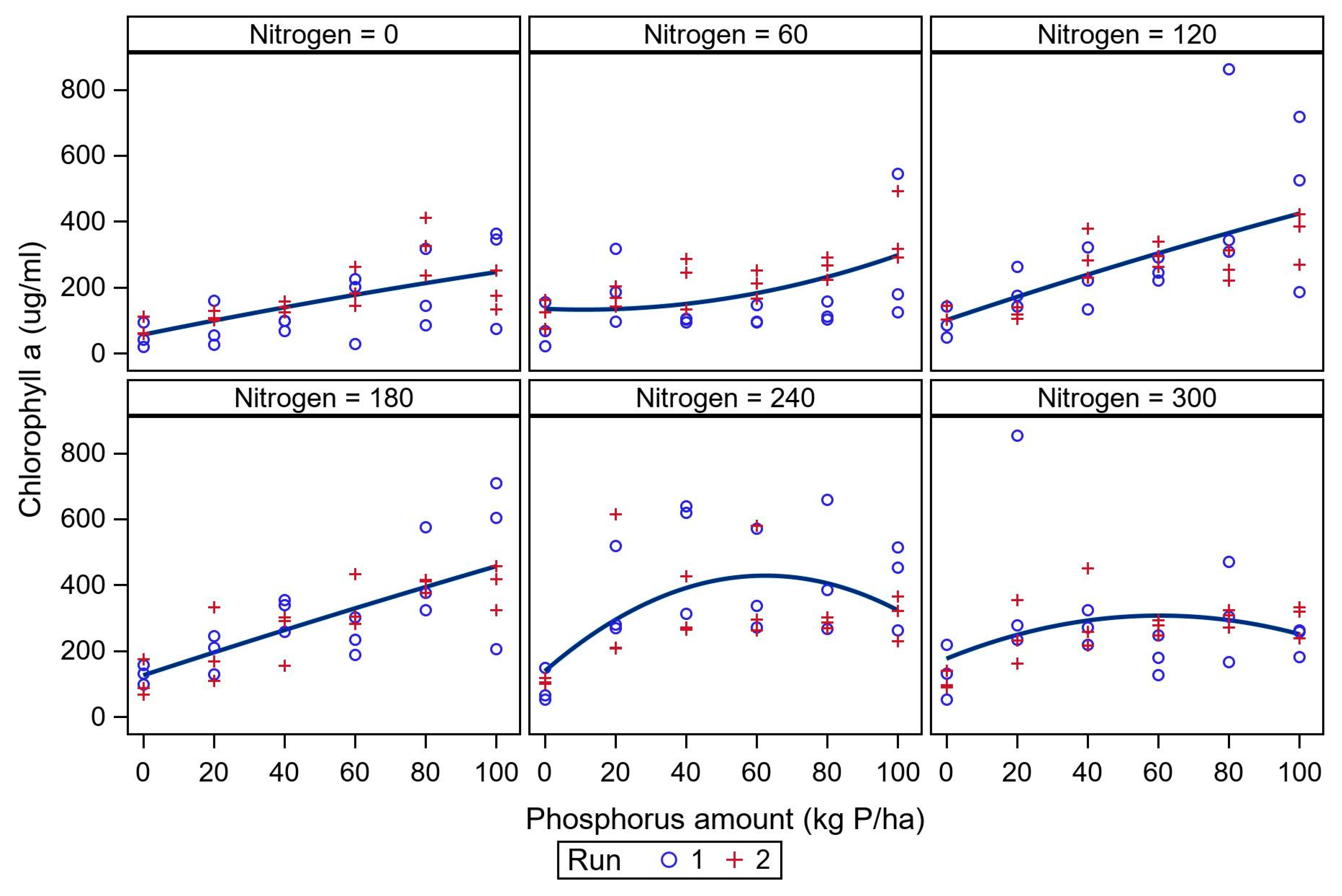
3.1. Nutrient Evaluation Study
3.2. Algaecide Evaluation Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McBride, W.D.; Skorbiansky, S.R.; Childs, N. US Rice Production in the New Millennium: Changes in Structure, Practices, and Costs. Econ. Res. Serv. Econ. Res. Bull. 2018, 1–62. [Google Scholar] [CrossRef]
- Hill, J.E.; Williams, J.; Mutters, R.; Greer, C. The California rice cropping system: Agronomic and natural resource issues for long-term sustainability. Paddy Water Environ. 2006, 4, 13–19. [Google Scholar] [CrossRef]
- Hill, J.E.; Brouder, S.M.; Roberts, S.R.; Williams, J.F.; Scardaci, S.C.; Wick, C.M. A survey of water management practices of California rice growers. J. Nat. Resour. Life Sci. Educ. 1994, 23, 119–124. [Google Scholar] [CrossRef]
- Spencer, D.F.; Lembi, C.A.; Blank, R. Spatial and Temporal Variation in the Composition and Biomass of Algae Present in Selected California Rice Fields. J. Freshwater Ecol. 2006, 21, 649–656. [Google Scholar] [CrossRef]
- Roger, P.A.; Reynaud, P.A. Ecology of Blue-Green Algae in Paddy Fields; International Rice Research Station Institute: Los Banos, Philipines, 1978; pp. 289–309. [Google Scholar]
- Ghosh, T.K.; Saha, K.C. Effects of inoculation with N2-fixing cyanobacteria on the nitrogenase activity in soil and rhizosphere of wetland rice (Oryza sativa L.). Biol. Fertil. Soils 1993, 16, 16–20. [Google Scholar] [CrossRef]
- Hashem, M.A. Problems and prospects of cyanobacterial biofertilizer for rice cultivation. Funct. Plant Biol. 2001, 8, 881–888. [Google Scholar] [CrossRef]
- Saha, K.C.; Mandal, L.N. A greenhouse study on the effect of inoculation of N-fixing blue-green algae in an alluvial soil treated with P and Mo on the yield of rice and changes in the N-content of soil. Plant Soil 1980, 57, 23–30. [Google Scholar] [CrossRef]
- Watanabe, I. Use of symbiotic and free-living blue-green algae in the rice culture. Outlook Agric. 1984, 13, 166–172. [Google Scholar] [CrossRef]
- Norman, R.J.; Wilson, C.E.; Slaton, A. Soil fertilization and mineral nutrition in US mechanized rice culture. In Rice: Origin, History, Technology and Production; John Wiley and Sons, Inc.: Hoboken, NJ, USA, 2002; pp. 331–412. [Google Scholar]
- Elser, J.J.; Marzolf, E.R.; Goldman, C.R. Phosphorus and nitrogen limitation of phytoplankton growth in the freshwaters of North America: A review and critique of experimental enrichments. Can. J. Fish Aquat. Sci. 1990, 47, 1468–1477. [Google Scholar] [CrossRef]
- Hecky, R.E.; Kilham, P. Nutrient limitation of phytoplankton in freshwater and marine environments: A review of recent evidence on the effects of enrichment. Limnol. Oceanogr. 1988, 33, 796–822. [Google Scholar] [CrossRef]
- Lundy, M.; Spencer, D.F.; Van Kessel, C.; Hill, J.; Linquist, B. Managing phosphorous fertilizer to reduce algae, maintain water quality, and sustain yields in water-seeded rice. Field Crops. Res. 2012, 131, 81–87. [Google Scholar] [CrossRef]
- Davis, T.W.; Bullerjahn, G.S.; Tuttle, T.; McKay, R.M.; Watson, S.B. Effects of increasing nitrogen and phosphorus concentrations on phytoplankton community growth and toxicity during Planktothrix blooms in Sandusky Bay, Lake Erie. Environ. Sci. Technol. 2015, 49, 7197–7207. [Google Scholar] [CrossRef]
- Julia, C.C.; Rose, T.J.; Pariasca-Tanaka, J.; Jeong, K.; Matsuda, T.; Wissuwa, M. Phosphorus uptake commences at the earliest stages of seedling development in rice. J. Exp. Bot. 2018, 69, 5233–5240. [Google Scholar] [CrossRef]
- Netherlands, M. Chemical control of aquatic weeds. In Biology and Control of Aquatic Plants: A Best Management Practices Handbook; Marietta, G.A., Ed.; Aquatic Ecosystem Restoration Foundation: Flint, MI, USA, 2014; pp. 71–88. [Google Scholar]
- Spencer, D.F.; Liow, P.S.; Lembi, C.A. Effect of a combination of two rice herbicides on the Caynobacterium, Nostoc. spongieforme. J. Aquat. Plant Manag. 2009, 47, 145–147. [Google Scholar]
- Spencer, D.F.; Liow, P.; Lembi, C.A. Influence of a non-copper algaecide on the cyanobacterium, Nostoc spongiaeforme, and the green alga, Hydrodictyon reticulatum, in field and laboratory experiments. Paddy Water Environ. 2013, 11, 611–617. [Google Scholar] [CrossRef]
- Mutters, R.G.; Greer, C.A.; Horwath, W.R. Rice Nutrient Management in California; University of California, Agriculture and Natural Resources: Berkeley, CA, USA, 2010; Publication 3516. [Google Scholar]
- Calomeni, A.J.; Kinley, C.M.; Geer, T.D.; Hendrikse, M.; Rodgers, J.H., Jr. Lyngbya wollei responses to copper algaecide exposures predicted using a concentration-exposure time (CET) model: Influence of initial biomass. J. Aquat. Plant Manag. 2018, 56, 73–83. [Google Scholar]
- Calomeni, A.J.; Geer, T.D.; Iwinski, K.J.; Rodgers, J.H.; Madsen, J.D.; Wersal, R.M. Monitoring for national pollutant discharge elimination system permit Requirements: Algaecides. J. Integr. Pest Manag. 2017, 27, 1–9. [Google Scholar] [CrossRef]
- Klausmeier, C.A.; Litchman, E.; Daufresne, T.; Levin, S.A. Optimal nitrogen-to-phosphorus stoichiometry of phytoplankton. Nature 2004, 429, 171–174. [Google Scholar] [CrossRef]
- Linquist, B.; Sengxua, P. Efficient and flexible management of nitrogen for rain fed lowland rice. Nutr. Cycl. Agroecosyst. 2003, 67, 107–115. [Google Scholar] [CrossRef]
- Spencer, D.F.; Lembi, C.A. Evaluation of Additional Alternative Methods for Managing Algae in California Rice Fields. Annual Report Comprehensive Research on Rice; California Rice Research Board: Biggs, CA, USA, 2006. [Google Scholar]
- Montgomery, G.B.; Bond, J.A.; Golden, B.R.; Gore, J.; Edwards, H.M.; Eubank, T.W.; Walker, T.W. Response of commercial rice cultivars to postemergence applications of saflufenacil. Weed Technol. 2014, 28, 679–684. [Google Scholar] [CrossRef]
- McKenzie, K.S. Oxyfluorfen Resistant Rice Lines. U.S. Patent Application 20180070548, 22 March 2018. [Google Scholar]
- Mikkelson, D.S. Zinc deficiency in California rice. Calif. Agric. 1975, 29, 8–9. [Google Scholar]
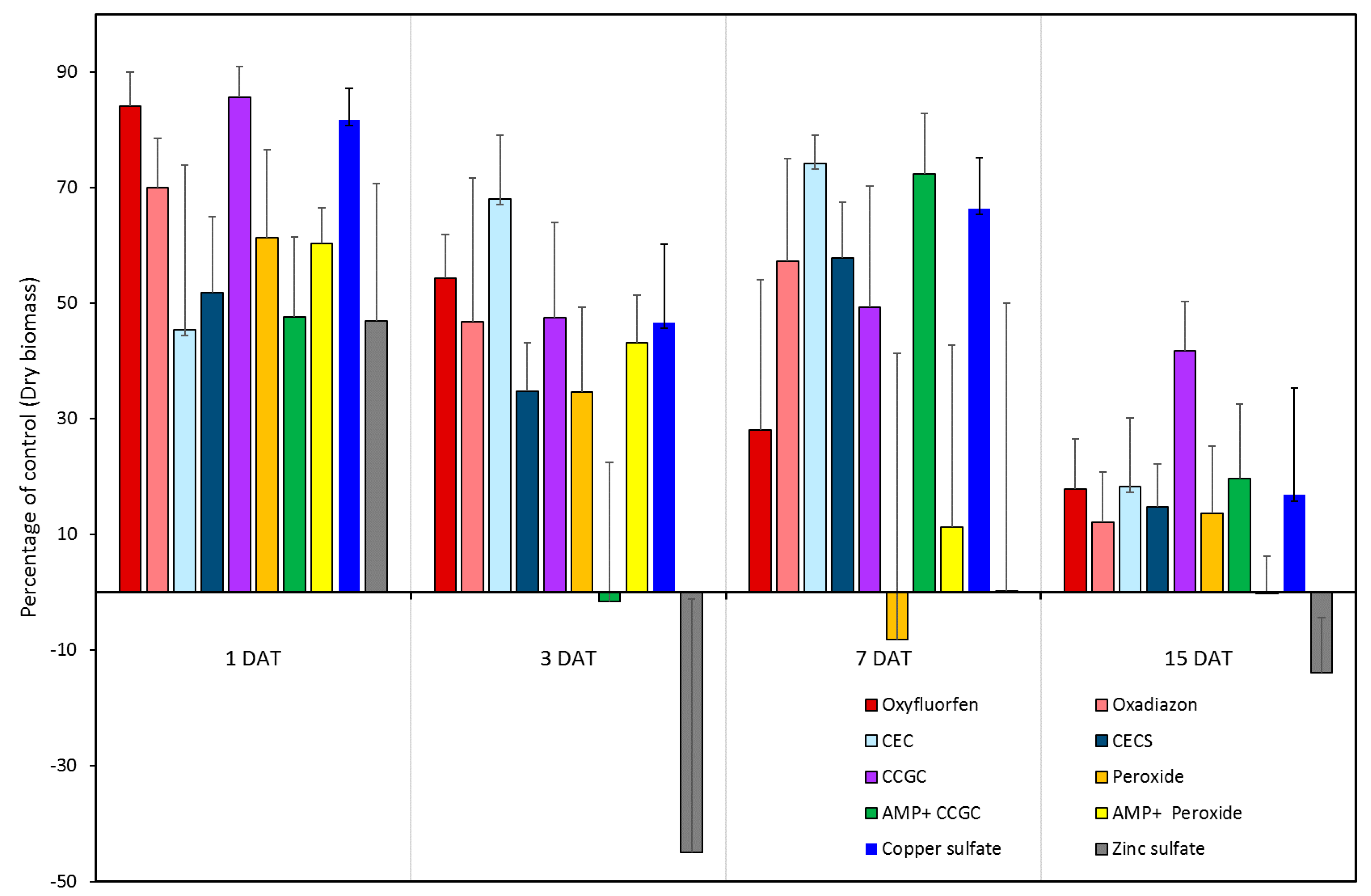
| Treatment | Trade Name | Application Rate | Abbreviation |
|---|---|---|---|
| No algaecide/herbicide | - | - | - |
| Oxyfluorfen | Goal® 2XL | 500 g ai ha−1 | oxyfluorfen |
| Oxadiazon | Ronstar®50 WSP | 500 g ai ha−1 | oxadiazon |
| Copper ethanolamine complex | Cutrine®-Plus | 1 ppm Cu | CEC |
| Copper ethanolamine complex with surfactant | Cutrine®-Ultra | 1 ppm Cu | CECS |
| Chelated copper gluconate and citrate | Algimycin® PWF | 1 ppm Cu | CCGC |
| Peroxide | LZA-peroxide | 30.8 mL ai ha−1 | peroxide |
| Yeast protein extract activator + CCGC | AMP® activator + Algimycin® PWF | 1.3 mL ai ha−1 + 1 ppm Cu | AMP+ CCGC |
| Yeast protein extract activator+ peroxide | AMP® activator + LZA-peroxide | 1.3 mL ai ha−1 + 30.8 mL ai ha−1 | AMP+ peroxide |
| Conventional copper sulfate | - | 1 ppm Cu | copper sulfate |
| Zinc sulfate | - | 18 ppm Zn | zinc sulfate |
| Sources of Variance | Fresh Biomass | Dry Biomass | Chlorophyll a Content | |||
|---|---|---|---|---|---|---|
| Mean Square | p | Mean Square | p | Mean Square | p | |
| Experiment | 45,136.22 | <0.0001 | 2664.48 | <0.0001 | 2383.87 | 0.6693 |
| Nitrogen | 1859.17 | 0.0021 | 55.72 | <0.0001 | 155,278.87 | <0.0001 |
| Phosphorous | 2965.55 | <0.0001 | 38.93 | <0.0001 | 248,929.93 | <0.0001 |
| Nitrogen × Phosphorous | 377.76 | 0.7259 | 7.80 | 0.1999 | 22,855.56 | 0.0215 |
| Nitrogen × Experiment | 576.78 | 0.2960 | 20.47 | 0.0073 | 27,391.81 | 0.0681 |
| Phosphorous × Experiment | 1402.83 | 0.0132 | 29.61 | 0.0004 | 14,451.50 | 0.3574 |
| Phosphorus × Nitrogen × Experiment | 391.60 | 0.6875 | 8.49 | 0.1273 | 11,207.83 | 0.6570 |
| Nitrogen Amount (kg N ha−1) | Model Parameters Estimate | R2 | ||
|---|---|---|---|---|
| a (SE) | b (SE) | c (SE) | ||
| 0 | 56.15 (29.94) | 2.18 (1.41) | −0.002 (0.01) | 0.41 |
| 60 | 134.46 (35.80) | −0.46 (1.68) | 0.02 (0.016) | 0.29 |
| 120 | 100.13 (51.86) | 3.59 (2.36) | −0.003 (0.022) | 0.43 |
| 180 | 125.48 (34.44) | 3.51 (1.71) | −0.002 (0.016) | 0.59 |
| 240 | 138.02 (51.94) | 9.27968 (2.44) | −0.07 (0.023) | 0.34 |
| 300 | 175.92 (49.19) | 4.32 (2.31) | −0.035 (0.022) | 0.11 |
| Division | Class | Order | Family | Species | Cell Concentration (Cell mL−1) |
|---|---|---|---|---|---|
| Adjacent rice field subsample | |||||
| Bacillariophyta | Bacillariophyceae | Bacillarales | Bacillariaceae | Nitzschi palea | 178,592 |
| N. gracillis | 847 | ||||
| Naviculales | Stauoneidaceae | Stauroneis phoenicenteron | 26 | ||
| Chlorophyta | Chlorophyceae | Chlorococcales | Chlorococcaceae | Chlorococcum spp. | 10,205.3 |
| Tetraedron minimum | 5102.7 | ||||
| Coelastraceae | Coelastrum microporum | 13,550.7 | |||
| Hydrodictyaceae | Pediastrum duplex | 5102.6 | |||
| Oocystaceae | Chlorella ellipsoidea | 17,527,526.9 | |||
| Monoraphidium arcuatum | 30,615.7 | ||||
| M. griffithii | 15,307.8 | ||||
| Scenedesmaceae | Desmodesmus brasiliensis | 51,025.7 | |||
| Scenedesmus acutus | 846.9 | ||||
| Oedogoniales | Oedogoniaceae | Oedogonium spp. | 383.4 | ||
| Volvocales | Volvocaceae | Volvox spp. | 30,489.2 | ||
| Chlamydomonadaceae | Chlamydomonas spp. | 10,205.2 | |||
| Zygnematales | Desmidiaceae | Cosmarium spp. | 846.9 | ||
| Zygnemataceae | Mougeotia spp. | 1693.8 | |||
| Chrysophyta | Chrysophyceae | Chromalinales | Chrysococcaceae | Chrysococcus minutus | 10,205.2 |
| Cyanophyta | Cyanophyceae | Chroococcales | Chroococcaceae | Synechococcus elongatus | 535,775.9 |
| Synechococcus spp. | 280,644.5 | ||||
| Nostocales | Nostocaceae | Anabaena spp. | 5,714,943.2 | ||
| Cylindrospermum spp. | 796,009.9 | ||||
| Dolichospermum macrosporum | 469,441.7 | ||||
| D. planctonicum | 67,753.9 | ||||
| Komvophoron spp. | 76,539.4 | ||||
| Pseudanabaena galeata | 8,761,966.3 | ||||
| Oscillatoriales | Oscillatoriaceae | Leptolyngbya subtilissima | 127,565.6 | ||
| Pleurocapsacea | Pleurocapsaceae | Pleurocapsa minor | 61,231.5 | ||
| Bucket algae subsample | |||||
| Chlorophyta | Chlorophyceae | Chlorococcales | Oocystaceae | Chlorella ellipsoidea | 17,527,526.9 |
| Monoraphidium arcuatum | 30,615.7 | ||||
| M. griffithii | 15,307.8 | ||||
| Scenedesmaceae | Desmodesmus brasiliensis | 15,244.6 | |||
| Scenedesmus acutus | 3387.6 | ||||
| Volvocales | Chlamydomonadaceae | Chlamydomonas spp. | 15,307.8 | ||
| Cyanophyta | Cyanophyceae | Chroococcales | Chroococcaceae | Synechococcus spp. | 612,315.3 |
| Nostocales | Nostocaceae | Pseudanabaena galeata | 42,346.1 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ohadi, S.; Godar, A.; Madsen, J.; Al-Khatib, K. Response of Rice Algal Assemblage to Fertilizer and Chemical Application: Implications for Early Algal Bloom Management. Agronomy 2021, 11, 542. https://doi.org/10.3390/agronomy11030542
Ohadi S, Godar A, Madsen J, Al-Khatib K. Response of Rice Algal Assemblage to Fertilizer and Chemical Application: Implications for Early Algal Bloom Management. Agronomy. 2021; 11(3):542. https://doi.org/10.3390/agronomy11030542
Chicago/Turabian StyleOhadi, Sara, Amar Godar, John Madsen, and Kassim Al-Khatib. 2021. "Response of Rice Algal Assemblage to Fertilizer and Chemical Application: Implications for Early Algal Bloom Management" Agronomy 11, no. 3: 542. https://doi.org/10.3390/agronomy11030542
APA StyleOhadi, S., Godar, A., Madsen, J., & Al-Khatib, K. (2021). Response of Rice Algal Assemblage to Fertilizer and Chemical Application: Implications for Early Algal Bloom Management. Agronomy, 11(3), 542. https://doi.org/10.3390/agronomy11030542





