Effects of Autotoxicity on Alfalfa (Medicago sativa): Seed Germination, Oxidative Damage and Lipid Peroxidation of Seedlings
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Experimental Conditions
2.3. Sampling and Preparation of Extracts
2.4. Effects of Extracts on Seed Germination and Seedling Growth
2.5. Measurement of Seedlings’ Physiological Parameters
2.5.1. Measurement of Soluble Protein, Soluble Sugar and Free Proline Content
2.5.2. Measurement of MDA and ROS (H2O2, OH• and O2•−) Levels
2.6. Statistical Analysis
3. Results
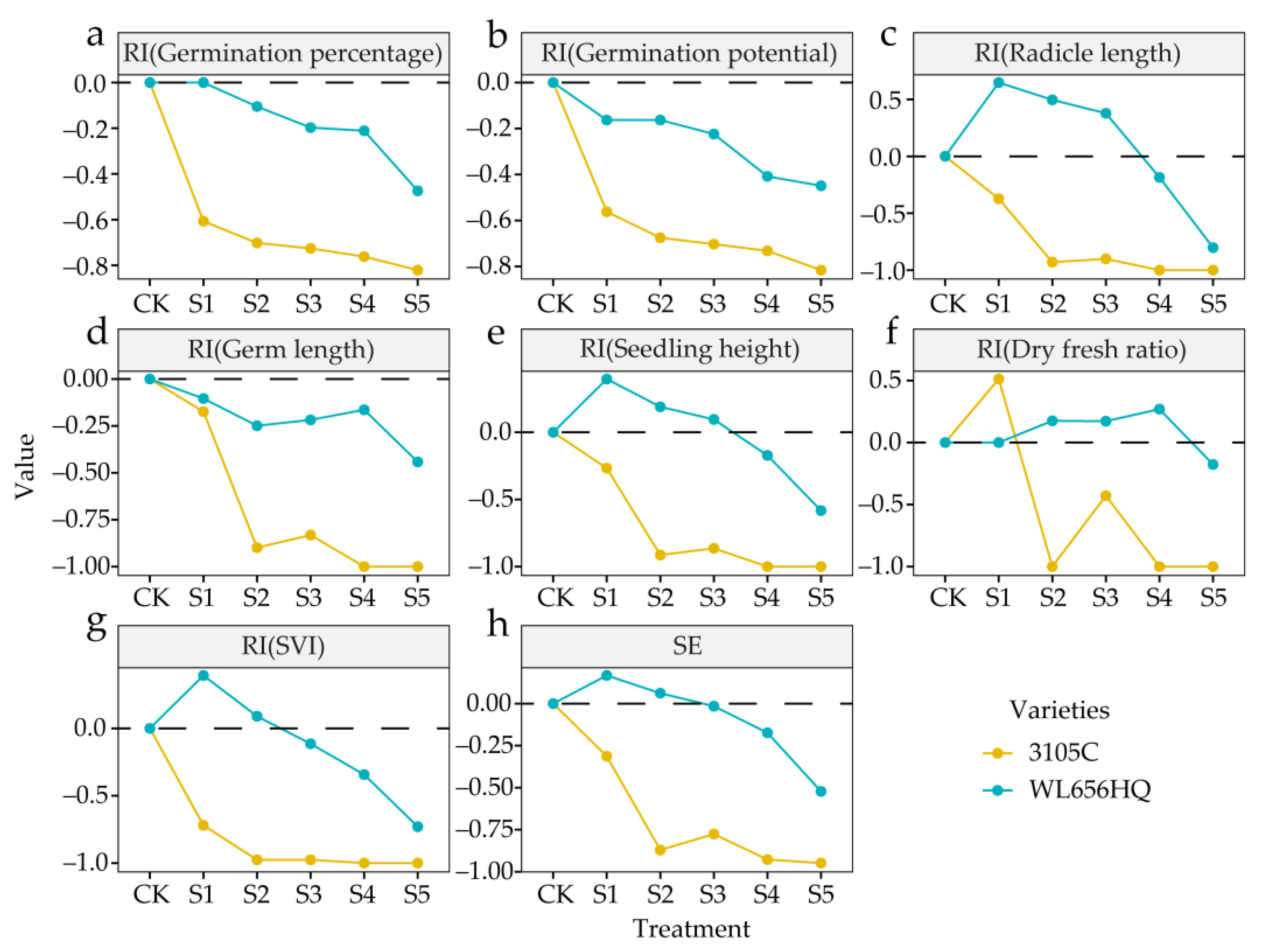
3.1. Effect of Autotoxicity on Seed Germination
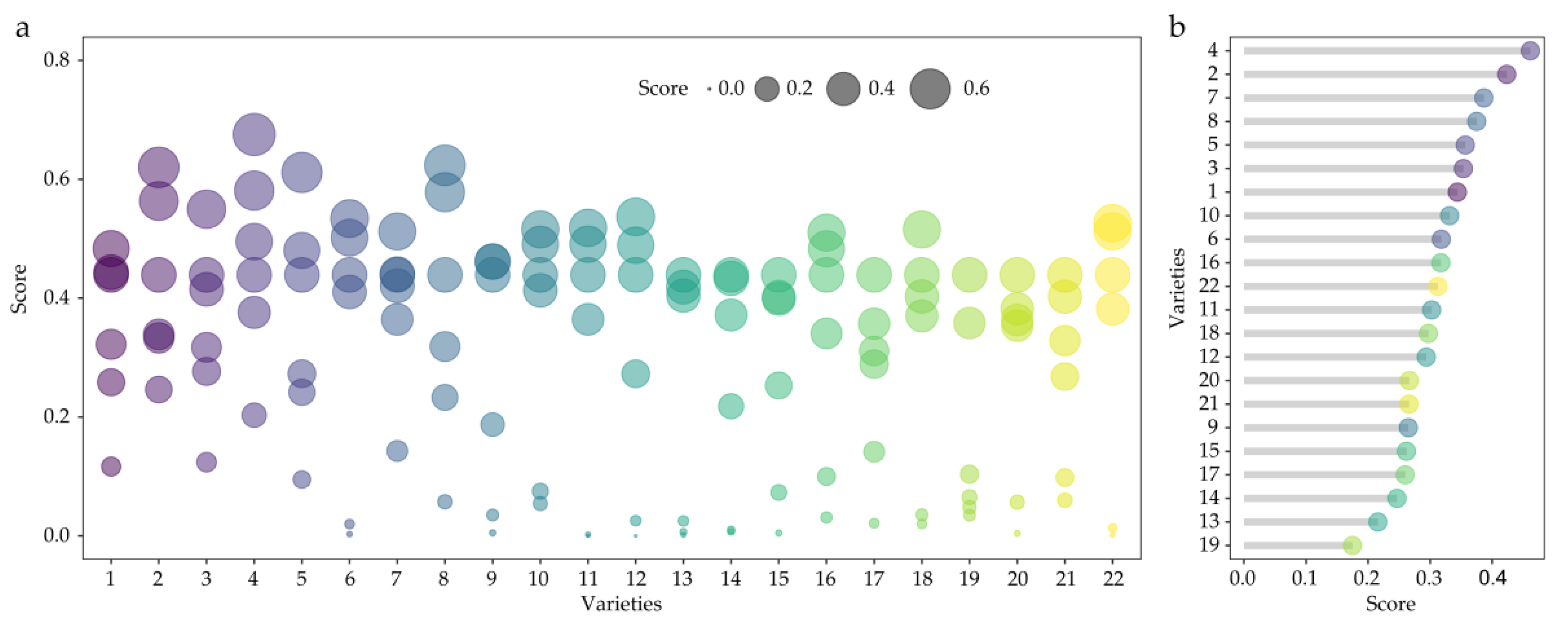
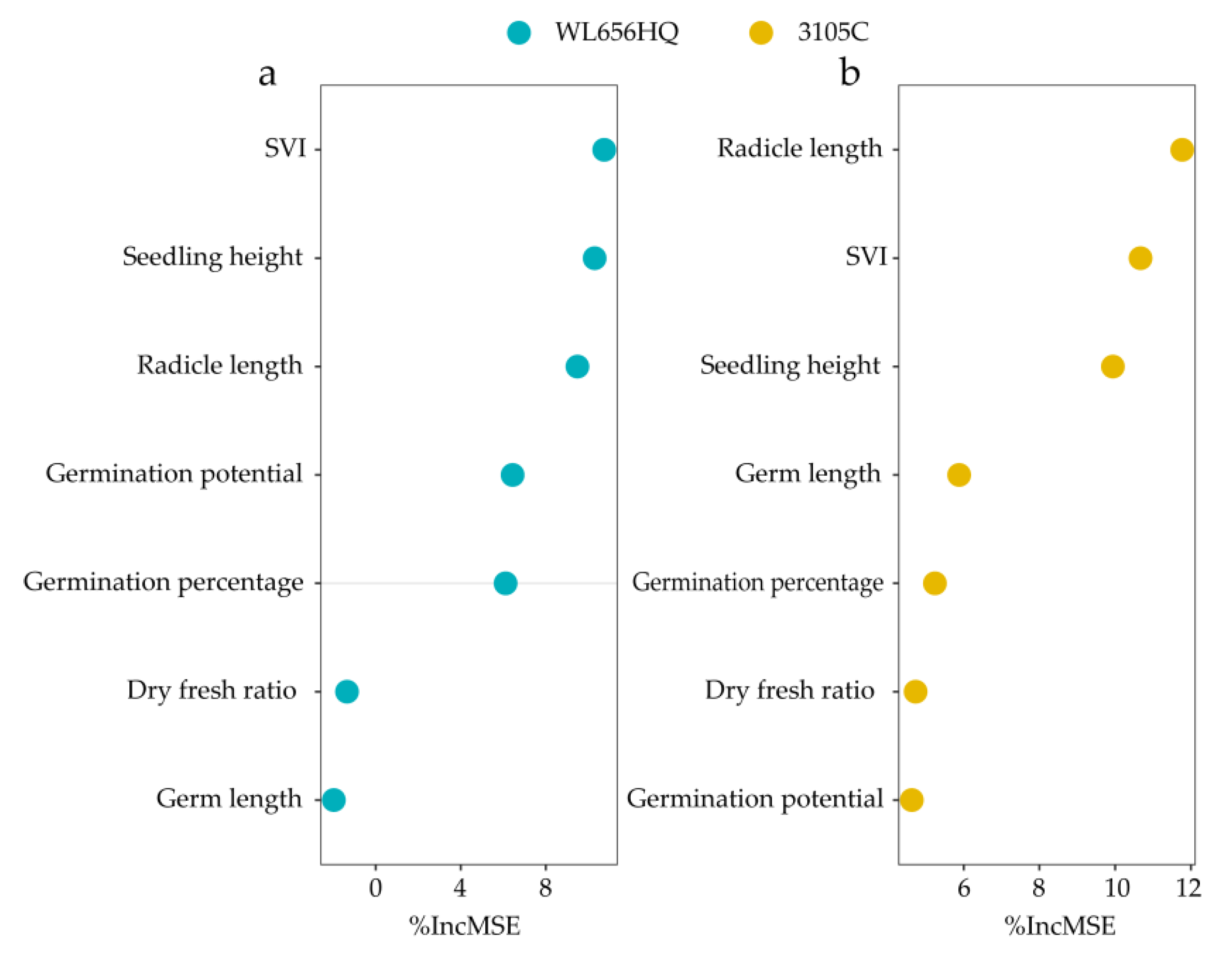
3.1.1. Technique for Order Preference by Similarity to an Ideal Solution (TOPSIS Variety Selection)
3.1.2. Effect of Autotoxicity on Allelopathic Comprehensive Effect Index of Alfalfa
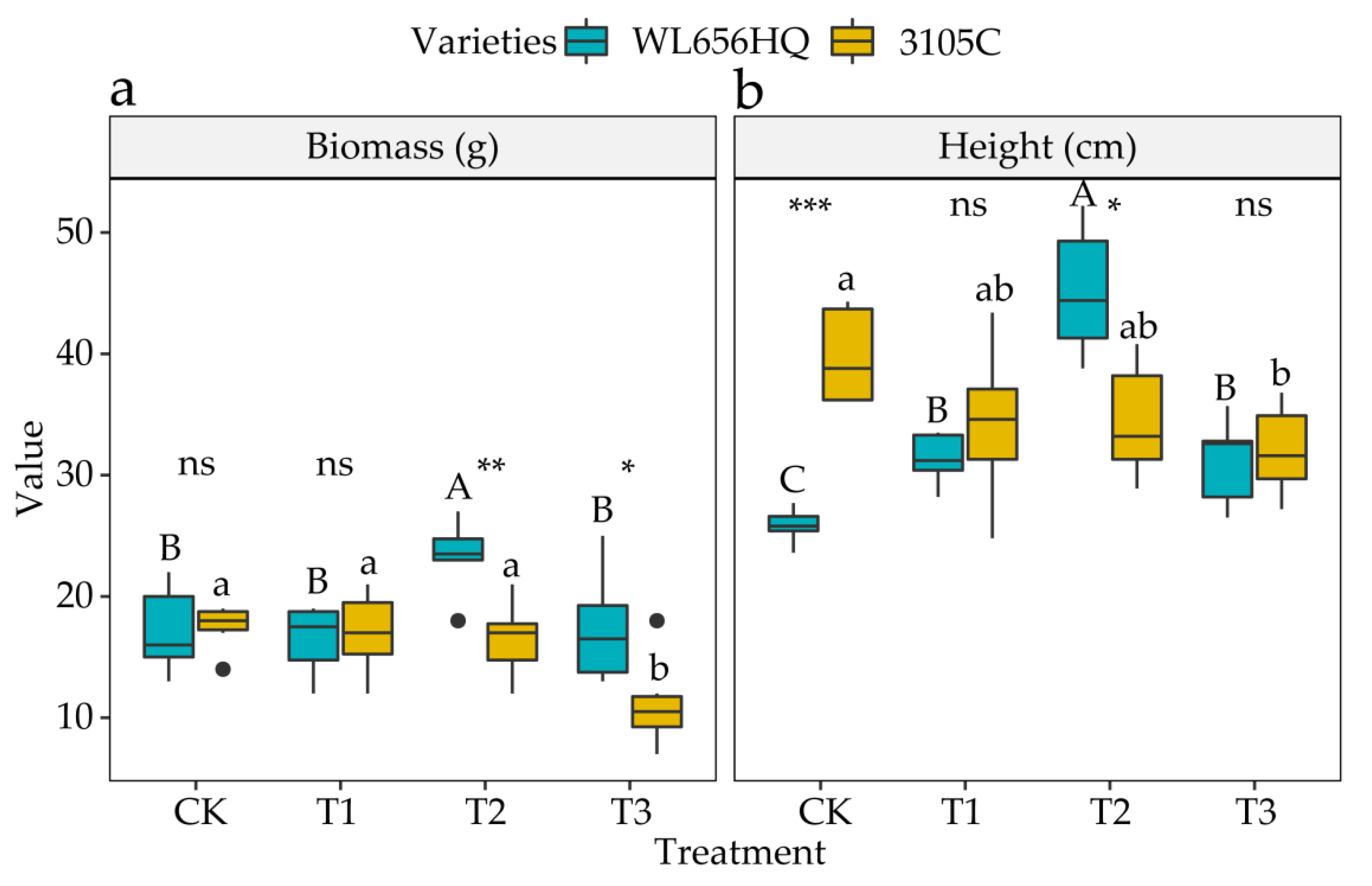
3.2. Effect of Autotoxicity on Aboveground Biomass and Alfalfa Height
3.3. Change of Osmotic Adjustment Substance of Alfalfa Leaves
3.4. Effect of Leaf Extract Autotoxicity on Lipid Peroxidation of Alfalfa
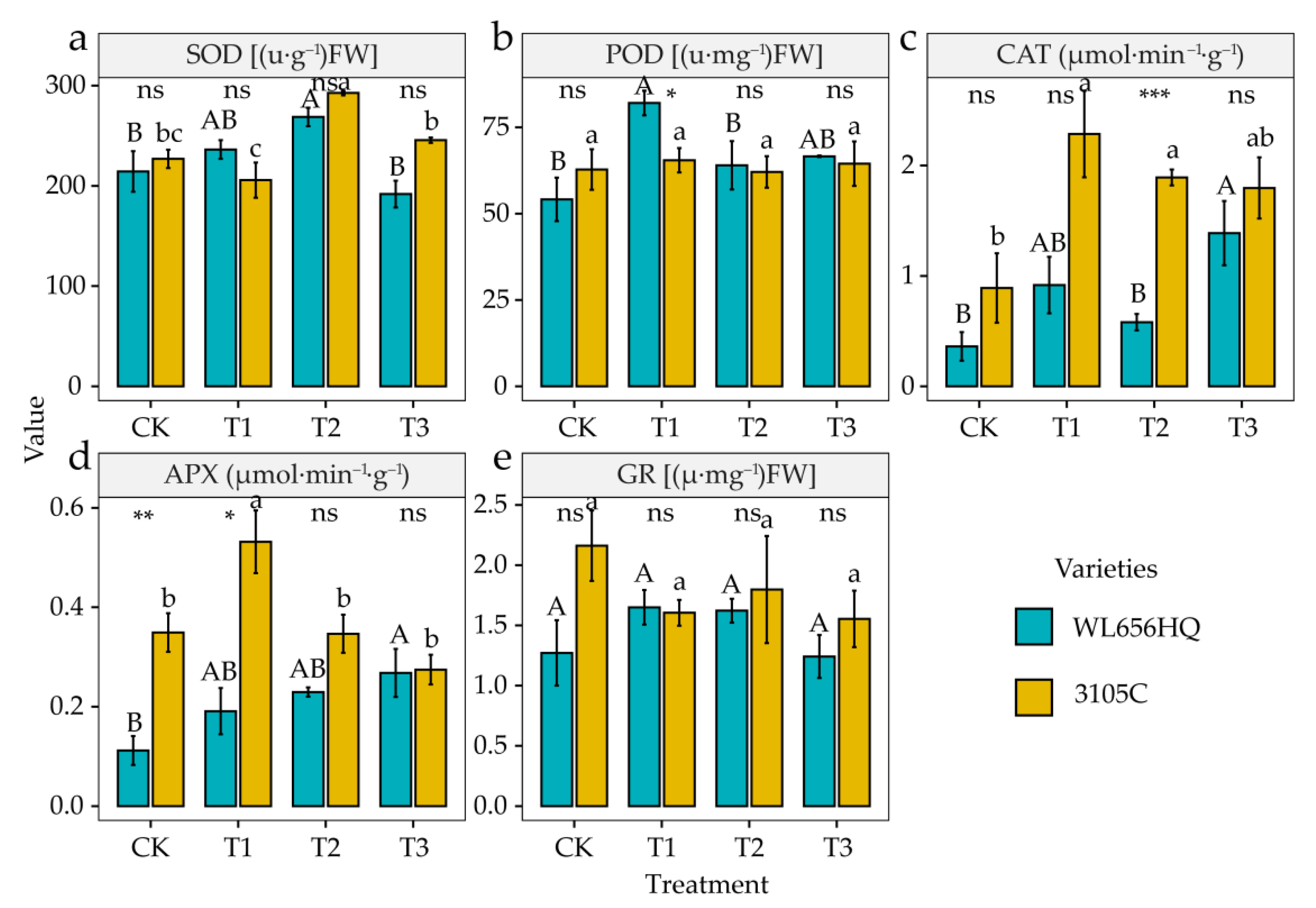
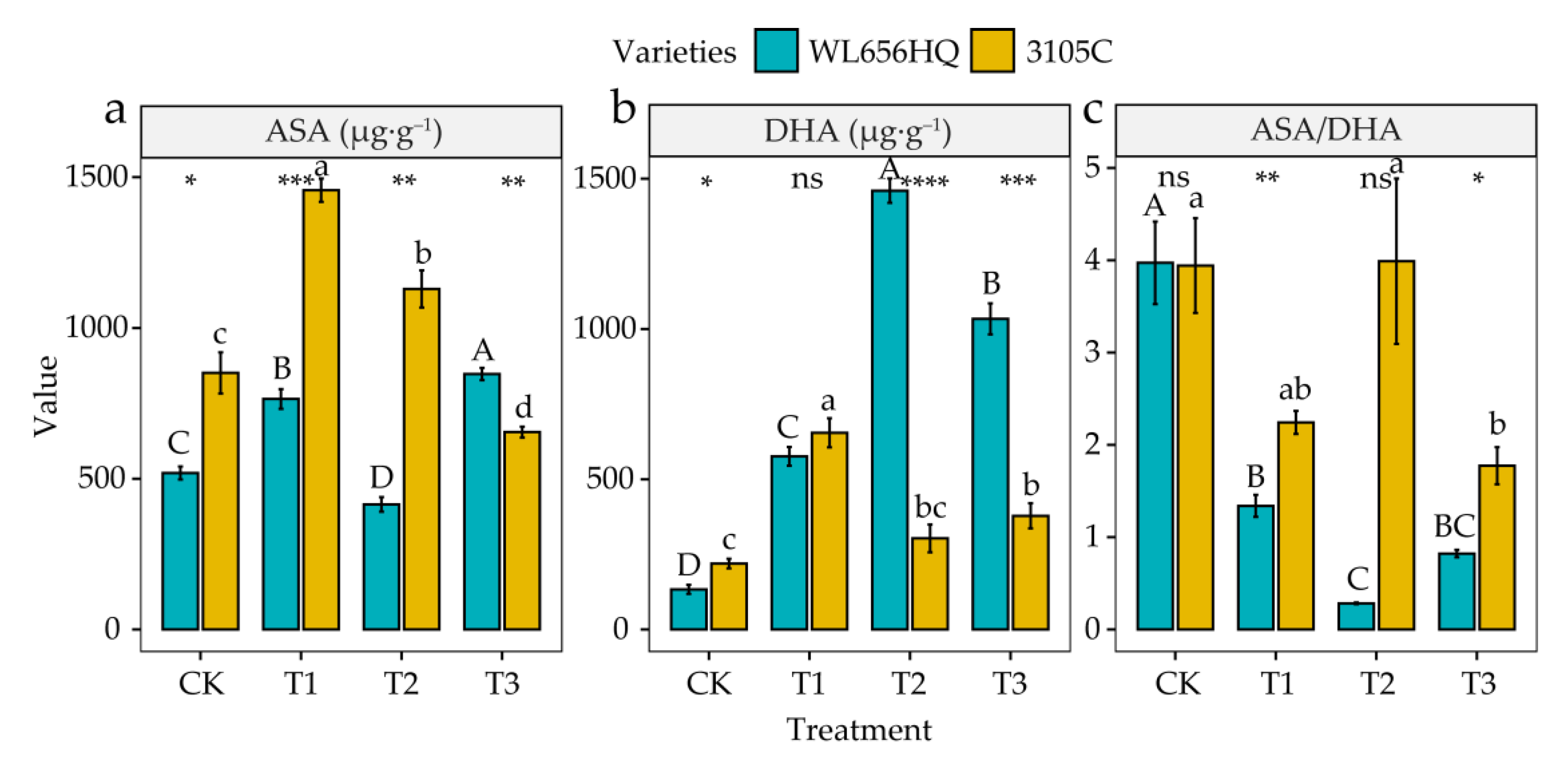
3.5. Alterations in Activities of Antioxidant Enzymes and Contents of Antioxidant
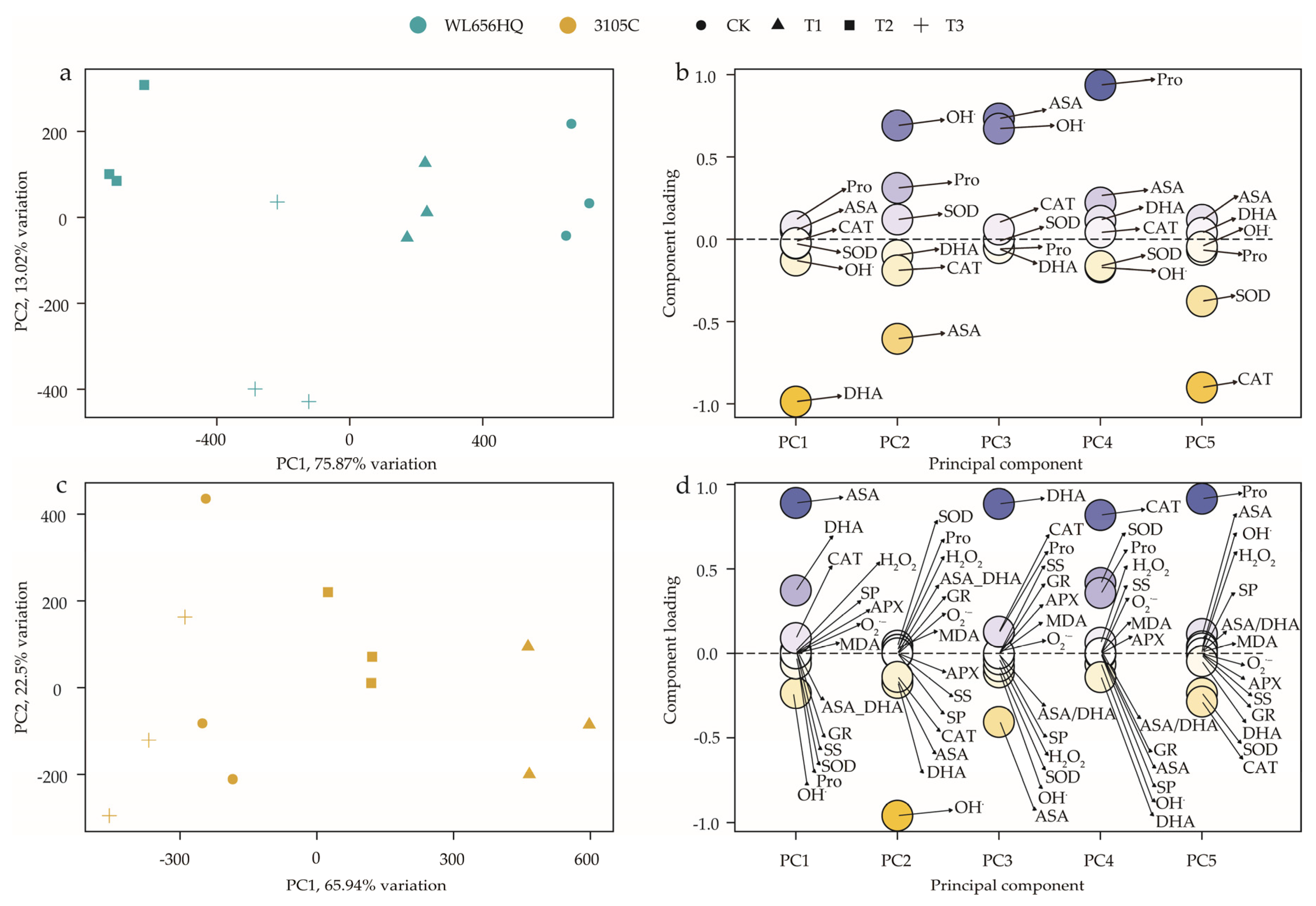
3.6. Principal Component Analysis of the Positive and Negative Contribution Indicators of the Two Varieties
4. Discussion
4.1. Effects of Autotoxicity on Seed Germination
4.2. Effect of Autotoxicity on Aboveground Biomass and Height
4.3. Change of Osmotic Adjustment Substance
4.4. Effect of Autotoxicity on Lipid Peroxidation
4.5. Alterations in Antioxidant Enzyme Activities and Antioxidant Contents
4.6. PCA Analysis of the Positive and Negative Contribution Indicators
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Zhao, Y.; Zhang, R.; Jiang, K.W.; Qi, J.; Hu, Y.; Guo, J.; Zhu, R.; Zhang, T.; Egan, A.N.; Yi, T.S.; et al. Nuclear Phylotranscriptomics/Phylogenomics Support Numerous Polyploidization Events and Hypotheses for the Evolution of Rhizobial Nitrogen-Fixing Symbiosis in Fabaceae. Mol. Plant 2021, 14, 748–773. [Google Scholar] [CrossRef]
- Chen, H.; Zeng, Y.; Yang, Y.; Huang, L.; Tang, B.; Zhang, H.; Hao, F.; Liu, W.; Li, Y.; Liu, Y.; et al. Allele-aware chromosome-level genome assembly and efficient transgene-free genome editing for the autotetraploid cultivated alfalfa. Nat. Commun. 2020, 11, 2494. [Google Scholar] [CrossRef] [PubMed]
- Mehrabi, Z.; Gill, M.; van Wijk, M.; Herrero, M.; Ramankutty, N. Livestock policy for sustainable development. Nat. Food 2020, 1, 160–165. [Google Scholar] [CrossRef]
- Li, J.; Essemine, J.; Shang, C.; Zhang, H.; Zhu, X.; Yu, J.; Chen, G.; Qu, M.; Sun, D. Combined Proteomics and Metabolism Analysis Unravels Prominent Roles of Antioxidant System in the Prevention of Alfalfa (Medicago sativa L.) against Salt Stress. Int. J. Mol. Sci. 2020, 21, 909. [Google Scholar] [CrossRef]
- Ghimire, B.K.; Ghimire, B.; Yu, C.Y.; Chung, I.M. Allelopathic and Autotoxic Effects of Medicago sativa—Derived Allelochemicals. Plants 2019, 8, 233. [Google Scholar] [CrossRef]
- Chung, I.M.; Miller, D.A. Effect of alfalfa plant and soil extracts on germination and growth of alfalfa. Agron. J. 1995, 87, 762–767. [Google Scholar] [CrossRef]
- Miller, D.A. Allelopathy in forage crop systems. Agron. J. 1996, 88, 854–859. [Google Scholar] [CrossRef]
- Singh, H.P.; Batish, D.R.; Kohli, R.K. Autotoxicity: Concept, organisms, and ecological significance. Crit. Rev. Plant Sci. 1999, 18, 757–772. [Google Scholar] [CrossRef]
- Chon, S.U.; Jennings, J.A.; Nelson, C.J. Alfalfa (Medicago sativa L.) autotoxicity: Current status. Allelopath. J. 2006, 18, 57–80. [Google Scholar]
- Wu, B.; Long, Q.; Gao, Y.; Wang, Z.; Shao, T.; Liu, Y.; Li, Y.; Ding, W. Comprehensive characterization of a time-course transcriptional response induced by autotoxins in Panax ginseng using RNA-Seq. BMC Genom. 2015, 16, 1010. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.F.; Song, L.X.; Xia, X.J.; Mao, W.H.; Kai, S.; Zhou, Y.H.; Yu, J.Q. Plant-soil feedbacks and soil sickness: From mechanisms to application in agriculture. J. Chem. Ecol. 2013, 39, 232–242. [Google Scholar] [CrossRef]
- Guong, V.T.; Rosling, A.; Alström, S.; Chai, B.; Högberg, N. Different crop rotation systems as drivers of change in soil bacterial community structure and yield of rice, Oryza sativa. Biol. Fertil. Soils. 2012, 48, 217–225. [Google Scholar]
- Yang, M.; Chuan, Y.; Guo, C.; Liao, J.; Xu, Y.; Mei, X.; Liu, Y.; Huang, H.; He, X.; Zhu, S. Panax notoginseng Root Cell Death Caused by the Autotoxic Ginsenoside Rg1 Is Due to Over-Accumulation of ROS, as Revealed by Transcriptomic and Cellular Approaches. Front. Plant Sci. 2018, 9, 264. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.F.; He, C.L.; Wang, Y.; Li, M.J.; Dai, Y.J.; Wang, T.; Lin, W.X. Enhancement of trichothecene mycotoxins of Fusarium oxysporum by ferulic acid aggravates oxidative damage in Rehmannia glutinosa Libosch. Sci. Rep. 2016, 6, 33962. [Google Scholar] [CrossRef]
- Chon, S.U.; Kim, Y.M. Herbicidal potential and quantification of suspected allelochemicals from four grass crop extracts. J. Agron. Crop Sci. 2010, 190, 145–150. [Google Scholar] [CrossRef]
- Chung, I.M.; Seigler, D.; Miller, D.A.; Kyung, S.H. Autotoxic Compounds from Fresh Alfalfa Leaf Extracts: Identification and Biological Activity. J. Chem. Ecol. 2000, 26, 315–327. [Google Scholar] [CrossRef]
- Zhang, C.M.; Shi, S.L. Physiological and Proteomic Responses of Contrasting Alfalfa (Medicago sativa L.) Varieties to PEG-Induced Osmotic Stress. Front. Plant Sci. 2018, 9, 242. [Google Scholar] [CrossRef]
- Wang, R.L.; Liu, S.W.; Xin, X.W.; Chen, S.; Peng, G.X.; Su, Y.J.; Song, Z.K. Phenolic acids contents and allelopathic potential of 10-cultivars of alfalfa and their bioactivity. Allelopath. J. 2017, 40, 63–70. [Google Scholar] [CrossRef]
- Chon, S.U.; Nelson, C.J. Effects of experimental procedures and conditions on bioassay sensitivity of alfalfa autotoxicity. Commun. Soil Sci. Plant Anal. 2001, 32, 1607–1619. [Google Scholar] [CrossRef]
- International Seed Testing Association (ISTA). International Rules for Seed Testing; ISTA Press: Basserdorf, Switzerland, 2012. [Google Scholar]
- Williamson, G.B.; Richardson, D. Bioassays for allelopathy: Measuring treatment responses with independent controls. J. Chem. Ecol. 1988, 14, 181–187. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The water culture method for growing plants without soil. Circ. Calif. Agric. Exp. Stn. 1950, 347, 357–359. [Google Scholar]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Buysse, J.; Merckx, R. An improve colorimetric method to quantify sugar content of plant tissue. J. Exp. Bot. 1993, 44, 1627–1629. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid Determination of Free Proline for Water-Stress Studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Draper, H.H.; Hadley, M. Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol. 1990, 186, 421–431. [Google Scholar] [PubMed]
- Willekens, H. Catalase is a sink for H2O2 and is indispensable for stress defence in C3 plants. Eur. Mol. Biol. Organ. J. 1997, 16, 4806–4816. [Google Scholar] [CrossRef]
- Liu, Y.J.; Zhao, Z.G.; Si, J.; Di, C.X.; Han, J.; An, L.Z. Brassinosteroids alleviate chilling-induced oxidative damage by enhancing antioxidant defense system in suspension cultured cells of Chorispora bungeana. Plant Growth Regul. 2009, 59, 207–214. [Google Scholar] [CrossRef]
- Elstner, E.F.; Heupel, A. Formation of hydrogen peroxide by isolated cell walls from horseradish (Armoracia lapathifolia Gilib.). Planta 1976, 130, 175–180. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Chance, B.; Maehly, A.C. Assay of catalase and peroxidases. Methods Enzymol. 1995, 2, 764–775. [Google Scholar]
- Havir, E.A.; Mchale, N.A. Biochemical and developmental characterization of multiple forms of catalase in tobacco leaves. Plant Physiol. 1987, 84, 450–455. [Google Scholar] [CrossRef]
- Murshed, R.; Lopezlauri, F.; Sallanon, H. Microplate quantification of enzymes of the plant ascorbate-glutathione cycle. Anal. Biochem. 2008, 383, 320–322. [Google Scholar] [CrossRef]
- Murshed, R.; Lopezlauri, F.; Sallanon, H. Effect of water stress on antioxidant systems and oxidative parameters in fruits of tomato (Solanumlycopersicon L cv. Micro-tom). Physiol. Mol. Biol. Plants 2013, 19, 363–378. [Google Scholar] [CrossRef] [PubMed]
- Tzeng, G.H.; Huang, J.J. Multiple Attributes Decision Making: Methods and Applications; Lecture Notes in Economics & Mathematical Systems; Springer: Berlin/Heidelberg, Germany, 2011; Volume 404, pp. 287–288. [Google Scholar]
- Amado, P.A.; Castro, A.H.F.; Zanuncio, V.S.S.; Stein, V.C.; da Silva, D.B.; dos Santos Lima, L.A.R. Assessment of allelopathic, cytotoxic, genotoxic and antigenotoxic potential of Smilax brasiliensis Sprengel leaves. Ecotoxicol. Environ. Saf. 2020, 192, 110310. [Google Scholar] [CrossRef] [PubMed]
- Dayan, F.E.; Romagni, J.G.; Duke, S.O. Investigating the Mode of Action of Natural Phytotoxins. J. Chem. Ecol. 2000, 26, 2079–2094. [Google Scholar] [CrossRef]
- Ladhari, A.; Gaaliche, B.; Zarrelli, A.; Ghannem, M.; Mimoun, M.B. Allelopathic potential and phenolic allelochemicals discrepancies in Ficus carica L. cultivars. S. Afr. J. Bot. 2020, 130, 30–44. [Google Scholar] [CrossRef]
- Chon, S.U.; Choi, S.K.; Jung, S.; Jang, H.G.; Pyo, B.S.; Kim, S.M. Effects of alfalfa leaf extracts and phenolic allelochemicals on early seedling growth and root morphology of alfalfa and barnyard grass. Crop Prot. 2002, 21, 1077–1082. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, X.; Xu, Y.; Wu, H.F.; Yu, J.B.; Xia, C.H. Autotoxic Ginsenosides in the Rhizosphere Contribute to the Replant Failure of Panax notoginseng. PLoS ONE. 2015, 10, e0118555. [Google Scholar] [CrossRef] [PubMed]
- Chi, W.C.; Chen, Y.A.; Hsiung, Y.C.; Fu, S.F.; Chou, C.H.; Trinh, N.N.; Chen, Y.C.; Huang, H.J. Autotoxicity mechanism of Oryza sativa: Transcriptome response in rice roots exposed to ferulic acid. BMC Genom. 2013, 14, 351. [Google Scholar] [CrossRef]
- Zhang, C.M.; Shi, S.L.; Liu, Z.; Yang, F. Drought tolerance in alfalfa (Medicago sativa L.) varieties is associated with enhanced antioxidative protection and declined lipid peroxidation. J. Plant Physiol. 2019, 232, 226–240. [Google Scholar] [CrossRef]
- Yu, J.; Sun, Y.; Zhang, Y.; Ding, J.; Xia, X.; Xiao, C.; Shi, K.; Zhou, Y. Selective trans-Cinnamic Acid Uptake Impairs [Ca2⁺](cyt) Homeostasis and Growth in Cucumis sativus L. J. Chem. Ecol. 2009, 35, 1471–1477. [Google Scholar] [CrossRef]
- Yang, C.Y.; Liu, S.J.; Zhou, S.W.; Wu, H.F.; Yu, J.B.; Xia, C.H. Allelochemical ethyl 2-methyl acetoacetate (EMA) induces oxidative damage and antioxidant responses in Phaeodactylum tricornutum. Pestic. Biochem. Physiol. 2011, 100, 93–103. [Google Scholar] [CrossRef]
- Lara-Nuñez, A.U.R.O.R.A.; Romero-Romero, T.E.R.E.S.A.; Ventura, J.L.; Blancas, V.; Anaya, A.L.; Cruz-Ortega, R.O.C.I.O. Allelochemical stress causes inhibition of growth and oxidative damage in Lycopersicon esculentum Mill. Plant Cell Environ. 2006, 29, 2009–2016. [Google Scholar] [CrossRef]
- Li, Q.; Yu, B.; Gao, Y.; Dai, A.H.; Bai, J.G. Cinnamic acid pretreatment mitigates chilling stress of cucumber leaves through altering antioxidant enzyme activity. J. Plant Physiol. 2011, 168, 927–934. [Google Scholar] [CrossRef]
- Ye, S.F.; Zhou, Y.H.; Sun, Y.; Zou, L.Y.; Yu, J.Q. Cinnamic acid causes oxidative stress in cucumber roots, and promotes incidence of Fusarium wilt. Environ. Exp. Bot. 2006, 56, 255–262. [Google Scholar] [CrossRef]
- Kapoor, D.; Singh, S.; Kumar, V.; Romero, R.; Prasad, R.; Singh, J. Antioxidant enzymes regulation in plants in reference to reactive oxygen species (ROS) and reactive nitrogen species (RNS). Plant Gene 2019, 19. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Bai, R.; Ma, F.; Liang, D.; Zhao, X. Phthalic Acid Induces Oxidative Stress and Alters the Activity of Some Antioxidant Enzymes in Roots of Malus prunifolia. J. Chem. Ecol. 2009, 35, 488–494. [Google Scholar] [CrossRef]


| Number | Alfalfa Varieties | Number | Alfalfa Varieties |
|---|---|---|---|
| 1 | WL-298HQ | 12 | SG-201 |
| 2 | WL-319 | 13 | Debao |
| 3 | WL-354HQ | 14 | Longdong |
| 4 | WL-656HQ | 15 | Jiasheng |
| 5 | WL-525 | 16 | Canon 429 |
| 6 | WL343HQ | 17 | 329 Wonder |
| 7 | WL-363HQ | 18 | Sanduli |
| 8 | WL-168HQ | 19 | 3105C |
| 9 | SG-601 | 20 | 218TR |
| 10 | SG-501 | 21 | 416WET |
| 11 | SG-401 | 22 | 420Ya |
| Enzymes | Buffer (PBS) | Reaction System | Measuring Wavelength | Reference |
|---|---|---|---|---|
| SOD | 0.1 mM EDTA 1% PVP pH7.8 | 50 mM PBS; 100 μL enzyme solution; 100 μL riboflavin | 560 nm | Giannopolitis et al., 1977 [30] |
| POD | 0.1 mM EDTA 1% PVP pH7.8 | 100 mM PBS; 20 mM guaiacol; 40 mM H2O2 | 470 nm | Chance et al., 1955 [31] |
| CAT | 0.1 mM EDTA 1% PVP pH7.8 | 50 mM PBS; 50 μL enzyme solution; 19 mM H2O2 | 240 nm | Havir et al., 1987 [32] |
| APX | - | 50 mM PBS; 0.25 mM AsA; 0.1 mM EDTA; 5 mM H2O2 | 290 nm | Murshed et al., 2008 [33] |
| GR | - | 50 mM PBS; 0.5 mM EDTA; 0.25 mM NADPH; 0.5 mM GSSG | 340 nm | Murshed et al., 2008 [30] |
| ASA plus DHA | 150 mM EDTA; 10 mM DTT; 0.5% (w/v) N-ethylmaleimide | 10% TCA; 44% orthophosphoric acid; 0.5% | 525 nm | Murshed et al., 2013 [34] |
| ASA | 150 mM EDTA; 0.3 mL water | BP-ethanol; 0.3% (w/v) FeCl3 | 525 nm | Murshed et al., 2013 [34] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.-Y.; Shi, S.-L.; Li, X.-L.; Li, C.-N.; Zhang, C.-M.; A, Y.; Kang, W.-J.; Yin, G.-L. Effects of Autotoxicity on Alfalfa (Medicago sativa): Seed Germination, Oxidative Damage and Lipid Peroxidation of Seedlings. Agronomy 2021, 11, 1027. https://doi.org/10.3390/agronomy11061027
Zhang X-Y, Shi S-L, Li X-L, Li C-N, Zhang C-M, A Y, Kang W-J, Yin G-L. Effects of Autotoxicity on Alfalfa (Medicago sativa): Seed Germination, Oxidative Damage and Lipid Peroxidation of Seedlings. Agronomy. 2021; 11(6):1027. https://doi.org/10.3390/agronomy11061027
Chicago/Turabian StyleZhang, Xiao-Yan, Shang-Li Shi, Xiao-Long Li, Chang-Ning Li, Cui-Mei Zhang, Yun A, Wen-Juan Kang, and Guo-Li Yin. 2021. "Effects of Autotoxicity on Alfalfa (Medicago sativa): Seed Germination, Oxidative Damage and Lipid Peroxidation of Seedlings" Agronomy 11, no. 6: 1027. https://doi.org/10.3390/agronomy11061027
APA StyleZhang, X.-Y., Shi, S.-L., Li, X.-L., Li, C.-N., Zhang, C.-M., A, Y., Kang, W.-J., & Yin, G.-L. (2021). Effects of Autotoxicity on Alfalfa (Medicago sativa): Seed Germination, Oxidative Damage and Lipid Peroxidation of Seedlings. Agronomy, 11(6), 1027. https://doi.org/10.3390/agronomy11061027





