Enhancing Irrigation Salinity Stress Tolerance and Increasing Yield in Tomato Using a Precision Engineered Protein Hydrolysate and Ascophyllum nodosum-Derived Biostimulant
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Chemical and Structural Characterisation of Biostimulant Treatment PSI-475
2.3. Evaluation of Salinity Stress Tolerance in Arabidopsis Thaliana
2.4. Tomato Material and Growth Conditions
2.5. Salinity Stress Tolerance Experimental Design in Tomato
2.6. Determination of Electrical Conductivity in Tomato Growth Medium
2.7. Evaluation of Tomato Plant Phenotypic Markers
2.8. Evaluation of Tomato Fruit Yield and Quality Parameters
2.9. Tomato Leaf Ion Content Analysis
2.10. Tomato RNA Extraction and RT-qPCR
2.11. Tomato Leaf RWC
2.12. Tomato Leaf Proline and Soluble Sugars Analysis Determination
2.13. Tomato Leaf Photosynthetic Pigments and Soluble Protein Content Analysis
2.14. Statistical Analysis
3. Results
3.1. Chemical and Structural Characterisation of PSI-475
3.2. Effect of Salinity Stress and PSI-475 on Arabidopsis Thaliana
3.3. Effect of Salinity and PSI-475 on Tomato Phenotypic, Fruit Yield and Fruit Quality Parameters
3.4. Effect of Salinity and PSI-475 on Tomato Leaf Sodium and Potassium Content
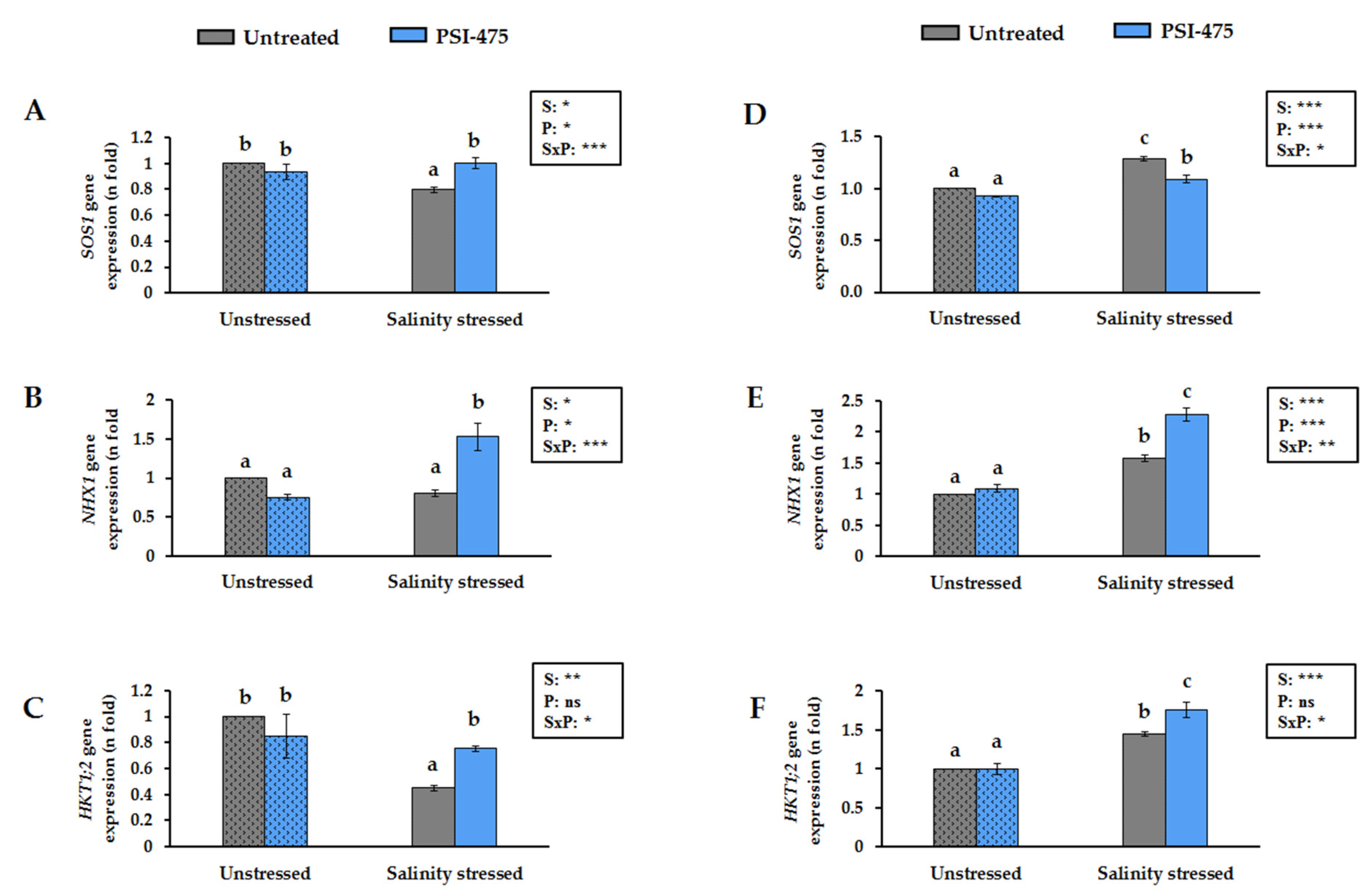
3.5. Effect of Salinity and PSI-475 on Tomato Leaf SOS1, NHX1 and HKT1;2 Gene Expression Levels
3.6. Effect of Salinity and PSI-475 on Tomato Leaf RWC, Proline, and Soluble Sugars Content
3.7. Effect of Salinity and PSI-475 on Tomato Leaf Soluble Protein and Photosynthetic Pigments
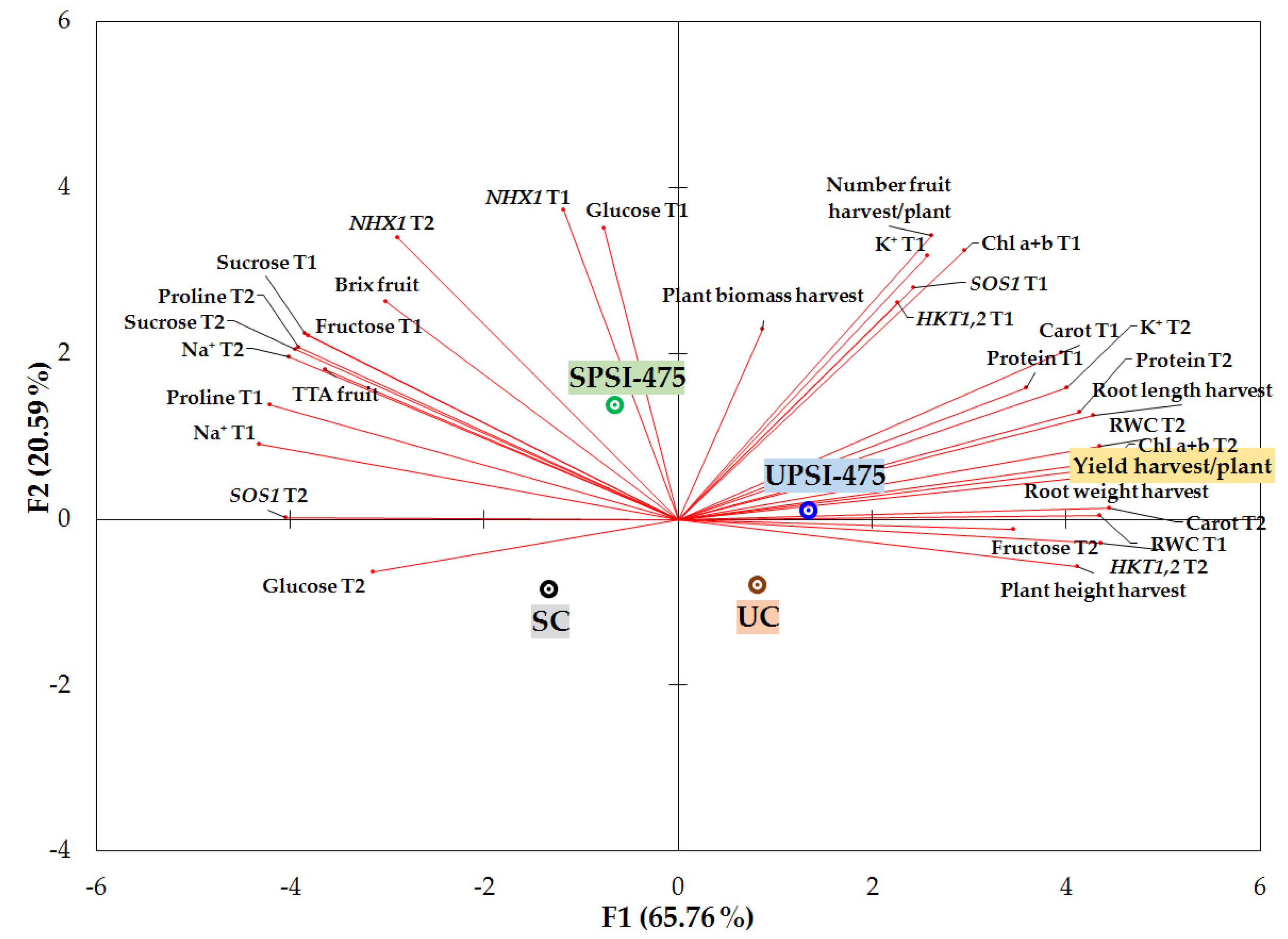
3.8. PCA Evaluation
4. Discussion
4.1. Impact of PSI-475 and Salinity Stress on Phenotypical, Yield, and Quality-Related Markers
4.2. Impact of PSI-475 and Salinity Stress on Physiological, Biochemical, and Molecular Markers
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Machado, R.; Serralheiro, R. Soil salinity: Effect on vegetable crop growth. Management practices to prevent and mitigate soil salinisation. Horticulturae 2017, 3, 30. [Google Scholar] [CrossRef]
- Ivushkin, K.; Bartholomeus, H.; Bregt, A.K.; Pulatov, A.; Kempen, B.; De Sousa, L. Global mapping of soil salinity change. Remote Sens. Environ. 2019, 231, 111260. [Google Scholar] [CrossRef]
- Shahid, S.A.; Zaman, M.; Heng, L. Soil salinity: Historical perspectives and a world overview of the problem. In Guideline for Salinity Assessment, Mitigation and Adaptation Using Nuclear and Related Techniques; Springer: Cham, Switzerland, 2018; pp. 43–53. [Google Scholar] [CrossRef] [Green Version]
- Corwin, D.L. Climate change impacts on soil salinity in agricultural areas. Eur. J. Soil Sci. 2021, 72, 842–862. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [Green Version]
- Choi, W.G.; Toyota, M.; Kim, S.H.; Hilleary, R.; Gilroy, S. Salt stress-induced Ca2+ waves are associated with rapid, long-distance root-to-shoot signaling in plants. Proc. Natl. Acad. Sci. USA 2014, 111, 6497–6502. [Google Scholar] [CrossRef] [Green Version]
- Negrão, S.; Schmöckel, S.M.; Tester, M. Evaluating physiological responses of plants to salinity stress. Ann. Bot. 2017, 119, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Isayenkov, S.; Maathuis, F.J.M. Plant Salinity Stress; Many Unanswered Questions Remain. Front. Plant Sci. 2019, 10, 80. [Google Scholar] [CrossRef] [Green Version]
- Shahid, M.A.; Sarkhosh, A.; Khan, N.; Balal, R.M.; Ali, S.; Rossi, L.; Gómez, C.; Mattson, N.; Nasim, W.; Garcia-Sanchez, F. Insights into the physiological and biochemical impacts of salt stress on plant growth and development. Agronomy 2020, 10, 938. [Google Scholar] [CrossRef]
- Van Zelm, E.; Zhang, Y.; Testerink, C. Salt tolerance mechanisms of plants. Annu. Rev. Plant Biol. 2020, 71, 403–433. [Google Scholar] [CrossRef] [Green Version]
- Panta, S.; Flowers, T.; Lane, P.; Doyle, R.; Haros, G.; Shabala, S. Halophyte agriculture: Success stories. Environ. Exp. Bot. 2014, 107, 71–83. [Google Scholar] [CrossRef]
- Qadir, M.; Quillérou, E.; Nangia, V.; Murtaza, G.; Singh, M.; Thomas, R.J.; Drechsel, P.; Noble, A.D. Economics of salt-induced land degradation and restoration. Nat. Resour. Forum 2014, 38, 282–295. [Google Scholar] [CrossRef]
- Zörb, C.; Geilfus, C.M.; Dietz, K.J. Salinity and crop yield. Plant Biol. 2019, 21, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Ondrasek, G.; Rengel, Z.; Veres, S. Soil salinisation and salt stress in crop production. In Abiotic Stress in Plants. Mechanisms and Adaptation; IntechOpen: London, UK, 2011; pp. 171–190. [Google Scholar]
- Ashraf, M.; Akram, N.A. Improving salinity tolerance of plants through conventional breeding and genetic engineering: An analytical comparison. Biotechnol. Adv. 2009, 27, 744–752. [Google Scholar] [CrossRef] [PubMed]
- García-Abellan, J.O.; Egea, I.; Pineda, B.; Sanchez-Bel, P.; Belver, A.; Garcia-Sogo, B.; Flores, F.B.; Atares, A.; Moreno, V.; Bolarin, M.C. Heterologous expression of the yeast HAL5 gene in tomato enhances salt tolerance by reducing shoot Na+ accumulation in the long term. Physiol. Plant. 2014, 152, 700–713. [Google Scholar] [CrossRef]
- Jaime-Pérez, N.; Pineda, B.; García-Sogo, B.; Atares, A.; Athman, A.; Byrt, C.S.; Olías, R.; Asins, M.J.; Gilliham, M.; Moreno, V.; et al. The sodium transporter encoded by the HKT1; 2 gene modulates sodium/potassium homeostasis in tomato shoots under salinity. Plant Cell Environ. 2017, 40, 658–671. [Google Scholar] [CrossRef] [Green Version]
- Ali, A.; Khan, I.U.; Jan, M.; Khan, H.A.; Hussain, S.; Nisar, M.; Chung, W.S.; Yun, D.J. The high-affinity potassium transporter EpHKT1; 2 from the extremophile Eutrema parvula mediates salt tolerance. Front. Plant Sci. 2018, 9, 1108. [Google Scholar] [CrossRef] [Green Version]
- Olías, R.; Eljakaoui, Z.; Li, J.U.N.; De Morales, P.A.; Marín-Manzano, M.C.; Pardo, J.M.; Belver, A. The plasma membrane Na+/H+ antiporter SOS1 is essential for salt tolerance in tomato and affects the partitioning of Na+ between plant organs. Plant Cell Environ. 2009, 32, 904–916. [Google Scholar] [CrossRef]
- Leidi, E.O.; Barragán, V.; Rubio, L.; El-Hamdaoui, A.; Ruiz, M.T.; Cubero, B.; Fernández, J.A.; Bressan, R.A.; Hasegawa, P.M.; Quintero, F.J.; et al. The AtNHX1 exchanger mediates potassium compartmentation in vacuoles of transgenic tomato. Plant J. 2010, 61, 495–506. [Google Scholar] [CrossRef] [Green Version]
- Salava, H.; Thula, S.; Mohan, V.; Kumar, R.; Maghuly, F. Application of Genome Editing in Tomato Breeding: Mechanisms, Advances, and Prospects. Int. J. Mol. Sci. 2021, 22, 682. [Google Scholar] [CrossRef]
- du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef] [Green Version]
- Research and Markets. Agriculture Biostimulant-Global Market Outlook (2019–2027); Research and Markets; European Union: Maastricht, The Netherlands, 2020. [Google Scholar]
- Regulation (EU) 2019/1009. 2019. Available online: https://eur-lex.europa.eu/eli/reg/2019/1009/oj (accessed on 22 September 2021).
- Colla, G.; Nardi, S.; Cardarelli, M.; Ertani, A.; Lucini, L.; Canaguier, R.; Rouphael, Y. Protein hydrolysates as biostimulants in horticulture. Sci. Hortic. 2015, 196, 28–38. [Google Scholar] [CrossRef]
- Colla, G.; Hoagland, L.; Ruzzi, M.; Cardarelli, M.; Bonini, P.; Canaguier, R.; Rouphael, Y. Biostimulant action of protein hydrolysates: Unraveling their effects on plant physiology and microbiome. Front. Plant Sci. 2017, 8, 2202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shukla, P.S.; Mantin, E.G.; Adil, M.; Bajpai, S.; Critchley, A.T.; Prithiviraj, B. Ascophyllum nodosum-based biostimulants: Sustainable applications in agriculture for the stimulation of plant growth, stress tolerance, and disease management. Front. Plant Sci. 2019, 10, 655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Łangowski, Ł.; Goñi, O.; Marques, F.S.; Hamawaki, O.T.; da Silva, C.O.; Nogueira, A.P.O.; Teixeira, M.A.J.; Glasenapp, J.S.; Pereira, M.; O’Connell, S. Ascophyllum nodosum Extract (SealicitTM) Boosts Soybean Yield Through Reduction of Pod Shattering-Related Seed Loss and Enhanced Seed Production. Front. Plant Sci. 2021, 12, 176. [Google Scholar] [CrossRef] [PubMed]
- Goñi, O.; Łangowski, Ł.; Feeney, E.; Quille, P.; O’Connell, S. Reducing Nitrogen Input in Barley Crops While Maintaining Yields Using an Engineered Biostimulant Derived from Ascophyllum nodosum to Enhance Nitrogen Use Efficiency. Front. Plant Sci. 2021, 12, 789. [Google Scholar] [CrossRef] [PubMed]
- Carmody, N.; Goñi, O.; Łangowski, Ł.; O’Connell, S. Ascophyllum nodosum extract biostimulant processing and its impact on enhancing heat stress tolerance during tomato fruit set. Front. Plant Sci. 2020, 11, 807. [Google Scholar] [CrossRef] [PubMed]
- Paul, K.; Sorrentino, M.; Lucini, L.; Rouphael, Y.; Cardarelli, M.; Bonini, P.; Miras Moreno, M.B.; Reynaud, H.; Canaguier, R.; Trtílek, M.; et al. A combined phenotypic and metabolomic approach for elucidating the biostimulant action of a plant-derived protein hydrolysate on tomato grown under limited water availability. Front. Plant Sci. 2019, 10, 493. [Google Scholar] [CrossRef]
- Goñi, O.; Quille, P.; O’Connell, S. Ascophyllum nodosum extract biostimulants and their role in enhancing tolerance to drought stress in tomato plants. Plant Physiol. Biochem. 2018, 126, 63–73. [Google Scholar] [CrossRef]
- Ertani, A.; Schiavon, M.; Muscolo, A.; Nardi, S. Alfalfa plant-derived biostimulant stimulate short-term growth of salt stressed Zea mays L. plants. Plant Soil 2013, 364, 145–158. [Google Scholar] [CrossRef]
- Lucini, L.; Rouphael, Y.; Cardarelli, M.; Canaguier, R.; Kumar, P.; Colla, G. The effect of a plant-derived biostimulant on metabolic profiling and crop performance of lettuce grown under saline conditions. Sci. Hortic. 2015, 182, 124–133. [Google Scholar] [CrossRef]
- Trevisan, S.; Manoli, A.; Quaggiotti, S. A Novel Biostimulant, Belonging to Protein Hydrolysates, Mitigates Abiotic Stress Effects on Maize Seedlings Grown in Hydroponics. Agronomy 2019, 9, 28. [Google Scholar] [CrossRef] [Green Version]
- Sitohy, M.Z.; Desoky, E.S.M.; Osman, A.; Rady, M.M. Pumpkin seed protein hydrolysate treatment alleviates salt stress effects on Phaseolus vulgaris by elevating antioxidant capacity and recovering ion homeostasis. Sci. Hortic. 2020, 271, 109495. [Google Scholar] [CrossRef]
- Carillo, P.; Ciarmiello, L.F.; Woodrow, P.; Corrado, G.; Chiaiese, P.; Rouphael, Y. Enhancing sustainability by improving plant salt tolerance through macro-and micro-algal biostimulants. Biology 2020, 9, 253. [Google Scholar] [CrossRef] [PubMed]
- FAOSTAT. 2020. Available online: http://www.fao.org/faostat/en/#data (accessed on 22 September 2021).
- Cuartero, J.; Fernández-Muñoz, R. Tomato and salinity. Sci. Hortic. 1998, 78, 83–125. [Google Scholar] [CrossRef]
- Campos, C.A.B.; Fernandes, P.D.; Gheyi, H.R.; Blanco, F.F.; Gonçalves, C.B.; Campos, S.A.F. Yield and fruit quality of industrial tomato under saline irrigation. Sci. Agric. 2006, 63, 146–152. [Google Scholar] [CrossRef] [Green Version]
- Del Amor, F.M.; Martinez, V.; Cerda, A. Salt tolerance of tomato plants as affected by stage of plant development. HortScience 2001, 36, 1260–1263. [Google Scholar] [CrossRef] [Green Version]
- Bao, H.; Li, Y. Effect of stage-specific saline irrigation on greenhouse tomato production. Irrig. Sci. 2010, 28, 421–430. [Google Scholar] [CrossRef]
- Zhang, P.; Yanyan, D.; Masateru, S.; Natsumi, M.; Kengo, I. Interactions of salinity stress and flower thinning on tomato growth, yield, and water use efficiency. Commun. Soil Sci. Plant Anal. 2017, 48, 2601–2611. [Google Scholar] [CrossRef]
- Moore, S.; Stein, W.H. A modified ninhydrin reagent for the photometric determination of amino acids and related compounds. J. Biol. Chem. 1954, 211, 907–913. [Google Scholar] [CrossRef]
- Gómez-Alonso, S.; Hermosín-Gutiérrez, I.; García-Romero, E. Simultaneous HPLC analysis of biogenic amines, amino acids, and ammonium ion as aminoenone derivatives in wine and beer samples. J. Agric. Food Chem. 2007, 55, 608–613. [Google Scholar] [CrossRef]
- Schägger, H. Tricine–sds-page. Nat. Protoc. 2006, 1, 16. [Google Scholar] [CrossRef] [PubMed]
- Burrell, T.; Fozard, S.; Holroyd, G.H.; French, A.P.; Pound, M.P.; Bigley, C.J.; Taylor, C.J.; Forde, B.G. The Microphenotron: A robotic miniaturized plant phenotyping platform with diverse applications in chemical biology. Plant Methods 2017, 13, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galkovskyi, T.; Mileyko, Y.; Bucksch, A.; Moore, B.; Symonova, O.; Price, C.A.; Topp, C.N.; Iyer-Pascuzzi, A.S.; Zurek, P.R.; Fang, S.; et al. GiA Roots: Software for the high throughput analysis of plant root system architecture. BMC Plant Biol. 2012, 12, 116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and carotenoids: Measurement and characterization by UV-VIS spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4-3. [Google Scholar] [CrossRef]
- VanderGheynst, J.S.; Pettygrove, S.; Dooley, T.M.; Arnold, K.A. Estimating electrical conductivity of compost extracts at different extraction ratios. Compos. Sci. Util. 2004, 12, 202–207. [Google Scholar] [CrossRef]
- Majidi, H.; Minaei, S.; Almasi, M.; Mostofi, Y. Total soluble solids, titratable acidity and repining index of tomato in various storage conditions. Aust. J. Basic Appl. Sci. 2011, 5, 1723–1726. [Google Scholar]
- Munns, R.; Wallace, P.A.; Teakle, N.L.; Colmer, T.D. Measuring soluble ion concentrations (Na+, K+, Cl−) in salt-treated plants. In Plant Stress Tolerance; Humana Press: Totowa, NJ, USA, 2010; pp. 371–382. [Google Scholar] [CrossRef]
- Løvdal, T.; Lillo, C. Reference gene selection for quantitative real-time PCR normalization in tomato subjected to nitrogen, cold, and light stress. Anal. Biochem. 2009, 387, 238–242. [Google Scholar] [CrossRef]
- Julkowska, M.M.; Hoefsloot, H.C.; Mol, S.; Feron, R.; de Boer, G.J.; Haring, M.A.; Testerink, C. Capturing Arabidopsis root architecture dynamics with ROOT-FIT reveals diversity in responses to salinity. Plant Physiol. 2014, 166, 1387–1402. [Google Scholar] [CrossRef] [Green Version]
- Aziz, N.A.; Mahgoub, M.H.; Siam, H.S. Growth, flowering and chemical constituents performence of Amaranthus tricolor plants as influenced by seaweed (Ascophyllum nodosum) extract application under salt stress conditions. J. Appl. Sci. Res. 2011, 7, 1472–1484. [Google Scholar]
- Corte, L.; Dell’Abate, M.T.; Magini, A.; Migliore, M.; Felici, B.; Roscini, L.; Sardella, R.; Tancini, B.; Emiliani, C.; Cardinali, G.; et al. Assessment of safety and efficiency of nitrogen organic fertilizers from animal-based protein hydrolysates—A laboratory multidisciplinary approach. J. Sci. Food Agric. 2014, 94, 235–245. [Google Scholar] [CrossRef]
- Rouphael, Y.; Carillo, P.; Cristofano, F.; Cardarelli, M.; Colla, G. Effects of vegetal-versus animal-derived protein hydrolysate on sweet basil morpho-physiological and metabolic traits. Sci. Hortic. 2021, 284, 110123. [Google Scholar] [CrossRef]
- Juan, M.; Rivero, R.M.; Romero, L.; Ruiz, J.M. Evaluation of some nutritional and biochemical indicators in selecting salt-resistant tomato cultivars. Environ. Exp. Bot. 2005, 54, 193–201. [Google Scholar] [CrossRef]
- Magán, J.J.; Gallardo, M.; Thompson, R.B.; Lorenzo, P. Effects of salinity on fruit yield and quality of tomato grown in soil-less culture in greenhouses in Mediterranean climatic conditions. Agric. Water Manag. 2008, 95, 1041–1055. [Google Scholar] [CrossRef]
- El-Mogy, M.M.; Garchery, C.; Stevens, R. Irrigation with salt water affects growth, yield, fruit quality, storability and marker-gene expression in cherry tomato. Acta Agric. Scand. Sect. B—Soil Plant Sci. 2018, 68, 727–737. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G.; Giordano, M.; El-Nakhel, C.; Kyriacou, M.C.; De Pascale, S. Foliar applications of a legume-derived protein hydrolysate elicit dose-dependent increases of growth, leaf mineral composition, yield and fruit quality in two greenhouse tomato cultivars. Sci. Hortic. 2017, 226, 353–360. [Google Scholar] [CrossRef]
- Maggio, A.; De Pascale, S.; Angelino, G.; Ruggiero, C.; Barbieri, G. Physiological response of tomato to saline irrigation in long-term salinized soils. Eur. J. Agron. 2004, 21, 149–159. [Google Scholar] [CrossRef]
- Ertani, A.; Pizzeghello, D.; Francioso, O.; Sambo, P.; Sanchez-Cortes, S.; Nardi, S. Capsicum chinensis L. growth and nutraceutical properties are enhanced by biostimulants in a long-term period: Chemical and metabolomic approaches. Front. Plant Sci. 2014, 5, 375. [Google Scholar] [CrossRef] [Green Version]
- Colla, G.; Cardarelli, M.; Bonini, P.; Rouphael, Y. Foliar applications of protein hydrolysate, plant and seaweed extracts increase yield but differentially modulate fruit quality of greenhouse tomato. HortScience 2017, 52, 1214–1220. [Google Scholar] [CrossRef]
- Tieman, D.; Bliss, P.; McIntyre, L.M.; Blandon-Ubeda, A.; Bies, D.; Odabasi, A.Z.; Rodríguez, G.R.; van der Knaap, E.; Taylor, M.G.; Goulet, C.; et al. The chemical interactions underlying tomato flavor preferences. Curr. Biol. 2012, 22, 1035–1039. [Google Scholar] [CrossRef] [Green Version]
- Shi, H.; Quintero, F.J.; Pardo, J.M.; Zhu, J.K. The putative plasma membrane Na+/H+ antiporter SOS1 controls long-distance Na+ transport in plants. Plant Cell 2002, 14, 465–477. [Google Scholar] [CrossRef] [Green Version]
- Horie, T.; Motoda, J.; Kubo, M.; Yang, H.; Yoda, K.; Horie, R.; Chan, W.Y.; Leung, H.Y.; Hattori, K.; Konomi, M.; et al. Enhanced salt tolerance mediated by AtHKT1 transporter-induced Na+ unloading from xylem vessels to xylem parenchyma cells. Plant J. 2005, 44, 928–938. [Google Scholar] [CrossRef]
- Cosentino, C.; Fischer-Schliebs, E.; Bertl, A.; Thiel, G.; Homann, U. Na+/H+ antiporters are differentially regulated in response to NaCl stress in leaves and roots of Mesembryanthemum crystallinum. New Phytol. 2010, 186, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Gálvez, F.J.; Baghour, M.; Hao, G.; Cagnac, O.; Rodríguez-Rosales, M.P.; Venema, K. Expression of LeNHX isoforms in response to salt stress in salt sensitive and salt tolerant tomato species. Plant Physiol. Biochem. 2012, 51, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Barragán, V.; Leidi, E.O.; Andrés, Z.; Rubio, L.; De Luca, A.; Fernández, J.A.; Cubero, B.; Pardo, J.M. Ion exchangers NHX1 and NHX2 mediate active potassium uptake into vacuoles to regulate cell turgor and stomatal function in Arabidopsis. Plant Cell 2012, 24, 1127–1142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maach, M.; Baghour, M.; Akodad, M.; Gálvez, F.J.; Sánchez, M.E.; Aranda, M.N.; Venema, K.; Rodríguez-Rosales, M.P. Overexpression of LeNHX4 improved yield, fruit quality and salt tolerance in tomato plants (Solanum lycopersicum L.). Mol. Biol. Rep. 2020, 47, 4145–4153. [Google Scholar] [CrossRef]
- Munns, R.; Passioura, J.B.; Colmer, T.D.; Byrt, C.S. Osmotic adjustment and energy limitations to plant growth in saline soil. New Phytol. 2020, 225, 1091–1096. [Google Scholar] [CrossRef] [Green Version]
- Afzal, I.; Hussain, B.; Basra, S.M.A.; Ullah, S.H.; Shakeel, Q.; Kamran, M. Foliar application of potassium improves fruit quality and yield of tomato plants. Acta Sci. Pol. Hortorum Cultus 2015, 14, 3–13. [Google Scholar]
- Ertani, A.; Schiavon, M.; Nardi, S. Transcriptome-wide identification of differentially expressed genes in Solanum lycopersicon L. in response to an alfalfa-protein hydrolysate using microarrays. Front. Plant Sci. 2017, 8, 1159. [Google Scholar] [CrossRef] [Green Version]
- Sestili, F.; Rouphael, Y.; Cardarelli, M.; Pucci, A.; Bonini, P.; Canaguier, R.; Colla, G. Protein Hydrolysate Stimulates Growth in Tomato Coupled with N-Dependent Gene Expression Involved in N Assimilation. Front. Plant Sci. 2018, 9, 1233. [Google Scholar] [CrossRef] [Green Version]
- Alfosea-Simón, M.; Zavala-Gonzalez, E.A.; Camara-Zapata, J.M.; Martínez-Nicolás, J.J.; Simón, I.; Simón-Grao, S.; García-Sánchez, F. Effect of foliar application of amino acids on the salinity tolerance of tomato plants cultivated under hydroponic system. Sci. Hortic. 2020, 272, 109509. [Google Scholar] [CrossRef]
- Lucini, L.; Miras-Moreno, B.; Rouphael, Y.; Cardarelli, M.; Colla, G. Combining molecular weight fractionation and metabolomics to elucidate the bioactivity of vegetal protein hydrolysates in tomato plants. Front. Plant Sci. 2020, 11, 976. [Google Scholar] [CrossRef] [PubMed]
- Goñi, O.; Quille, P.; O’Connell, S. Seaweed carbohydrates. In The Chemical Biology of Plant Biostimulants; Geelen, D., Xu, L., Stevens, C.V., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2020; pp. 57–95. [Google Scholar]
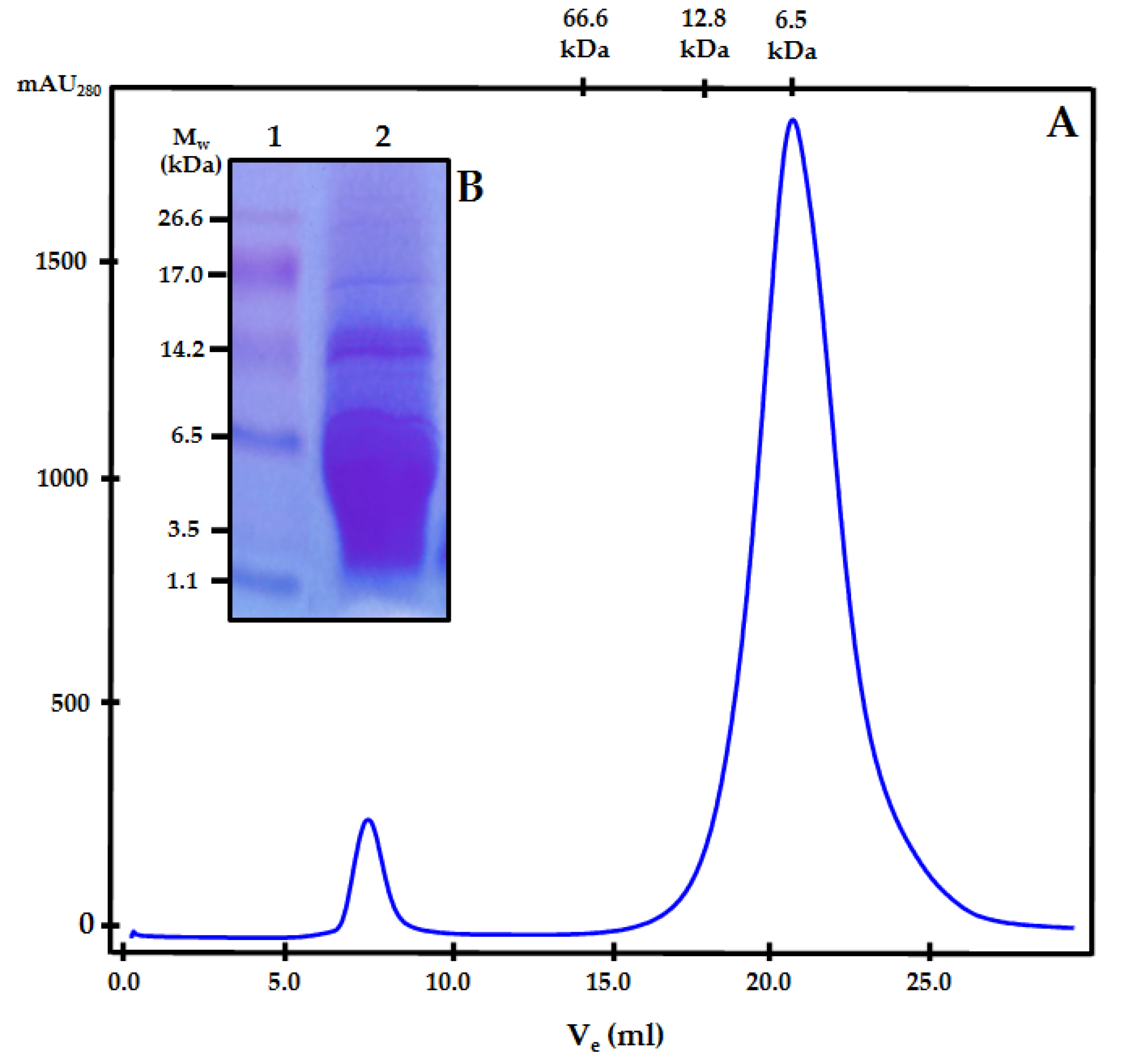
| Component | Concentration | |
|---|---|---|
| Ash % (w v−1) | 3.00 ± 0.15 | |
| Uronics acids % (w v−1) | 0.85 ± 0.07 | |
| Fucose % (w v−1) | 0.81 ± 0.05 | |
| Laminarin % (w v−1) | 0.29 ± 0.02 | |
| Free mannitol % (w v−1) | 0.56 ± 0.06 | |
| Polyphenol % (w v−1) | 1.22 ± 0.08 | |
| Total free amino acids % (w v−1) | 1.90 ± 0.03 | |
| Soluble peptides % (w v−1) | 1.55 ± 0.02 | |
| Elements % (w v−1) | N | 1.03 ± 0.04 |
| P | 0.13 ± 0.01 | |
| K | 1.65 ± 0.05 | |
| S | 0.26 ± 0.02 | |
| Microelements (ppm) | Mg | 561.60 ± 6.34 |
| B | 443.15 ± 5.87 | |
| Zn | 236.40 ± 7.75 | |
| Fe | 117.61 ± 4.30 | |
| Ca | 68.80 ± 1.45 |
| Source of Variance | Root Length (mm) | Root Network (mm) | Chl (a + b) (mg g−1 FW) | Carotenoids (mg g−1 FW) |
|---|---|---|---|---|
| Salinity (S) | *** | *** | *** | *** |
| PSI-475 (P) | *** | ns | *** | *** |
| S × P | ** | ns | *** | *** |
| Salinity (S) | ||||
| Unstressed | 11.03 b | 32.03 b | 0.14 b | 0.05 b |
| Stressed | 9.77 a | 26.93 a | 0.06 a | 0.02 a |
| PSI-475 (mg L−1) (P) | ||||
| 0 | 9.72 a | 28.96 | 0.09 a | 0.03 a |
| 2.5 | 10.21 b | 28.27 | 0.10 b | 0.03 a |
| 5 | 10.47 b | 30.44 | 0.11 b | 0.04 b |
| 10 | 11.03 c | 29.63 | 0.10 b | 0.03 a |
| 25 | 10.57 b | 30.09 | 0.11 b | 0.04 b |
| S × P | ||||
| Unstressed × 0 | 10.4 bc | 31.57 | 0.12 d | 0.04 d |
| Unstressed × 2.5 | 10.84 bcd | 31.25 | 0.14 de | 0.04 d |
| Unstressed × 5 | 11.75 d | 32.61 | 0.14 de | 0.05 d |
| Unstressed × 10 | 11.21 bcd | 32.91 | 0.15 e | 0.05 d |
| Unstressed × 25 | 10.97 cd | 31.81 | 0.17 f | 0.06 e |
| Stressed × 0 | 9.04 a | 26.35 | 0.05 a | 0.02 a |
| Stressed × 2.5 | 9.58 ab | 25.29 | 0.07 bc | 0.03 bc |
| Stressed × 5 | 9.19 a | 28.27 | 0.08 c | 0.03 c |
| Stressed × 10 | 10.86 bcd | 26.34 | 0.05 ab | 0.02 ab |
| Stressed × 25 | 10.17 abc | 28.37 | 0.05 a | 0.02 ab |
| Source of Variance | Plant Height (cm) | Plant Biomass (g) | Root Length (cm) | Root Biomass (mg) | Fruit Number | Fruit Yield (g) | Sugar Cont. (°Brix) | TTA (% w v−1) |
|---|---|---|---|---|---|---|---|---|
| Salinity (S) | *** | ns | *** | *** | ns | *** | *** | * |
| PSI-475 (P) | ns | ns | * | ns | *** | * | * | ns |
| S × P | ns | ns | ns | ns | ns | ns | ns | ns |
| Salinity (S) | ||||||||
| Unstressed | 29.21 b | 14.34 | 20.45 b | 65 b | 13.57 | 28.76 b | 5.44 a | 0.61 a |
| Stressed | 25.38 a | 14.24 | 18.62 a | 49 a | 12.22 | 17.98 a | 6.65 b | 0.73 b |
| PSI-475 (P) | ||||||||
| Untreated | 27.24 | 14.33 | 19.01 a | 55 | 10.91 a | 21.01 a | 5.76 a | 0.63 |
| PSI-475 | 27.36 | 14.25 | 20.05 b | 59 | 14.88 b | 25.73 b | 6.33 b | 0.59 |
| S × P | ||||||||
| Unstressed × Untreated | 28.77 | 14.46 | 20.02 | 64 | 12.01 | 26.51 | 4.97 | 0.63 |
| Unstressed × PSI-475 | 29.66 | 14.22 | 20.87 | 65 | 15.13 | 31.01 | 5.92 | 0.59 |
| Stressed × Untreated | 25.70 | 14.21 | 18.01 | 46 | 9.81 | 15.51 | 6.55 | 0.73 |
| Stressed × PSI-475 | 25.06 | 14.28 | 19.23 | 53 | 14.62 | 20.45 | 6.75 | 0.72 |
| Source of Variance | T1 (87-Day-Old Plants) | T2 (101-Day-Old Plants) | ||||
|---|---|---|---|---|---|---|
| K+ | Na+ | K+/Na+ | K+ | Na+ | K+/Na+ | |
| (mg g−1 DW) | (mg g−1 DW) | |||||
| Salinity (S) | ns | *** | *** | ** | *** | *** |
| PSI-475 (P) | ns | *** | ns | * | ns | ns |
| S × P | ns | *** | ns | ns | ns | ns |
| Salinity (S) | ||||||
| Unstressed | 53.74 | 1.45 a | 39.69 b | 44.72 b | 1.12 a | 40.62 b |
| Stressed | 51.31 | 16.36 b | 3.06 a | 38.95 a | 30.23 b | 1.31 a |
| PSI-475 (P) | ||||||
| Untreated | 50.14 | 9.69 b | 21.67 | 39.54 a | 14.65 | 18.50 |
| PSI-475 | 54.91 | 8.12 a | 21.08 | 44.47 b | 17.01 | 23.43 |
| S × P | ||||||
| Unstressed × Untreated | 51.13 | 1.26 a | 40.71 | 42.03 | 1.02 | 35.67 |
| Unstressed × PSI-475 | 56.36 | 1.64 a | 38.68 | 47.41 | 1.22 | 45.57 |
| Stressed × Untreated | 49.16 | 18.12 c | 2.63 | 37.05 | 28.29 | 1.33 |
| Stressed × PSI-475 | 53.46 | 14.60 b | 3.48 | 41.12 | 32.81 | 1.29 |
| Source of Variance | T1 (87-Day-Old Plants) | T2 (101-Day-Old Plants) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| RWC | Proline | Fructose | Glucose | Sucrose | RWC | Proline | Fructose | Glucose | Sucrose | |
| % | (mg g−1 DW) | % | (mg g−1 DW) | |||||||
| Salinity (S) | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** |
| PSI-475 (P) | *** | ns | ns | *** | ns | *** | *** | * | * | *** |
| S × P | * | ns | * | ns | ns | * | ns | *** | *** | *** |
| Salinity (S) | ||||||||||
| Unstressed | 78.14 b | 1.67 a | 1.80 a | 2.20 a | 1.16 a | 76.97 b | 1.35 a | 2.33 b | 1.50 a | 1.51 a |
| Stressed | 74.32 a | 6.20 b | 3.41 b | 2.64 b | 2.68 b | 71.39 a | 5.57 b | 1.97 a | 1.82 b | 3.84 b |
| PSI-475 (P) | ||||||||||
| Untreated | 75.36 a | 3.96 | 2.56 | 2.00 a | 1.87 | 72.60 a | 3.08 a | 2.06 a | 1.73 b | 2.51 a |
| PSI-475 | 77.10 b | 3.91 | 2.65 | 2.84 b | 1.98 | 75.75 b | 3.85 b | 2.24 b | 1.58 a | 2.84 b |
| S × P | ||||||||||
| Unstressed × Untreated | 77.02 c | 1.51 | 1.98 a | 1.69 | 1.23 | 75.82 c | 1.01 | 2.10 a | 1.40 a | 1.50 a |
| Unstressed × PSI-475 | 79.26 d | 1.83 | 1.62 a | 2.71 | 1.09 | 78.11 d | 1.70 | 2.57 b | 1.59 a | 1.51 a |
| Stressed × Untreated | 73.70 a | 6.41 | 3.15 b | 2.31 | 2.50 | 69.37 a | 5.15 | 2.03 a | 2.06 b | 3.52 b |
| Stressed × PSI-475 | 74.94 b | 5.99 | 3.67 b | 2.96 | 2.86 | 73.40 b | 5.99 | 1.92 a | 1.57 a | 4.16 c |
| Source of Variance | T1 (87-Day-Old Plants) | T2 (101-Day-Old Plants) | ||||
|---|---|---|---|---|---|---|
| Protein | Chl (a + b) | Carot. | Protein | Chl (a + b) | Carot. | |
| (mg g−1 DW) | (mg g−1 DW) | |||||
| Salinity (S) | * | ns | * | * | *** | *** |
| PSI-475 (P) | * | ** | * | ns | * | ns |
| S × P | ns | ns | ns | ns | ns | ns |
| Salinity (S) | ||||||
| Unstressed | 82.25 b | 15.88 | 1.90 b | 81.31 b | 16.15 b | 2.09 b |
| Stressed | 65.12 a | 14.86 | 1.69 a | 62.52 a | 12.66 a | 1.67 a |
| PSI-475 (P) | ||||||
| Untreated | 65.46 a | 14.30 a | 1.72 a | 68.37 | 13.74 a | 1.83 |
| PSI-475 | 81.91 b | 16.44 b | 1.87 b | 75.46 | 15.08 b | 1.93 |
| S × P | ||||||
| Unstressed × Untreated | 69.88 | 14.97 | 15.76 | 80.56 | 1.87 | 2.06 |
| Unstressed × PSI-475 | 94.61 | 16.78 | 16.55 | 82.05 | 1.93 | 2.12 |
| Stressed × Untreated | 61.03 | 13.63 | 11.72 | 56.17 | 1.58 | 1.59 |
| Stressed × PSI-475 | 69.21 | 16.09 | 13.61 | 68.86 | 1.81 | 1.74 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ikuyinminu, E.; Goñi, O.; O’Connell, S. Enhancing Irrigation Salinity Stress Tolerance and Increasing Yield in Tomato Using a Precision Engineered Protein Hydrolysate and Ascophyllum nodosum-Derived Biostimulant. Agronomy 2022, 12, 809. https://doi.org/10.3390/agronomy12040809
Ikuyinminu E, Goñi O, O’Connell S. Enhancing Irrigation Salinity Stress Tolerance and Increasing Yield in Tomato Using a Precision Engineered Protein Hydrolysate and Ascophyllum nodosum-Derived Biostimulant. Agronomy. 2022; 12(4):809. https://doi.org/10.3390/agronomy12040809
Chicago/Turabian StyleIkuyinminu, Elomofe, Oscar Goñi, and Shane O’Connell. 2022. "Enhancing Irrigation Salinity Stress Tolerance and Increasing Yield in Tomato Using a Precision Engineered Protein Hydrolysate and Ascophyllum nodosum-Derived Biostimulant" Agronomy 12, no. 4: 809. https://doi.org/10.3390/agronomy12040809
APA StyleIkuyinminu, E., Goñi, O., & O’Connell, S. (2022). Enhancing Irrigation Salinity Stress Tolerance and Increasing Yield in Tomato Using a Precision Engineered Protein Hydrolysate and Ascophyllum nodosum-Derived Biostimulant. Agronomy, 12(4), 809. https://doi.org/10.3390/agronomy12040809







