High Level of Iron Inhibited Maize Straw Decomposition by Suppressing Microbial Communities and Enzyme Activities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Soil Microbial Suspension and Maize Straw
2.2. Experimental Design
2.3. Decomposition Rate and Main Component Content of Maize Straw
2.4. Enzyme Activity Assays
2.5. DNA Extraction and High Throughput Sequencing
2.6. Bioinformatic Analysis
2.7. Statistical Analysis
3. Results
3.1. Impact of Fe Addition on Maize Straw Decomposition
3.2. Impact of Fe Addition on Lignocellulose Components in Maize Straw
3.3. Microbial Community Composition
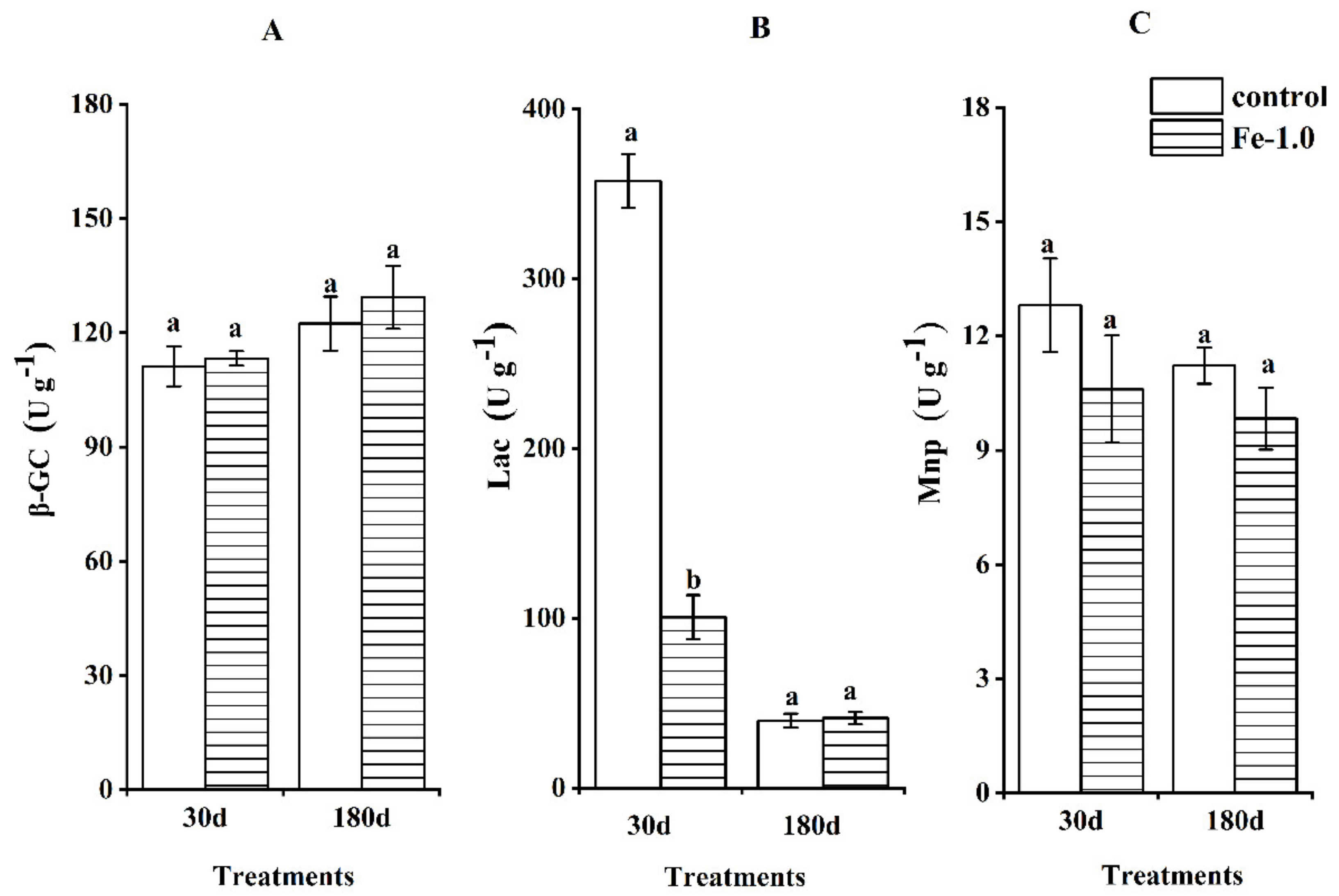
3.4. Enzyme Activities
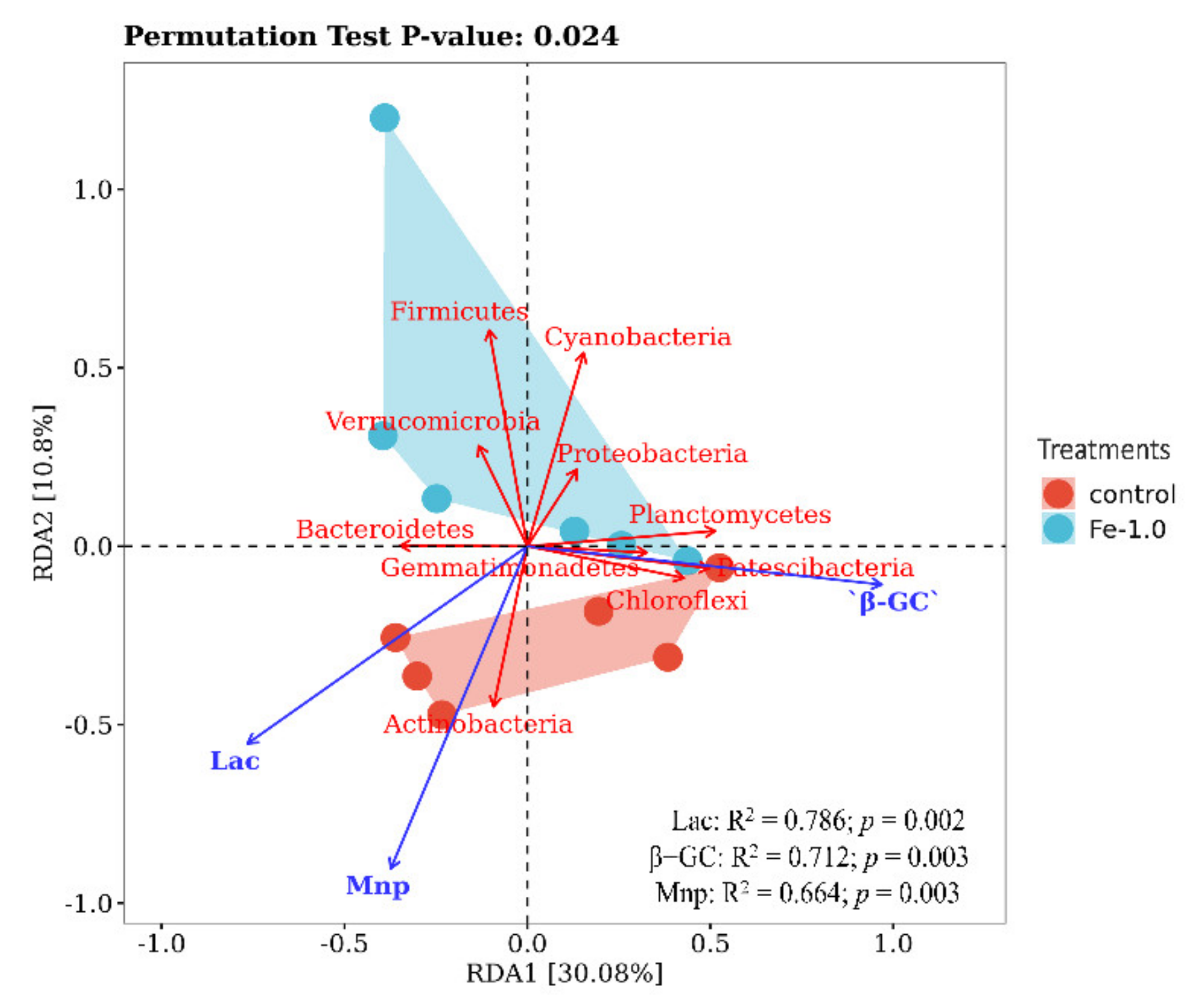
3.5. RDA Analysis of Correlation between Bacteria and Enzyme Activities
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xia, L.; Lam, S.K.; Wolf, B.; Kiese, R.; Chen, D.; Butterbach-Bahl, K. Trade-offs between soil carbon sequestration and reactive nitrogen losses under straw return in global agroecosystems. Glob. Chang. Biol. 2018, 24, 5919–5932. [Google Scholar] [CrossRef] [PubMed]
- Berhane, M.; Xu, M.; Liang, Z.; Shi, J.; Wei, G.; Tian, X. Effects of long-term straw return on soil organic carbon storage and sequestration rate in North China upland crops: A meta-analysis. Glob. Chang. Biol. 2020, 26, 2686–2701. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, X.; Geng, P.; Yang, Q.; Chen, K.; Liu, N.; Fan, Y.; Zhan, X.; Han, X. Effects of different returning method combined with decomposer on decomposition of organic components of straw and soil fertility. Sci. Rep. 2021, 11, 15495. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xu, Y.J.; Gao, H.J.; Mao, J.D.; Chu, W.Y.; Thompson, M.L. Biochemical stabilization of soil organic matter in straw-amended, anaerobic and aerobic soils. Sci. Total Environ. 2018, 625, 1065–1073. [Google Scholar] [CrossRef]
- Su, P.; Brookes, P.C.; He, Y.; Wu, J.; Xu, J. An evaluation of a microbial inoculum in promoting organic C decomposition in a paddy soil following straw incorporation. J. Soil Sediments 2016, 16, 1776–1786. [Google Scholar] [CrossRef]
- Xun, W.; Wang, B.; Ran, W. Research progress on the effect of different fertilizations on microbiome structure and function in upland red soil in southern China. J. Agric. Resour. Environ. 2021, 38, 537–544. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, S. Linkages between straw decomposition rate and the change in microbial fractions and extracellular enzyme activities in soils under different long-term fertilization treatments. PLoS ONE 2018, 13, e0202660. [Google Scholar] [CrossRef] [Green Version]
- Zhao, S.; Qiu, S.; Xu, X.; Ciampitti, I.A.; Zhang, S.; He, P. Change in straw decomposition rate and soil microbial community composition after straw addition in different long-term fertilization soils. Appl. Soil Ecol. 2019, 138, 123–133. [Google Scholar] [CrossRef]
- Xu, Y.; Fan, J.; Ding, W.; Bol, R.; Chen, Z.; Luo, J.; Bolan, N. Stage-specific response of litter decomposition to N and S amendments in a subtropical forest soil. Biol. Fert. Soils 2016, 52, 711–724. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, J.; Zhao, B.; Yan, P.; Zhou, G.; Xin, X. Effects of straw amendment and moisture on microbial communities in Chinese fluvo-aquic soil. J. Soil Sediments 2014, 14, 1829–1840. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, X.; Jiang, L. Influence of the Metal Ions on the Activity of Endo-1,4-β-D-glucanase and Exo-1,4-β-D-glucanase. J. Cellul. Sci. Technol. 2011, 19, 6–13. [Google Scholar] [CrossRef]
- Huang, C.; Lai, C.; Zeng, G.; Huang, D.; Xu, P.; Zhang, C.; Cheng, M.; Wan, J. Manganese-enhanced degradation of lignocellulosic waste by Phanerochaete chrysosporium: Evidence of enzyme activity and gene transcription. Appl. Microbiol. Biotechnol. 2017, 101, 6541–6549. [Google Scholar] [CrossRef] [PubMed]
- Hermosilla, E.; Schalchli, H.; Mutis, A.; Diez, M.C. Combined effect of enzyme inducers and nitrate on selective lignin degradation in wheat straw by Ganoderma lobatum. Environ. Sci. Pollut. Res. Int. 2017, 24, 21984–21996. [Google Scholar] [CrossRef] [PubMed]
- Van Kuijk, S.J.A.; Sonnenberg, A.S.M.; Baars, J.J.P.; Hendriks, W.H.; Cone, J.W. The effect of adding urea, manganese and linoleic acid to wheat straw and wood chips on lignin degradation by fungi and subsequent in vitro rumen degradation. Anim. Feed Sci. Technol. 2016, 213, 22–28. [Google Scholar] [CrossRef]
- Tang, X.; Li, X.; Liu, X.; Hashmi, M.Z.; Xu, J.; Brookes, P.C. Effects of inorganic and organic amendments on the uptake of lead and trace elements by Brassica chinensis grown in an acidic red soil. Chemosphere 2015, 119, 177–183. [Google Scholar] [CrossRef]
- Wyman, V.; Serrano, A.; Fermoso, F.G.; Villa Gomez, D.K. Trace elements effect on hydrolytic stage towards biogas production of model lignocellulosic substrates. J. Environ. Manag. 2019, 234, 320–325. [Google Scholar] [CrossRef]
- Xu, J.B.; Wang, Y.L.; Luo, X.S.; Feng, Y.Z. Influence of Fe3O4 nanoparticles on lettuce (Lactuca sativa L.) growth and soil bacterial community structure. Chin. J. Appl. Ecol. 2017, 28, 3003–3010. [Google Scholar]
- Bandyopadhyay, T.; Prasad, M. IRONing out stress problems in crops: A homeostatic perspective. Physiol. Plant. 2021, 171, 559–577. [Google Scholar] [CrossRef]
- Canessa, P.; Munoz-Guzman, F.; Vicuna, R.; Larrondo, L.F. Characterization of PIR1, a GATA family transcription factor involved in iron responses in the white-rot fungus Phanerochaete chrysosporium. Fungal Genet. Biol. 2012, 49, 626–634. [Google Scholar] [CrossRef]
- Wang, M.; Mu, Z.; Wang, J.; Hou, S.; Han, L.; Dong, Y.; Xiao, L.; Xia, R.; Fang, X. The identification of and relief from Fe3+ inhibition for both cellulose and cellulase in cellulose saccharification catalyzed by cellulases from Penicillium decumbens. Bioresour. Technol. 2013, 133, 507–512. [Google Scholar] [CrossRef]
- Tejirian, A.; Xu, F. Inhibition of cellulase-catalyzed lignocellulosic hydrolysis by iron and oxidative metal ions and complexes. Appl. Environ. Microbiol. 2010, 76, 7673–7682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zong, M.; Lin, C.; Li, S.; Li, H.; Duan, C.; Peng, C.; Guo, Y.; An, R. Tillage activates iron to prevent soil organic carbon loss following forest conversion to cornfields in tropical acidic red soils. Sci. Total Environ. 2021, 761, 143253. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Su, Y.; He, X.; Wu, J.; Zheng, H.; Li, Y.; Wang, A. Response of soil organic carbon mineralization in typical Karst soils following the addition of 14C-labeled rice straw and CaCO3. J. Sci. Food Agric. 2012, 92, 1112–1118. [Google Scholar] [CrossRef] [PubMed]
- Song, X.Y.; Spaccini, R.; Pan, G.; Piccolo, A. Stabilization by hydrophobic protection as a molecular mechanism for organic carbon sequestration in maize-amended rice paddy soils. Sci. Total Environ. 2013, 458, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Chen, S.S.; Feng, Z.Y.; Deng, Z.L.; LI, Z.; Wang, S.M. Combined effects of soil microbes and organic matter on aggregate formation in saline-alkali soil. J. Agro-Environ. Sci. 2017, 36, 2080–2085. [Google Scholar]
- Berg, B. Decomposition patterns for foliar litter–A theory for influencing factors. Soil Biol. Biochem. 2014, 78, 222–232. [Google Scholar] [CrossRef]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Symposium carbohydrate methodology, metabolism, and nutritional implications in dairy cattle. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Baraznenok, V.A.; Becker, E.G.; Ankudimova, N.V.; Okunev, N.N. Characterization of neutral xylanases from Chaetomium cellulolyticum and their biobleaching effect on eucalyptus pulp. Enzyme Microb. Tech. 1999, 25, 651–659. [Google Scholar] [CrossRef]
- Xu, X.; Xu, Z.; Shi, S.; Lin, M. Lignocellulose degradation patterns, structural changes, and enzyme secretion by Inonotus obliquus on straw biomass under submerged fermentation. Bioresour. Technol. 2017, 241, 415–423. [Google Scholar] [CrossRef]
- Feng, S.; Su, Y.; Dong, M.; He, X.; Kumaresan, D.; O’Donnell, A.G.; Wu, J.; Chen, X. Laccase activity is proportional to the abundance of bacterial laccase-like genes in soil from subtropical arable land. World J. Microbiol. Biotechnol. 2015, 31, 2039–2045. [Google Scholar] [CrossRef]
- Li, H.; Li, Z.; Qu, J.; Wang, J. Bacterial diversity in traditional Jiaozi and sourdough revealed by high-throughput sequencing of 16S rRNA amplicons. LWT-Food Sci. Technol. 2017, 81, 319–325. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Dillon, M.R.; Zhang, Y.; Rideout, J.R.; Bolyen, E.; Li, H.; Albert, P.S.; Caporaso, J.G. q2-longitudinal: Longitudinal and Paired-Sample Analyses of Microbial Data. Msystems 2018, 3, e00219-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katoh, K.; Misawa, K.; Kuma, K.; Miyata, T. MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Price, M.N.; Dehal, P.S.; Arkin, A.P. FastTree 2–Approximately maximum-likelihood trees for large alignments. PLoS ONE 2010, 5, e9490. [Google Scholar] [CrossRef]
- Su, Y.; Lv, J.L.; Yu, M.; Ma, Z.H.; Xi, H.; Kou, C.L.; He, Z.C.; Shen, A.L. Long-term decomposed straw return positively affects the soil microbial community. J. Appl. Microbiol. 2020, 128, 138–150. [Google Scholar] [CrossRef] [Green Version]
- Monteiro, C.M.; Castro, P.M.; Malcata, F.X. Metal uptake by microalgae: Underlying mechanisms and practical applications. Biotechnol. Prog. 2012, 28, 299–311. [Google Scholar] [CrossRef]
- Cai, Y.; Zhao, X.; Zhao, Y.; Wang, H.; Yuan, X.; Zhu, W.; Cui, Z.; Wang, X. Optimization of Fe2+ supplement in anaerobic digestion accounting for the Fe-bioavailability. Bioresour. Technol. 2018, 250, 163–170. [Google Scholar] [CrossRef]
- Chen, J.L.; Ortiz, R.; Steele, T.W.; Stuckey, D.C. Toxicants inhibiting anaerobic digestion: A review. Biotechnol. Adv. 2014, 32, 1523–1534. [Google Scholar] [CrossRef]
- Wei, L.; Li, Y.; Noguera, D.R.; Zhao, N.; Song, Y.; Ding, J.; Zhao, Q.; Cui, F. Adsorption of Cu2+ and Zn2+ by extracellular polymeric substances (EPS) in different sludges: Effect of EPS fractional polarity on binding mechanism. J. Hazard. Mater. 2017, 321, 473–483. [Google Scholar] [CrossRef]
- Wu, D.; Wei, Z.; Zhao, Y.; Zhao, X.; Mohamed, T.A.; Zhu, L.; Wu, J.; Meng, Q.; Yao, C.; Zhao, R. Improved lignocellulose degradation efficiency based on Fenton pretreatment during rice straw composting. Bioresour. Technol. 2019, 294, 122132. [Google Scholar] [CrossRef] [PubMed]
- Hassan, W.; Chen, W.; Cai, P.; Huang, Q. Oxidative enzymes, the ultimate regulator: Implications for factors affecting their efficiency. J. Environ. Qual. 2013, 42, 1779–1790. [Google Scholar] [CrossRef]
- Tang, W.; Wu, X.; Huang, C.; Huang, C.; Lai, C.; Yong, Q. Humic acid-assisted autohydrolysis of waste wheat straw to sustainably improve enzymatic hydrolysis. Bioresour. Technol. 2020, 306, 123103. [Google Scholar] [CrossRef]
- Navada, K.K.; Kulal, A. Kinetic characterization of purified laccase from Trametes hirsuta: A study on laccase catalyzed biotransformation of 1,4-dioxane. Biotechnol. Lett. 2021, 43, 613–626. [Google Scholar] [CrossRef] [PubMed]
- Christopher, L.P.; Yao, B.; Ji, Y. Lignin Biodegradation with Laccase-Mediator Systems. Front. Energy Res. 2014, 2, 12. [Google Scholar] [CrossRef]
- Rajapaksha, R.M.; Tobor-Kaplon, M.A.; Baath, E. Metal toxicity affects fungal and bacterial activities in soil differently. Appl. Environ. Microbiol. 2004, 70, 2966–2973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Zhang, M.; You, S.; Ma, D.; Zhao, J.; Chen, Z. Effect of Fe3+ on the sludge properties and microbial community structure in a lab-scale A2O process. Sci. Total Environ. 2021, 780, 146505. [Google Scholar] [CrossRef] [PubMed]
- Dunning, J.C.; Ma, Y.; Marquis, R.E. Anaerobic killing of oral streptococci by reduced, transition metal cations. Appl. Environ. Microbiol. 1998, 64, 27–33. [Google Scholar] [CrossRef] [Green Version]
- Kugler, S.; Cooper, R.E.; Wegner, C.E.; Mohr, J.F.; Wichard, T.; Kusel, K. Iron-organic matter complexes accelerate microbial iron cycling in an iron-rich fen. Sci. Total Environ. 2019, 646, 972–988. [Google Scholar] [CrossRef]
- Bennett, B.D.; Gralnick, J.A. Mechanisms of toxicity by and resistance to ferrous iron in anaerobic systems. Free Radic. Biol. Med. 2019, 140, 167–171. [Google Scholar] [CrossRef]
- Nakamura, K.; Go, N. Function and molecular evolution of multicopper blue proteins. Cell. Mol. Life Sci. 2005, 62, 2050–2066. [Google Scholar] [CrossRef] [PubMed]
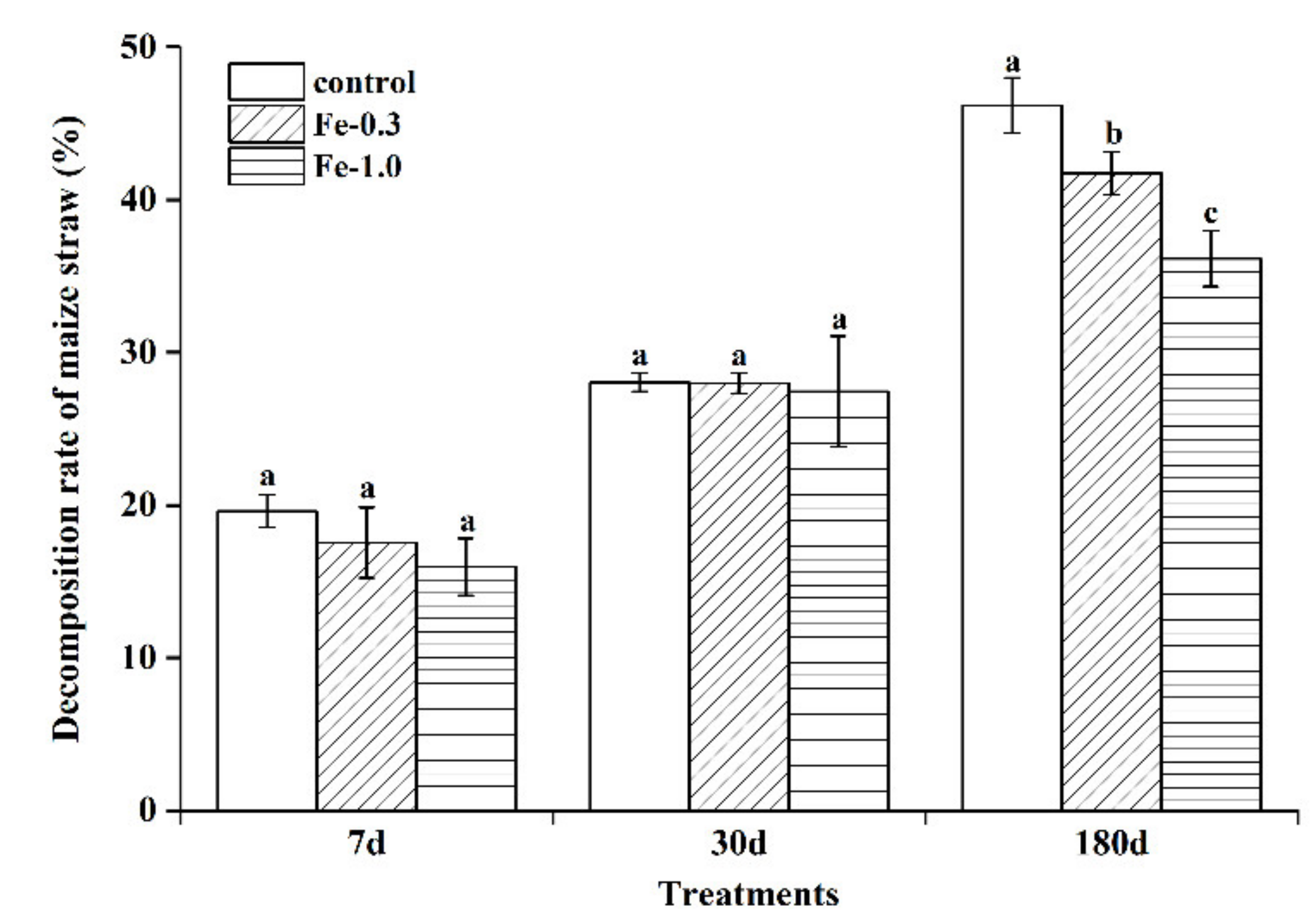
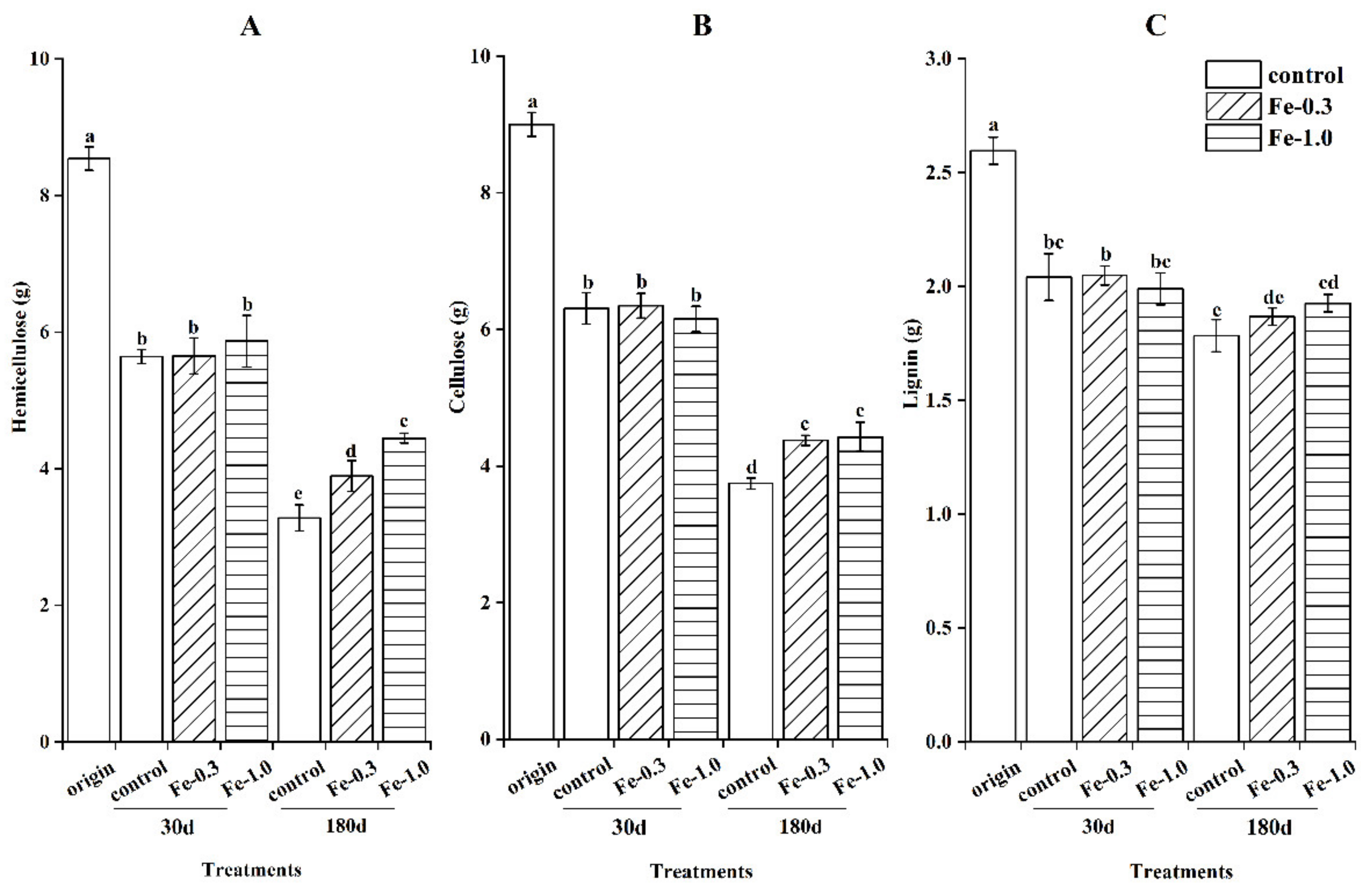
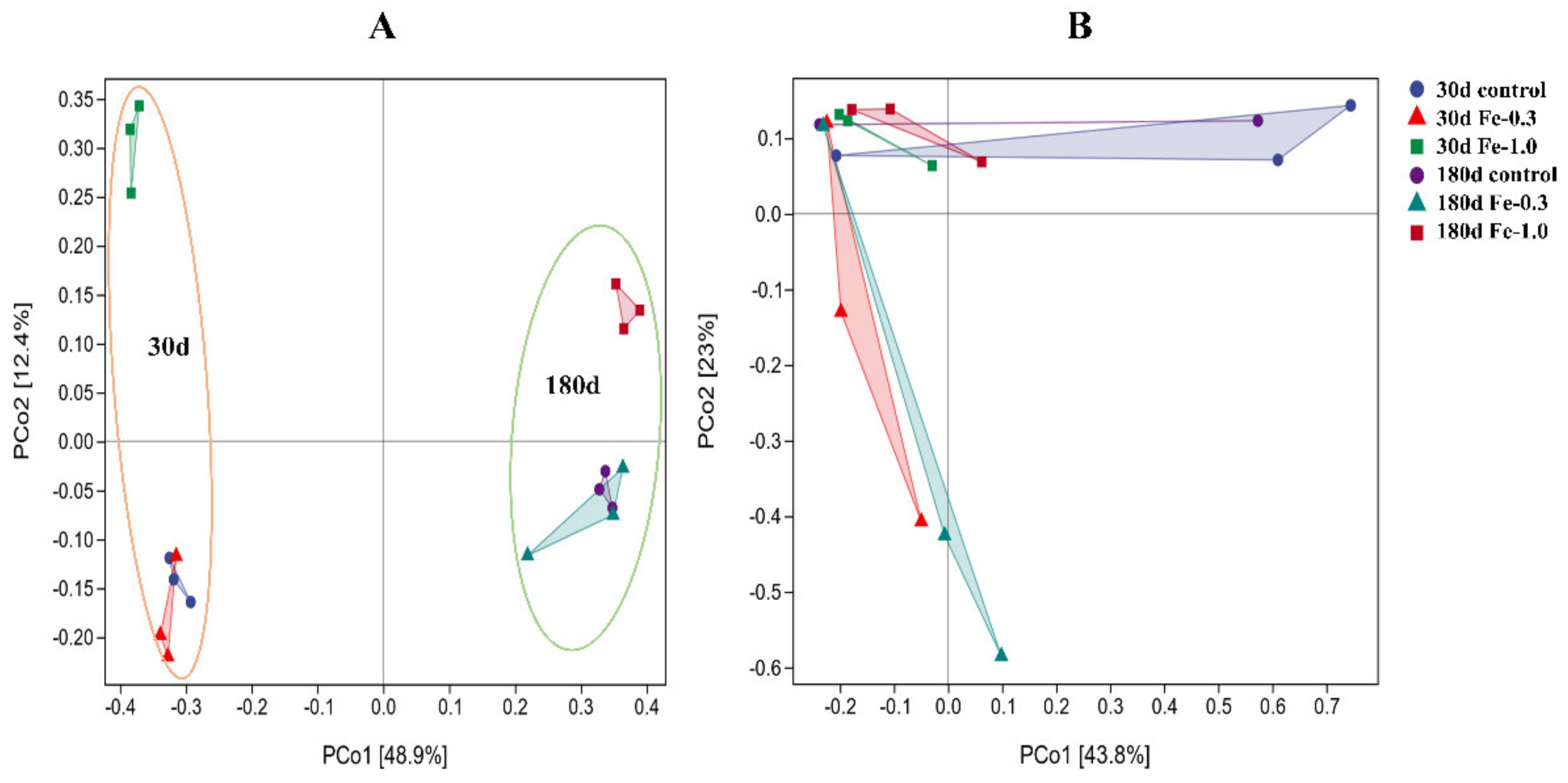
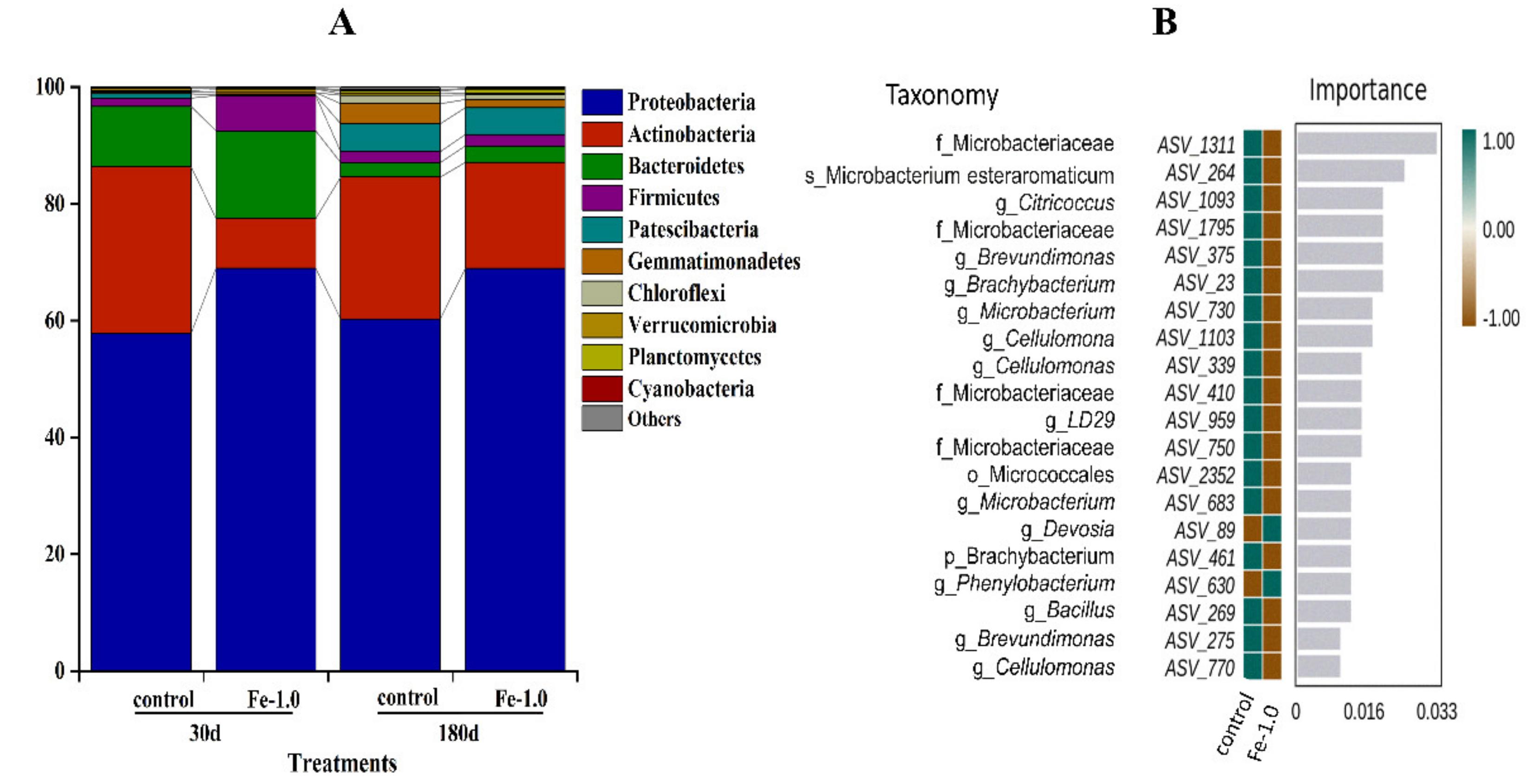
| Treatments | M = Mf × exp (−kf × t) + Ms × exp (−ks × t) | ||||
|---|---|---|---|---|---|
| Mf | kf | Ms | ks | R2 | |
| control | 23.79 | 0.2170 | 76.21 | 0.0019 | 0.9968 |
| Fe-0.3 | 25.14 | 0.1582 | 74.86 | 0.0014 | 0.9963 |
| Fe-1.0 | 26.27 | 0.1281 | 73.73 | 0.0008 | 0.9805 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, M.; Guan, H.; Zhang, W.; Tian, D.; Wei, J.; Kalkhajeh, Y.K.; Gao, H. High Level of Iron Inhibited Maize Straw Decomposition by Suppressing Microbial Communities and Enzyme Activities. Agronomy 2022, 12, 1286. https://doi.org/10.3390/agronomy12061286
Jin M, Guan H, Zhang W, Tian D, Wei J, Kalkhajeh YK, Gao H. High Level of Iron Inhibited Maize Straw Decomposition by Suppressing Microbial Communities and Enzyme Activities. Agronomy. 2022; 12(6):1286. https://doi.org/10.3390/agronomy12061286
Chicago/Turabian StyleJin, Mengcan, Hao Guan, Wenjie Zhang, Da Tian, Junling Wei, Yusef Kianpoor Kalkhajeh, and Hongjian Gao. 2022. "High Level of Iron Inhibited Maize Straw Decomposition by Suppressing Microbial Communities and Enzyme Activities" Agronomy 12, no. 6: 1286. https://doi.org/10.3390/agronomy12061286






