Evaluation of the Effect of Different Co-Solvent Mixtures on the Supercritical CO2 Extraction of the Phenolic Compounds Present in Moringa oleifera Lam. Leaves
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biological Material
2.2. Reagents and Solvents
2.3. Selecting the Co-Solvent
2.4. Moringa Leaf Characterization
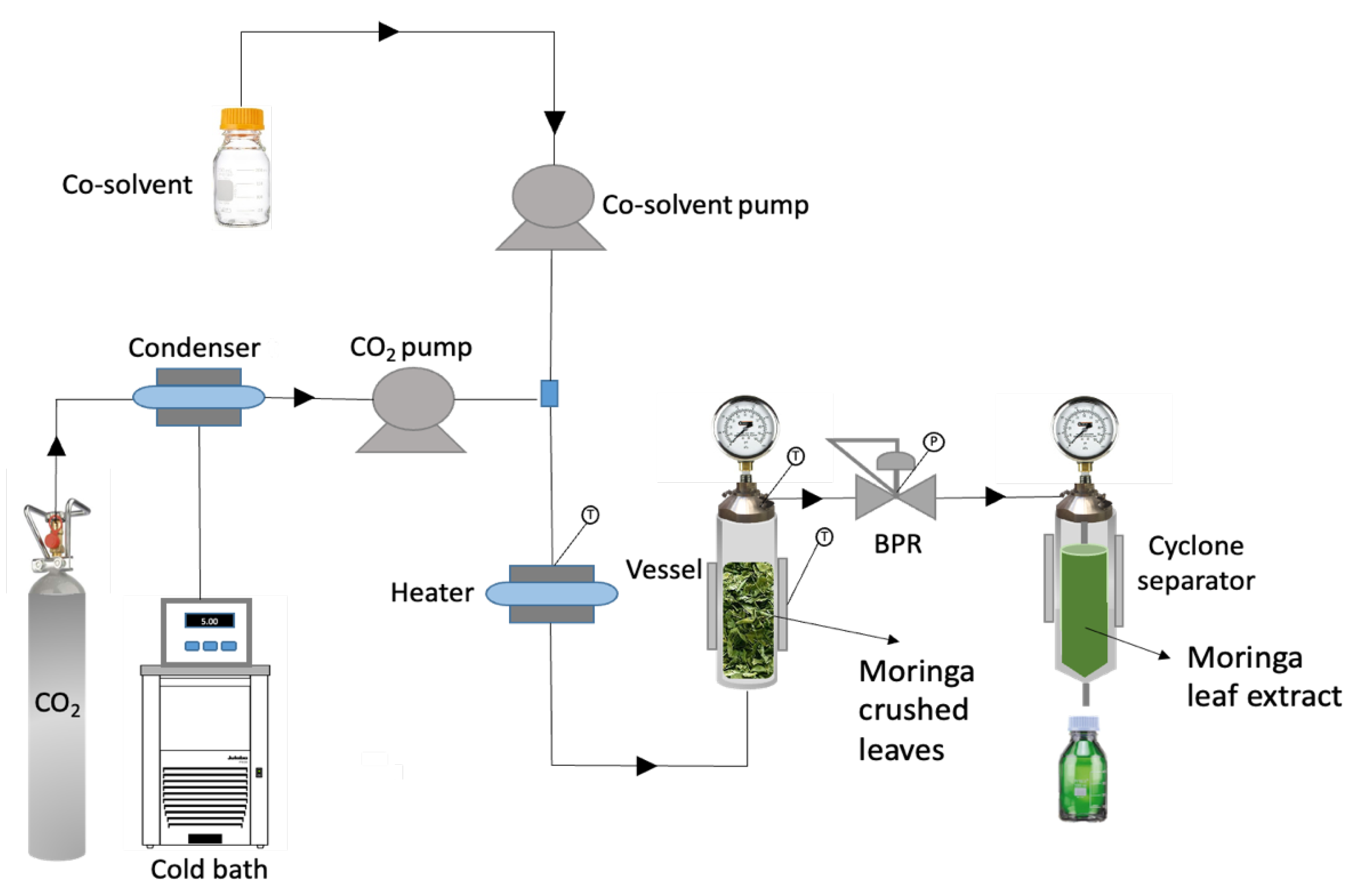
2.5. High-Pressure Extraction Procedure
2.6. Identification of Flavonoids by UHPLC-Q-ToF-MS
2.7. Quantification of Flavonoids by UHPLC-PDA
2.8. Statistical Analyses
3. Results and Discussion
3.1. Effect of the Co-Solvent on the Extraction Yields and Their Flavonoid Content
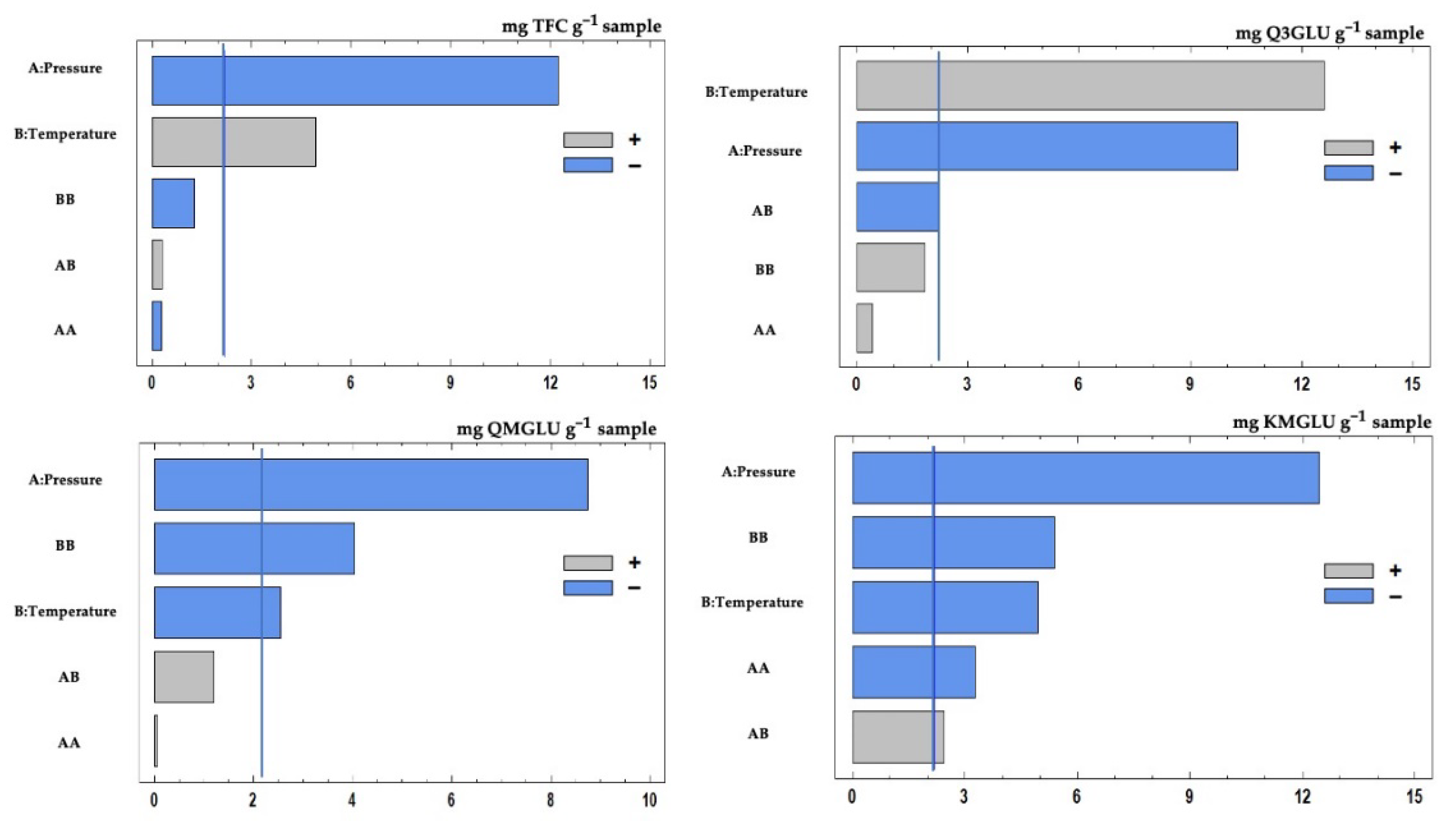
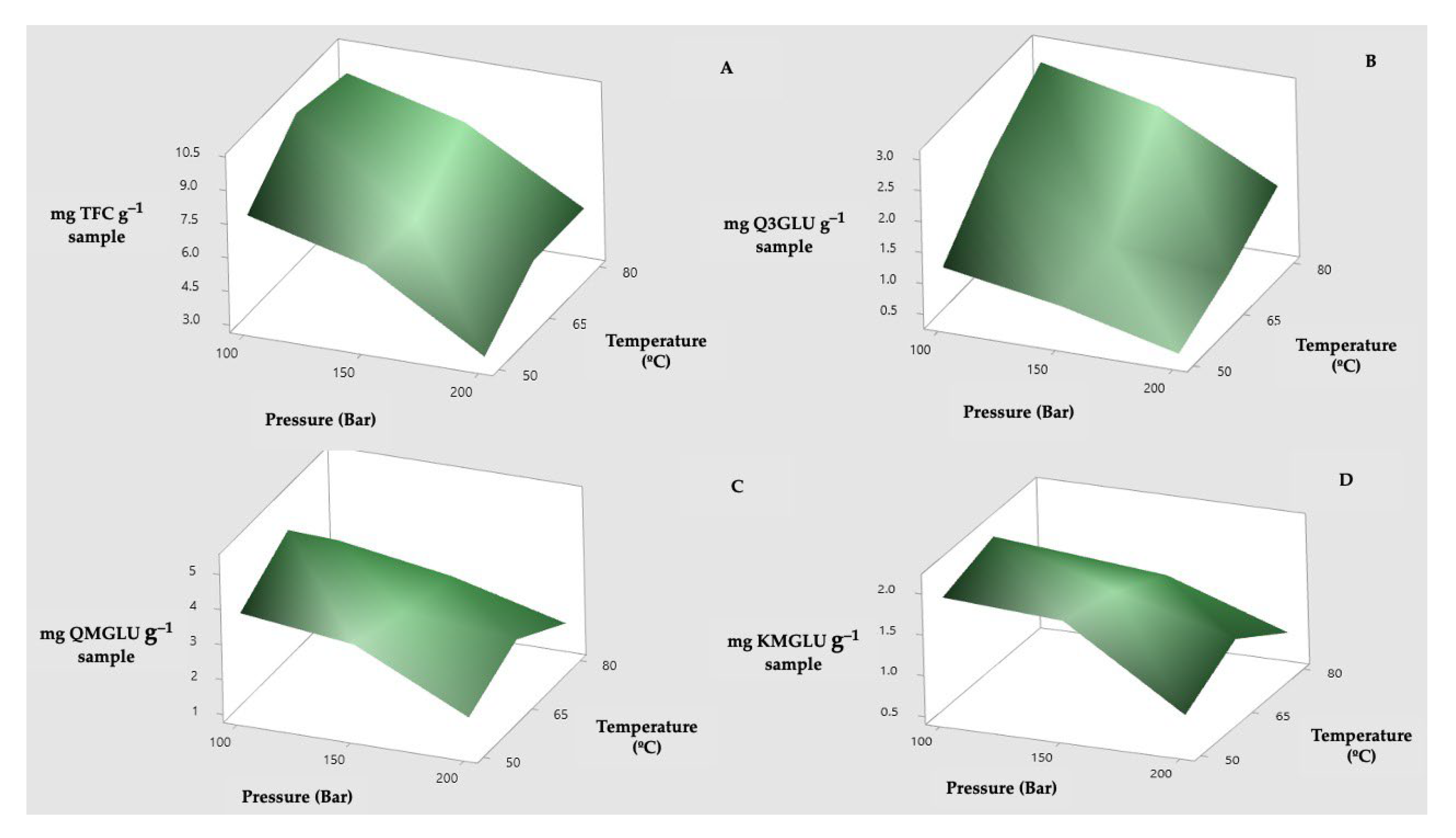
3.2. Effect of Pressure and Temperature on the Extract’s Flavonoid Composition
3.3. Optimal Extraction Conditions
3.4. Extraction Kinetics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Olson, M.E.; Fahey, J.W. Moringa oleifera: A multipurpose tree for the dry tropics. Rev. Mex. Biodivers. 2011, 82, 1071–1082. [Google Scholar]
- Stohs, S.J.; Hartman, M.J. Review of the safety and efficacy of Moringa oleifera. Phytother. Res. 2015, 29, 796–804. [Google Scholar] [CrossRef]
- Anwar, F.; Latif, S.; Ashraf, M.; Gilani, A.H. Moringa oleifera: A Food Plant with Multiple Medicinal Uses. Phytother. Res. 2007, 21, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Manguro, L.O.A.; Lemmen, P. Phenolics of Moringa oleifera leaves. Nat. Prod. Res. 2007, 21, 56–68. [Google Scholar] [CrossRef]
- Kekuda, T.R.; Mallikarjun, N.; Swathi, D.; Nayana, K.V.; Aiyar, M.B.; Rohini, T.R. Antibacterial and Antifungal efficacy of steam distillate of Moringa oleifera Lam. J. Pharm. Sci. Res. 2010, 2, 34–37. [Google Scholar]
- Mahajan, S.G.; Mali, R.G.; Mehta, A.A. Protective effect of ethanolic extract of seeds of Moringa oleifera Lam. against inflammation associated with development of arthritis in rats. J. Immunotoxicol. 2007, 4, 39–47. [Google Scholar] [CrossRef] [Green Version]
- Jaiswal, D.; Kumar Rai, P.; Kumar, A.; Mehta, S.; Watal, G. Effect of Moringa oleifera Lam. leaves aqueous extract therapy on hyperglycemic rats. J. Ethnopharmacol. 2009, 123, 392–396. [Google Scholar] [CrossRef]
- Mehta, K.; Balaraman, R.; Amin, A.H.; Bafna, P.A.; Gulati, O.D. Effect of fruits of Moringa oleifera on the lipid profile of normal and hypercholesterolaemic rabbits. J. Ethnopharmacol. 2003, 86, 191–195. [Google Scholar] [CrossRef]
- Singh, B.N.; Singh, B.R.; Singh, R.L.; Prakash, D.; Dhakarey, R.; Upadhyay, G. Oxidative DNA damage protective activity, antioxidant and anti-quorum sensing potentials of Moringa oleifera. Food Chem. Toxicol. 2009, 47, 1109–1116. [Google Scholar] [CrossRef]
- Verma, A.R.; Vijayakumar, M.; Mathela, C.S.; Rao, C.V. In vitro and in vivo antioxidant properties of different fractions of Moringa oleifera leaves. Food Chem. Toxicol. 2009, 47, 2196–2201. [Google Scholar] [CrossRef]
- Sreelatha, S.; Jeyachitra, A.; Padma, P.R. Antiproliferation and induction of apoptosis by Moringa oleifera leaf extract on human cancer cells. Food Chem. Toxicol. 2011, 49, 1270–1275. [Google Scholar] [CrossRef]
- Rodriguez- Perez, C.; Gilbert-López, B.; Mendiola, J.A.; Segura-Carretero, A.; Quirantes-Pin, R.; Elena, I.B. Optimization of microwave-assisted extraction and pressurized liquid extraction of phenolic compounds from Moringa oleifera leaves by multiresponse surface. Electrophoresis 2016, 37, 1938–1946. [Google Scholar] [CrossRef] [PubMed]
- Yerena-Prieto, B.J.; Gonzalez-Gonzalez, M.; Vázquez-Espinosa, M.; González-de-Peredo, A.V.; García-Alvarado, M.Á.; Palma, M.; Rodriguez-Jimenes, G.D.C.; Barbero, G.F. Optimization of an Ultrasound-Assisted Extraction Method Applied to the Extraction of Flavonoids from Moringa Leaves (Moringa oleífera Lam.). Agronomy 2022, 12, 261. [Google Scholar] [CrossRef]
- Nuapia, Y.; Cukrowska, E.; Tutu, H.; Chimuka, L. Statistical comparison of two modeling methods on pressurized hot water extraction of vitamin C and phenolic compounds from Moringa oleifera leaves. S. Afr. J. Bot. 2020, 129, 9–16. [Google Scholar] [CrossRef]
- Del Valle, J.M.; Aguilera, J.M. Review: High pressure CO2 extraction. Fundamentals and applications in the food industry. Int. J. Food Sci. 1999, 5, 1–24. [Google Scholar] [CrossRef]
- Reyes, F.A.; Mendiola, J.A.; Ibañez, E.; Del Valle, J.M. Astaxanthin extraction from Haematococcus pluvialis using CO2-expanded ethanol. J. Supercrit. Fluids 2014, 92, 75–83. [Google Scholar] [CrossRef] [Green Version]
- Khoddami, A.; Wilkes, M.A.; Roberts, T.H. Techniques for analysis of plant phenolic compounds. Molecules 2013, 18, 2328–2375. [Google Scholar] [CrossRef]
- Fernández-Ponce, M.T.; Casas, L.; Mantell, C.; De La Ossa, E.M. Use of high pressure techniques to produce Mangifera indica L. leaf extracts enriched in potent antioxidant phenolic compounds. Innov. Food Sci. Emerg. Technol. 2015, 29, 94–106. [Google Scholar] [CrossRef]
- Castro-Vargas, H.I.; Rodríguez-Varela, L.I.; Ferreira, S.R.S.; Parada-Alfonso, F. Extraction of phenolic fraction from guava seeds (Psidium guajava L.) using supercritical carbon dioxide and co-solvents. J. Supercrit. Fluids 2010, 51, 319–324. [Google Scholar] [CrossRef]
- Brunner, G. Supercritical fluids: Technology and application to food processing. J. Food Eng. 2005, 67, 21–33. [Google Scholar] [CrossRef]
- Chinnarasu, C.; Montes, A.; Pereyra, C.; Casas, L.; Fernández-Ponce, M.T.; Mantell, C. Preparation of polyphenol fine particles potent antioxidants by a supercritical antisolvent process using different extracts of Olea europaea leaves. Korean J. Chem. Eng. 2016, 33, 594–602. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, D. Supercritical fluid extraction and characterisation of Moringa oleifera leaves oil. Sep. Purif. Technol. 2013, 118, 497–502. [Google Scholar] [CrossRef]
- Rodríguez-Pérez, C.; Mendiola, J.A.; Quirantes-Piné, R.; Ibáñez, E.; Segura-Carretero, A. Green downstream processing using supercritical carbon dioxide, CO2-expanded ethanol and pressurized hot water extractions for recovering bioactive compounds from Moringa oleifera leaves. J. Supercrit. Fluids 2016, 116, 90–100. [Google Scholar] [CrossRef]
- Fernández-Ponce, M.T.; Parjikolaei, B.R.; Lari, H.N.; Casas, L.; Mantell, C.; Martínez de la Ossa, E.J. Pilot-plant scale extraction of phenolic compounds from mango leaves using different green techniques: Kinetic and scale up study. Chem. Eng. J. 2016, 299, 420–430. [Google Scholar] [CrossRef]
- Guamán-Balcázar, M.C.; Montes, A.; Fernández-Ponce, M.T.; Casas, L.; Mantell, C.; Pereyra, C. Generation of potent antioxidant nanoparticles from mango leaves by supercritical antisolvent extraction. J. Supercrit. Fluids 2018, 138, 92–101. [Google Scholar] [CrossRef]
- Ting, S.S.T.; Tomasko, D.L.; Macnaughton, S.J.; Foster, N.R. Chemical-Physical Interpretation of Cosolvent Effects in Supercritical Fluids. Ind. Eng. Chem. Res. 1993, 32, 1482–1487. [Google Scholar] [CrossRef]
- Hwang, R.J.; Ortiz, J. Mitigation of asphaltics deposition during CO2 flood by enhancing CO2 solvency with chemical modifiers. Org. Geochem. 2000, 31, 1451–1462. [Google Scholar] [CrossRef]
- Herrero, M.; Castro-Puyana, M.; Mendiola, J.A.; Ibañez, E. Compressed fluids for the extraction of bioactive compounds. TrAC-Trends Anal. Chem. 2013, 43, 67–83. [Google Scholar] [CrossRef]
- Cejudo Bastante, C.; Casas Cardoso, L.; Fernández Ponce, M.T.; Mantell Serrano, C.; Martínez de la Ossa-Fernández, E.J. Characterization of olive leaf extract polyphenols loaded by supercritical solvent impregnation into PET/PP food packaging films. J. Supercrit. Fluids 2018, 140, 196–206. [Google Scholar] [CrossRef]
- Coppin, J.P.; Xu, Y.; Chen, H.; Pan, M.H.; Ho, C.T.; Juliani, R. Determination of flavonoids by LC/MS and anti-inflammatory activity in Moringa oleifera. J. Funct. Foods 2013, 5, 1892–1899. [Google Scholar] [CrossRef]
- Oldoni, T.L.C.; Merlin, N.; Karling, M.; Carpes, S.T.; De Alencar, S.M.; Morales, R.G.F. Bioguided extraction of phenolic compounds and UHPLC-ESI-Q-TOF-MS/MS characterization of extracts of Moringa oleifera leaves collected in Brazil. Food Res. Int. 2019, 125, 108647. [Google Scholar] [CrossRef] [PubMed]
- Amaglo, N.K.; Bennett, R.N.; Curto, R.B.L.; Rosa, E.A.; Turco, V.; Giuffrida, A. Profiling selected phytochemicals and nutrients in different tissues of the multipurpose tree Moringa oleifera Lam., grown in Ghana. Food Chem. 2010, 122, 1047–1054. [Google Scholar] [CrossRef]
- Rodríguez-Pérez, C.; Quirantes-Piné, R.; Fernández-Gutiérrez, A.; Segura-Carretero, A. Optimization of extraction method to obtain a phenolic compounds-rich extract from Moringa oleifera Lam leaves. Ind. Crops Prod. 2015, 66, 246–254. [Google Scholar] [CrossRef]
- Garcia-Salas, P.; Morales-Soto, A.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Phenolic-compound-extraction systems for fruit and vegetable samples. Molecules 2010, 15, 8813–8826. [Google Scholar] [CrossRef] [PubMed]
- Goli, A.H.; Barzegar, M.; Sahari, M.A. Antioxidant activity and total phenolic compounds of pistachio (Pistachia vera) hull extracts. Food Chem. 2005, 92, 521–525. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, Y.G.; Park, J.G.; Lee, J. Supercritical fluid extracts of Moringa oleifera and their unsaturated fatty acid components inhibit biofilm formation by Staphylococcus aureus. Food Control 2017, 80, 74–82. [Google Scholar] [CrossRef]
- Toews, K.L.; Shroll, R.M.; Wai, C.M.; Smart, N.G. pH-Defining Equilibrium between Water and Supercritical CO2. influence on SFE of Organics and Metal Chelates. Anal. Chem. 1995, 67, 4040–4043. [Google Scholar] [CrossRef]
- Solana, M.; Boschiero, I.; Dall’Acqua, S.; Bertucco, A. A comparison between supercritical fluid and pressurized liquid extraction methods for obtaining phenolic compounds from Asparagus officinalis L. J. Supercrit. Fluids 2015, 100, 201–208. [Google Scholar] [CrossRef]
- Paes, J.; Dotta, R.; Barbero, G.F.; Martínez, J. Extraction of phenolic compounds and anthocyanins from blueberry (Vaccinium myrtillus L.) residues using supercritical CO2 and pressurized liquids. J. Supercrit. Fluids 2014, 95, 8–16. [Google Scholar] [CrossRef]
- Ting, S.S.T.; Tomasko, D.L.; Macnaughton, S.J.; Foster, N.R. Solubility of naproxen in supercritical carbon dioxide with and without cosolvents. Ind. Eng. Chem. Res. 1993, 32, 1471–1481. [Google Scholar] [CrossRef]
- Garcia-Mendoza, M.D.P.; Espinosa-Pardo, F.A.; Baseggio, A.M.; Barbero, G.F.; Maróstica Junior, M.R.; Rostagno, M.A. Extraction of phenolic compounds and anthocyanins from juçara (Euterpe edulis Mart.) residues using pressurized liquids and supercritical fluids. J. Supercrit. Fluids 2017, 119, 9–16. [Google Scholar] [CrossRef]
- Seabra, I.J.; Braga, M.E.M.; Batista, M.T.; de Sousa, H.C. Effect of solvent (CO2/ethanol/H2O) on the fractionated enhanced solvent extraction of anthocyanins from elderberry pomace. J. Supercrit. Fluids 2010, 54, 145–152. [Google Scholar] [CrossRef]
- Wissam, Z.; Ghada, B.; Wassim, A.; Warid, K. Effective extraction of polyphenols and proanthocyanidins from pomegranate’s peel. Int. J. Pharm. Pharm. Sci. 2012, 4, 675–682. [Google Scholar]
- Jayaprakasha, G.K.; Singh, R.P.; Sakariah, K.K. Antioxidant activity of grape seed (Vitis vinifera) extracts on peroxidation models in vitro. Food Chem. 2001, 73, 285–290. [Google Scholar] [CrossRef]
- Pappa, G.D.; Perakis, C.; Tsimpanogiannis, I.N.; Voutsas, E.C. Thermodynamic modeling of the vapor-liquid equilibrium of the CO2/H2O mixture. Fluid Phase Equilibria 2009, 284, 56–63. [Google Scholar] [CrossRef]
- Espinosa-Pardo, F.A.; Nakajima, V.M.; Macedo, G.A.; Macedo, J.A.; Martínez, J. Extraction of phenolic compounds from dry and fermented orange pomace using supercritical CO2 and cosolvents. Food Bioprod. Process. 2017, 101, 1–10. [Google Scholar] [CrossRef]

| % CO2 | % EtOH | % H2O | mg TFC g–1 Sample |
|---|---|---|---|
| 75.0 | 25.0 | 0.0 | 0.0 e |
| 50.0 | 50.0 | 0.0 | 0.0 e |
| 25.0 | 75.0 | 0.0 | 0.0 e |
| 75.0 | 0.0 | 25.0 | 2.478 ± 0.104 d |
| 50.0 | 0.0 | 50.0 | 6.931 ± 0.258 a |
| 25.0 | 0.0 | 75.0 | 4.749 ± 0.148 b |
| 50.0 | 40.0 | 10.0 | 3.828 ± 0.355 c |
| 50.0 | 10.0 | 40.0 | 2.010 ± 0.123 d |
| Variable | mg Flavonoid g–1 Sample | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| P (Bar) | T (°C) | AP1 | AP2 | Q3GLU | QMGLU | QAGLU | K3GLU | KMGLU | TFC |
| 100 | 50 | 0.18 ± 0.03 b | 0.12 ± 0.05 b | 1.21 ± 0.015 e | 3.79 ± 0.05 b | 0.09 ± 0.00 a | 0.46 ± 0.04 e | 1.98 ± 0.09 b | 7.85 ± 0.16 c |
| 100 | 65 | 0.12 ± 0.03 c | 0.12 ± 0.00 b | 2.18 ± 0.07 c | 4.77 ± 0.02 a | 0.05 ± 0.01 b | 0.62 ± 0.03 d | 2.12 ± 0.05 a | 9.98 ± 0.22 a |
| 100 | 80 | ND | 0.29 ± 0.05 a | 2.83 ± 0.02 a | 3.10 ± 0.01 cd | ND | 1.77 ± 0.07 a | 1.46 ± 0.09 d | 9.47 ± 0.14 b |
| 150 | 50 | ND | ND | 0.86 ± 0.03 g | 3.40 ± 0.02 c | ND | 0.21 ± 0.03 f | 1.79 ± 0.05 b | 6.27 ± 0.12 d |
| 150 | 65 | 0.12 ± 0.05 c | ND | 1.01 ± 0.04 f | 3.09 ± 0.20 de | ND | 0.35 ± 0.04 ef | 1.63 ± 0.04 c | 6.23 ± 0.16 d |
| 150 | 80 | 0.30 ± 0.02 a | ND | 2.44 ± 0.06 b | 2.64 ± 0.07 f | ND | 1.51 ± 0.04 b | 1.33 ± 0.05 de | 8.23 ± 0.12 c |
| 200 | 50 | ND | ND | 0.42 ± 0.05 h | 1.80 ± 0.05 g | ND | ND | 0.84 ± 0.03 f | 3.07 ± 0.08 f |
| 200 | 65 | 0.07 ± 0.03 d | ND | 0.89 ± 0.03 g | 2.68 ± 0.08 ef | ND | 0.22 ± 0.03f | 1.28 ± 0.02 e | 5.16 ± 0.15 e |
| 200 | 80 | 0.16 ± 0.01 b | ND | 1.49 ± 0.04 d | 1.78 ± 0.02 g | ND | 0.96 ± 0.06c | 0.82 ± 0.08 f | 5.22 ± 0.07 e |
| Observed Values | Estimated Values | |||||||
|---|---|---|---|---|---|---|---|---|
| Q3GLU | QMGLU | KMGLU | TFC | Q3GLU | QMGLU | KMGLU | TFC | |
| Exp./R2 | 0.94788 | 0.84352 | 0.92937 | 0.90605 | ||||
| 1 | 1.211 | 3.798 | 1.986 | 7.851 | 1.277 | 4.070 | 2.001 | 8.078 |
| 2 | 2.187 | 4.770 | 2.124 | 9.998 | 1.959 | 4.374 | 2.049 | 9.350 |
| 3 | 2.839 | 3.102 | 1.467 | 9.471 | 3.002 | 3.242 | 1.462 | 9.790 |
| 4 | 0.864 | 3.403 | 1.793 | 6.275 | 0.807 | 3.008 | 1.669 | 5.808 |
| 5 | 1.017 | 3.098 | 1.632 | 6.230 | 1.337 | 3.465 | 1.818 | 7.150 |
| 6 | 2.442 | 2.645 | 1.333 | 8.238 | 2.229 | 2.484 | 1.333 | 8.158 |
| 7 | 0.425 | 1.802 | 0.848 | 3.075 | 0.422 | 1.967 | 0.952 | 3.140 |
| 8 | 0.893 | 2.682 | 1.284 | 5.165 | 0.801 | 2.576 | 1.203 | 5.095 |
| 9 | 1.496 | 3.798 | 0.820 | 5.220 | 1.542 | 1.747 | 0.820 | 5.342 |
| Factors | TFC | Q3GLU | QMGLU | KMGLU |
|---|---|---|---|---|
| Pressure (bar) | 100 | 100 | 100 | 100 |
| Temperature (°C) | 80 | 80 | 55.8 | 53.8 |
| Maximum concentration (mg g−1 sample) | 9.791 | 3.003 | 4.435 | 2.107 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yerena-Prieto, B.J.; Gonzalez-Gonzalez, M.; García-Alvarado, M.Á.; Casas, L.; Palma, M.; Rodríguez-Jimenes, G.d.C.; Barbero, G.F.; Cejudo-Bastante, C. Evaluation of the Effect of Different Co-Solvent Mixtures on the Supercritical CO2 Extraction of the Phenolic Compounds Present in Moringa oleifera Lam. Leaves. Agronomy 2022, 12, 1450. https://doi.org/10.3390/agronomy12061450
Yerena-Prieto BJ, Gonzalez-Gonzalez M, García-Alvarado MÁ, Casas L, Palma M, Rodríguez-Jimenes GdC, Barbero GF, Cejudo-Bastante C. Evaluation of the Effect of Different Co-Solvent Mixtures on the Supercritical CO2 Extraction of the Phenolic Compounds Present in Moringa oleifera Lam. Leaves. Agronomy. 2022; 12(6):1450. https://doi.org/10.3390/agronomy12061450
Chicago/Turabian StyleYerena-Prieto, Beatriz Juliana, Monserrat Gonzalez-Gonzalez, Miguel Ángel García-Alvarado, Lourdes Casas, Miguel Palma, Guadalupe del Carmen Rodríguez-Jimenes, Gerardo F. Barbero, and Cristina Cejudo-Bastante. 2022. "Evaluation of the Effect of Different Co-Solvent Mixtures on the Supercritical CO2 Extraction of the Phenolic Compounds Present in Moringa oleifera Lam. Leaves" Agronomy 12, no. 6: 1450. https://doi.org/10.3390/agronomy12061450