Yield and Quality of Processing Tomato as Improved by Biostimulants Based on Trichoderma sp. and Ascophyllum nodosum and Biodegradable Mulching Films
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design, Growing Conditions, and Tomato Cultivar
2.2. Mulch Management and Biostimulant Characteristics and Application
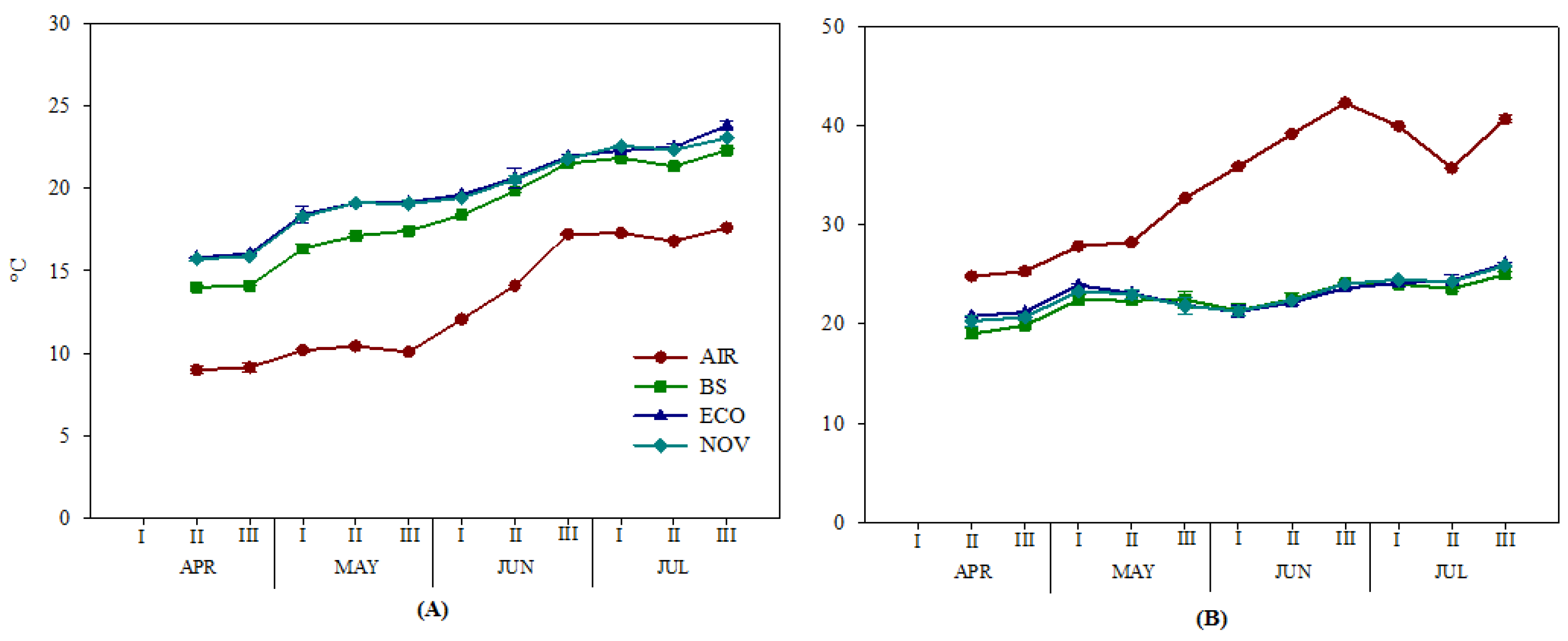
2.3. Soil and Air Temperatures
2.4. Yield, Morphological Parameters, and Tomato Fruit Colorimetry
2.5. Lipophilic and Hydrophilic Antioxidant Activity and Total Ascorbic Acid Analysis
2.6. Carotenoid Analysis and Nitrate Content
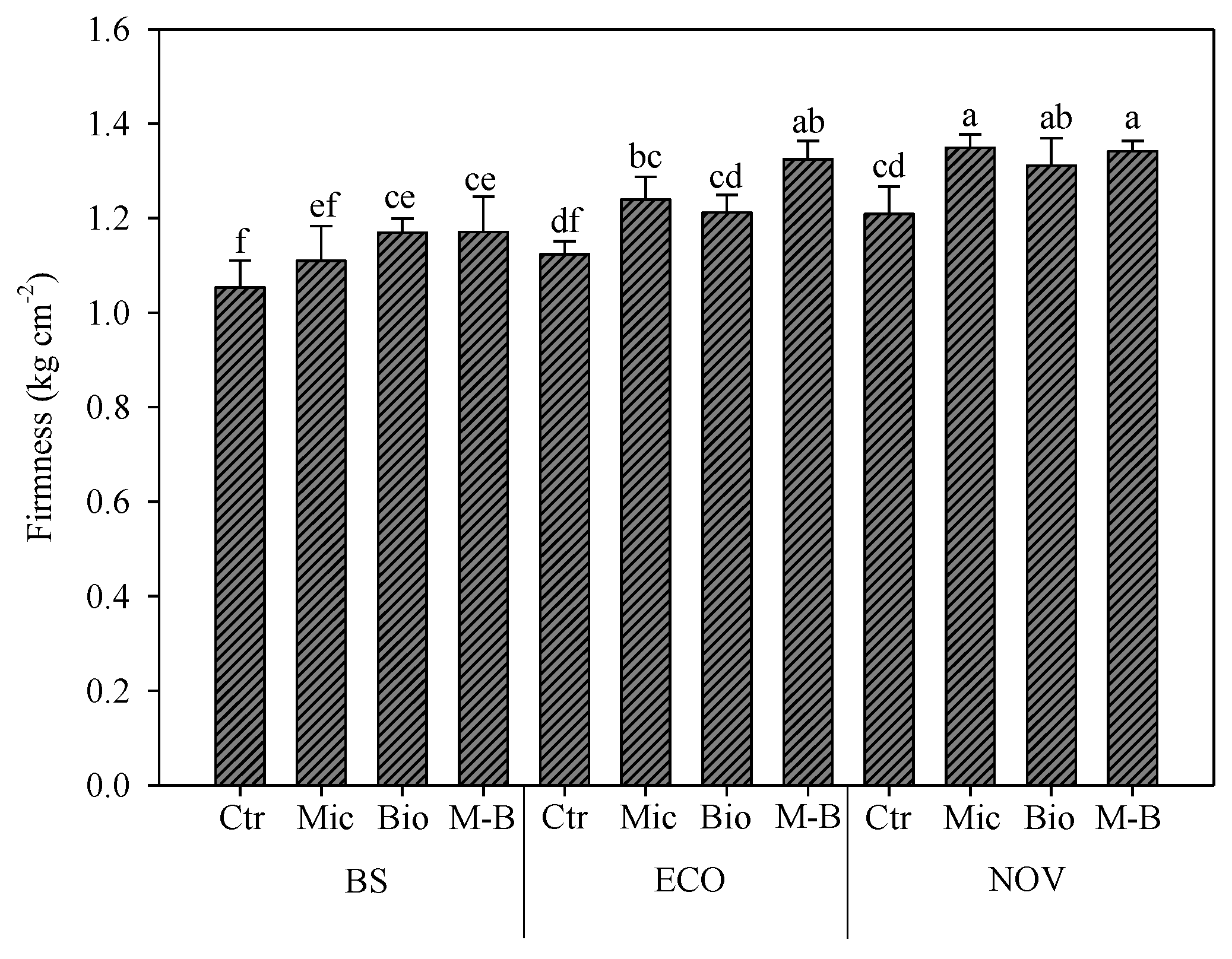
2.7. Total Soluble Solids Content and Firmness Determination
2.8. Statistical Processing
3. Results
3.1. Soil Temperatures as Affected by Mulching Films
3.2. Yield and Yield Components of Tomato Fruits
3.3. Color Parameters and Carotenoids of Tomato Fruits
3.4. Total Soluble Solids Content and Firmness of Tomato Fruits
3.5. Antioxidant Activity and Compounds in Tomato Fruits
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Istat 2021. Available online: http://dati.istat.it/Index.aspx?QueryId=33703 (accessed on 14 December 2022).
- Godfray, H.C.J.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Pretty, J.; Robinson, S.; Thomas, S.M. Food security: The challenge of feeding 9 billion people. Science 2010, 327, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Lopes, W.A.R.; Negreiros, M.Z.; Dombroski, J.L.D.; Rodrigues, G.S.O.; Soares, A.M.; Araújo, A.P. An’alise do crescimento de tomate ‘SM-16′ cultivado sob diferentes coberturas de solo. Hortic. Bras. 2011, 29, 554–561. [Google Scholar] [CrossRef]
- Briassoulis, D.; Giannoulis, A. Evaluation of the functionality of bio-based plastic mulching films. Polym. Test. 2018, 67, 99–109. [Google Scholar] [CrossRef]
- Zhang, X.; You, S.; Tian, Y.; Li, J. Comparison of plastic film, biodegradable paper and bio-based film mulching for summer tomato production: Soil properties, plant growth, fruit yield and fruit quality. Sci. Hortic. 2019, 249, 38–48. [Google Scholar] [CrossRef]
- Touchaleaume, F.; Martin-Closas, L.; Angellier-Coussy, H.; Chevillard, A.; Cesar, G.; Gontard, N.; Gastaldi, E.J.C. Performance and environmental impact of biodegradable polymers as agricultural mulching films. Curr. Biol. 2016, 144, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Martin-Closas, L.; Costa, J.; Pelacho, A.M. Agronomic effects of biodegradable films on crop and field environment. In Soil Degradable Bioplastics for a Sustainable Modern Agriculture; Malinconico, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 67–104. [Google Scholar] [CrossRef]
- Shen, M.; Song, B.; Zeng, G.; Zhang, Y.; Huang, W.; Wen, X.; Tang, W. Are biodegradable plastics a promising solution to solve the global plastic pollution? Environ. Pollut. 2020, 263, 114469. [Google Scholar] [CrossRef]
- Shruti, V.C.; Kutralam-Muniasamy, G. Bioplastics: Missing link in the era of microplastics. Sci. Total Environ. 2019, 697, 134139. [Google Scholar] [CrossRef]
- Cowan, J.S.; Miles, C.A.; Andrews, P.K.; Inglis, D.A. Biodegradable mulch performed comparably to polyethylene in high tunnel tomato (Solanum lycopersicum L.) production. J. Sci. Food Agric. 2014, 94, 1854–1864. [Google Scholar] [CrossRef]
- DeVetter, L.W.; Zhang, H.; Ghimire, S.; Watkinson, S.; Miles, C.A. Plastic biodegradable mulches reduce weeds and promote crop growth in day-neutral strawberry in western Washington. HortScience 2017, 52, 1700–1706. [Google Scholar] [CrossRef]
- Ghimire, S.; Wszelaki, A.L.; Moore, J.C.; Inglis, D.A.; Miles, C. The use of biodegradable mulches in pie pumpkin crop production in two diverse climates. HortScience 2018, 53, 288–294. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, C.; Cao, G.; Wang, D.; Ho, S.H. A sustainable solution to plastics pollution: An eco-friendly bioplastic film production from high-salt contained Spirulina sp. residues. J. Hazard. Mater. 2020, 388, 121773. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, D.; Nagarajan, S.; Aggarwal, P.; Gupta, V.K.; Tomar, R.K.; Garg, R.N.; Sahoo, R.N.; Sarkar, A.; Chopra, U.K.; Sarma, K.S.S.; et al. Effect of mulching on soil and plant water status, and the growth and yield of wheat (Triticum aestivum L.) in a semi-arid environment. Agric. Water Manag. 2008, 95, 1323–1334. [Google Scholar] [CrossRef]
- Sharma, R.; Bhardwaj, S. Effect of mulching on soil and water conservation-A review. Agric. Rev. 2017, 38, 311–315. [Google Scholar] [CrossRef]
- Acharya, C.L.; Hati, K.M.; Bandyopadhyay, K.K. Mulches. In Encyclopedia of Soils in the Environment; Hillel, D., Rosenzweig, C., Pawlson, D.S., Scow, K.M., Sorger, M.J., Sparks, D.L., Hatfield, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2005; pp. 521–532. [Google Scholar]
- Jordán, A.; Zavala, L.M.; Gil, J. Effects of mulching on soil physical properties and runoff under semi-arid conditions in southern Spain. Catena 2010, 81, 77–85. [Google Scholar] [CrossRef]
- Cozzolino, E.; Giordano, M.; Fiorentino, N.; El-Nakhel, C.; Pannico, A.; Di Mola, I.; Rouphael, Y. Appraisal of biodegradable mulching films and vegetal-derived biostimulant application as eco-sustainable practices for enhancing lettuce crop performance and nutritive value. Agronomy 2020, 10, 427. [Google Scholar] [CrossRef]
- Di Mola, I.; Cozzolino, E.; Ottaiano, L.; Duri, L.G.; Riccardi, R.; Spigno, P.; Mori, M. The effect of novel biodegradable films on agronomic performance of zucchini squash grown under open-field and greenhouse conditions. Aust. J. Crop Sci. 2019, 13, 1810–1818. [Google Scholar]
- Carillo, P.; Colla, G.; Fusco, G.M.; Dell’Aversana, E.; El-Nakhel, C.; Giordano, M.; Pannico, A.; Cozzolino, E.; Mori, M.; Reynaud, H.; et al. Morphological and Physiological Responses Induced by Protein Hydrolysate-Based Biostimulant and Nitrogen Rates in Greenhouse Spinach. Agronomy 2019, 9, 450. [Google Scholar] [CrossRef]
- Du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Du Jardin, P.; Xu, L.; Geelen, D. Agricultural functions and action mechanisms of plant biostimulants (PBs). In The Chemical Biology of Plant Biostimulants; Geelen, D., Xu, L., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2020; pp. 1–30. [Google Scholar]
- Battacharyya, D.; Babgohari, M.Z.; Rathor, P.; Prithiviraj, B. Seaweed extracts as biostimulants in horticulture. Sci. Hortic. 2015, 196, 39–48. [Google Scholar] [CrossRef]
- Khan, W.; Rayirath, U.P.; Subramanian, S.; Jithesh, M.N.; Rayorath, P.; Hodges, D.M.; Critchley, A.T.; Craigie, J.S.; Norrie, J.; Prithiviraj, B. Seaweed extracts as biostimulants of plant growth and development. J. Plant Growth Regul. 2009, 28, 386–399. [Google Scholar] [CrossRef]
- Harman, G.E.; Howell, C.R.; Viterbo, A.; Chet, I.; Lorito, M. Trichoderma species—Opportunistic, avirulent plant symbionts. Nat. Rev. Microbiol. 2004, 2, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Lorito, M.; Woo, S.L.; Harman, G.E.; Monte, E. Translational research on Trichoderma: From ‘omics to the field. Annu. Rev. Phytopathol. 2010, 48, 395–417. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.L.; Ruocco, M.; Vinale, F.; Nigro, M.; Marra, R.; Lombardi, N.; Pascale, A.; Lanzuise, S.; Manganiello, G.; Lorito, M. Trichoderma-based products and their widespread use in agriculture. Open Mycol. J. 2014, 8, 71–126. [Google Scholar] [CrossRef]
- Shoresh, M.; Harman, G.E.; Mastouri, F. Induced systemic resistance and plant responses to fungal biocontrol agents. Annu. Rev. Phytopathol. 2010, 48, 21–43. [Google Scholar] [CrossRef] [PubMed]
- Carillo, P.; Woo, S.L.; Comite, E.; El-Nakhel, C.; Rouphael, Y.; Fusco, G.M.; Borzacchiello, A.; Lanzuise, S.; Vinale, F. Application of Trichoderma harzianum, 6-Pentyl-α-pyrone and Plant Biopolymer Formulations Modulate Plant Metabolism and Fruit Quality of Plum Tomatoes. Plants 2020, 9, 771. [Google Scholar] [CrossRef] [PubMed]
- Harman, G.E. Overview of mechanisms and uses of Trichoderma spp. Phytopathology 2006, 96, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Marra, R.; Lombardi, N.; Piccolo, A.; Bazghaleh, N.; Prashar, P.; Vandenberg, A.; Woo, S. Mineral biofortification and growth stimulation of lentil plants inoculated with Trichoderma strains and metabolites. Microorganisms 2021, 10, 87. [Google Scholar] [CrossRef]
- Marra, R.; Lombardi, N.; d’Errico, G.; Troisi, J.; Scala, G.; Vinale, F.; Lorito, M. Application of Trichoderma strains and metabolites enhances soybean productivity and nutrient content. J. Agri. Food Chem. 2019, 67, 1814–1822. [Google Scholar] [CrossRef] [PubMed]
- Di Mola, I.; Ottaiano, L.; Cozzolino, E.; Senatore, M.; Sacco, A.; El-Nakhel, C.; Mori, M. Trichoderma spp. and mulching films differentially boost qualitative and quantitative aspects of greenhouse lettuce under diverse N conditions. Horticulturae 2020, 6, 55. [Google Scholar] [CrossRef]
- Hargreaves, G.; Samani, Z. Reference crop evapotranspiration from temperature. Appl. Eng. Agric. 1985, 1, 96–99. [Google Scholar] [CrossRef]
- Tradecorp. Available online: https://tradecorp.it/product/phylgreen (accessed on 5 February 2021).
- Koppert Italia. Available online: https://www.koppert.it/trianum-p (accessed on 5 February 2021).
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Fogliano, V.; Verde, V.; Randazzo, G.; Ritieni, A. Method for measuring antioxidant activity and its application to monitoring the antioxidant capacity of wines. J. Agric. Food Chem. 1999, 47, 1035–1040. [Google Scholar] [CrossRef] [PubMed]
- Kampfenkel, K.; Van Montagu, M.; Inzé, D. Extraction and determination of ascorbate and dehydroascorbate from plant tissue. Anal. Biochem. 1995, 225, 165–167. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Sah, R.N. Nitrate-nitrogen determination: A critical review. Commun. Soil Sci. Plant Anal. 1994, 25, 2841–2869. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Horwitz, B.A.; Herrera-estrella, A.; Schmoll, M.; Kenerley, C.M. Trichoderma research in the genome era. Annu. Rev. Phytopathol. 2012, 51, 105–129. [Google Scholar] [CrossRef]
- Nicolás, C.; Hermosa, R.; Rubio, B.; Mukherjee, P.K.; Monte, E. Trichoderma genes in plants for stress tolerance-status and prospects. Plant Sci. 2014, 228, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Bulgari, R.; Morgutti, S.; Cocetta, G.; Negrini, N.; Farris, S.; Calcante, A.; Spinardi, A.; Ferrari, E.; Mignani, I.; Oberti, R.; et al. Evaluation of Borage Extracts As Potential Biostimulant Using a Phenomic, Agronomic, Physiological, and Biochemical Approach. Front. Plant Sci. 2017, 8, 935. [Google Scholar] [CrossRef]
- Bulgari, R.; Franzoni, G.; Ferrante, A. Biostimulants Application in Horticultural Crops under Abiotic Stress Conditions. Agronomy 2019, 9, 306. [Google Scholar] [CrossRef]
- Bonini, P.; Rouphael, Y.; Miras-Moreno, B.; Lee, B.; Cardarelli, M.; Erice, G.; Cirino, V.; Lucini, L.; Colla, G. A Microbial-Based Biostimulant Enhances Sweet Pepper Performance by Metabolic Reprogramming of Phytohormone Profile and Secondary Metabolism. Front. Plant Sci. 2020, 11, 567388. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G. Toward a Sustainable Agriculture Through Plant Biostimulants: From Experimental Data to Practical Applications. Agronomy 2020, 10, 1461. [Google Scholar] [CrossRef]
- Kasirajan, S.; Ngouajio, M. Polyethylene and biodegradable mulches for agricultural applications: A review. Agron. Sustain. Dev. 2012, 32, 501–529. [Google Scholar] [CrossRef]
- Kyrikou, I.; Briassoulis, D. Biodegradation of Agricultural Plastic Films: A Critical Review. J. Polym. Environ. 2007, 15, 125–150. [Google Scholar] [CrossRef]
- Briassoulis, D.; Babou, E.; Hiskakis, M.; Kyrikou, I. Degradation in soil behavior of artificially aged polyethylene films with pro-oxidants. J. Appl. Polym. Sci. 2015, 132, 42289. [Google Scholar] [CrossRef]
- Muroi, F.; Tachibana, Y.; Kobayashi, Y.; Sakurai, T.; Kasuya, K.I. Influences of poly (butylene adipate-co-terephthalate) on soil microbiota and plant growth. Polym. Degrad. Stab. 2016, 129, 338–346. [Google Scholar] [CrossRef]
- Taromi Aliabadi, B.; Hassandokht, M.R.; Etesami, H.; Alikhani, H.A.; Dehghanisanij, H. Effect of mulching on some characteristics of tomato (Lycopersicon esculentum Mill.) under deficit irrigation. J. Agric. Sci. Technol. 2019, 21, 927–941. [Google Scholar]
- Moreno, M.M.; Cirujeda, A.; Aibar, J. Soil thermal and productive responses of biodegradable mulch materials in a processing tomato (Lycopersicon esculentum Mill.) crop. Research 2016, 54, 207–221. [Google Scholar] [CrossRef]
- Di Mola, I.; Cozzolino, E.; Ottaiano, L.; Riccardi, R.; Spigno, P.; Fagnano, M.; Mori, M. Agronomic and environmental benefits of ‘re-using’a biodegradable mulching film for two consecutive lettuce cycles. Ital. J. Agron. 2022, 17, 2061. [Google Scholar]
- Cozzolino, E.; Di Mola, I.; Ottaiano, L.; Bilotto, M.; Petriccione, M.; Ferrara, E.; Morra, L. Assessing Yield and Quality of Melon (Cucumis melo L.) Improved by Biodegradable Mulching Film. Plants 2023, 12, 219. [Google Scholar] [CrossRef]
- Costa, R.; Saraiva, A.; Carvalho, L.; Duarte, E. The use of biodegradable mulch films on strawberry crop in Portugal. Sci. Hortic. 2014, 173, 65–70. [Google Scholar] [CrossRef]
- Waterer, D. Evaluation of biodegradable mulches for production of warm-season vegetable crops. Can. J. Plant Sci. 2010, 90, 737–743. [Google Scholar] [CrossRef]
- Caruso, G.; El-Nakhel, C.; Rouphael, Y.; Comite, E.; Lombardi, N.; Cuciniello, A.; Woo, S.L. Diplotaxis tenuifolia (L.) DC. Yield and Quality as Influenced by Cropping Season, Protein Hydrolysates, and Trichoderma applications. Plants 2020, 9, 697. [Google Scholar] [CrossRef] [PubMed]
- Fiorentino, N.; Ventorino, V.; Woo, S.L.; Pepe, O.; De Rosa, A.; Gioia, L.; Romano, I.; Lombardi, N.; Napolitano, M.; Colla, G.; et al. Trichoderma-based biostimulants modulate rhizosphere microbial populations and improve N uptake efficiency, yield, and nutritional quality of leafy vegetables. Front. Plant. Sci. 2018, 9, 743. [Google Scholar] [CrossRef]
- Vinale, F.; Sivasithamparam, K.; Ghisalberti, E.L.; Marra, R.; Woo, S.L.; Lorito, M. Trichoderma–plant–pathogen interactions. Soil Biol. Biochem. 2008, 40, 1–10. [Google Scholar] [CrossRef]
- Vinale, F.; Sivasithamparam, K. Beneficial effects of Trichoderma secondary metabolites on crops. Phytother. Res. 2020, 34, 2835–2842. [Google Scholar] [CrossRef] [PubMed]
- Vinale, F.; Mazzei, P.; Woo, S.L.; Pascale, A.; Lorito, M.; Piccolo, A. Metabolomics by Proton High-Resolution Magic-Angle-Spinning Nuclear Magnetic Resonance of Tomato Plants Treated with Two Secondary Metabolites Isolated from Trichoderma. J. Agric. Food Chem. 2016, 64, 3538–3545. [Google Scholar]
- Larkin, R.P.; Roberts, D.P.; Gracia-Garza, J.A. Biological control of fungal diseases. In Fungicidal Activity, Chemical and Biological Approaches; Wiley: Hoboken, NJ, USA, 1998. [Google Scholar]
- Meyer, S.L.; Roberts, D.P. Combinations of biocontrol agents for management of plant parasitic nematodes and soilborne plant-pathogenic fungi. J. Nematol. 2002, 34, 1. [Google Scholar]
- Huang, H.C.; Bremer, E.; Hynes, R.K.; Erickson, R.S. Foliar application of fungal biocontrol agents for the control of white mold of dry bean caused by Sclerotinia sclerotiorum. Biol. Control. 2000, 18, 270–276. [Google Scholar] [CrossRef]
- Fleming, T.R.; Fleming, C.C.; Levy, C.C.; Repiso, C.; Hennequart, F.; Nolasco, J.B.; Liu, F. Biostimulants enhance growth and drought tolerance in Arabidopsis thaliana and exhibit chemical priming action. Ann. Appl. Biol. 2019, 174, 153–165. [Google Scholar] [CrossRef]
- Ali, N.; Farrell, A.; Ramsubhag, A.; Jayaraman, J. The effect of Ascophyllum nodosum extract on the growth, yield and fruit quality of tomato grown under tropical conditions. J. Appl. Phyc. 2016, 28, 1353–1362. [Google Scholar] [CrossRef]
- Ruiz-Cisneros, M.F.; Ornelas-Paz, J.; Olivas-Orozco, G.I.; Acosta-Muñiz, C.H.; Sepúlveda-Ahumada, D.R.; Pérez-Corral, D.A.; Rios-Velasco, C.; Salas-Marina, M.A.; Fernández-Pavía, P. Effect of Trichoderma spp. and phytopathogenic fungi on plant growth and tomato fruit quality. Mex. J. Phytop. 2018, 36, 444–456. [Google Scholar]
- Huang, Y.; Lu, R.; Chen, K. Prediction of firmness parameters of tomatoes by portable visible and near-infrared spectroscopy. J. Food Eng 2018, 222, 185–198. [Google Scholar] [CrossRef]
- Shafique, H.A.; Sultana, V.; Ehteshamul-Haque, S.; Athar, M. Management of soil-borne diseases of organic vegetables. J. Plant Prot. Res. 2016, 56, 221–230. [Google Scholar] [CrossRef]
- Cozzolino, E.; Di Mola, I.; Ottaiano, L.; El-Nakhel, C.; Rouphael, Y.; Mori, M. Foliar application of plant-based biostimulants improve yield and upgrade qualitative characteristics of processing tomato. Ita. J. Agron. 2021, 16, 1825. [Google Scholar] [CrossRef]
- Davies, J.N.; Hobson, G.E. The constituents of tomato fruit. The influence of environment, nutrition and genotype. Crit. Rev. Food Sci. Nutr. 1981, 15, 205–280. [Google Scholar] [CrossRef] [PubMed]
- Hobson, G.E.; Adams, P.; Dixon, T.J. Assessing the color of tomato fruit during the ripening. J. Sci. Food Agric. 1983, 34, 286–292. [Google Scholar] [CrossRef]
- Dixon, T.J.; Hobson, G.E. A general method for the instrumental assessment of the colour of tomato fruit during ripening. J. Sci. Food Agric. 1984, 35, 1277–1281. [Google Scholar] [CrossRef]
- Riquelme, F. Postcosecha del tomate para consumo en fresco. In El Cultivo del Tomate; Prensa, M., Ed.; Dialnet: Madrid, Spain, 1995; pp. 590–623. [Google Scholar]
- Gordon, S.; Lindstrom, J.; Loy, B.; Rudd, D.; Wells, O. Theory and development of wavelength selective mulches. Proc. Natl. Agric. Plast. Congr. 1989, 21, 193–197. [Google Scholar]
- Giovanelli, G.; Paradiso, A. Paradise Stability of dried and intermediate moisture tomato pulp during storage. J. Agri. Food Chem. 2002, 50, 7277–7281. [Google Scholar] [CrossRef]
- Clevidence, B.A.; Judd, J.T.; Schaefer, E.J.; Jenner, J.L.; Lichtenstein, A.H.; Muesing, R.A.; Sunkin, M.E. Plasma lipoprotein (a) levels in men and women consuming diets enriched in saturated, cis-, or trans-monounsaturated fatty acids. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 1657–1661. [Google Scholar] [CrossRef] [PubMed]
- Maye, S.T.; Zhang, P. Phytochemical Interactions: B-Carotene. In Phytochemicals: A New Paradigm; CRC Press: Boca Raton, FL, USA, 1998; p. 53. [Google Scholar]
- Kyriacou, M.C.; Rouphael, Y. Towards a new definition of quality for fresh fruits and vegetables. Sci. Hortic. 2018, 234, 463–469. [Google Scholar] [CrossRef]
- Khanam, U.K.S.; Oba, S.; Yanase, E.; Murakami, Y. Phenolic acids, flavonoids and total antioxidant capacity of selected leafy vegetables. J. Funct. Foods 2012, 4, 979–987. [Google Scholar] [CrossRef]
- Kris-Etherton, P.M.; Hecker, K.D.; Bonanome, A.; Coval, S.M.; Binkoski, A.E.; Hilpert, K.F.; Etherton, T.D. Bioactive compounds in foods: Their role in the prevention of cardiovascular disease and cancer. Am. J. Med. 2002, 113, 71–88. [Google Scholar] [CrossRef] [PubMed]
- Morra, L.; Cozzolino, E.; Salluzzo, A.; Modestia, F.; Bilotto, M.; Baiano, S.; del Piano, L. Plant Growth, Yields and Fruit Quality of Processing Tomato (Solanum lycopersicon L.) as Affected by the Combination of Biodegradable Mulching and Digestate. Agronomy 2021, 11, 100. [Google Scholar] [CrossRef]
- Ayyar, S. Mulching and fertigation on the yield and quality of tomato. IJCS 2019, 7, 2539–2541. [Google Scholar]
- Dumas, Y.; Dadomo, M.; Di Lucca, G.; Grolier, P. Effects of environmental factors and agricultural techniques on antioxidant content of tomatoes. J. Sci. Food Agric. 2003, 83, 369–382. [Google Scholar] [CrossRef]
- Gautier, H.; Massot, C.; Stevens, R.; Sérino, S.; Génard, M. Regulation of tomato fruit ascorbate content is more highly dependent on fruit irradiance than leaf irradiance. Ann. Bot. 2009, 103, 495–504. [Google Scholar] [CrossRef]
- Raffo, A.; La Malfa, G.; Fogliano, V.; Maiani, G.; Quaglia, G. Seasonal variations in antioxidant components of cherry tomatoes (Lycopersicon esculentum cv. Naomi F1). J. Food Comp. Anal. 2006, 19, 11–19. [Google Scholar] [CrossRef]




| Parameters | Measure Unit | Soil |
|---|---|---|
| Particle Size Distribution | ||
| Coarse sand | % | 16.6 |
| Fine sand | % | 31.4 |
| Silt | % | 29.0 |
| Clay | % | 23.0 |
| N—total (Kjeldahl method) | % | 0.118 |
| P2O5 (Olsen method) | ppm | 161.7 |
| K2O (Tetraphenylborate method) | ppm | 1539.1 |
| Organic matter (Bichromate method) | % | 1.39 |
| NO3-N | ppm | 5.0 |
| NH4-N | ppm | 15.1 |
| pH | 7.2 | |
| Electrical conductivity | dS m−1 | 0.118 |
| Treatment | Fruits | |||||||
|---|---|---|---|---|---|---|---|---|
| Marketable | Green | Rotten | ||||||
| n m−2 | g fruit−1 | t ha−1 | n m−2 | g fruit−1 | t ha−1 | n m−2 | g fruit−1 | |
| Mulching | ||||||||
| BS | 258.3 ± 23.5 b | 42.5 ± 1.6 | 24.1 ± 9.2 | 72.1 ± 23.4 | 31.0 ± 4.6 | 0.84 ± 0.58 | 2.5 ± 0.9 | 23.1 ± 10.6 |
| ECO | 320.4 ± 10.9 a | 44.3 ± 2.3 | 30.2 ± 8.3 | 88.2 ± 21.2 | 32.9 ± 1.6 | 0.89 ± 0.50 | 3.0 ± 1.0 | 24.3 ± 7.5 |
| NOV | 311.0 ± 12.3 a | 43.5 ± 1.1 | 26.9 ± 6.2 | 73.3 ± 14.7 | 35.8 ± 4.6 | 0.86 ± 0.24 | 3.0 ± 0.8 | 29.1 ± 6.3 |
| Biostimulant | ||||||||
| Control | 266.6 ± 14.9b | 41.2 ± 2.0 b | 19.6 ± 6.8 b | 57.8 ± 15.4 b | 32.4 ± 3.4 | 0.65 ± 0.46 | 2.8 ± 0.9 | 17.4 ± 7.5 |
| Mic | 299.1 ± 11.5a | 45.3 ± 1.8 a | 37.6 ± 12.4 a | 104.4 ± 28.9 a | 33.8 ± 4.1 | 0.68 ± 0.22 | 2.1 ± 0.8 | 31.1 ± 5.8 |
| Bio | 298.8 ± 22.2a | 45.0 ± 1.6 a | 21.8 ± 5.4 ab | 66.8 ± 15.8 ab | 32.3 ± 2.3 | 1.46 ± 0.84 | 4.2 ± 1.1 | 29.0 ± 9.3 |
| M-B | 321.7 ± 13.5a | 42.3 ± 1.3ab | 29.2 ± 7.4 ab | 82.5 ± 19.0 ab | 34.5 ± 4.5 | 0.66 ± 0.23 | 2.2 ± 0.8 | 24.5 ± 9.9 |
| Significance | ||||||||
| Mulching (M) | ** | ns | ns | ns | ns | ns | ns | ns |
| Biostimulant (B) | ** | * | * | * | ns | ns | ns | ns |
| M × B | ns | ns | ns | ns | ns | ns | ns | ns |
| Treatments | L* | a* | b* |
|---|---|---|---|
| Mulching | |||
| BS | 25.7 ± 0.7 c | 38.4 ± 0.7 a | 44.3 ± 0.9 a |
| ECO | 32.7 ± 1.2 a | 32.6 ± 0.9 c | 36.9 ± 2.3 b |
| NOV | 29.7 ± 1.0 b | 35.6 ± 1.0 b | 39.6 ± 1.7 b |
| Biostimulant | |||
| Control | 27.7 ± 0.7 b | 36.5 ± 0.9 a | 42.9 ± 1.5 a |
| Mic | 30.7 ± 1.1 a | 34.1 ± 1.1 b | 38.1 ± 1.7 b |
| Bio | 28.4 ± 0.9 b | 36.2 ± 0.7 a | 43.2 ± 1.5 a |
| M-B | 30.7 ± 1.0 a | 35.4 ± 0.8 ab | 36.9 ± 1.9 b |
| Significance | |||
| Mulching (M) | ** | ** | ** |
| Biostimulant (B) | ** | * | ** |
| M × B | ns | ns | ns |
| Treatments | LAA | Phenols | AsA |
|---|---|---|---|
| mM Trolox 100 g−1 dw | mg gallic acid g−1 dw | mg 100 g−1 fw | |
| Mulching | |||
| BS | 5.52 ± 0.63 c | 1.64 ± 0.09 | 21.20 ± 0.81 b |
| ECO | 8.45 ± 0.71 b | 1.74 ± 0.06 | 29.74 ± 1.52 a |
| NOV | 32.92 ± 1.07 a | 1.73 ± 0.07 | 29.06 ± 1.23 a |
| Biostimulant | |||
| Control | 14.03 ± 4.67 | 1.38 ± 0.06 b | 26.21 ± 2.34 |
| Mic | 16.82 ± 4.44 | 1.83 ± 0.04 a | 28.17 ± 2.32 |
| Bio | 16.73 ± 4.30 | 1.88 ± 0.07 a | 27.11 ± 1.53 |
| M-B | 14.96 ± 4.30 | 1.74 ± 0.06 a | 25.17 ± 1.39 |
| Significance | |||
| Mulching (M) | ** | ns | ** |
| Biostimulant (B) | ns | ** | ns |
| M × B | ns | ns | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Mola, I.; Ottaiano, L.; Cozzolino, E.; Marra, R.; Vitale, S.; Pironti, A.; Fiorentino, N.; Mori, M. Yield and Quality of Processing Tomato as Improved by Biostimulants Based on Trichoderma sp. and Ascophyllum nodosum and Biodegradable Mulching Films. Agronomy 2023, 13, 901. https://doi.org/10.3390/agronomy13030901
Di Mola I, Ottaiano L, Cozzolino E, Marra R, Vitale S, Pironti A, Fiorentino N, Mori M. Yield and Quality of Processing Tomato as Improved by Biostimulants Based on Trichoderma sp. and Ascophyllum nodosum and Biodegradable Mulching Films. Agronomy. 2023; 13(3):901. https://doi.org/10.3390/agronomy13030901
Chicago/Turabian StyleDi Mola, Ida, Lucia Ottaiano, Eugenio Cozzolino, Roberta Marra, Stefania Vitale, Angela Pironti, Nunzio Fiorentino, and Mauro Mori. 2023. "Yield and Quality of Processing Tomato as Improved by Biostimulants Based on Trichoderma sp. and Ascophyllum nodosum and Biodegradable Mulching Films" Agronomy 13, no. 3: 901. https://doi.org/10.3390/agronomy13030901
APA StyleDi Mola, I., Ottaiano, L., Cozzolino, E., Marra, R., Vitale, S., Pironti, A., Fiorentino, N., & Mori, M. (2023). Yield and Quality of Processing Tomato as Improved by Biostimulants Based on Trichoderma sp. and Ascophyllum nodosum and Biodegradable Mulching Films. Agronomy, 13(3), 901. https://doi.org/10.3390/agronomy13030901










