Abstract
The sweet oranges [C. sinensis (L.) Osbeck] produced in India are mainly consumed fresh as a table fruit and in the form of freshly extracted juice. Currently, a fraction of the fruit is processed into products like orange juice, concentrates, pulp, and other value-added products. Seedless varieties are preferred both by the consumers and by the processing industry; however, indigenously developed seedless sweet orange cultivars are not available. Citrus triploids are usually seedless due to their abnormal meiosis and embryo abortion. A research study was undertaken at CCRI Nagpur to develop triploid seedless plants of the local sweet orange cultivar Mosambi through endosperm culture in the shortest possible time by dovetailing various techniques. Various steps, viz., endosperm excision, standardization of media for callus initiation, somatic embryogenesis, shoot/root differentiation, mini-grafting, and validation of the triploid status through flow cytometry, chromosome counting, and other morphological parameters, were standardized, and seven confirmed triploid plants were finally produced. An evaluation of fruit quality parameters during the 2022 and 2023 fruiting seasons revealed that the fruits of the triploid sweet orange trees were commercially seedless. This is the first reported comprehensive study on the successful development of commercially seedless plants of the sweet orange cultivar Mosambi. The fruits of the triploid plants showed desirable fruit quality parameters in terms of their seed number (3 to 5.9/fruit), higher vitamin C content (34.4 to 42.66 mg), and lower limonene content (7.77 to 11.34 µg/mL). These triploid plants have the potential to gain recognition as a distinct variety.
1. Introduction
Sweet orange [C. sinensis (L.) Osbeck] is commercially grown in India in the states of Andhra Pradesh, Karnataka, Maharashtra, Punjab, Rajasthan, and Telangana, among others, over an area of 188,000 ha with a production of 3,580,000 million tonnes [1]. The traditionally grown cultivars are Mosambi (Maharashtra), Sathgudi, Batavian (Andhra Pradesh, Telangana), and Malta and Jaffa (Punjab). The fruiting season of these cultivars spans from October to March. The sweet oranges produced in India are mainly consumed fresh, while a smaller percentage is processed into products like orange juice, concentrates, pulp, and other value-added products. The freshly extracted juice of sweet oranges is a choice drink for both healthy and recuperating persons. The popularity of orange juice has increased due to its nutraceutical components and associated health benefits. The pulp of sweet orange is rich in vitamin C, dietary fibers, and other essential nutrients.
Several Indian companies such as Pepsico India (Tropicana), Coco-Cola India (Minute maid), Dabur India (Real), and ITC (B-Natural) are engaged in the production of citrus juices, including orange juice (brand names are mentioned in parentheses). The food processing sector is identified as a thrust area of the economy by the Government of India, and the Ministry of Food Processing Industries has introduced various schemes to promote processing and exports. The availability of suitable citrus varieties with desirable attributes, viz., high yields, disease resistance, fewer seeds, lower limonene contents, higher juice contents, and early/late fruiting seasons, would boost the processing industry. Research organizations and entrepreneurs have introduced promising varieties, including seedless ones from the U.S.A., Brazil, and other countries, to bridge the gap. A strong varietal breeding program is a perpetual necessity to improve the indigenous cultivars as they are well acclimated to local conditions and are popular among consumers.
Both fresh fruit consumers and the processing industry prefer seedless fruits. The seeds are the prime source of the bitterness components “limonene” and naringin; hence, seedless cultivars are preferred by the processing industry. Seeded cultivars are accepted in the absence of seedless cultivars or when the fruit quality parameters of seeded cultivars are far superior to those of seedless cultivars. Many citrus cultivars with desirable quality characteristics have not attained commercial importance because of their seededness [2]. The Washington navel orange, Marsh seedless grapefruit, and Satsuma mandarin are preferred and prized varieties because they are seedless. Hence, incorporating seedlessness has become one of the most important breeding objectives of many citrus research projects around the world.
The development of seedless cultivars through conventional breeding methods, viz., natural selection and mutation breeding, is laborious and time-consuming due to the long juvenility, incompatibility, and high degree of apomixes [3,4]. The frequency of natural seedless mutants is negligible; further, natural mutants may or may not be superior horticulturally.
Mutation breeding through irradiation can enhance the frequency of seedless/triploid mutants. The need to maintain a large mutant population, the efficient screening methods needed to isolate desirable mutants, and the long juvenility limit the use of this method. Ploidy manipulation (through colchicine) and interploid hybridization (2n × 4n: 4n × 2n) followed by embryo reuse is one possible approach to produce triploids. The somatic hybridization of haploids and diploids via protoplast fusion can lead to the development of triploids. The development of transgenic plants containing genes for decreased seed set and their identification by PCR techniques can also lead to the development of triploids, but these techniques are technology- and cost-intensive.
Advanced biotechnological pathways (interploid hybridization, somatic hybridization, transgenics, etc.) are tedious [5,6,7,8]; hence, breeders have harnessed endosperm culture, which is a single-step approach and the most efficient method for triploid plant production. The endosperm is a unique tissue in ploidy level, origin and development [5,9,10] and is the product of the union of three haploid nuclei, two from a female gametophyte and one originating from pollen via double fertilization; hence, the endosperm contains three sets of chromosomes and is triploid in nature.
The first attempt to achieve in vitro endosperm culture was made in 1930 [11]. Endosperm culture was attempted in around 64 species for triploid regeneration, and the differentiation of shoots/roots/plants from the endosperm was reported in only 32 species. Confirmed triploid plants were reported in only 15 species. The genotype, sampling time, and medium formulation were found to be the factors influencing endosperm culture and successful regeneration [12].
Sweet orange [C. sinensis (L.) Osbeck] cv. Mosambi is a popular cultivar traditionally grown in Central India and is well-acclimated to local conditions. A study conducted during the Ambia season of 2011–2012 in the Jalna district of Maharashtra reported 26 seeds per fruit in a nine-year-old orchard of Mosambi [13].
The present research was conducted in order to create stable triploid seedless plants of the Mosambi cultivar in the shortest possible time by dovetailing various techniques, viz., endosperm culture, somatic embryogenesis, and mini-grafting, and by confirming the triploid status through flow cytometry, chromosome counting, and other morphological parameters.
2. Materials and Methods
2.1. Plant Materials
A few promising trees of C. sinensis (L.) Osbeck cv. Mosambi were selected from an experimental block of the CCRI in Nagpur as a parent source. Around 1500 flowers of sweet orange cv. Mosambi were tagged at the time of anthesis in January, during the Ambia bahar season. The tagged open-pollinated immature fruitlets were harvested 75–84 days after anthesis. Fruitlets were sterilized using sodium hypochlorite 1% (w/v) for 10 min, followed by rinsing three times with autoclaved distilled water. Fruitlets were dissected aseptically under a zoom stereo dissection microscope. Immature seeds were dried on filter paper, peeled, and dissected, and the endosperms were carefully squeezed out. After the endosperm was excised, the nucellus tissue and associated embroid tissues were also peeled off under the microscope. One uninured endosperm was cultured in each test tube.
2.2. Primary Callus Induction, Proliferation, and Embryogenesis
Five different media formulations, viz., (i) Murashige and Tucker (MT) + malt extract (ME) (500 mg/L), (ii) MT + ME + casein hydrolysate (CH) (500 mg/L) + benzyl adenine (BA) (5 mg/L) + 2,4-dichlorophenoxyacetic acid (2,4-D) (2 mg/L), (iii) MT + ME + CH (500 mg /L) + 2,4-D (2 mg/L), (iv) Murashige and Skoog (MS) + ME (500 mg/L), and (v) MT + ME + CH (500 mg/L) + 2,4-D (2 mg/L) were tested to determine the response of the cellular endosperm for primary callus induction. Cultures were grown in darkness at 26 ± 1 °C to induce callus formation. After callus initiation and proliferation in darkness, all cultures were grown under a 16 h fluorescent light photoperiod. The observations recorded were the endosperm response to callus induction, percentage of callus induction, and number of days taken for initiation of the primary callus.
Proliferated primary calli were separated and cultured in 25 mm × 150 mm tubes containing 25 mL of MT [14] medium supplemented with 2,4-D (2 mg/L), CH, and ME and MS [15] medium supplemented with only CH for the initiation of somatic embryogenesis and morphogenesis. The observations recorded were the numbers of globular, heart-shaped, torpedo, and cotyledonary embryoids.
The embryoids from embryogenic calli were isolated and cultured on six medium formulations containing growth regulators. MT medium was supplemented with growth regulators, viz., gibberellic acid (GA3) (1mg/L), IBA (2 mg/L), adenine sulphate (10 mg/L), and benzyl adenine (BA) (2 mg/L), for shoot and root regeneration.
2.3. Lab-to-Land Transfer
To facilitate the successful transfer of prospective triploid plants from the lab to an external environment, elongated strong shoots regenerated from in vitro endosperm cultures were mini-grafted onto greenhouse-grown, five-month-old vigorous rough lemon rootstock in the year 2017. The rootstock seedlings were decapitated at a height of 9 inches from the ground, and endosperm-derived shoots were inserted into small vertical incisions made at the top of the decapitated rootstock. Successful grafts were acclimatized in the same screen house. Thereafter, the plants were planted in the experimental block for field evaluation in the year 2019.
2.4. Ploidy Analysis
The ploidy analysis of the endosperm-regenerated plants was conducted using flow cytometry (Partec Gmbh, Munster, Germany). Tender leaves were collected from the field-transferred plants. The nuclear cells were extracted from the leaves, stained with a fluorescent dye (4′,6-diamidino-2-phenylindole dihydrochloride (DAPI)), and illuminated with UV light. The fluorescent light intensity was measured and presented in the form of a histogram, proportional to the DNA content of the labeled cells. Peaks in the histogram depict the ploidy levels of the respective samples. More than 5000 nuclei were analyzed in each sample. The use of flow cytometry and specialized software facilitated the accurate analysis of a large number of nuclei in each sample. Leaf samples from diploid sweet orange were used as controls.
2.5. Cytology
The protoplast dropping method developed by [16] was used with some modifications for counting the chromosomes [17]. Shoot tips from new flushes collected from field-transferred plants of endosperm origin were used for ploidy determination. The enzymatic digestion (cellulase and pectinase) of shoot tips was used to prepare metaphase chromosomes. The resulting protoplasts were treated hypotonically and dropped onto the microscopic slide, and the chromosome sets were then counted.
2.6. Stomatal Morphology
Stomatal analysis was carried out by collecting fully developed leaf samples of field-planted triploid and diploid plants between 10.30 and 11.00 a.m. A thin layer of transparent nail polish was applied on the abaxial surface of the leaves. The dried nail polish layer was removed from the leaves with the help of transparent sticky tape and transferred to a glass slide. An analysis of stomatal characteristics, such as their size and density, was performed using a Leica phase-contrast microscope (40× and 100× magnification) equipped with a video camera connected to a computer. The stoma number per 500 µm area was recorded.
2.7. Analysis of Fruit Quality Parameters
Fruits were harvested from field-planted triploid and diploid plants during the fruiting seasons of 2022 and 2023. Yield and fruit quality parameters, viz., fruit weight, fruit diameter, peel thickness, number of segments, seed number, juice (%), TSS (°Brix), acidity (%), and vitamin C (mg/100 gm) and limonene contents (ppm), were recorded and analyzed as per the standard procedures.
2.8. Statistical Analysis
A completely randomized statistical design (CRD) with 4 replications comprising 10 explants for each replication was employed for the studies on primary callus induction, the morphogenesis of endosperm calli, and plantlet regeneration. The stoma morphometry (stoma length, width, and density) was estimated via t-test analysis.
3. Results
The development of seedless triploid plants of sweet orange cv. Mosambi through endosperm culture involved various steps, viz., (i) the identification of the stage at which the endosperm is most responsive; (ii) a technique of endosperm rescue; (iii) the identification of the optimal culture medium for callus induction, somatic embryogenesis, and shoot/root differentiation; (iv) the transfer of regenerated plants to the field by mini-grafting and acclimatization; (v) a confirmation of the ploidy status; and (vi) an analysis of fruit quality parameters and count of the seed content of field-transferred stable/viable triploid plants. The results of this study are described below.
3.1. Age of Responsive Endosperm
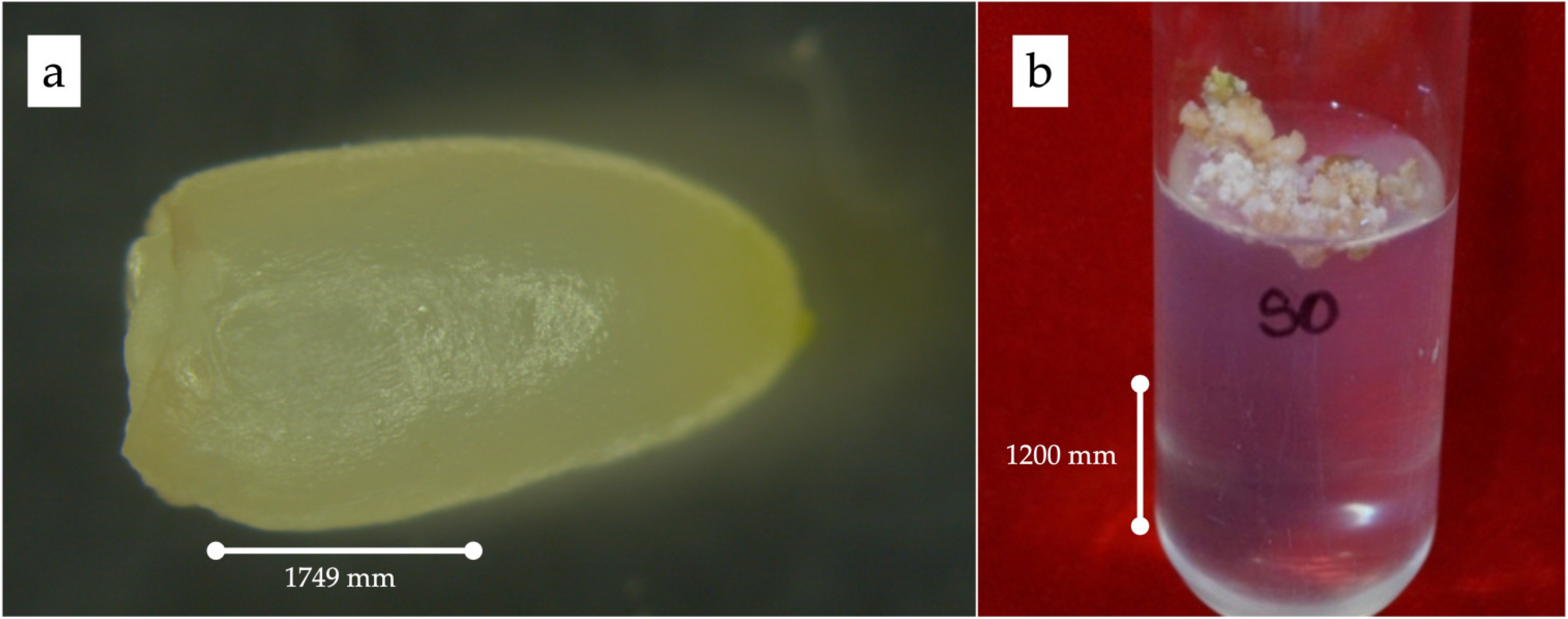
The endosperms were cultured, and complete plants were regenerated successfully, indicating that 11- to 12-week-old endosperms were responsive to triploid plant production. It was observed that the primary endosperm callus had a creamy texture and was compact but not hard (Figure 1).
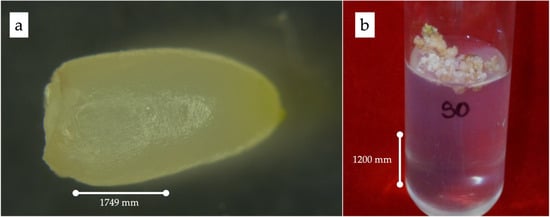
Figure 1.
(a) Rescued endosperm; (b) induction of primary calli.
3.2. Callus Induction and Proliferation
The data pertaining to the influence of the medium on the endosperm survival percentage, the endosperm response to callus induction, and the number of days taken for the initiation of primary calli are presented in Table 1. The data indicate that the callus induction response (78.16%) and survival (70.40%) were at their maximum in the MT + ME (500 mg/L) + CH (500 mg/L) + 2,4-D (2 mg/L) medium. The callus initiation response in different media ranged from 15.08% to 47.57%, and it was significantly higher (47.57%) in the MT + ME + CH (500 mg/L) + 2,4-D (2 mg/L) medium, followed by the MS + ME (500 mg/L) medium (35.72%) (Table 1).

Table 1.
Influence of the medium on the induction of primary calli/embryoids of sweet orange.
3.3. Effect of Media on Morphogenesis of Embryogenic Callus
Proliferating callus cultures were sub-cultured on five different medium formulations after one month of incubation in the dark (Table 2). The callus cultures transformed from amorphous to a greenish embryogenic form when the cultures were shifted to different medium formulations. The maximum stimulation for embryogenesis and morphogenesis was observed in the medium consisting of 2MT + ME (500 mg/L) + adenine sulphate (Ad.S) (2 mg/L) + GA3 (2 mg/L) + BA (2 mg/L), followed by MT + CH (500 mg/L) + GA3 (1 mg/L) + BA (0.25 mg/L). The results of this experiment highlighted the influence of different medium formulations on embryogenesis, morphogenesis, and cotyledonary embryoid production.

Table 2.
Influence of the medium on the morphogenesis of sweet orange endosperm calli.
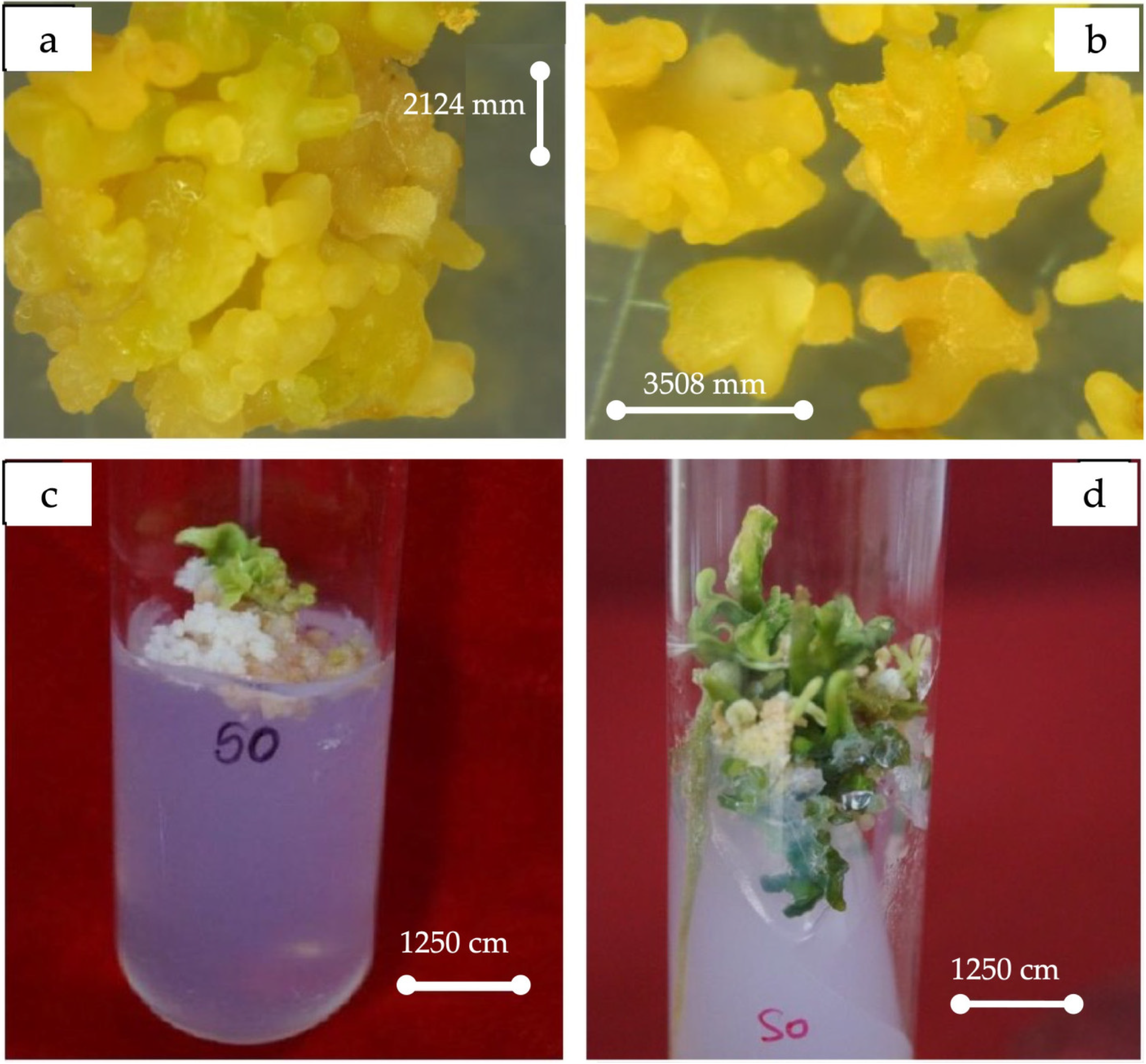
The best stimulation for callus induction, embryogenesis, and later morphogenesis to embryoids (Figure 2) occurred when the medium was supplemented with CH (500 mg/L) at all stages. The highest number of cotyledonary embryoids was observed in 2MT + ME (500 mg/L) + Ad.S (2 mg/L) + GA3 (2 mg/L) + BA (2 mg/L), followed by MT + CH (500 mg/L) + GA3 (1 mg/L) + BA (0.25 mg/L). The regeneration potential of the proliferating embryogenic calli was recorded for an additional year by sub-culturing.
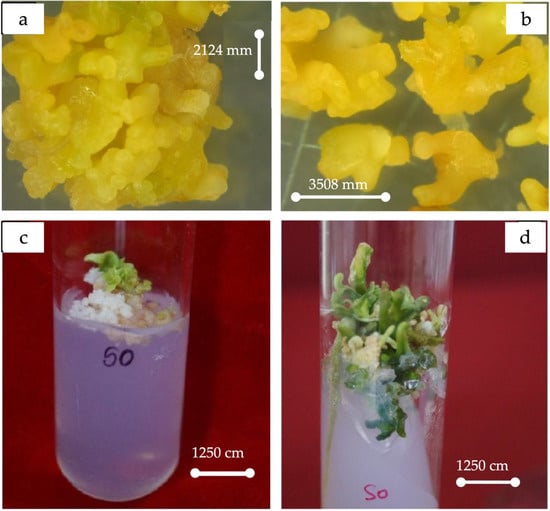
Figure 2.
(a) Morphogenesis of somatic embryos. (b) Magnified view of embryoids. (c) Initiation. (d) Morphogenesis to various stages of embryoids.
3.4. Effect of Phytohormones on Shoot and Root Differentiation
The data on the effect of phytohormones on shoot and root differentiation from cotyledonary embryoids are presented in Table 3 and Figure 3. The maximum response (92.54%) in terms of shoot differentiation occurred in MT + ME + Ad.S (10 mg/L) + GA3 (2 mg/L), followed by MT + ME + GA3 (1 mg/L) (88.63%). The number of shoots and roots was the highest and the elongation of shoots and roots was at its maximum in MT + ME (500 mg/L) + Ad.S (10 mg/L) + BA (2 mg/L) + GA3 (2 mg/L). Shoot bud differentiation from green cotyledonary embryoids occurred within 7 to 19 days after culturing. The shoots attained a length of 1.05 to 3.3 cm in 60–75 days, depending on the medium used (Figure 4).

Table 3.
Complete plantlet regeneration from sweet orange endosperm via somatic embryogenesis.

Figure 3.
Shoot and root initiation in cotyledonary embryoids (a,b).
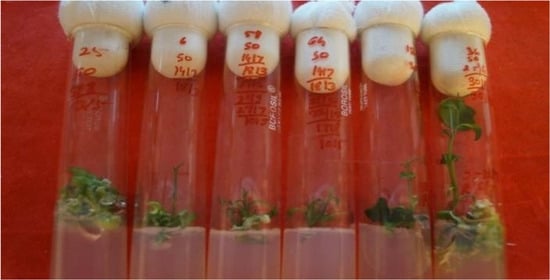
Figure 4.
Complete plantlet regeneration from endosperm rescue via somatic embryogenesis.
3.5. Morphological Characterization
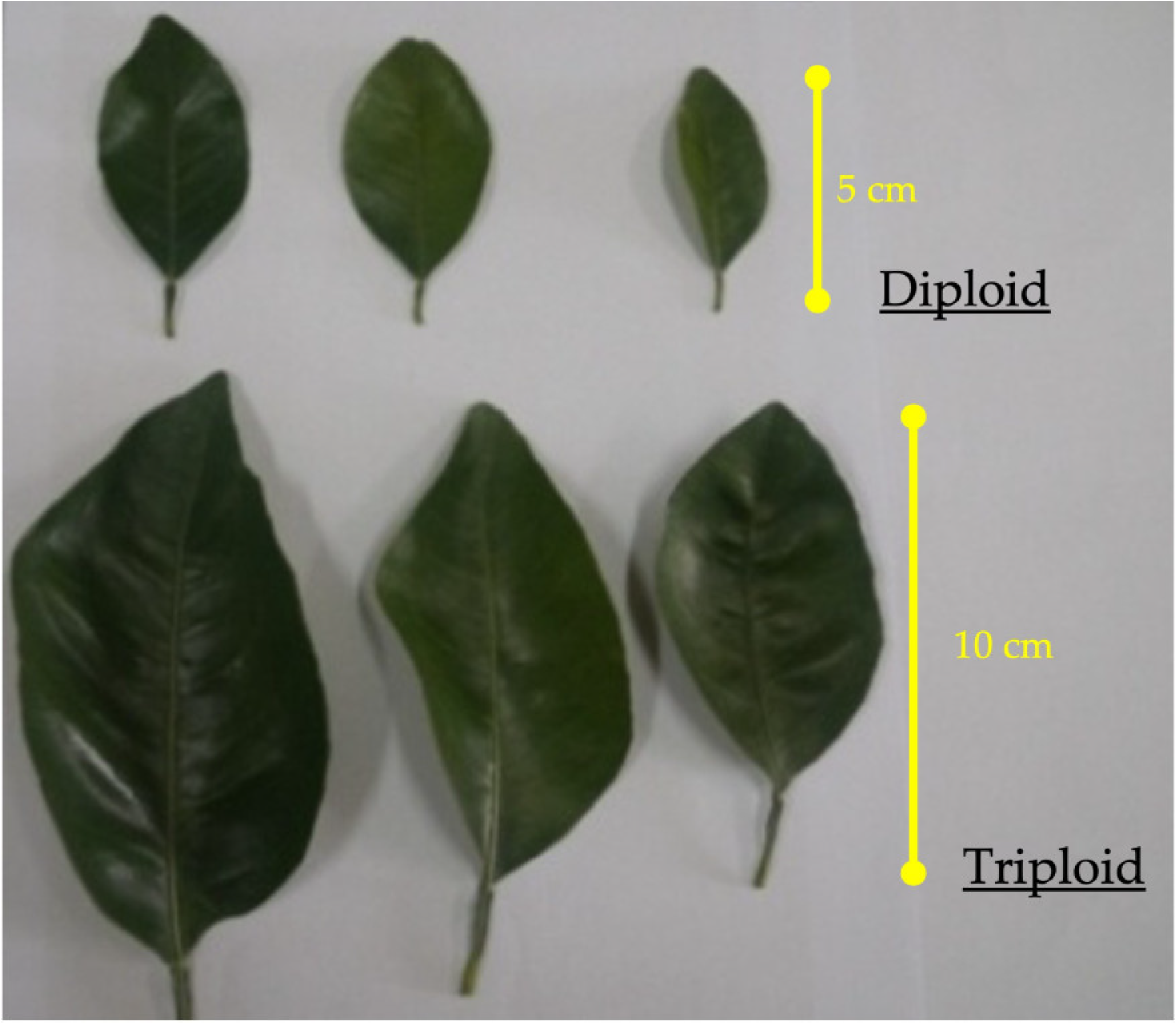
The sweet orange triploid plants displayed more thorny, robust, and vigorous growth than did the diploid plants. The data concerning the leaf morphology characteristics, viz., the length, width, and area of the leaves of triploids and diploids, are presented in Table 4 and Figure 5. The length, width, and area were higher in triploids than in diploids. It was observed that the leaves of triploids rolled upwards at the outer margin, giving a spoon-like shape, whereas the leaves of diploid plants were flat (Figure 5).

Table 4.
Statistical analysis of the leaf area.
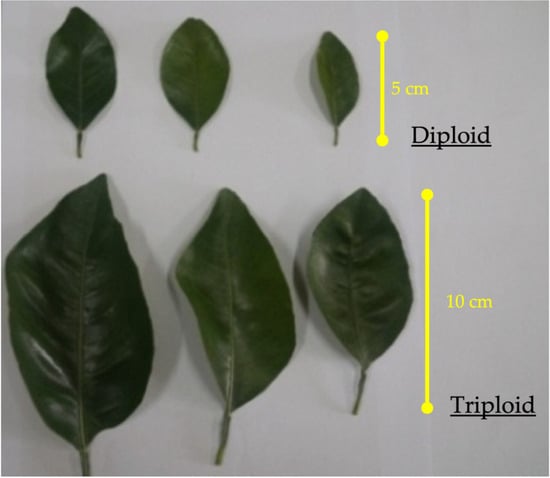
Figure 5.
Leaf morphology from diploid and triploid plants of C. sinensis (L.) Osbeck cv. Mosambi.
3.6. Triploid Induction Ratio in Sweet Orange
Initially, 120 tender seeds were aseptically extracted from 25 tagged fruits of selected elite sweet oranges. Out of the 120 seeds, 90 endosperms were aseptically excised and cultured (Figure 1). Out of the 90 rescued endosperms, 70 putative triploids were regenerated and mini-grafted onto rough lemon rootstocks, with 57% success.
Out of the 70 pot-transferred grafts, 40 survived in the screen house. The ploidy status of these 40 pot-transferred plants was ascertained using flow cytometric, cytogenetic, and morphological studies, and 7 plants were certified/validated as triploids. The observed ratio of tender seeds to stable triploids was 17:1 (120:7). The ratio of rescued endosperm to stable triploids was 13:1 (90:7) (Table 5). The success ratio of the lab-to-land-transferred putative triploids from mini-grafting to confirmed triploids was 6:1 (40:7), equivalent to 17.5%.

Table 5.
Triploid induction ratio in sweet orange [Citrus sinensis (L.) Osbeck] cv. Mosambi.
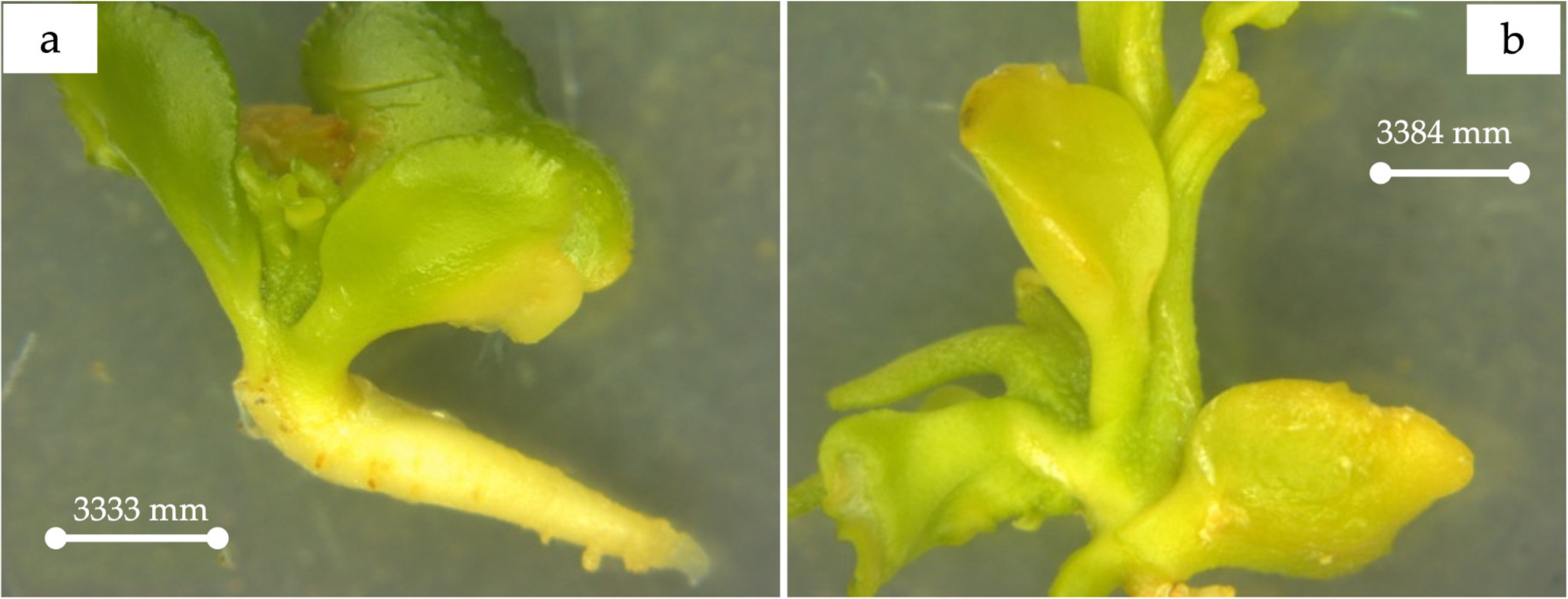
The confirmed triploid plants exhibited variations in important morphological traits, viz., leaf size and shape, stomatal characteristics, and the appearance of prominent thorns, compared to those of diploid plants (Figure 6 and Figure 7).

Figure 6.
(a) Mini-grafting of endosperm-regenerated shoots. (b) Pot-transferred confirmed triploid plant. (c) Field-transferred sweet orange plant. (d) Fruiting branch without thorns. (e) Thorns on the main stem of a triploid plant.

Figure 7.
(a) Comparison of diploid and triploid flowers. (b) Seedless characteristic of C. sinensis (L.) Osbeck cv. Mosambi.
3.7. Characterization of Triploid Plants by Flow Cytometry
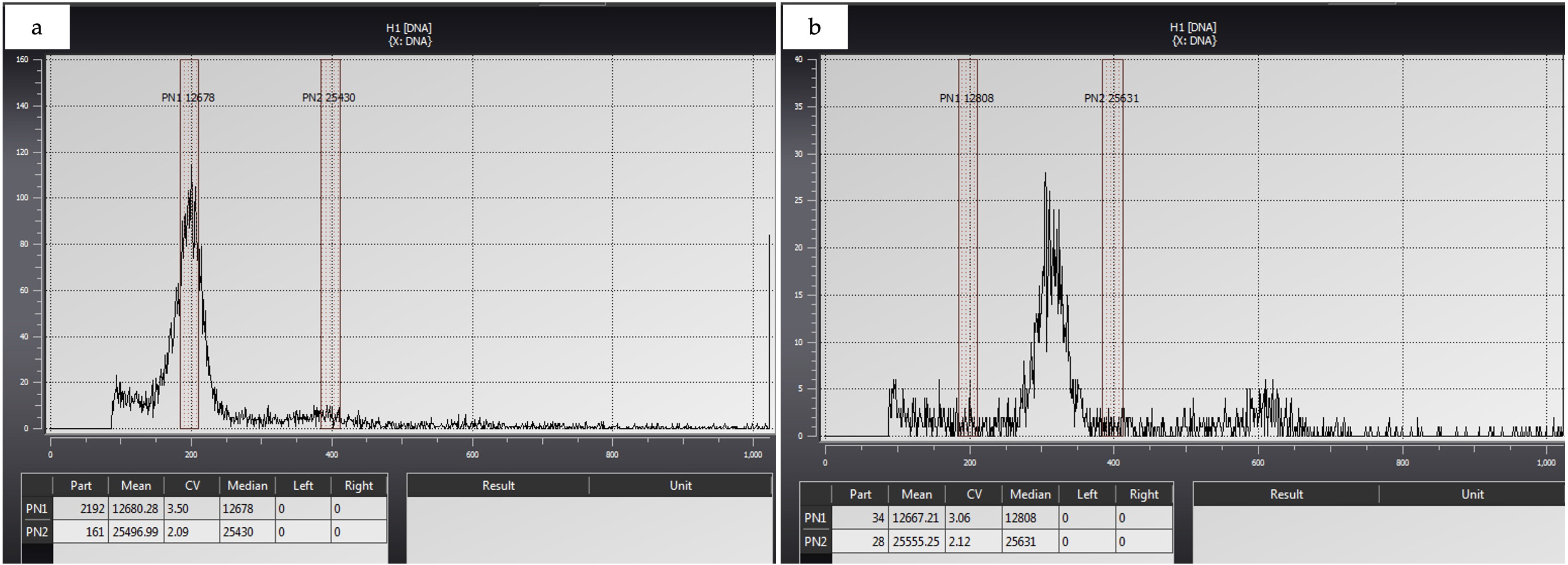
Nuclear DNA histograms were constructed by using CyView software (serial no. 140500541), and the relative ploidy levels of the tested diploid and triploid samples were determined. Flow cytometry analysis validated the triploid nature of the endosperm-rescued plants (Figure 8).
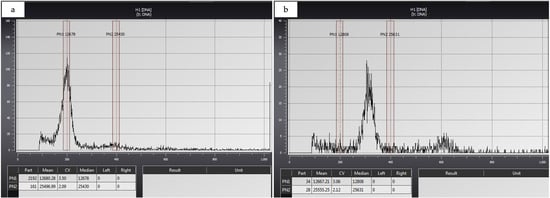
Figure 8.
Flow cytometry histograms represent (a) diploid and (b) triploid C. sinensis (L.) Osbeck cv. Mosambi.
3.8. Triploid Characterization by Cytology
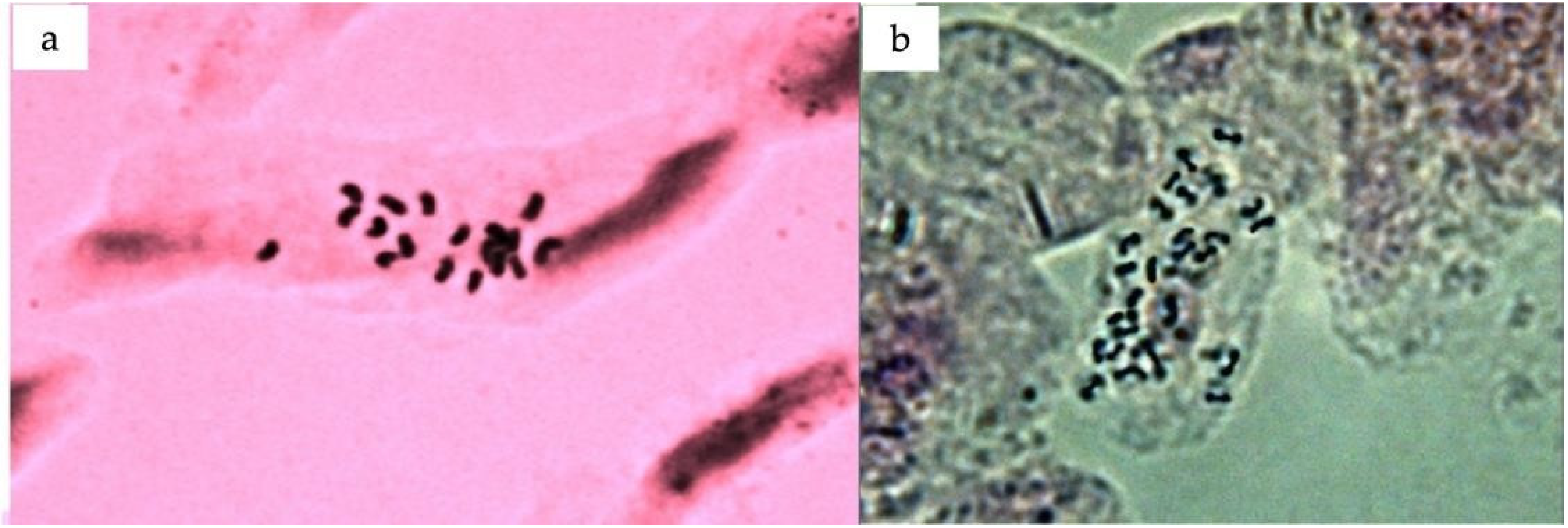
The chromosome number in emerging leaf tissue was counted using enzyme digestion and protoplast dropping techniques to further confirm the ploidy results obtained through flow cytometry. The chromosome number of the tested triploid plants was 2n = 3x = 27, confirming their triploid nature (Figure 9).
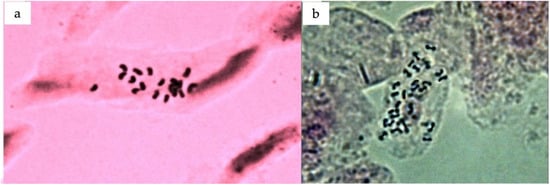
Figure 9.
Processed metaphase cells of Citrus sinensis: (a) diploid; (b) triploid.
3.9. Stomatal Analysis
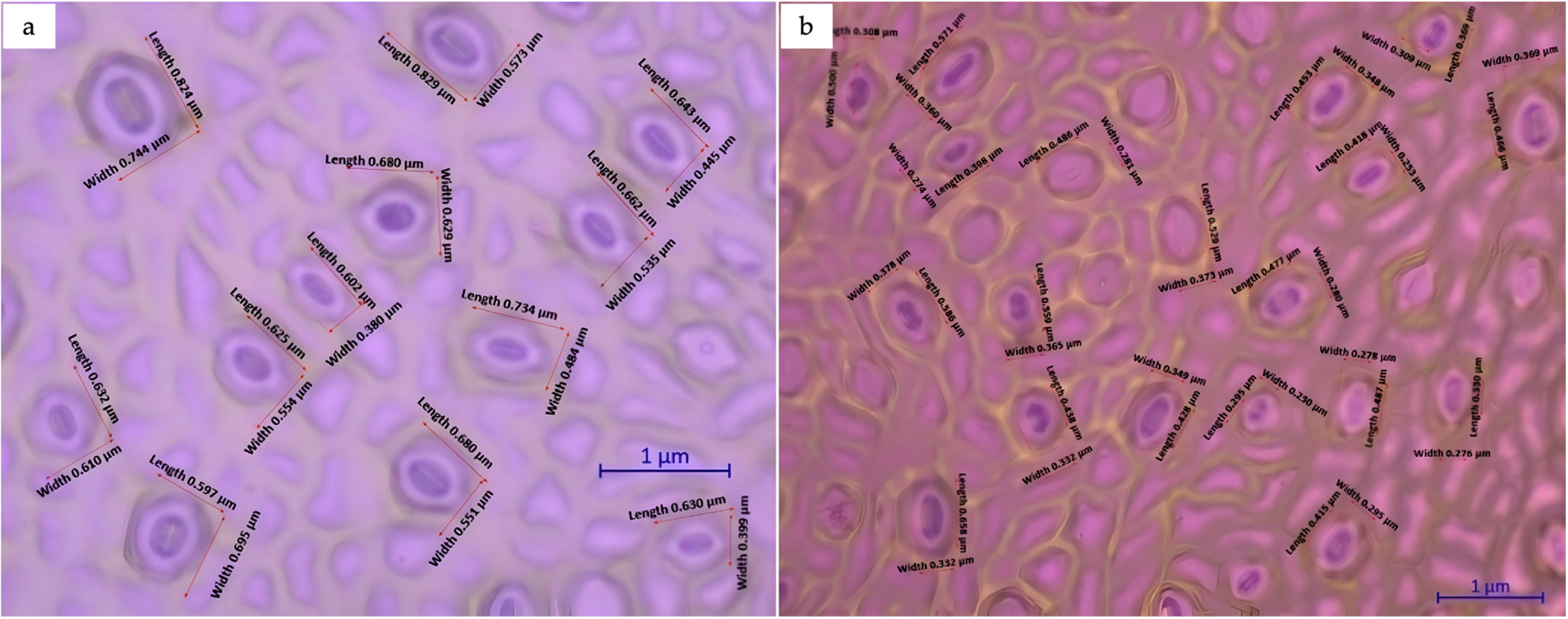
Significant differences were observed as regards the size and number of guard cells between diploid and triploid plants. Variations in the ploidy level affected the size and frequency of stomata in C. sinensis (L.) Osbeck cv. Mosambi. The stomata in triploid plants were longer and wider (Figure 10) compared to those in diploids. The numbers of stomata per 500 µm leaf area in triploids and diploids were 12 and 19, respectively. The average stomatal length in triploids was 0.683 µm, whereas the average stomatal length in diploids was 0.463 µm. The stomatal length in triploid samples was 47.51% longer compared to that in diploid samples (Table 6).
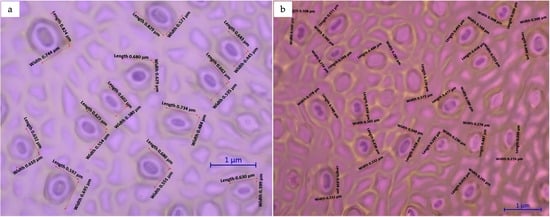
Figure 10.
Effect of ploidy level on leaf morphology and stomatal characteristics in Citrus sinensis: (a) triploid; (b) diploid.

Table 6.
Morphometric analysis of stomata in triploid and diploid Citrus sinensis (L.) Osbeck cv. Mosambi.
Student’s t-test was conducted to assess the statistical significance of the differences observed in the mean length and width of stomata between diploid and triploid samples. The t-test indicated a significant difference in the stoma mean length between diploid and triploid samples and also suggested a positive correlation between the ploidy level and stoma length (Figure 10).
3.10. Fruit Quality Parameters of Field-Transferred Sweet Orange Triploid Plants
It was observed that the field-transferred triploid plants displayed a stronger spreading growth habit with drooping branches. Prominent thorns were present all over the tree, except on fruit-bearing branches. The fruits of triploid plants showed desirable fruit quality parameters in terms of their seed number (2 to 5.9, commercially seedless), high vitamin C content (34.4 to 42.66 mg /100 g), and lower limonene content (7.77 to 11.34 µg/mL) (Table 7).

Table 7.
Initial fruit quality parameters of field-transferred Citrus sinensis (L.) Osbeck cv. Mosambi triploid plants.
4. Discussion
In India, sweet orange [C. sinensis (L.) Osbeck] cv. Mosambi fruit is consumed as table fruit and used for fresh juice extraction. The quantity of processed sweet orange fruit is increasing due to the acknowledgement of the nutritive value of the juice by consumers and due to the entry of corporations in this sector. An increased processing volume promotes price stabilization and protect the interests of growers. The availability of seedless varieties enhances consumer acceptance, provides greater ease of processing, minimizes the amount of limonene in extracted juice, and favors the quality of the produced juice; hence, there is a need to develop seedless varieties of sweet orange.
The development of seedless varieties of Citrus spp. has been an important objective of breeding programs worldwide. Citrus triploids are seedless due to their seed sterility caused by improper meiotic division. Breeders have used different approaches like the selection of natural mutants, interploid hybridization [4], mutation breeding [18], and the somatic hybridization of haploids and diploids via protoplast fusion [18] for the production of seedless triploid varieties. The use of these methods is restricted by various genetic limitations, such as the long juvenility, incompatibility, and high degree of apomixes found in Citrus species. The regeneration of plants from endosperm, a naturally occurring triploid tissue, offers a direct one-step approach to produce triploids [19] and helps to overcome genetic limitations. It is important to combine both conventional and innovative in vitro and in vivo techniques to achieve the desired results.
The present study was undertaken to develop triploids of C. sinensis (L.) Osbeck cv. Mosambi in a cost-effective manner and within a reasonable timeframe by dovetailing various techniques, viz., endosperm culture, micrografting, and flow cytometry.
The production of stable triploids in citrus via endosperm rescue is technically challenging and requires proper advance planning and high skill. The flowering period of individual sweet orange cv. Mosambi trees lasts for a few days; hence, the tagging of flowers at anthesis is required to decide the most appropriate time of fruitlet harvesting and the age of responsive endosperms. Our studies indicated that sweet orange cv. Mosambi endosperm rescued from pre-tagged fruitlets at 75–84 days post-anthesis exhibited a better response for callus initiation, somatic embryogenesis, morphogenesis, and shoot bud differ entiation [10]. Exciting endosperms beyond 11–12 weeks was difficult as the seeds had matured further.
Squeezing and separating the endosperm from the immature seeds of 11–12-week-old fruitlets was easy and convenient compared to squeezing the endosperm from the mature seeds of older fruitlets, as the seed coats of the latter were thicker. Endosperms at this stage were tender and elastic, with a liquid-to-soft texture, and were more responsive. Cotyledonary embryoids/zygotic embryos were observed at 14 weeks post-anthesis or beyond; hence, the right stage for endosperm rescue in sweet orange cv. Mosambi was up to 12 weeks. Similar findings were reported in previous studies [10,20,21].
In the present study, endosperms were cultured in five (5) different medium compositions to achieve callus induction and proliferation. The best callus induction response was observed in MT + ME (500 mg/L) + CH (500 mg/L) + 2,4-D (2 mg/L) medium. These results indicated that a concentration of 2,4-D greater than 1.0 mg/L was required for callus induction with stronger plant regeneration ability. The majority of prior studies on endosperm culture also reported that 2,4-D was the most effective phytohormone for callus induction from endosperm [10,22,23,24,25]. The genotype, sampling time, and culture medium composition determine the successful development of triploid plants [10,23,26,27].
The proliferating endosperm calli were sub-cultured after one month of incubation in the dark, in five (5) different medium combinations, and shifted to light conditions in order to induce somatic embryogenesis and morphogenesis.
The maximum stimulation for embryogenesis and morphogenesis was observed in the medium with 2MT + ME (500 mg/L) + Ad.S (2 mg/L) + GA3 (2 mg/L) + BA (2 mg/L). Creamish calli transitioned to a greenish color in about 17 to 25 days on the embryogenesis stimulation medium, followed by morphogenesis, leading to the development of various kinds of embryoids [21,26]. BA was found to be the most effective cytokinin for the induction of morphogenesis and various kinds of embryoids. All the previous reports on endosperm culture also indicated BA as an indispensable cytokinin [10,21,22,23,27,28].
Enhanced nutrition and GA3 were both essential for globular callus induction and the stimulation of embryogenesis from primary calli leading to embryoid development. Cotyledonary embryoids of endosperm calli underwent a regeneration process involving shoot and root differentiation in different medium formulations. The number and length of roots and shoots were greater in MT + ME (500 mg/L) + Ad.S (10 mg/L) + BA (1 mg/L) + GA3 (2 mg/L).
The activation of endosperm metabolism and shoot bud differentiation occurred when the media were supplemented with GA3, Ad.S, and BA. Similar results were reported in endosperm culture studies of pomelo and sweet orange [10,29], Nagpur mandarin [21], kiwifruit [22], mulberry [26], passion fruit [27], neem [30], and tangor dweet [31].
The characteristic feature of triploid shoots of endosperm origin is the presence of a large number of multicellular glands/organ fasciation; young shoots, especially, appear to be fused, a morphology which can be observed in the figures. The successful mini-grafting of such shoots with variable vigor, the transfer of mini-grafts from the lab to the greenhouse, and their acclimatization in the soil were real challenges faced in our studies [10,21,30].
The morphometric analysis of the stomata revealed significant differences between diploids and triploids in terms of the number and dimensions of guard cells, as ploidy level variation leads to changes in the size and frequency of stomata. Triploid plants exhibited longer and wider stomata compared to their diploid counterparts. The t-test indicated a significant difference in the mean length of stomata between diploid and triploid samples. The results suggested a positive correlation between the ploidy level and stoma length and an inverse relationship between the stoma frequency and ploidy level [32].
The modern analytical method of flow cytometry with accompanying software was used to construct nuclear DNA histograms to provide a visual representation of the relative ploidy levels of the tested diploid and triploid samples. Flow cytometry analysis was found to be a much easier and faster method to validate the triploid nature of endosperm-derived plants, which is crucial for ensuring the accuracy of ploidy confirmation [5,33]. Additionally, chromosomal counting was performed to further corroborate the results obtained through flow cytometry. The chromosome numbers of the triploid plants provided additional support for the accuracy of the ploidy analysis conducted in this study. These results were consistent with the results obtained in previous reports on clementine [34], passion fruit [27], and kiwifruit [22].
The observed regeneration ratio of confirmed triploids from the tagged harvested fruitlets was 1:3.5 (28%), and the ratio of successfully surviving stable triploid plantlets from endosperm rescue was 1:13.
Several studies have also reported that polyploidization increases the size and area of leaves. The triploid leaves rolled upwards at the edges, displaying a spoon-like leaf shape. The unbalanced growth of two different tissues in the same leaf might have resulted in variations in the leaf shape and leaf rolling. This observation was consistent with previous reports [35,36].
The fruits collected from triploid trees (aged 5–6 years) planted in the experimental block of CCRI contained fewer seeds, numbering 2 to 5.9, i.e., commercially seedless, whereas in the research conducted by Patil and Panchal [13] during the Ambia bahar season of 2011–2012 in the Jalna District of Maharashtra, 26 seeds were reported per fruit harvested from a 9-year-old local sweet orange tree.
The observed physical features of endosperm-regenerated plants, viz., morphological differences in the leaves, the density and dimensions of stomata, the non-thorny nature of fruiting shoots, and the thorny nature of the main stems of the trees, indicate the distinct characteristics of the triploids [28,37]. The cytological confirmation of triploid status, the stable nature of field-transferred triploid plants, and the desirable fruit parameters, viz., fewer seeds (2 to 5.9/fruit), a high vitamin C content (34.4 to 42.66 mg/100 g), and a lower limonene content (7.77 to 11.34 µg/mL), proved the successful achievement of our objective in this study.
5. Conclusions
This study demonstrated that triploid seedless Citrus sinensis (L.) Osbeck cv. Mosambi plants with desirable fruit quality parameters can be successfully produced through endosperm culture. The complete protocol for successful triploid plant regeneration through endosperm rescue was standardized. These seedless plants can be further multiplied vegetatively and have the potential to be registered as a distinct variety.
This is the first and the most comprehensive study reported from India on the successful production of commercially seedless triploid plants of Citrus sinensis (L.) Osbeck cv. Mosambi.
Author Contributions
Conceptualization, planning, supervision, methodology, data interpretation, original draft preparation, review and editing, visualization, project administration, funding acquisition, V.N.; lab and field work, data collection, formal analysis, Y.L., S.P., K.F., P.U. and K.K.; review, editing, correspondence V.Z.; project intermittent handling, project administration, P.T.K.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by ICAR-CCRI under project no. CCRI/2.7. Vijayakumari N. is thankful to the ICAR for their financial help in the execution of this research work.
Data Availability Statement
Data are available from the lead author and the PI of the project.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of this study; in the collection, analysis, or interpretation of data; in the writing of this manuscript; or in the decision to publish this result.
References
- Available online: https://agriwelfare.gov.in/en/StatHortEst (accessed on 12 March 2024).
- Fatta, D.B.; Matrango, S.G.; Geraci, G. Micro and macro sporogenesis of two triploid hybrids of citrus. Proc. Int. Soc. Citric. 1992, 1, 122–124. [Google Scholar]
- Grosser, J.W.; Ollitrault, P.; Olivares-Fuster, O. Somatic hybridization in Citrus: An effective tool to facilitate cultivar improvement. In Vitro Cellular and Development Biology. Plant 2000, 36, 434–449. [Google Scholar]
- Soost, R.K.; Cameron, J.W. Citrus. In Advances in Fruit Breeding; Janick, J., Moore, J.N., Eds.; Purdue University Press: West Lafayette, IN, USA, 1975; pp. 507–540. [Google Scholar]
- Germana, M.A.; Chiancone, B.; Lain, O.; Testolin, R. Anther culture in Citrus clementina: A way to regenerate tri-haploids. Aust. J. Agric. Res. 2005, 56, 839–845. [Google Scholar] [CrossRef]
- Esen, A.; Soost, R.K. Tetraploid progenies from 2x × 4x crosses of citrus and their origin. J. Am. Soc. Hortic. Sci. 1971, 97, 410–414. [Google Scholar] [CrossRef]
- Ollitrault, P.; Dambier, D.; Luro, F.; Froelicher, Y. Ploidy manipulation for breeding seedless triploid citrus. Plant Breed. Rev. 2008, 30, 323–354. [Google Scholar]
- Soost, R.K.; Cameron, J.W. ‘Melogold’, a triploid pummelo-grapefruit hybrid. HortScience 1985, 20, 1134–1135. [Google Scholar] [CrossRef]
- Thomas, T.D.; Rakhi, C. Endosperm culture: A novel method for triploid plant production. Plant Cell Tissue Organ Cult. 2008, 93, 1–14. [Google Scholar] [CrossRef]
- Gmitter, F.G., Jr.; Hu, X. The possible role of Yunan, China, in the origin of contemporary Citrus species (Rutaceae). Econ. Bot. 1990, 44, 267–277. [Google Scholar] [CrossRef]
- Lampe, L.; Mills, C.O. Growth and development of isolated endosperm and embryo of maize. Bull. Torrey Bot. Club 1936, 63, 365–382. [Google Scholar]
- Popielarska-Konieczna, M.; Kleszcz, I. Preliminary studies on plants regenerated from endosperm-derived callus of kiwifruit (Actinidia deliciosa var. deliciosa). Mod. Phytomorphology 2015, 7, 55–57. [Google Scholar]
- Patil, M.B.; Panchal, V.M. Comparative studies on ‘Nucellar’, ‘Sathgudi’ and ‘Local’ sweet orange (Mosambi) (Citrus sinensis Osbeck.) under Marathwada conditions. J. Hortic. Sci. 2016, 11, 44–46. [Google Scholar] [CrossRef]
- Murashige, T.; Tucker, D.P.H. Growth factor requirements of citrus tissue culture. Proc. First Int. Citrus Symp. 1969, 3, 1155–1161. [Google Scholar]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Kesara, A.J. Preparation of chromosome from plant leaf meristems for karyotype analysis and in situ hybridization. Methods Cell Sci. 2003, 25, 91–95. [Google Scholar]
- Krug, C.A. Chromosome number in the subfamily Aurantioideae with special reference to the genus Citrus. Bot. Gazzette 1943, 104, 602–611. [Google Scholar] [CrossRef]
- Vardi, A.; Levin, I.; Carmi, N. Induction of Seedlessness in Citrus: From Classical Techniques to emerging Biotechnological Approaches. J. Am. Soc. Hortic. Sci. 2008, 133, 117–126. [Google Scholar] [CrossRef]
- Bhojwani, S.S.; Razdan, M.K. Plant Tissue Culture: Theory and Practice. A Revised Edition; Elsevier: Amsterdam, The Netherlands; Lausanne, Switzerland; New York, NY, USA; Oxford, UK; Shannon, Ireland; Tokyo, Japan, 1996; pp. 1–767. [Google Scholar]
- Mooney, P.; Watson, M.; Harty, A. Developing New Seedless Citrus Triploid Cultivars. Hort. Res. Publ. 2007, 1–13. [Google Scholar]
- Vijayakumari, N.; Pooja, K. In vitro regeneration from hybrid endosperm tissue of Nagpur mandarin: A new research inroad towards seedless Citrus triploid cultivars. Indian Hortic. 2013, 18–19. [Google Scholar]
- Asakura, I.; Hoshino, Y. Endosperm-derived triploid plant regeneration in diploid ctinidiakolomikta, a cold-hardy kiwifruit relative. Sci. Hortic. 2017, 219, 53–59. [Google Scholar] [CrossRef]
- Wang, X.; Cheng, Z.M.; Zhi, S.X.F. Breeding Triploid Plants: A Review. Czech. J. Gen. Plant Breed. 2016, 52, 41–54. [Google Scholar] [CrossRef]
- Wang, D.Y. In vitro culture of Citrus embryos. Chin. J. Bot. 1975, 17, 149–152. [Google Scholar]
- Góralski, G.; Popielarska, M.; Slesak, H.; Siwinska, D.; Batycka, M. Organogenesis in endosperm of Actinidia deliciosa cv. Hayward cultured in vitro. Acta Biol. Cracov. Bot. 2005, 47, 121–128. [Google Scholar]
- Thomas, T.D.; Bhatnagar, A.K.; Bhojwani, S.S. Production of triploid plants of mulberry (Morus alba L.) by endosperm culture. Plant Cell Rep. 2000, 19, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Silva, N.T.D.; Silva, L.A.S.; Rels, A.C.; Machado, M.; Matos, E.M.D.; Ciccini, L.F.; Otoni, W.C.; Carvalho, I.F.D.; Rocha, D.I.; Silva, M.L.D. Endosperm culture: A facile and efficient biotechnological tool to generate passion fruit (Passiflora cincinnata Mast.) triploid plants. Plant Cell Tissue Organ Cult. 2020, 142, 613–624. [Google Scholar] [CrossRef]
- Raza, H.; Mumtaz, K.; Asifi, A.K. Review seedless in citrus. Int. J. Agric. Biol. 2003, 5, 388–391. [Google Scholar]
- Sajid, A.; Ahmad, S.K.; Syed, A.R.; Rana, N.U.R. Innovative breeding methods to develop seedless citrus cultivars. Int. J. Biosci. 2013, 3, 191–201. [Google Scholar]
- Rakhi, C.; Maharaj, K.R.; Sant, S.B. An efficient protocol for the production of triploid plants from endosperm callus of Neem, Azadirachta indica A. Juss. J. Plant Physiol. 2003, 160, 557–564. [Google Scholar]
- Mooney, P.; Richardson, A.C.; Marsh, K.B. Applications of biotechnology to cultivar improvement in New Zealand. Acta Hortic. 2000, 535, 207–212. [Google Scholar] [CrossRef]
- Beck, S.L.; Dunlop, R.; Fossey, A. Stomatal length and frequency as a measure of ploidy level in black wattle, Acacia mearnsii (de Wild). Bot. J. Linn. Soc. 2003, 141, 177–181. [Google Scholar] [CrossRef]
- Alzea, P.; Juarez, J.; Cuenca, J.; Ollitrault, P.; Navarro, L. Recovery of citrus triploid hybrids by embryo rescue and flow Cytometry from 2x × 2x sexual hybridisation and its application to extensive breeding programs. Plant Cell Rep. 2010, 29, 1023–1034. [Google Scholar] [CrossRef]
- Diego, P.; Amr, M.; Benedetta, C.; Maria, A.G.; Patan, S.S.V.K. Ploidy levels in Citrus clementine affects leaf morphology, stomatal density and water content. Theor. Exp. Plant Physiol. 2013, 25, 283–290. [Google Scholar]
- Zhou, J.; Hirata, Y.; Nou, I.S.; Shiotani, H.; Ito, T. Interactions between different genotypic tissues in citrus graft chimeras. Euphytica 2002, 126, 355–364. [Google Scholar] [CrossRef]
- Ye, Y.M.; Tong, J.; Shi, X.P.; Yuan, W.; Li, G.R. Morphological and cytological studies of diploid and colchicine-induced tetraploid lines of crape myrtle (Lagerstroemia indica L.). Sci. Hortic. 2010, 124, 95–101. [Google Scholar] [CrossRef]
- Spiegel, R.P.; Varid, A. Shani, Drah and Winsla three new selections from our breeding program. Proc. Int. Soc. Citric. 1992, 1, 72–73. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).