Response Surface Methodology for Development of Nutrient Solution Formula for Hydroponic Lettuce Based on the Micro-Elements Fertilizer Requirements at Different Growth Stages
Abstract
:1. Introduction
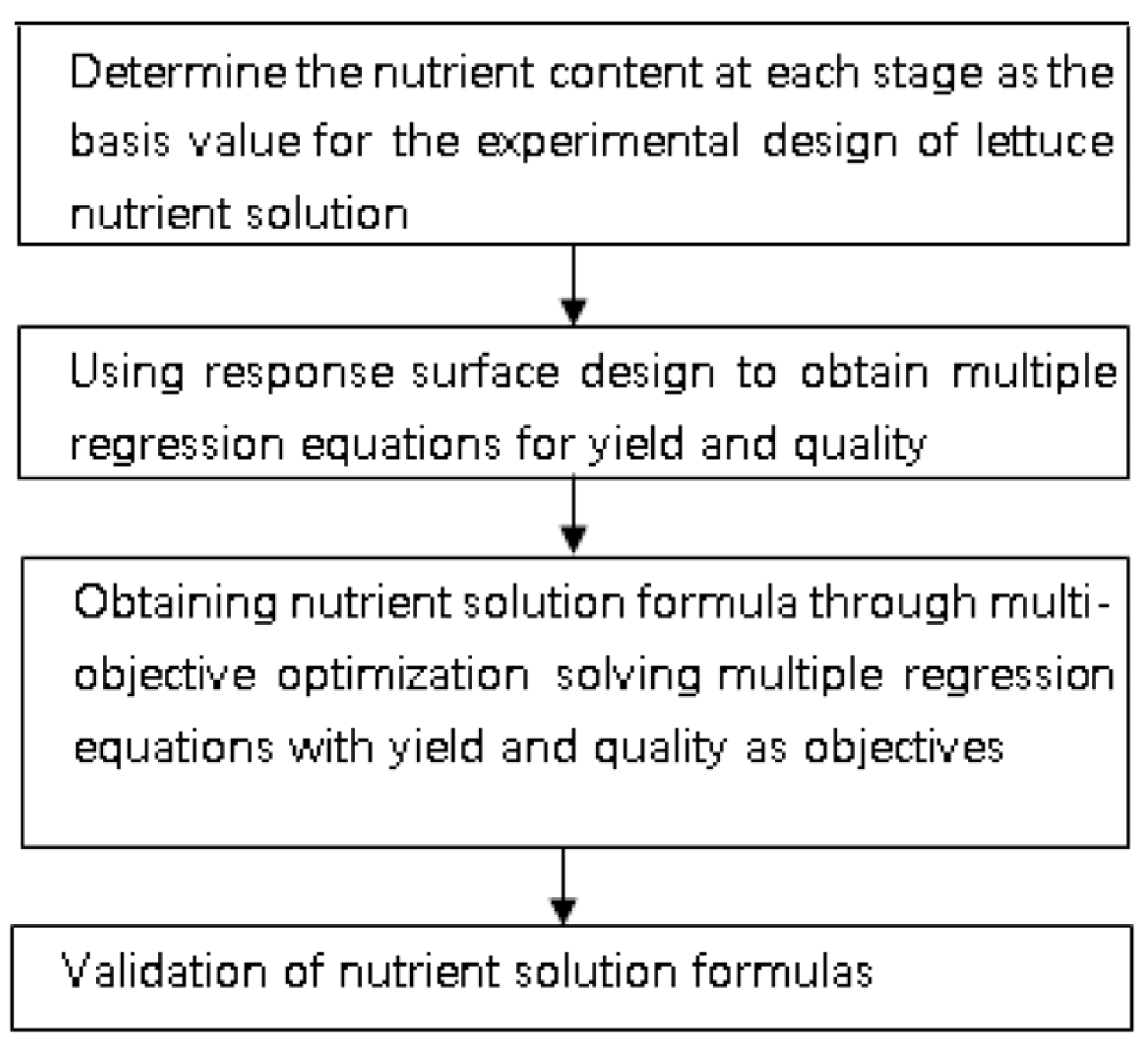
2. Materials and Methods
2.1. Plant Material and Plant Cultivation
2.2. Measurements
2.2.1. Nutrient Contents in Lettuce Plants
2.2.2. Nutrient Solution Response Surface Method Design
2.2.3. Growth and Quality Indices under Different P, K, Ca, and Mg Concentrations and Nutrient Solution Formulas
2.2.4. Establishment of the Regression Equation for Shoot Fresh Weight and Quality Indexes
2.2.5. Multiobjective Optimization of the Nutrient Solution Formulas
2.3. Data Analysis and Statistics
3. Results
3.1. Nutrient Content in Leaves of Lettuce Plants at Different Growth Stages
3.2. Shoot Fresh Weight, SPAD Value, Soluble Sugar Content, and Vc and Nitrate Content in the Lettuce at Different Nutrient Concentrations
3.3. Quadratic Regression Models between Shoot Fresh Weight, SPAD Value, Soluble Sugar Content, Vc Content and Nutrient Content
3.4. Optimization of Nutrient Concentrations for the Shoot Fresh Weight, SPAD Value, Soluble Sugar Content and Vc Content in Lettuce
3.5. The Seven Selected Nutrient Solution Formulas for the Validation Experiment
3.6. Validation of Nutrient Solution Formulas
3.6.1. Effects of Different Formulations of Nutrient Solutions on the Growth Parameters of Lettuce
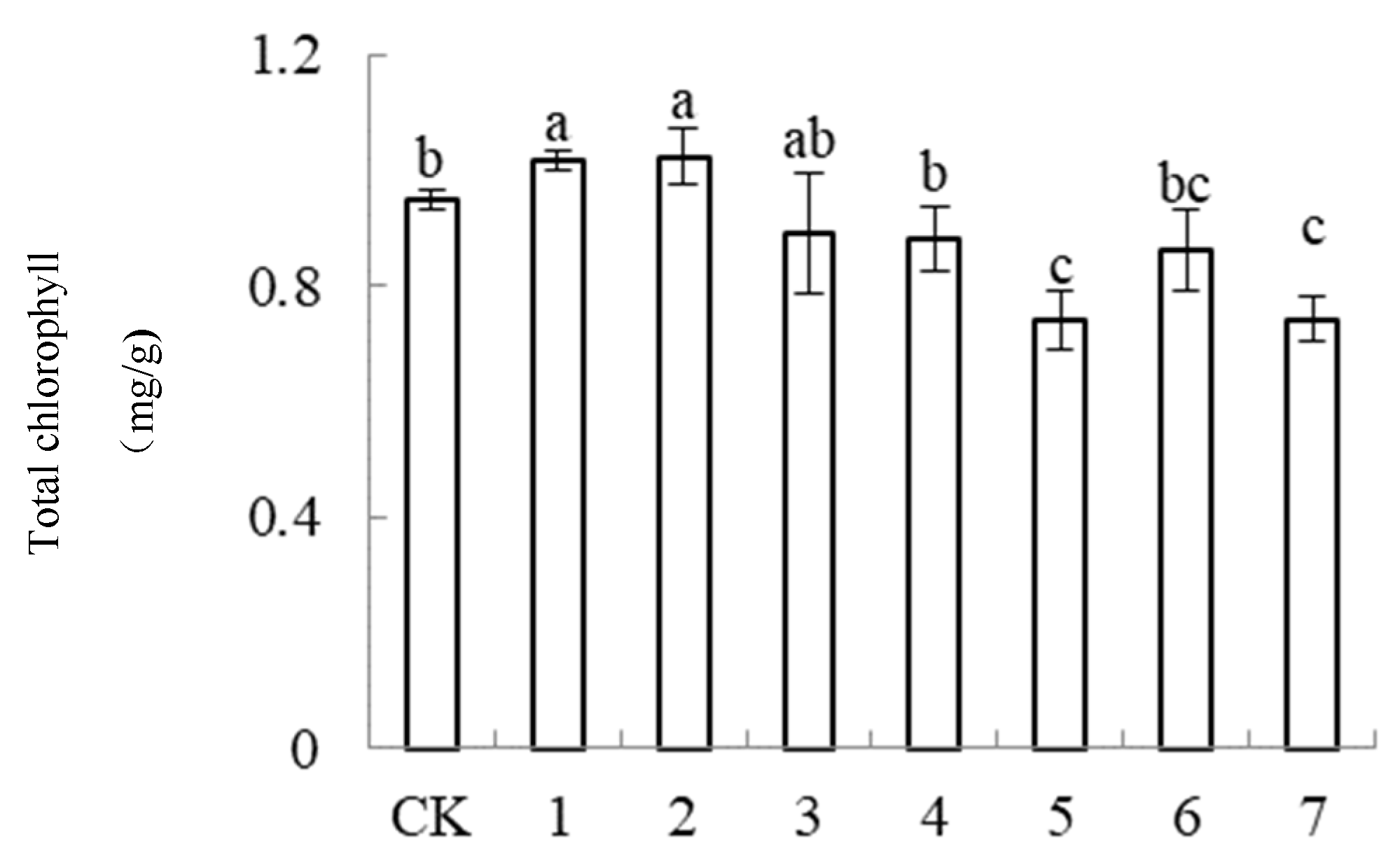
3.6.2. Effects of Different Formulations of Nutrient Solutions on the Chlorophyll Content in the Lettuce
3.6.3. Effects of Different Formulations of Nutrient Solutions on the Quality Parameters of Lettuce
3.6.4. Effects of Different Formulations of Nutrient Solutions on the Amino Acid Content in Lettuce
4. Discussion
4.1. Plant Chemical Analysis Method
4.2. Nutrient Solution Response Surface Method Design
4.3. Multiobjective Optimization of the Nutrient Solution Formulas
4.4. Nutrient Contents in Different Formulations of Nutrient Solutions for Lettuce
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Richa, A.; Touil, S.; Fizir, M.; Martinez, V. Recent advances and perspectives in the treatment of hydroponic wastewater: A review. Rev. Environ. Sci. Biotechnol. 2020, 19, 945–966. [Google Scholar] [CrossRef]
- Sun, J.; Li, Q.S.; Yue, D. Research status and application prospects of soilless culture technology in the world. J. Nanjing Agric. Univ. 2022, 45, 898–915. [Google Scholar]
- Majid, M.; Khan, J.N.; Shah, Q.A.; Masoodi, K.Z.; Afroza, B.; Parvaze, S. Evaluation of hydroponic systems for the cultivation of Lettuce (Lactuca sativa L. var. Longifolia) and comparison with protected soil-based cultivation. Agric. Water Manag. 2021, 245, 106572. [Google Scholar] [CrossRef]
- Keller, R.; Perin, K.; Souza, W.G.; Cruz, L.S.; Goncalves, R.F. Use of polishing pond effluents to cultivate lettuce (Lactuca sativa) in a hydroponic system. Water Sci. Technol. 2008, 58, 2051–2057. [Google Scholar] [CrossRef]
- Short, T.H.; El-Attal, A.; Keener, H.M.; Fynn, R.P. A decision model for hydroponic greenhouse tomato production. Acta Hortic. 1998, 456, 493–504. [Google Scholar] [CrossRef]
- Ikeuraa, H.; Tsukadab, K.; Tamaki, M. Effect of microbubbles in deep flow hydroponic culture on spinach growth. J. Plant Nutr. 2017, 40, 2358–2364. [Google Scholar] [CrossRef]
- Ren, X.W.; Lu, N.; Xu, W.S.; Zhuang, Y.F.; Takagaki, M. Optimization of the Yield, Total Phenolic Content, and Antioxidant Capacity of Basil by Controlling the Electrical Conductivity of the Nutrient Solution. Horticulturae 2022, 8, 216. [Google Scholar] [CrossRef]
- Song, J.; Huang, H.; Hao, Y.; Song, S.; Zhang, Y.; Su, W.; Liu, H. Nutritional quality, mineral and antioxidant content in lettuce affected by interaction of light intensity and nutrient solution concentration. Sci. Rep. 2020, 10, 2796. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Acharya, S.; Kumar, K.; Singh, N. Hydroponics as an advanced technique for vegetable production: An overview. J. Soil Water Conserv. 2019, 17, 364–371. [Google Scholar] [CrossRef]
- Gillespie, D.P.; Papio, G.; Kubota, C. High nutrient concentrations of hydroponic solution can improve growth and nutrient uptake of spinach (Spinacia oleracea L.) grown in acidic nutrient solution. Hortscience 2021, 56, 687–694. [Google Scholar] [CrossRef]
- Cortella, G.; Saro, O.; Angelis, A.D.; Ceccotti, L.; Tomasi, N.; Costa, L.D. Temperature control of nutrient solution in floating system cultivation. Appl. Therm. Eng. 2014, 73, 1055–1065. [Google Scholar] [CrossRef]
- Frasetya, B.; Taofik, A.; Sholehah, M. The evaluation of various nutrient formulation on the growth of lettuce (Lactuca sativa Var. Arista) in hydroponic raft system at tropic region. J. Phys. Conf. Ser. 2019, 1402, 033025. [Google Scholar] [CrossRef]
- Xiao, S.; Liu, L.T.; Zhang, Y.J. Review on new methods of in situ observation of plant micro-roots and interpretation of root images. J. Plant Nutr. Fertil. 2020, 26, 370–385. [Google Scholar]
- Guo, J.T.; Dong, L.D.; Jiao, Y.G. Optimum Formula of Hydroponic Nutrient Solution for Low Nitrate Leaf Vegetables. J. Agric. 2017, 7, 28–32. [Google Scholar]
- Liu, C.W.; Sung, Y.; Chen, B.C.; Lai, H.Y. Effects of Nitrogen Fertilizers on the Growth and Nitrate Content of Lettuce (Lactuca sativa L.). Int. J. Environ. Res. Public Health 2014, 11, 4427–4444. [Google Scholar] [CrossRef] [PubMed]
- Yuki, S.; Airi, S. Quantitative nutrient management reduces nitrate accumulation in hydroponic butterhead lettuces grown under artificial lighting. Hortsci. A Publ. Am. Soc. Hortic. Sci. 2018, 53, 963–967. [Google Scholar] [CrossRef]
- Maneejantra, N.; Tsukagoshi, S.; Lu, N. A quantitative analysis of nutrient requirements for hydroponic spinach (Spinacia Oleracea L.) production under artificial light in a plant factory. J. Fertil. Pestic. 2016, 7, 170. [Google Scholar] [CrossRef]
- Ren, X.W.; Lu, N.; Xu, W.S.; Zhuang, Y.F.; Tsukagoshi, S.; Takagaki, M. Growth and Nutrient Utilization in Basil Plant as Affected by Applied Nutrient Quantity in Nutrient Solution and Light Spectrum. Biology 2022, 11, 991. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Guo, Y.Z.; Qiu, L. Effects of ozone-treated domestic sludge on hydroponic lettuce growth and nutrition. J. Integr. Agric. 2018, 17, 593–602. [Google Scholar] [CrossRef]
- Qu, M.S.; Dong, H.Q.; Xing, W.X.; Guo, N.; Chen, B.H.; Ji, W. Effects of Adjustment of Yamazaki Lettuce Nutrient Solution Formula on Yield and Quality of Lettuce. J. Hebei Agric. Sci. 2012, 16, 31–35. [Google Scholar]
- Weerakkody, W.A.P.; Wakui, K.; Nukaya, A. Plant nutrient uptake in recirculation culture of tomato under growth stage based electrical conductivity adjustments. J. Natl. Sci. Found. Sri Lanka 2011, 39, 139–147. [Google Scholar] [CrossRef]
- Kagohashi, S.; Kano, H.; Kageyama, M. Effects of controlling the nutrient uptake on the plant growth and the fruit qualities of muskmelons cultivated in autumn and spring. J. Jpn. Soc. Hortic. Sci. 2007, 50, 306–316. [Google Scholar] [CrossRef]
- Kuronuma, T.; Ando, M.; Watanabe, H. Tipburn Incidence and Ca Acquisition and Distribution in Lisianthus (Eustoma grandiflorum (Raf.) Shinn.) Cultivars under Di_erent Ca Concentrations in Nutrient Solution. Agronomy 2020, 10, 216. [Google Scholar] [CrossRef]
- Stefanelli, D.; Brady, S.; Winkler, S.; Jones, R.B. Lettuce (Lactuca sativa L.) growth and quality response to applied nitrogen under hydroponic conditions. Acta Hortic. 2012, 927, 353–360. [Google Scholar] [CrossRef]
- Monforte, A.R.; Oliveira, C.; Martins SIFSFerreira, A.C.S. Response surface methodology: A tool to minimize aldehydes formation and oxygen consumption in wine model system. Food Chem. 2019, 283, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.J.; Zhang, Y.H.; Guo, S.P. Optimization of boron fertilizer application method using response surface design model for high-quality upper leaves of flue-cured tobacco. J. Plant Nutr. 2022, 28, 366–374. [Google Scholar]
- Wang, J.; Shen, L.Z.; Yang, Y.Z. Optimizing calibration of design points for non-road high pressure common rail diesel engine base on response surface methodology. Trans. Chin. Soc. Agric. Eng. 2017, 33, 31–39. [Google Scholar]
- Jokić, A.; Pajčin, I.; Lukić, N.; Vlajkov, V.; Kiralj, A.; Dmitrović, S.; Grahovac, J. Modeling and optimization of gas sparging-assisted bacterial cultivation broth microfiltration by response surface methodology and genetic algorithm. Membranes 2021, 11, 681. [Google Scholar] [CrossRef]
- Derossi, A.; Severini, C.; Mastro, A.D.; Pilli, T.D. Study and optimization of osmotic dehydration of cherry tomatoes in complex solution by response surface methodology and desirability approach. LWT—Food Sci. Technol. 2015, 60, 641–648. [Google Scholar] [CrossRef]
- Abreu, M.; Beiro-Da-Costa, S.; Gonalves, E.M.; Beiro-Da-Costa, M.L.; Moldo-Martins, M. Use of mild heat pre-treatments for quality retention of fresh-cut ‘Rocha’ pear. Postharvest Biol. Technol. 2003, 30, 153–160. [Google Scholar] [CrossRef]
- Song, X.X.; Shu, S.; Guo, S.R. Optimization of nutrient solution formula applied in cucumber cultivation with substrate. J. Nanjing Agric. Univ. 2015, 38, 197–204. [Google Scholar]
- Qu, F.; Zhang, J.; Wang, J.Z. Genetic algorithm-based optimization of nutrient solution formula for substrate-cultivated cucumber. Trans. Chin. Soc. Agric. Eng. 2021, 37, 96–104. [Google Scholar] [CrossRef]
- Ma, S.; Chen, Z.; Yang, F.J. Optimizing fertilization scheme of N,P2O5 and K2O concentration for eggplant under soilless culture. Chin. J. Appl. Ecol. 2018, 29, 2935–2942. [Google Scholar]
- Ruangraka, E.; Khummueng, W. Effects of artificial light sources on accumulation of phytochemical contents in hydroponic lettuce. J. Hortic. Sci. Biotechnol. Trust. 2018, 94, 378–388. [Google Scholar] [CrossRef]
- Eshkabilov, S.; Stenger, J.; Knutson, E.N.; Küçüktopcu, E. Hyperspectraln Image Data and Waveband Indexing Methods to Estimate Nutrient Concentration on Lettuce (Lactuca sativa L.) Cultivars. Sensors 2022, 22, 8158. [Google Scholar] [CrossRef] [PubMed]
- Ezziddine, M.; Liltved, H.; Seljåsen, R. Hydroponic Lettuce Cultivation Using Organic Nutrient Solution from Aerobic Digested Aquacultural Sludge. Agronomy 2021, 11, 1484. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The Water-Culture Method for Growing Plants without Soil. In Circular; California Agricultural Experiment Station: Davis, CA, USA, 2018. [Google Scholar]
- Pohl, P.; Stecka, H.; Greda, K.; Jamroz, P. Determination of the hydrophobic fraction of Ca, Fe, mg and Zn in dark color honeys using solid phase extraction and flame atomic absorption spectrometry. J. Braz. Chem. Soc. 2012, 23, 1098–1103. [Google Scholar] [CrossRef]
- Lee, J.G.; Lee, B.Y.; Lee, H.J. Accumulation of phytotoxic organic acids in reused nutrient solution during hydroponic cultivation of lettuce (Lactuca sativa L.). Sci. Hortic. 2006, 110, 119–128. [Google Scholar] [CrossRef]
- Moraes, V.H.; Giongo, P.R.; Giongo, A.M.M.; Cavalcante, T.J.; Arantes, B.H.T. Electrical conductivity in nutritive solution and influence on hydroponic production in lettuce culture (Lactuta sativa L.). Aust. J. Basic Appl. Sci. 2018, 12, 32–35. [Google Scholar]
- Li, S.; Cui, T.J.; Viriyasitavat, W. Edge Device Fault Probability Based Intelligent Calculations for Fault Probability of Smart Systems. Tsinghua Sci. Technol. 2024, 29, 1023–1036. [Google Scholar] [CrossRef]
- Shailendra, R.; Jayapalan, A.; Velayutham, S.; Baladhandapani, A.; Srivastava, A.; Gupta, S.K.; Kumar, M. An IoT and machine learning based intelligent system for the classification of therapeutic plants. Neural Process. Lett. 2022, 54, 4465–4493. [Google Scholar] [CrossRef]
- Yang, S.Y.; Lin, W.Y.; Hsiao, Y.M.; Chiou, T.J. Milestones in understanding transport, sensing, and signaling of the plant nutrient phosphorus. Plant Cell 2024, 36, 1504–1523. [Google Scholar] [CrossRef] [PubMed]
- Neeser, C.; Savidov, N.; Driedger, D. Production of hydroponically grown calcium fortified lettuce. Acta Hortic. 2007, 744, 317–322. [Google Scholar] [CrossRef]
- Meagy, M.J.; Eaton, T.E.; Barker, A.V. Nutrient density in lettuce cultivars grown with organic or conventional fertilization with elevated calcium concentrations. Hortsci. A Publ. Am. Soc. Hortic. Sci. 2013, 48, 670–680. [Google Scholar] [CrossRef]
- Jezek, M.; Geilfus, C.M.; Bayer, A.; Muhling, K.H. Photosynthetic capacity, nutrient status, and growth of maize (Zea mays L.) upon MgSO4 leaf application. Front. Plant. Sci. 2015, 5, 781. [Google Scholar] [CrossRef]
| Treatment | Variable Values | |||
|---|---|---|---|---|
| X1(P) | X2(K) | X3(Ca) | X4(Mg) | |
| CK 1 | — +1 | — +1 | — +1 | — +1 |
| 1 | +1 | +1 | +1 | +1 |
| 2 | +1 | +1 | −1 | +1 |
| 3 | +1 | −1 | +1 | −1 |
| 4 | +1 | −1 | −1 | −1 |
| 5 | −1 | +1 | +1 | −1 |
| 6 | −1 | +1 | −1 | −1 |
| 7 | −1 | −1 | +1 | +1 |
| 8 | −1 | −1 | −1 | +1 |
| 9 | −1.682 | 0 | 0 | 0 |
| 10 | +1.682 | 0 | 0 | 0 |
| 11 | 0 | −1.682 | 0 | 0 |
| 12 | 0 | +1.682 | 0 | 0 |
| 13 | 0 | 0 | −1.682 | 0 |
| 14 | 0 | 0 | +1.682 | 0 |
| 15 | 0 | 0 | 0 | −1.682 |
| 16 | 0 | 0 | 0 | +1.682 |
| 17 | 0 | 0 | 0 | 0 |
| 18 | 0 | 0 | 0 | 0 |
| 19 | 0 | 0 | 0 | 0 |
| 20 | 0 | 0 | 0 | 0 |
| 21 | 0 | 0 | 0 | 0 |
| Growth Stage | Nutrients | ||||
|---|---|---|---|---|---|
| N (mg·g−1 DW) | P (mg·g−1 DW) | K (mg·g−1 DW) | Ca (mg·g−1 DW) | Mg (mg·g−1 DW) | |
| 30 days | 21.05 ± 4.78 | 4.67 ± 0.31 | 36.33 ± 1.15 | 12.94 ± 0.81 | 8.76 ± 0.83 |
| 50 days | 22.56 ± 3.45 | 4.06 ± 0.03 | 31.88 ± 0.75 | 13.37 ± 1.10 | 9.81 ± 0.45 |
| 65 days | 22.37 ± 5.90 | 4.78 ± 0.13 | 35.53 ± 0.25 | 14.32 ± 1.02 | 9.69 ± 0.23 |
| Average | 21.99 | 4.50 | 34.58 | 13.54 | 9.42 |
| Variation | Unit | Coding Level | ||||
|---|---|---|---|---|---|---|
| −1.628 | −1 | 0 | +1 | +1.628 | ||
| N | mmol·L−1 | 22.00 | 22.00 | 22.00 | 22.00 | 22.00 |
| P | 0.65 | 1.20 | 2.00 | 2.80 | 3.35 | |
| K | 5.31 | 6.40 | 8.00 | 9.60 | 10.69 | |
| Ca | 3.65 | 4.40 | 5.50 | 6.60 | 7.35 | |
| Mg | 1.47 | 2.90 | 5.00 | 7.10 | 8.53 | |
| Treatments | Nutrient Concentration (mmol·L−1) | Shoot Fresh Weight (g·Plant−1) | SPAD | Soluble Sugar (mg·g−1) | Vc (mg·100 g−1) | Nitrate (mg·g−1) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | P | K | Ca | Mg | ||||||
| CK | 16.00 | 2.00 | 6.00 | 4.00 | 1.00 | 181.26 | 33.45 | 1.74 | 6.42 | 10.41 |
| 1 | 22.00 | 2.80 | 9.60 | 6.60 | 7.10 | 134.75 | 32.42 | 1.38 | 5.32 | 6.36 |
| 2 | 22.00 | 2.80 | 9.60 | 4.40 | 7.10 | 201.85 | 33.21 | 2.36 | 6.62 | 4.32 |
| 3 | 22.00 | 2.80 | 6.40 | 6.60 | 2.90 | 134.75 | 35.65 | 2.89 | 8.31 | 5.98 |
| 4 | 22.00 | 2.80 | 6.40 | 4.40 | 2.90 | 117.15 | 27.07 | 2.56 | 5.22 | 12.13 |
| 5 | 22.00 | 1.20 | 9.60 | 6.60 | 2.90 | 132.55 | 36.44 | 3.67 | 4.23 | 4.79 |
| 6 | 22.00 | 1.20 | 9.60 | 4.40 | 2.90 | 183.15 | 26.65 | 1.97 | 8.03 | 8.25 |
| 7 | 22.00 | 1.20 | 6.40 | 6.60 | 7.10 | 145.75 | 29.44 | 1.90 | 5.02 | 14.51 |
| 8 | 22.00 | 1.20 | 6.40 | 4.40 | 7.10 | 286.00 | 36.55 | 3.35 | 6.12 | 5.96 |
| 9 | 22.00 | 0.65 | 8.00 | 5.50 | 5.00 | 78.10 | 30.67 | 2.10 | 5.62 | 4.89 |
| 10 | 22.00 | 3.35 | 8.00 | 5.50 | 5.00 | 149.62 | 31.67 | 3.48 | 7.24 | 6.79 |
| 11 | 22.00 | 2.00 | 5.31 | 5.50 | 5.00 | 121.00 | 29.88 | 2.36 | 5.59 | 8.25 |
| 12 | 22.00 | 2.00 | 10.69 | 5.50 | 5.00 | 159.50 | 34.52 | 2.16 | 4.84 | 4.63 |
| 13 | 22.00 | 2.00 | 8.00 | 3.65 | 5.00 | 116.05 | 29.83 | 1.71 | 7.88 | 7.16 |
| 14 | 22.00 | 2.00 | 8.00 | 7.35 | 5.00 | 88.00 | 29.81 | 1.84 | 4.42 | 5.63 |
| 15 | 22.00 | 2.00 | 8.00 | 5.50 | 1.47 | 211.75 | 30.31 | 1.57 | 4.92 | 4.63 |
| 16 | 22.00 | 2.00 | 8.00 | 5.50 | 8.53 | 145.97 | 28.73 | 1.77 | 6.01 | 6.74 |
| 17 | 22.00 | 2.00 | 8.00 | 5.50 | 5.00 | 225.50 | 28.33 | 1.38 | 5.38 | 8.59 |
| 18 | 22.00 | 2.00 | 8.00 | 5.50 | 5.00 | 200.20 | 28.54 | 1.44 | 5.22 | 8.56 |
| 19 | 22.00 | 2.00 | 8.00 | 5.50 | 5.00 | 215.60 | 28.10 | 1.67 | 4.44 | 8.58 |
| 20 | 22.00 | 2.00 | 8.00 | 5.50 | 5.00 | 195.80 | 28.33 | 1.51 | 6.23 | 8.77 |
| 21 | 22.00 | 2.00 | 8.00 | 5.50 | 5.00 | 191.40 | 28.21 | 1.57 | 6.23 | 8.61 |
| Objective Function | Equation | R2 | F Value | p Value |
|---|---|---|---|---|
| Shoot fresh weight | Y1 = 227.48 + 19.23 × 1 − 15.27 × 2 + 32.10 × 3 + 28.03 × 4 − 10.00 × 1×2 − 48.42 × 1×3 + 12.61 × 1×4 − 32.83 × 2×3 − 33.47 × 2×4 − 33.47 × 12 − 15.17 × 22 − 10.10 × 32 + 4.85 × 42 | 0.91 | 3.92 | 0.039 |
| SPAD | Y2 = 28.11 + 0.04 × 1 + 1.38 × 2 + 0.50 × 3 − 0.03 × 4 + 0.6887 × 1×3 − 1.33 × 1×4 + 0.89 × 2×3 − 2.83 × 3×4 + 1.22 × 12 + 1.60 × 22 + 0.76 × 32 + 0.65 × 42 | 0.95 | 13.87 | 0.0005 |
| Soluble sugar | Y3 = 1.49 + 0.41 × 1 − 0.06 × 2 − 0.01 × 3 + 0.06 × 4 − 0.32 × 1×2 − 0.11 × 1×3 − 0.10 × 1×4 + 0.23 × 2×3 − 0.62 × 2×4 + 0.48 × 12 + 0.29 × 22 + 0.12 × 32 + 0.08 × 42 | 0.98 | 33.99 | 0.0002 |
| Vc | Y4 = 5.85 + 0.48 × 1 − 0.22 × 2 − 0.65 × 3 + 0.32 × 4 − 0.66 × 1×2 + 0.84 × 1×3 + 0.16 × 1×4 − 0.88 × 2×3 − 0.22 × 2×4 − 0.21 X3×4 | 0.81 | 3.22 | 0.038 |
| Factors-Independent Variables | Goal | Optimized Value |
|---|---|---|
| N(mmol·L−1) | - | 22.00 |
| P(mmol·L−1) | In range | 2.71 |
| K(mmol·L−1) | In range | 6.42 |
| Ca(mmol·L−1) | In range | 5.58 |
| Mg(mmol·L−1) | In range | 7.11 |
| Responses—Dependent Variables | Goal | Optimized Value |
| Shoot fresh weight | maximum | 246.15 g |
| SPAD | maximum | 31.96 |
| Soluble sugar | maximum | 3.42 mg·g−1 |
| Vc | maximum | 6.84 mg·100 g−1 |
| Desirability value | 0.851 | |
| Treatments | NH4H2PO4 (mmol L−1) | KNO3 (mmol L−1) | Ca (NO3)2 (mmol L−1) | MgSO4 (mmol L−1) | Desirability Value |
|---|---|---|---|---|---|
| CK | 1.00 | 6.00 | 4.00 | 2.00 | - |
| T1 | 2.70 | 6.40 | 5.60 | 7.10 | 0.851 |
| T2 | 2.80 | 6.40 | 5.80 | 7.30 | 0.823 |
| T3 | 3.35 | 8.00 | 5.50 | 5.00 | 0.769 |
| T4 | 2.80 | 9.60 | 4.40 | 7.10 | 0.755 |
| T5 | 2.00 | 8.00 | 5.50 | 5.00 | 0.712 |
| T6 | 2.00 | 8.00 | 3.65 | 5.00 | 0.554 |
| T7 | 1.20 | 6.40 | 4.40 | 7.10 | 0.501 |
| Treatments | Shoot Fresh Weight (g) | Root Fresh Weight (g) | Shoot Dry Weight (g) | Root Dry Weight (g) |
|---|---|---|---|---|
| CK | 213.99 ± 7.65 b | 41.60 ± 3.32 b | 22.38 ± 1.67 b | 1.70 ± 0.08 c |
| T1 | 301.02 ± 6.03 a | 51.21 ± 2.57 a | 32.12 ± 1.63 a | 3.40 ± 0.19 a |
| T2 | 280.54 ± 4.32 a | 49.74 ± 3.22 ab | 30.52 ± 2.76 a | 2.98 ± 0.40 ab |
| T3 | 209.88 ± 8.05 b | 42.36 ± 2.21 b | 23.40 ± 2.11 b | 2.28 ± 0.46 b |
| T4 | 183.47 ± 7.34 bc | 43.78 ± 3.16 b | 21.04 ± 0.92 b | 1.94 ± 0.35 bc |
| T5 | 204.39 ± 8.02 b | 40.20 ± 1.28 bc | 20.60 ± 1.64 bc | 2.30 ± 0.13 b |
| T6 | 159.30 ± 6.64 d | 39.28 ± 2.39 bc | 13.86 ± 1.53 d | 1.70 ± 0.21 c |
| T7 | 154.65 ± 7.91 c | 40.61 ± 2.11 bc | 13.88 ± 0.89 d | 2.36 ± 0.15 b |
| Treatments | Soluble Sugar (mg g−1) | Soluble Protein (mg g−1) | Vc (mg 100 g−1) | Nitrate Content (mg g−1) | Amino Acid (μg g−1) |
|---|---|---|---|---|---|
| CK | 2.89 ± 0.36 bc | 5.07 ± 0.25 c | 5.53 ± 0.31 c | 10.45 ± 0.61 b | 1.71 ± 0.10 d |
| T1 | 3.23 ± 0.67 a | 7.05 ± 0.12 a | 6.67 ± 0.50 b | 7.29 ± 0.52 e | 2.75 ± 0.10 a |
| T2 | 3.08 ± 0.45 ab | 5.86 ± 0.13 b | 5.54 ± 0.42 c | 4.72 ± 0.54 f | 2.34 ± 0.02 b |
| T3 | 2.21 ± 0.26 d | 4.92 ± 0.27 c | 5.56 ± 0.16 c | 8.70 ± 0.41 d | 1.12 ± 0.05 ef |
| T4 | 1.65 ± 0.12 e | 4.98 ± 0.17 c | 8.46 ± 0.23 a | 9.34 ± 0.32 c | 1.22 ± 0.13 e |
| T5 | 1.98 ± 0.03 d | 4.48 ± 0.09 d | 4.60 ± 0.20 c | 16.95 ± 0.78 a | 1.74 ± 0.01 d |
| T6 | 2.04 ± 0.11 d | 4.91 ± 0.27 c | 4.73 ± 0.23 c | 9.89 ± 0.33 bc | 0.99 ± 0.03 f |
| T7 | 2.42 ± 0.22 c | 5.50 ± 0.02 b | 8.20 ± 0.40 ab | 11.00 ± 0.47 b | 1.93 ± 0.05 c |
| Treatments | Glutamate (ug g−1) | Glycine (ug g−1) | Alanine (ug g−1) | GABA (ug g−1) | Total Amino Acid (ug g−1) |
|---|---|---|---|---|---|
| CK | 6.07 ± 0.41 a | 4.19 ± 0.27 bc | 15.75 ± 0.68 d | 55.52 ± 3.85 c | 1.71 ± 0.10 d |
| T1 | 6.03 ± 0.20 a | 6.38 ± 0.26 a | 21.71 ± 1.45 bc | 66.03 ± 3.29 ab | 2.75 ± 0.10 a |
| T2 | 4.87 ± 0.48 bc | 6.17 ± 0.32 a | 25.60 ± 1.45 ab | 66.02 ± 4.86 ab | 2.34 ± 0.02 b |
| T3 | 4.83 ± 0.22 bc | 5.06 ± 0.25 b | 17.48 ± 1.21 cd | 53.71 ± 4.46 c | 1.12 ± 0.05 ef |
| T4 | 5.18 ± 0.13 abc | 5.02 ± 0.30 b | 15.97 ± 1.26 d | 51.03 ± 2.16 c | 1.22 ± 0.13 e |
| T5 | 5.84 ± 0.26 ab | 5.11 ± 0.28 b | 17.82 ± 0.71 cd | 56.87 ± 3.21 c | 1.74 ± 0.01 d |
| T6 | 5.56 ± 0.55 ab | 4.81 ± 0.20 bc | 27.76 ± 2.38 a | 59.02 ± 6.22 c | 0.99 ± 0.03 f |
| T7 | 4.23 ± 0.28 c | 6.21 ± 0.72 a | 25.45 ± 2.35 ab | 75.91 ± 7.04 a | 1.93 ± 0.05 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, B.; Ren, X.; Hao, W.; Li, J.; Hou, S.; Yang, K.; Wu, X.; Gao, H. Response Surface Methodology for Development of Nutrient Solution Formula for Hydroponic Lettuce Based on the Micro-Elements Fertilizer Requirements at Different Growth Stages. Agronomy 2024, 14, 1160. https://doi.org/10.3390/agronomy14061160
Gong B, Ren X, Hao W, Li J, Hou S, Yang K, Wu X, Gao H. Response Surface Methodology for Development of Nutrient Solution Formula for Hydroponic Lettuce Based on the Micro-Elements Fertilizer Requirements at Different Growth Stages. Agronomy. 2024; 14(6):1160. https://doi.org/10.3390/agronomy14061160
Chicago/Turabian StyleGong, Binbin, Xiaowei Ren, Wenyu Hao, Jingrui Li, Shenglin Hou, Kun Yang, Xiaolei Wu, and Hongbo Gao. 2024. "Response Surface Methodology for Development of Nutrient Solution Formula for Hydroponic Lettuce Based on the Micro-Elements Fertilizer Requirements at Different Growth Stages" Agronomy 14, no. 6: 1160. https://doi.org/10.3390/agronomy14061160
APA StyleGong, B., Ren, X., Hao, W., Li, J., Hou, S., Yang, K., Wu, X., & Gao, H. (2024). Response Surface Methodology for Development of Nutrient Solution Formula for Hydroponic Lettuce Based on the Micro-Elements Fertilizer Requirements at Different Growth Stages. Agronomy, 14(6), 1160. https://doi.org/10.3390/agronomy14061160






