Brackish Water, Phosphate Fertilization and Trichoderma in the Agronomic Performance of Beet Crops
Abstract
:1. Introduction
2. Materials and Methods
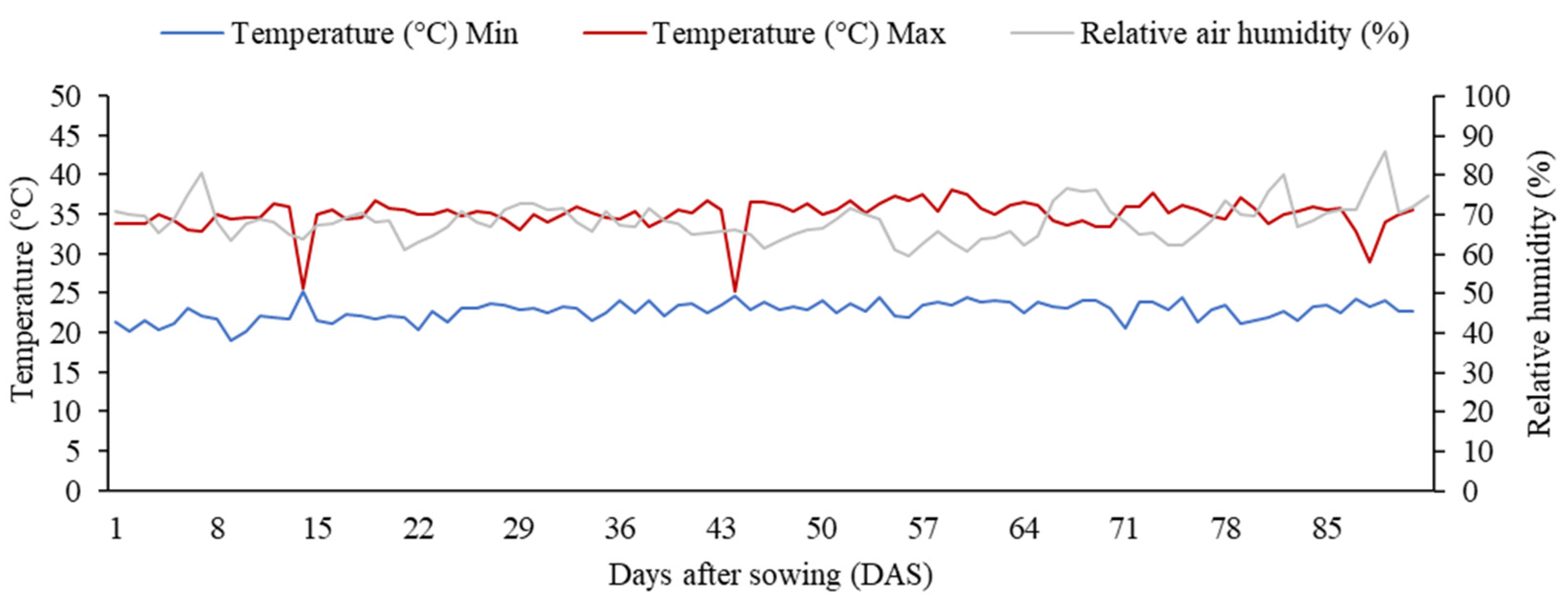
2.1. Experimental Area and Weather Conditions
2.2. Plant Material and Substrate for Cultivation
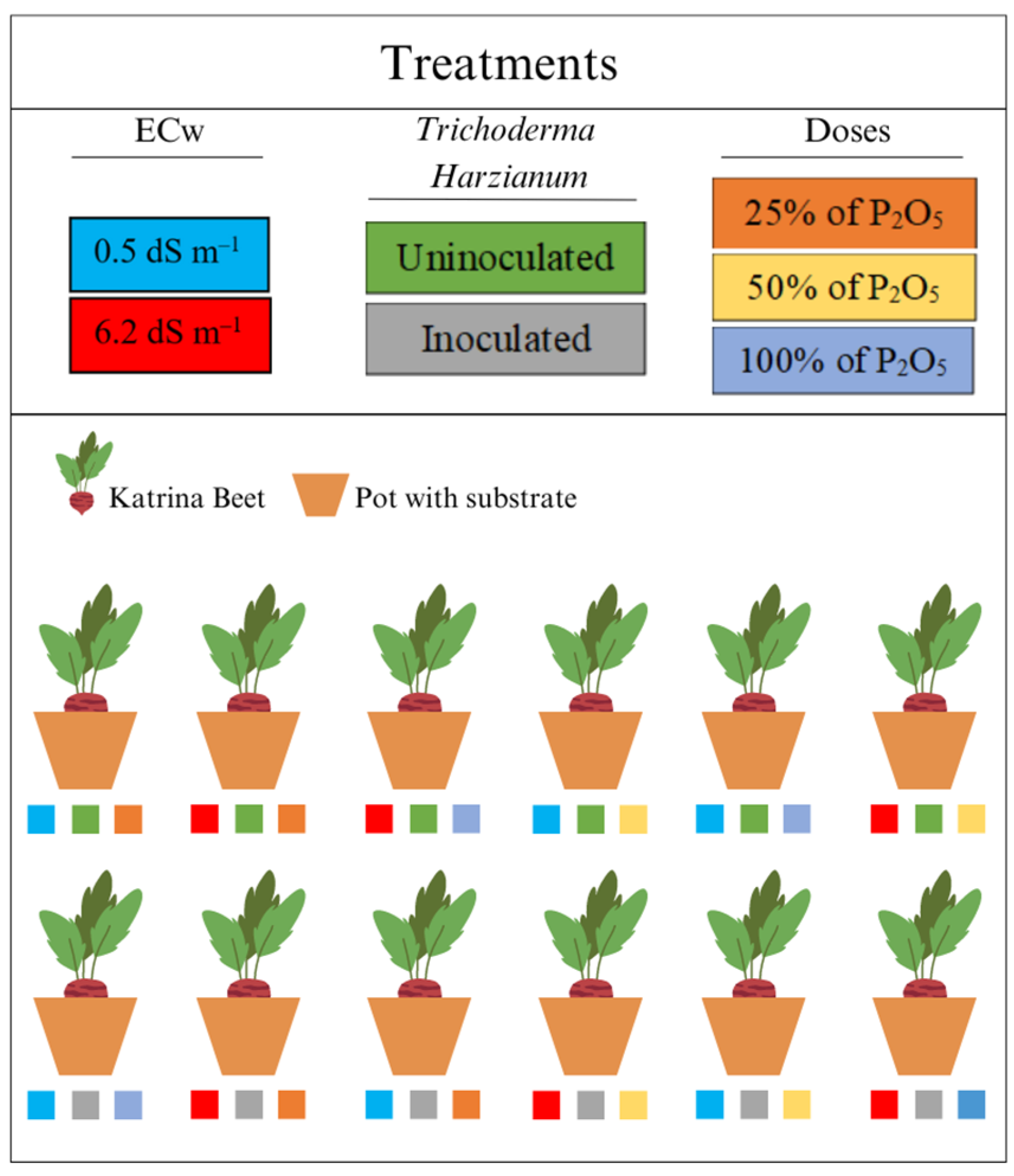
2.3. Experimental Design and Description of Treatments
2.4. Irrigation Management
2.5. Fertilization and Inoculation
2.6. Plant Analysis
2.7. Data Analysis
3. Results
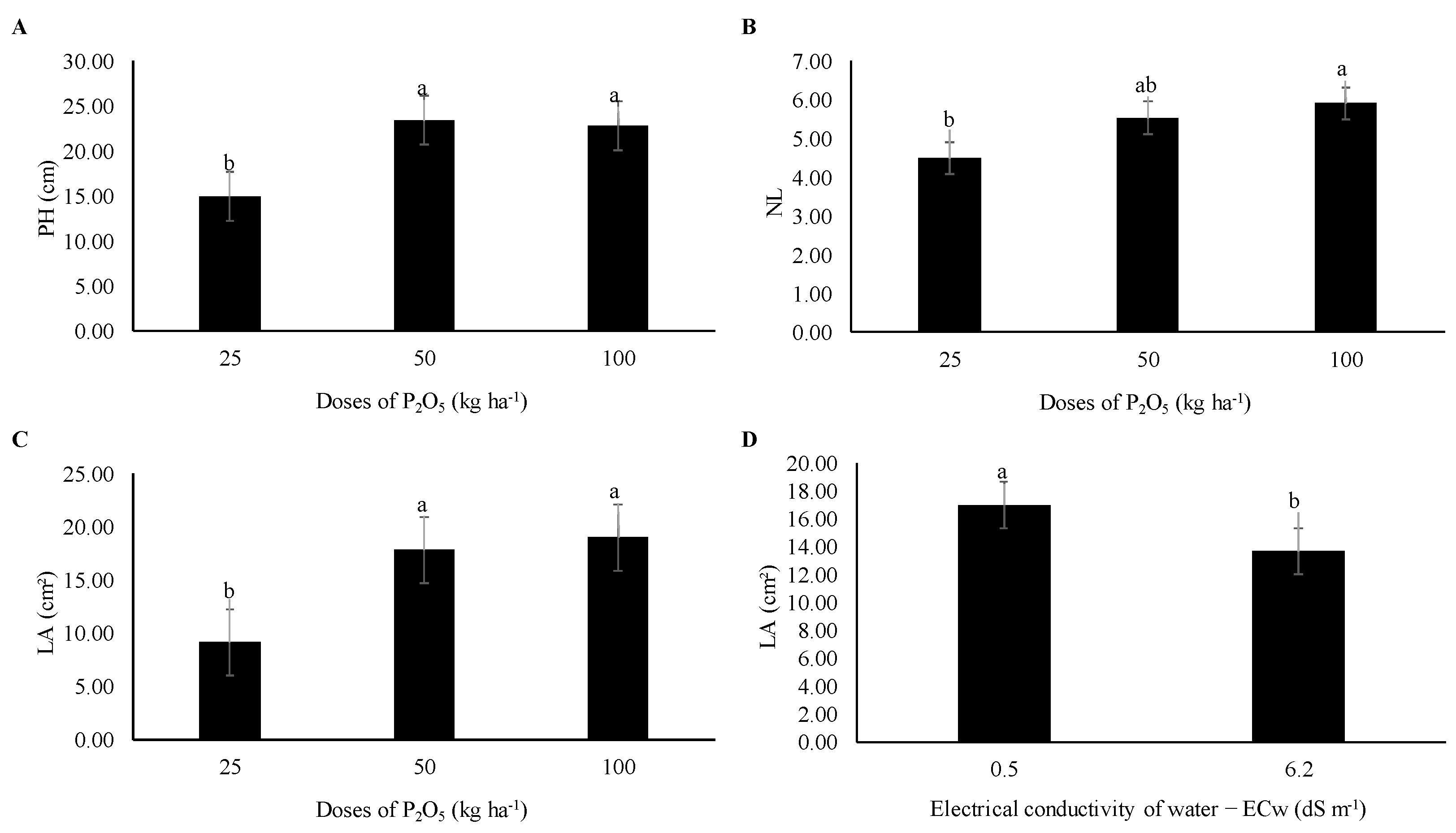
3.1. Growth and Biomass Variables
3.1.1. Growth
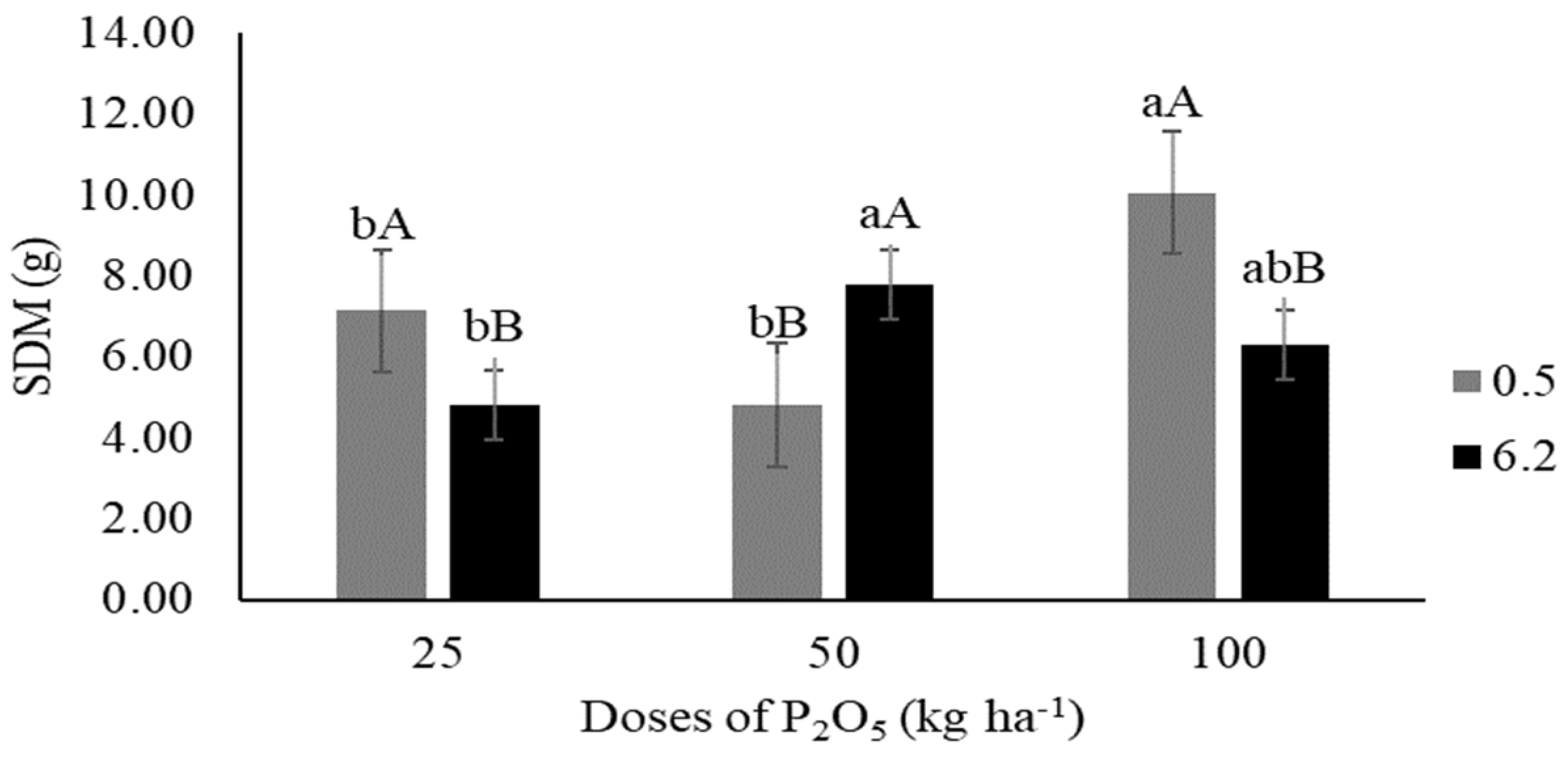
3.1.2. Biomass
3.2. Leaf Gas Exchange
3.3. Post-Harvest and Yield
4. Discussion
4.1. Growth and Biomass Variables
4.1.1. Growth
4.1.2. Biomass
4.2. Leaf Gas Exchange
4.3. Post-Harvest and Yield
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pacheco, B.R.O.; Makoski, J.R.; Lima, C.S.M.; Rosa, G.G. Classificação comercial e caracterização físico-química de beterrabas oriundas de sistema de plantio direto de hortaliças sob diferentes densidades de palhada de milho. Rev. Iberoam. De Tecnol. Postcosecha 2021, 22, 212–225. [Google Scholar]
- Lacerda, Z.C.; Turco, J.E.P. Métodos de estimação de referência evapotranspiração (ETo) para Uberlândia—MG. Eng. Agríc. 2015, 35, 27–38. [Google Scholar] [CrossRef]
- Gadelha, B.B.; Freire, M.H.D.C.; Sousa, H.C.; Costa, F.H.; Lessa, C.I.; Sousa, G.G.D. Crescimento e produção de beterraba irrigada com água salina em diferentes tipos de cobertura vegetal. Rev. Bras. De Eng. Agrícola E Ambient. 2021, 25, 847–852. [Google Scholar] [CrossRef]
- Holanda, J.D.; Amorim, J.D.; Ferreira Neto, M.; Holanda, A.D.; Sá, F.D.S. Qualidade da água para irrigação. In Manejo da Salinidade na Agricultura: Estudos Básicos e Aplicados; Gheyi, H.R., Dias, N.D.A.S., Lacerda, C.F., Gomes Filho, E., Eds.; Systems–Digital Solutions: Fortaleza, Brazil, 2016; Volume 2, pp. 35–46. [Google Scholar]
- Silva, E.B.; Viana, T.V.A.; Sousa, G.G.; Sousa, J.T.M.; Santos, M.F.; Azevedo, B.M. Growth and nutrition of peanut crop subjected to saline stress and organomineral fertilization. Rev. Bras. De Eng. Agrícola E Ambient. 2022, 26, 495–501. [Google Scholar] [CrossRef]
- Dias, A.S.; Lima, G.S.; Gheyi, H.R.; Soares, L.A.A.; Souza, L.P.; Bezerra, I.L. Crescimento do algodoeiro ‘BRS rubi’ em função da irrigação com águas salinas e adubação nitrogenada. Rev. Bras. De Agric. Irrig. 2017, 11, 1945–1955. [Google Scholar] [CrossRef]
- Sousa, M.S.; Echer, M.M. Growth promoting microorganisms: A sustainable alternative in beet agronomic performance: Microrganismos promotores de crescimento: Uma alternativa sustentável no desempenho agronômico da beterraba. Concilium 2023, 23, 529–541. [Google Scholar] [CrossRef]
- Sá, F.D.S.; Gheyi, H.R.; Lima, G.S.; Ferreira Neto, M.; Paiva, E.P.; Silva, L.D.A.; Moreira, R.C.L. Cultivation of West Indian cherry irrigated with saline water under phosphorus and nitrogen proportions. Semin. Ciênc. Agrár. 2020, 41, 395–406. [Google Scholar] [CrossRef]
- Oliveira, F.R.D.; Sousa, G.G.D.; Sousa, J.T.M.D.; Leite, K.N.; Guilherme, J.M.D.S.; Nogueira, R.D.S. Physiological responses of the beet crop under agricultural environment and saline stress. Rev. Ambiente Água 2022, 17, 2868. [Google Scholar] [CrossRef]
- Lessa, C.I.N.; Sousa, G.G.; Sousa, H.C.; Silva, F.D.B.; Gomes, S.P.; Araújo Viana, T.V. Agricultural ambience and salt stress in production of yellow passion fruit seedlings. Comun. Sci. 2022, 13, e3703. [Google Scholar] [CrossRef]
- Bouras, H.; Bouaziz, A.; Bouazzama, B.; Hirich, A.; Choukr-Allah, R. How phosphorus fertilization alleviates the effect of salinity on sugar beet (Beta vulgaris L.) productivity and quality. Agronomy 2021, 11, 1491. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E.; Moller, I.M.; Murphy, A. Fisiologia e Desenvolvimento Vegetal, 6th ed.; Artmed: Porto Alegre, Brazil, 2017; p. 888. [Google Scholar]
- Ribeiro, R.M.R.; Sousa, G.G.; Barbosa, A.S.; Lacerda, C.F.; Freire, M.H.C.; Moraes, J.G.L. Irrigation strategies with saline waterand phosphate fertilization in cowpea culture. Rev. Bras. De Ciências 2022, 17, e2572. [Google Scholar] [CrossRef]
- Sood, M.; Kapoor, D.; Kumar, V.; Sheteiwy, M.S.; Ramakrishnan, M.; Landi, M.; Sharma, A. TRICHODERMA: The “secrets” of a multitalented biocontrol agent. Plants 2020, 9, 762. [Google Scholar] [CrossRef]
- Jing, Z.; Chen, R.; Wei, S.; Feng, Y.; Zhang, J.; Lin, X. Response and feedback of C mineralization to P availability driven by soil microorganisms. Soil Biol. Biochem. 2017, 105, 111–120. [Google Scholar] [CrossRef]
- Ji, S.; Liu, Z.; Liu, B.; Wang, Y.; Wang, J. The effect of Trichoderma biofertilizer on Chinese cabbage flowering quality and soil environment. Sci. Hortic. 2020, 262, 109069. [Google Scholar] [CrossRef]
- Diniz, G.L.; Costa, C.C.; Sousa, V.F.O.; Lopes, K.P.; Bomfim, M.P.; Santos, J.B. Uso de Trichoderma spp e estresse salino na produção de mudas de melancia. Rev. Em Agronegócio E Meio Ambiente 2022, 15, 1–16. [Google Scholar] [CrossRef]
- Santos, H.G.; Jacomine, P.K.T.; Anjos, L.H.C.; Oliveira, V.Á.; Lumbreras, J.F.; Coelho, M.R.; Almeida, J.A.; Araújo Filho, J.C.; Oliveira, J.B.; Cunha, T.J.F. Sistema Brasileiro De Classificação do Solo, 6th ed.; Sistema Brasileiro de Classificação de Solos: Brasília, Brazil, 2018. [Google Scholar]
- Ayers, R.S.; Westcot, D.W.A. Qualidade Da Água Na Agricultura; UFPB: Campina Grande, Brazil, 1999; p. 153. [Google Scholar]
- Bernardo, S.; Mantovani, E.C.; Silva, D.D.; Soares, A.A. Manual De Irrigação, 9th ed.; Editora UFV: Viçosa, Brazil, 2019; p. 545. [Google Scholar]
- Rhoades, J.D.; Kandiah, A.; Mashali, A.M. Uso De Águas Salinas Para Produção Agrícola; UFPB: Campina Grande, Brazil, 2000; p. 117. [Google Scholar]
- Marrocos, S.T.P.; Dantas, M.S.M.; Dombroski, J.L.D.; Lucena, R.R.M.; Batista, T.M.V. Análise comparativa de métodos de estimativa de área foliar em beterraba. Rev. Verde De Agroecol. E Desenvolv. Sustentável 2010, 5, 140–146. [Google Scholar]
- Resende, G.M.; Cordeiro, G.G. Uso De Água Salina e Condicionador do Solo na Produtividade de Beterraba e Cenoura No Semi-Árido Do Submédio São Francisco; Embrapa Semi-Árido: Petrolina, Brazil, 2007; p. 4. [Google Scholar]
- Silva, F.A.S.; Azevedo, C.A.V. The Assistat Software Version 7.7 and its use in theanalysis of experimental data. Afr. J. Agric. Res. 2016, 39, 3733–3740. [Google Scholar]
- Bechtaoui, N.; Rabiu, M.K.; Raklami, A.; Oufdou, K.; Hafidi, M.; Jemo, M. Phosphate-Dependent Regulation of Growth and Stresses Management in Plants. Front. Em Plant Sci. 2021, 12, 679916. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.A.; Grangeiro, L.C.; Sousa, V.D.F.; Silva, L.R.; Jesus, P.M.; Silva, J.L. Desempenho agronômico de cultivares de beterraba em função da adubação fosfatada. Rev. Bras. De Eng. Agríc. E Ambient. 2019, 23, 518–523. [Google Scholar] [CrossRef]
- Bonfim-Silva, E.M.; Silva, I.D.F.; Ribeiro, J.M.; Souza Fernandes, W.; Nonato, J.J. Calagem e adubação fosfatada no cultivo rabanete em Latossolo Vermelho Liming and phosphate fertilization for rabanette cultivated in Red Oxisol. Braz. J. Dev. 2021, 7, 78970–78986. [Google Scholar] [CrossRef]
- Avalhaes, C.C.; Mello Prado, R.; Oliveira Gondim, A.R.; Alves, A.U.; Correia, M.A.R. Rendimento e crescimento da beterraba em função da adubação com fósforo. Sci. Agrár. 2009, 10, 75–80. [Google Scholar] [CrossRef]
- Cordeiro, C.F.D.S.; Echer, F.R.; Batista, G.D.; Fernandes, A.M. Sweet potato yield and quality as a function of phosphorus fertilization in different soils. Rev. Bras. De Eng. Agrícola E Ambient. 2023, 27, 487–495. [Google Scholar] [CrossRef]
- Dey, G.; Banerjee, P.; Sharma, R.K.; Maity, J.P.; Etesami, H.; Shaw, A.K.; Huang, Y.H.; Huang, H.B.; Chen, C.Y. Management of phosphorus in salinity-stressed agriculture for sustainable crop production by salt-tolerant phosphate-solubilizing bacteria—A review. Agronomy 2021, 11, 1552. [Google Scholar] [CrossRef]
- Santos, D.P.D.; Santos, C.S.D.; Silva, P.F.D.; Pinheiro, M.P.M.A.; Santos, J.C. Crescimento e fitomassa da beterraba sob irrigação suplementar com água de diferentes concentrações salinas. Rev. Ceres 2016, 63, 509–516. [Google Scholar] [CrossRef]
- Sá, F.V.S.; Ferreira Neto, M.; Lima, Y.M.; Paiva, E.P.; Prata, R.C.; Lacerda, C.F.; Brito, M.E.B. Growth, gas exchange and photochemical efficiency of the cowpea bean under salt stress and phosphorus fertilization. Comun. Sci. 2018, 9, 668–679. [Google Scholar] [CrossRef]
- Dourado, P.R.M.; Souza, E.R.; Santos, M.A.D.; Lins, C.M.T.; Monteiro, D.R.; Paulino, M.K.S.S.; Schaffer, B. Stomatal regulation and osmotic adjustment in sorghum in response to salinity. Agriculture 2022, 12, 658. [Google Scholar] [CrossRef]
- Chagas, L.F.B.; Martins, A.L.L.; Carvalho Filho, M.R.; Oliveira Miller, L.; Oliveira, J.C.; Junior, A.F.C. Bacillus subtilis e Trichoderma sp. no incremento da biomassa em plantas de soja, feijão-caupi, milho e arroz. Agri-Environ. Sci. 2017, 3, 10–18. [Google Scholar] [CrossRef]
- Mupambi, G.; Anthony, B.M.; Layne, D.R.; Musacchi, S.; Serra, S.; Schmidt, T.; Kalcsits, L.A. The influence of the protection network on the physiology of the tree and the quality of the apple fruit: A review. Sci. Hortic. 2018, 236, 60–72. [Google Scholar] [CrossRef]
- Silva, L.V.D.; Oliveira, S.B.R.D.; Azevedo, L.A.D.; Rodrigues, A.C.; Bonifácio, A. Coinoculação com bradyrhizobium e trichoderma altera os efeitos do estresse salino em feijão-caupi. Rev. Caatinga 2019, 32, 336–344. [Google Scholar] [CrossRef]
- Pinho, L.L.; Lacerda, C.F.D.; Sousa, J.A.D.; Santos, A.M.; Bezerra, A.M.E.; Cavalcante, E.S. Effects of artificial shading and irrigation with brackish water on the initial development of Anadenanthera colubrina (vell.) brenan plants. Rev. Árvore 2022, 46, 1–12. [Google Scholar] [CrossRef]
- Ahmad, P.; Hashem, A.; Abd-Allah, E.F.; Alqarawi, A.A.; John, R.; Egamberdieva, D.; Gucel, S. Papel de Trichoderma harzianum na mitigação do estresse por NaCl em mostarda-da-índia (Brassica juncea L.) por meio do sistema de defesa antioxidante. Front. Na Ciência Veg. 2015, 6, 868. [Google Scholar]
- Rodrigues, V.D.S.; Sousa, G.G.D.; Soares, S.D.C.; Leite, K.N.; Ceita, E.D.A.R.D.; Sousa, J.T.M.D. Gas exchanges and mineral content of corn crops irrigated with saline water. Rev. Ceres 2021, 68, 453–459. [Google Scholar] [CrossRef]
- Razaq, M.; Zhang, P.; Shen, H.; Salahuddin. Influence of nitrogen and phosphorus on the growth and root morphology of Acer mono. PLoS ONE 2017, 12, e0171321. [Google Scholar] [CrossRef] [PubMed]
- Lima, G.S.; Félix, C.M.; Silva, S.S.; Soares, L.A.A.; Gheyi, H.R.; Soares, M.D.M.; Sousa, P.F.N.; Fernandes, P.D. Gas exchange, growth, and production of mini-watermelon under saline water irrigation and phosphate fertilization. Semina 2020, 41, 3039–3052. [Google Scholar] [CrossRef]
- Sousa, A.E.C.; Lacerda, C.F.; Gheyi, H.R.; Soares, F.A.L.; Uyeda, C.A. Teores de nutrientes foliares e respostas fisiológicas em pinhão manso submetido a estresse salino e adubação fosfatada. Rev. Caatinga 2012, 25, 144–152. [Google Scholar]
- Masrahi, A.S.; Alasmari, A.; Shahin, M.G.; Qumsani, A.T.; ORABY, H.F.; Awad-Allah, M.M. Role of Arbuscular Mycorrhizal Fungi and Phosphate Solubilizing Bacteria in Improving Yield, Yield Components, and Nutrients Uptake of Barley under Salinity Soil. Agriculture 2023, 13, 537. [Google Scholar] [CrossRef]
- Frasca, L.L.D.M.; Nascente, A.S.; Lanna, A.C.; Carvalho, M.C.S.; Costa, G.G. Biostimulants in plant growth and agronomic performance of common bean super-cycle cycle. Agrarian 2020, 13, 27–41. [Google Scholar] [CrossRef]
- Mendoza, A.; Zaid, R.; Lawry, R.; Hermosa, R.; Monte, E.; Horwitz, B.; Mukherjee, P.K. Molecular dialogues between Trichoderma and roots: Role of the fungal secretome. Fungal Biol. Rev. 2018, 32, 62–85. [Google Scholar] [CrossRef]
- Lima, A.F.S.; Santos, M.F.; Oliveira, M.L.; Sousa, G.G.; Mendes Filho, P.F.; Luz, L.N. Physiological responses of inoculated and uninoculated peanuts under saline stress. Rev. Ambiente Água 2021, 16, e2643. [Google Scholar] [CrossRef]
- Luz, J.M.Q.; Queiroz, A.A.; Borges, M.; Oliveira, C.R.; Leite, S.S.; Cardoso, R.R. Influence of phosphate fertilization on phosphorus levels in the production of foliage and tubers of the potato cv. Agate. Semin. Agric. Sci. 2013, 2, 649–656. [Google Scholar] [CrossRef]
- Gashash, E.A.; Ashmawi, A.E.; El-Taher, A.M.; Osman, N.A.; Elkelish, A. Effect of fertilizing with different levels of phosphorous and zinc on the botanical characteristics of table beet (Beta vulgaris L.). Not. Bot. Horti Agrobot. Cluj-Napoca 2022, 50, 12579. [Google Scholar] [CrossRef]
- Freire, J.L.O.; Nascimento, G.S.; Medeiros, A.K.A. Teores e acúmulos de nutrientes em mudas de maracujazeiros sob salinidade hídrica e uso de urina de vaca. Nativa 2020, 8, 464–475. [Google Scholar] [CrossRef]
- Ghaly, F.; Abd-Hady, M.; Abd-Elhamied, A. Effect of varieties, phosphorus and boron fertilization on sugar beet yield and its quality. J. Soil Sci. Agric. Eng. 2019, 10, 115–122. [Google Scholar] [CrossRef]
- Nascimento, M.V.; Fernandes, L.R.S.G.; Xavier, R.C.; Benett, K.S.S.; Silva, L.M. Adubação fosfatada no cultivo de hortaliças produtoras de raízes. Rev. De Agric. Neotrop. 2017, 4, 8–16. [Google Scholar] [CrossRef]
- Silva, S.S.; Lima, G.S.; Lima, V.L.A.; Gheyi, H.R.; Soares, L.A.A.; Melo, J.P. Production and quality of watermelon fruits under salinity management strategies and nitrogen fertilization. Semin Ciências Agrárias Londrina 2020, 41, 2923–2936. [Google Scholar] [CrossRef]
- Paiva, F.I.G.; Oliveira, F.A.; Medeiros, J.F.; Tarjino, A.J.O.; Santos, S.T.; Silva, R.C.P. Qualidade de tomate em função da salinidade da água de irrigação e relações K/Ca via fertirrigação. Irriga 2018, 23, 168–193. [Google Scholar] [CrossRef]
- Santos, F.L.; Costa, E.S.; Lima, C.S.M. Diferentes substratos no desenvolvimento e na pós-colheita de microverdes de beterraba (Beta vulgaris L.). Rev. Iberoam. De Tecnol. Postcosecha 2020, 21, 2. [Google Scholar]
- Nascimento, J.A.M.; Cavalcante, L.F.; Dantas, S.A.G.; Silva, S.A.; Dias, T.J. Biofertilizante e adubação mineral na qualidade de frutos de maracujazeiro irrigado com água salina. Irriga 2015, 20, 220–232. [Google Scholar] [CrossRef]



| O.M | N | Ca²+ | K+ | Mg2+ | Na+ | P | pH | ESP | ECw |
|---|---|---|---|---|---|---|---|---|---|
| (g kg−1) | (cmolc dm−3) | (mg kg−1) | (H2O) | (dS m−1) | |||||
| 4.86 | 0.29 | 1 | 0.24 | 1.1 | 0.1 | 1.00 | 5.8 | 2 | 0.29 |
| Mean Squares | |||||
|---|---|---|---|---|---|
| SV | DF | PH | NL | LA | SDM |
| Doses—D | 2 | 19.1556 ** | 4.6411 * | 14.5094 ** | 4.7904 ns |
| Water Salinity—WS | 1 | 3.0910 ns | 1.1216 ns | 4.0535 * | 2.6982 ns |
| Inoculant—I | 1 | 0.2804 ns | 0.6032 ns | 1.0641 ns | 0.0012 ns |
| D × W | 2 | 1.5163 ns | 1.9890 ns | 2.8658 ns | 10.8139 * |
| D × I | 2 | 0.7334 ns | 2.6072 ns | 0.9479 ns | 1.3841 ns |
| WS × I | 1 | 0.5673 ns | 0.4038 ns | 0.0143 ns | 0.4317 ns |
| D × WS × I | 2 | 1.0031 ns | 0.5533 ns | 0.5239 ns | 1.1728 ns |
| Treatments | 11 | ||||
| Residue | 60 | ||||
| Total | 71 | ||||
| CV (%) | 25.8 | 31.38 | 25.31 | 31.78 | |
| OM | 20.42516 | 5.31944 | 15.33859 | 6.83042 | |
| Mean Squares | ||||||
|---|---|---|---|---|---|---|
| SV | DF | A | E | gs | Ci | LT |
| Doses—D | 2 | 7.3539 ** | 11.9426 ** | 9.0021 ** | 0.4968 ns | 111.8156 ** |
| Water Salinity—WS | 1 | 25.4585 ** | 22.4801 ** | 17.2475 ** | 0.0856 ns | 1.7193 ns |
| Inoculant—I | 1 | 0.9285 ns | 8.1310 ** | 1.8596 ns | 0.9240 ns | 31.5789 ** |
| D × WS | 2 | 4.8148 * | 0.1094 ns | 0.3288 ns | 5.1102 * | 32.6405 ** |
| D × I | 2 | 2.2854 ns | 0.4389 ns | 1.3958 ns | 1.1812 ns | 36.6581 ** |
| WS × I | 1 | 6.6086 * | 6.4680 * | 1.6944 ns | 0.2698 ns | 4.2456 * |
| D × WS × I | 2 | 5.5218 ** | 3.8545 * | 2.1010 ns | 0.6703 ns | 0.9561 ns |
| Treatments | 11 | |||||
| Residue | 36 | |||||
| Total | 47 | |||||
| CV (%) | 27.65 | 20.12 | 39.08 | 15.57 | 5.60 | |
| OM | 1.235.056 | 285.472 | 0.15889 | 206.97222 | 3.413.333 | |
| Mean Square | |||||||
|---|---|---|---|---|---|---|---|
| SV | DF | TRM | PROD | TRD | TRL | °BRIX | pH |
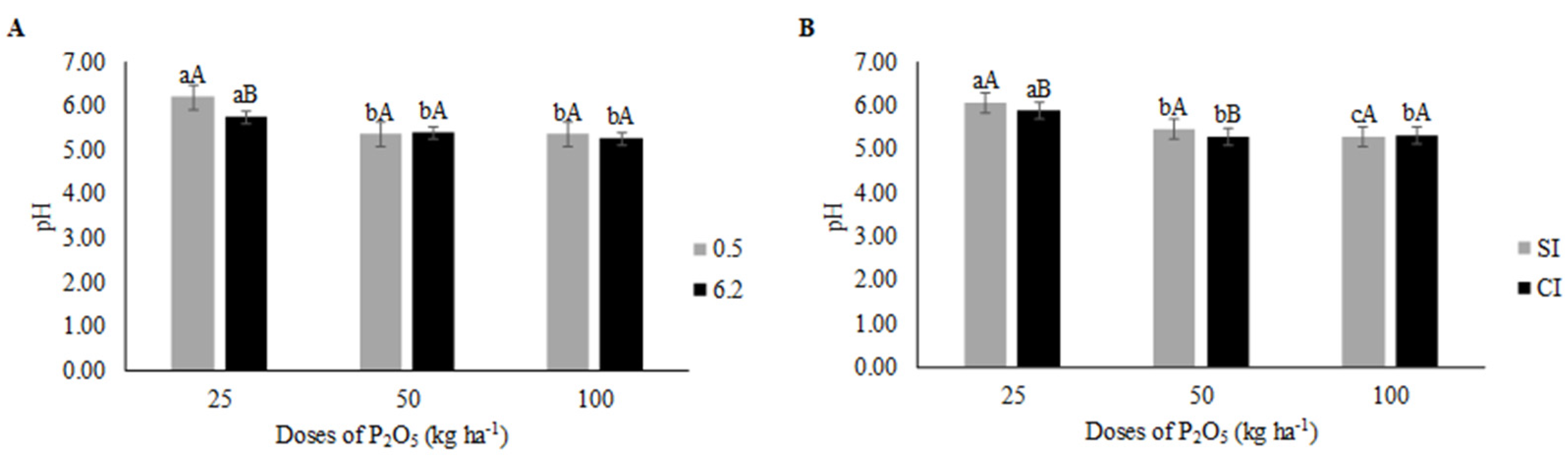
| Doses—D | 2 | 5.2064 * | 5.2064 * | 4.3043 * | 5.2746 ** | 1.9528 ns | 178.6896 ** |
| Water Salinity—WS | 1 | 1.8959 ns | 1.8959 ns | 2.1453 ns | 0.7942 ns | 0.0196 ns | 34.4705 ** |
| Inoculant—I | 1 | 0.2946 ns | 0.2946 ns | 0.5125 ns | 0.5805 ns | 0.5818 ns | 12.7840 ** |
| D × WS | 2 | 0.3422 ns | 0.3422 ns | 1.8221 ns | 0.3966 ns | 0.1577 ns | 22.0982 ** |
| D × I | 2 | 3.1136 ns | 3.1136 ns | 1.7582 ns | 1.2701 ns | 2.5779 ns | 5.1356 * |
| WS × I | 1 | 0.2685 ns | 0.2685 ns | 0.2407 ns | 0.8729 ns | 0.0700 ns | 0.0720 ns |
| D × WS × I | 2 | 1.0202 ns | 1.0202 ns | 5.7425 ** | 1.1188 ns | 0.8914 ns | 2.4745 ns |
| Treatments | 11 | ||||||
| Residue | 36 | ||||||
| Total | 47 | ||||||
| CV (%) | 26.29 | 26.29 | 23.31 | 22.15 | 27.62 | 11.97 | |
| OM | 33.89549 | 753.23302 | 39.4173 | 33.2729 | 2.46458 | 5.55632 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbosa, A.S.; Silva, A.O.d.; Sousa, G.G.d.; Souza, M.V.P.d.; Freire, M.H.d.C.; Goes, G.F.; Pereira, A.P.d.A.; Viana, T.V.d.A.; Costa, R.N.T.; Lacerda, C.F.d.; et al. Brackish Water, Phosphate Fertilization and Trichoderma in the Agronomic Performance of Beet Crops. Agronomy 2024, 14, 1306. https://doi.org/10.3390/agronomy14061306
Barbosa AS, Silva AOd, Sousa GGd, Souza MVPd, Freire MHdC, Goes GF, Pereira APdA, Viana TVdA, Costa RNT, Lacerda CFd, et al. Brackish Water, Phosphate Fertilization and Trichoderma in the Agronomic Performance of Beet Crops. Agronomy. 2024; 14(6):1306. https://doi.org/10.3390/agronomy14061306
Chicago/Turabian StyleBarbosa, Andreza Silva, Alexsandro Oliveira da Silva, Geocleber Gomes de Sousa, Maria Vanessa Pires de Souza, Márcio Henrique da Costa Freire, Geovana Ferreira Goes, Arthur Prudêncio de Araújo Pereira, Thales Vinícius de Araújo Viana, Raimundo Nonato Távora Costa, Claudivan Feitosa de Lacerda, and et al. 2024. "Brackish Water, Phosphate Fertilization and Trichoderma in the Agronomic Performance of Beet Crops" Agronomy 14, no. 6: 1306. https://doi.org/10.3390/agronomy14061306
APA StyleBarbosa, A. S., Silva, A. O. d., Sousa, G. G. d., Souza, M. V. P. d., Freire, M. H. d. C., Goes, G. F., Pereira, A. P. d. A., Viana, T. V. d. A., Costa, R. N. T., Lacerda, C. F. d., Silva, G. F. d., & Rolim, M. M. (2024). Brackish Water, Phosphate Fertilization and Trichoderma in the Agronomic Performance of Beet Crops. Agronomy, 14(6), 1306. https://doi.org/10.3390/agronomy14061306








