Effect of Soil pH on the Uptake of Essential Elements by Tea Plant and Subsequent Impact on Growth and Leaf Quality
Abstract
1. Introduction
2. Materials and Methods
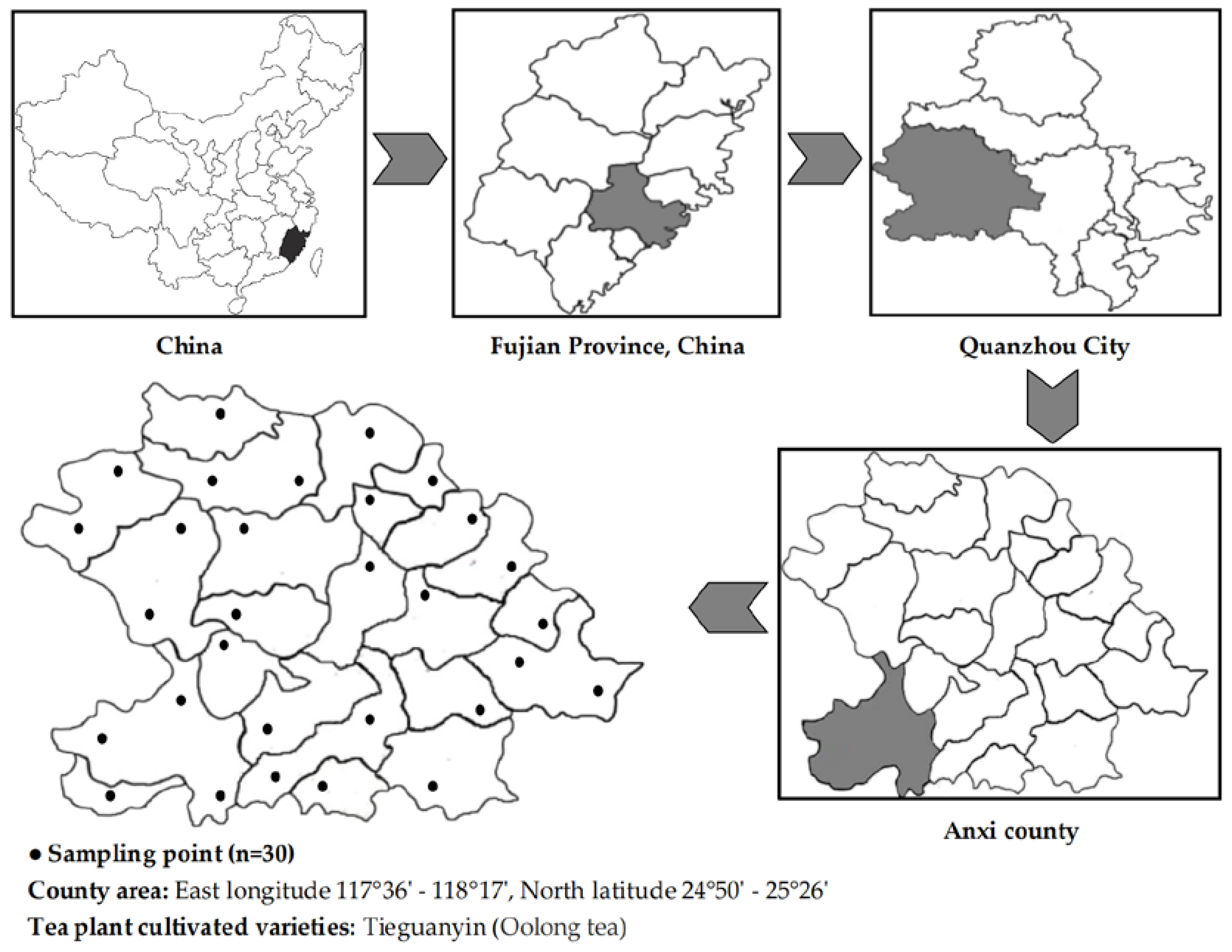
2.1. Test Tea Plantation and Sample Collection
2.2. Determination of Soil pH
2.3. Multielement Determination of Soil and Tea Leaves
2.4. Tea Plant Seedling Planting and Sample Sampling
2.5. Statistical Analyses
3. Results and Discussion
3.1. Validation of Quality Control Methods
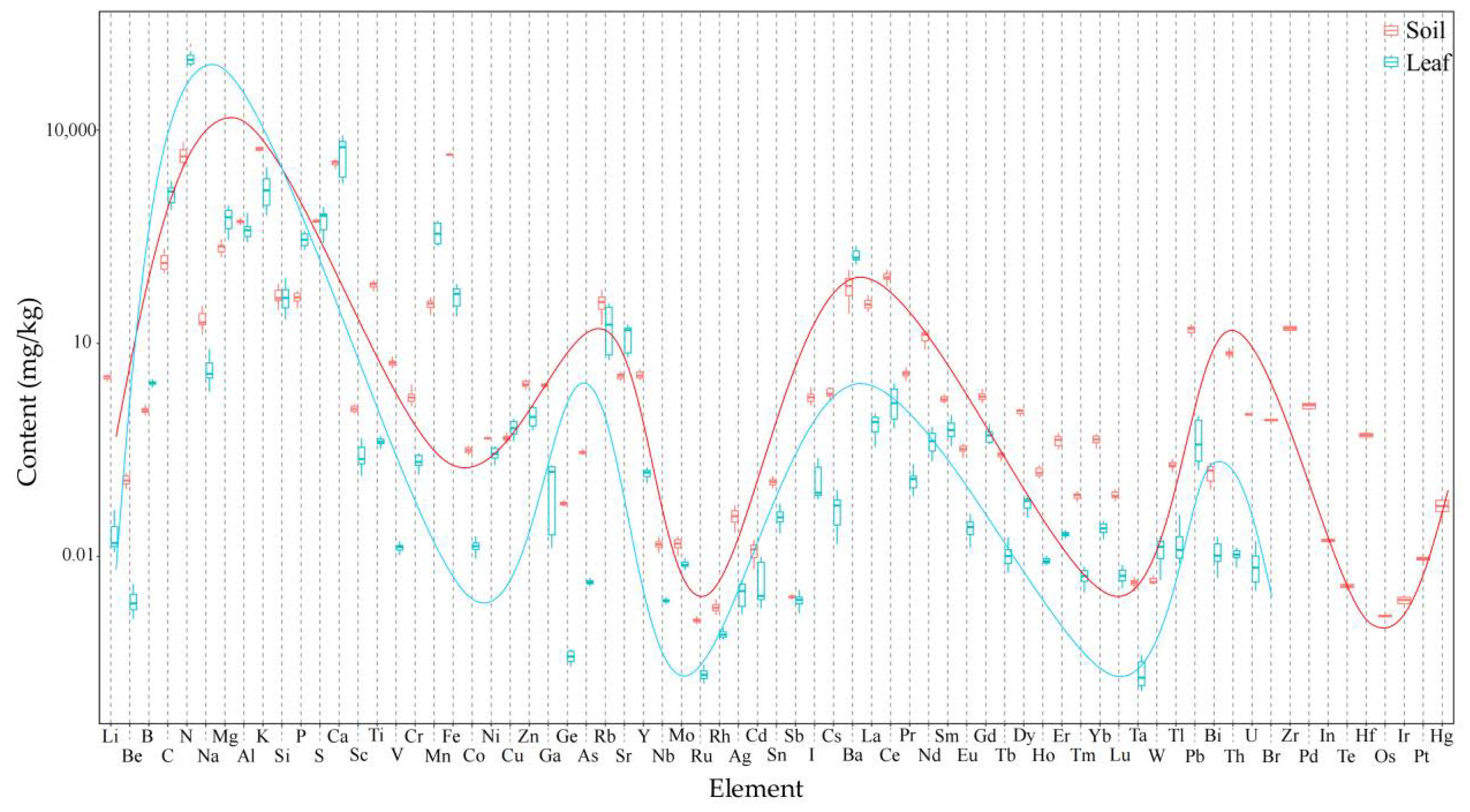
3.2. Analysis of Soil pH and Tea and Soil Elements
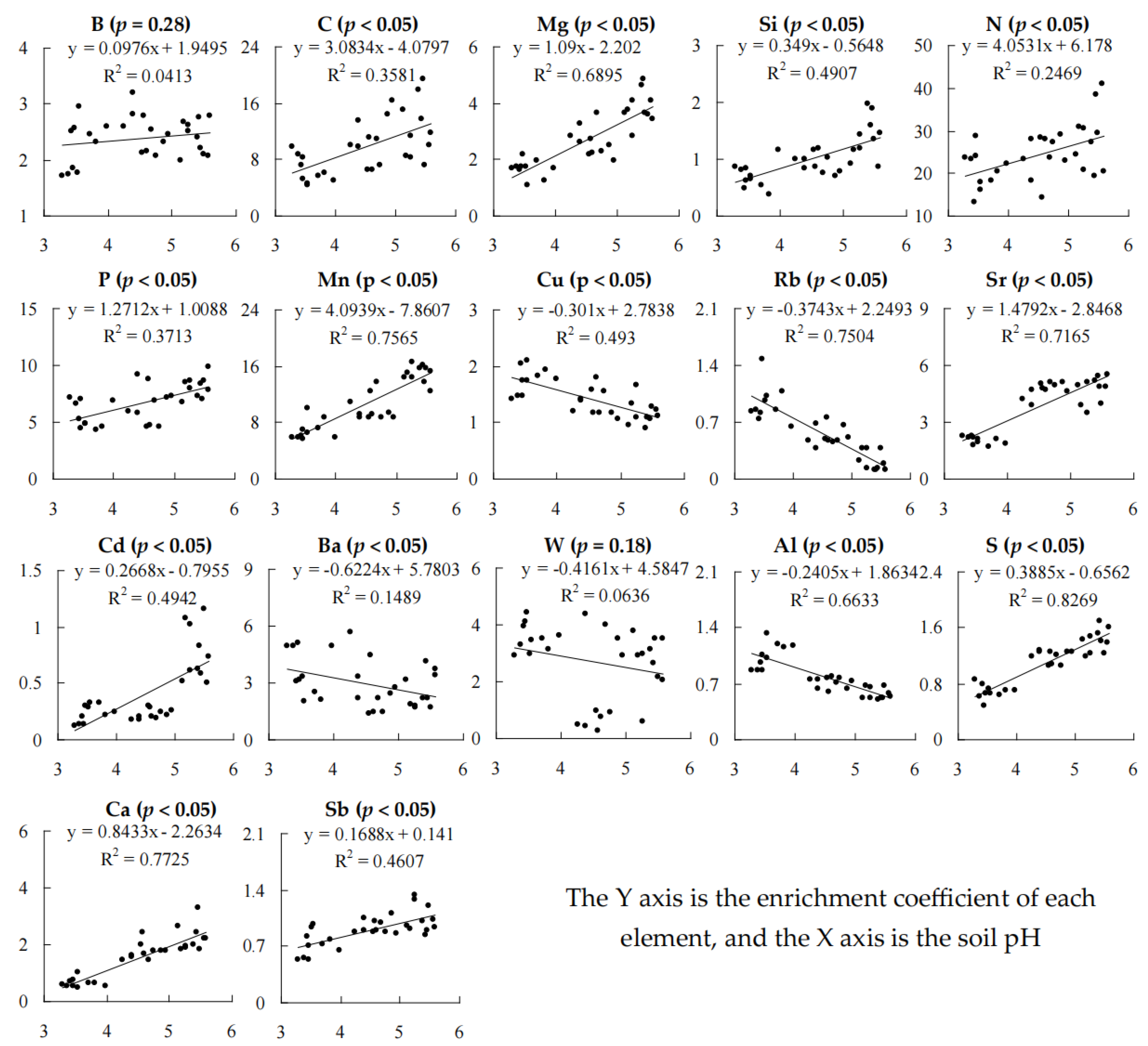
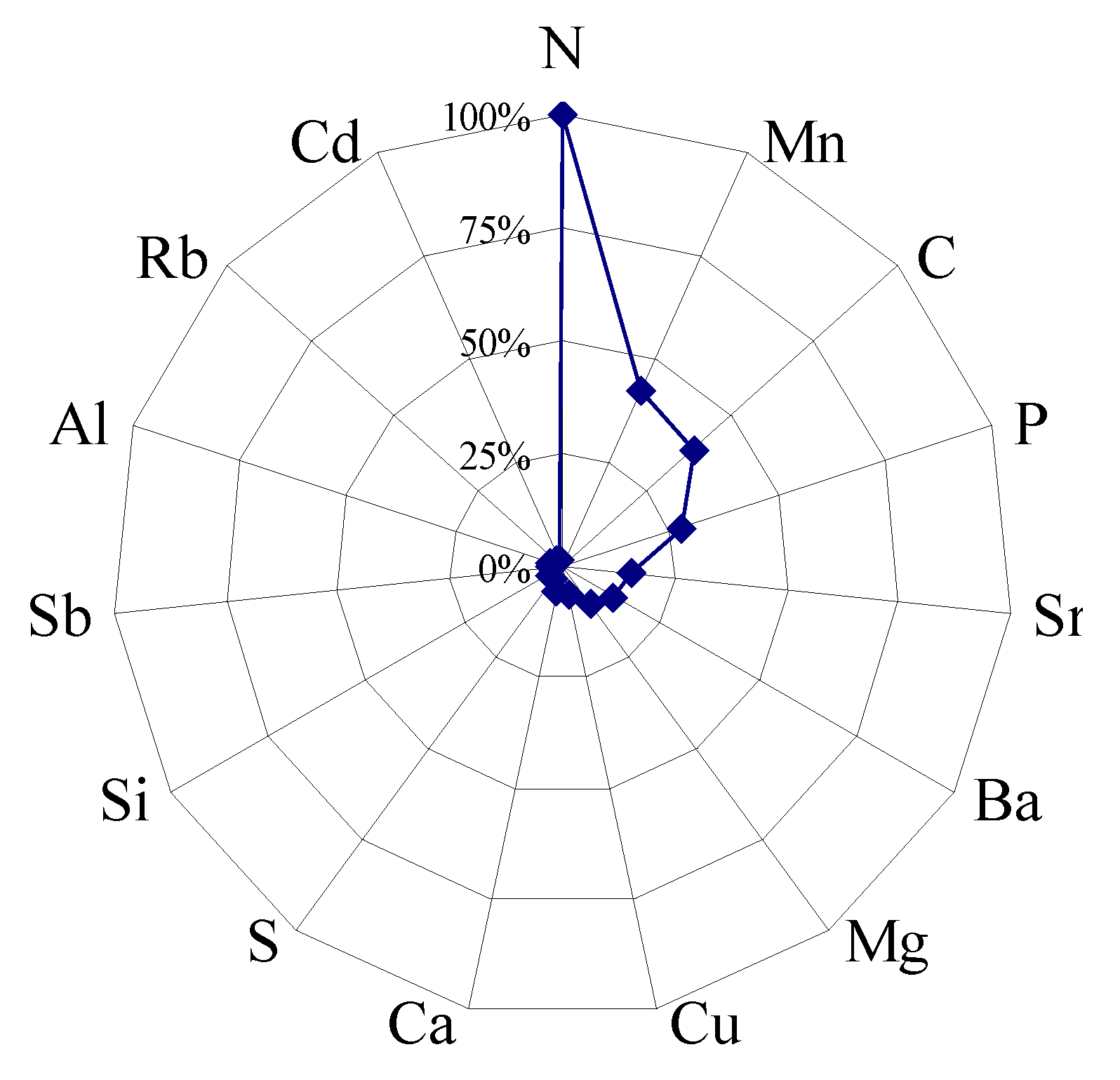
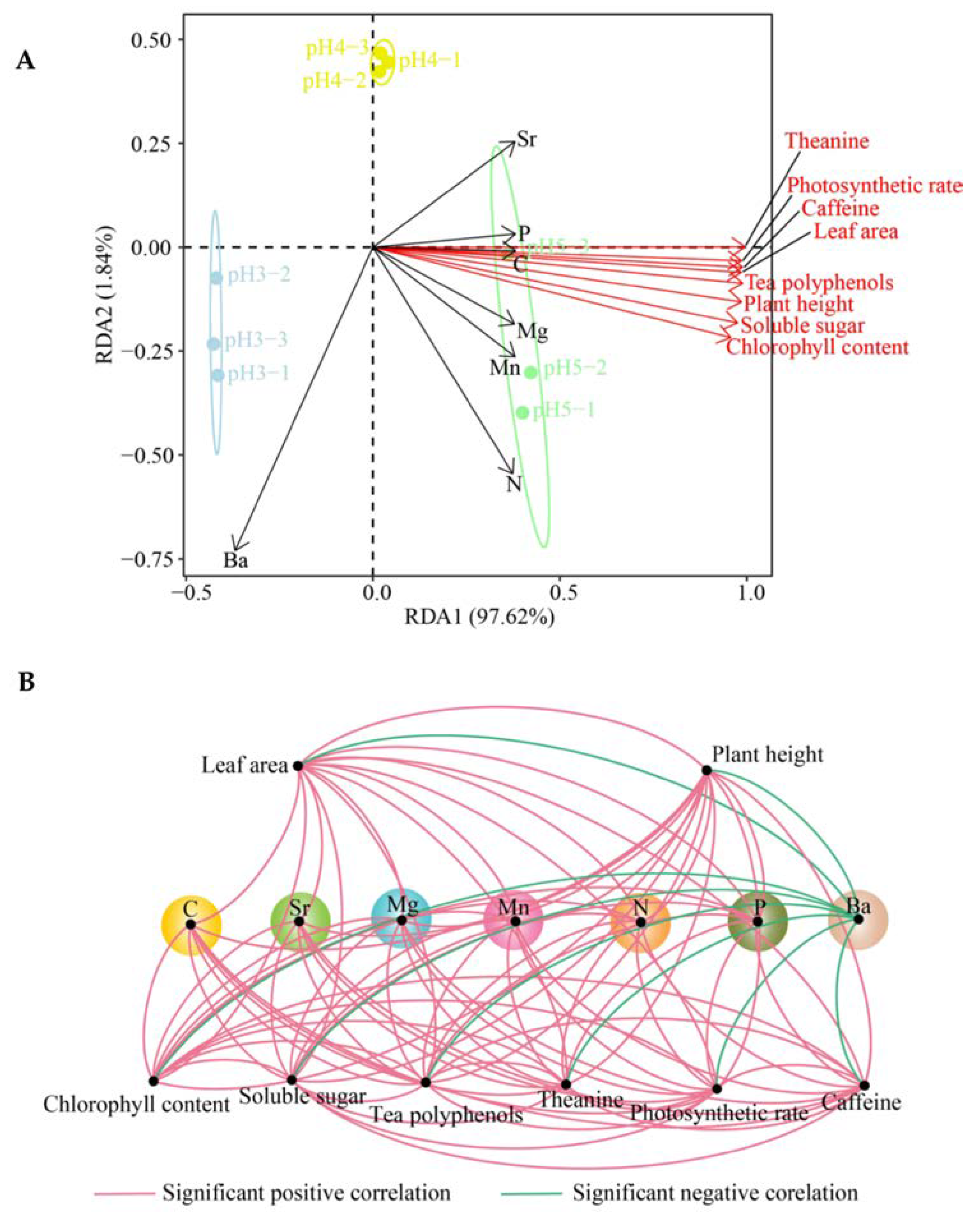
3.3. Effect of Soil pH on Elemental Enrichment Coefficients of Tea Plant Leaves
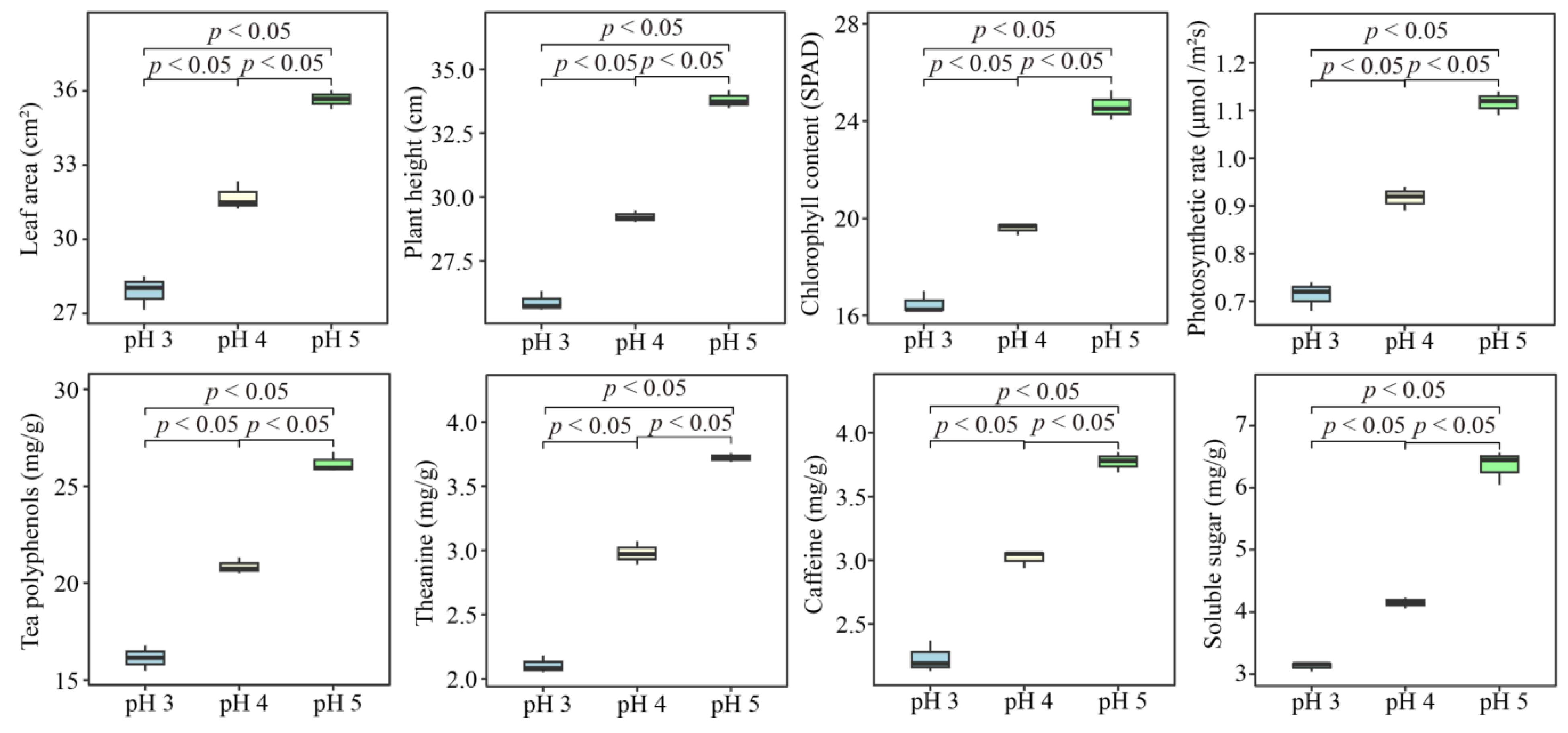
3.4. Effect of Soil pH on the Growth, Photosynthetic Capacity and Quality of Tea Plant Seedlings
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Naz, F. Plant nutrition, transport, mechanism and sensing in plants. In Sustainable Plant Nutrition; Academic Press: Cambridge, MA, USA, 2023; pp. 209–228. [Google Scholar] [CrossRef]
- Jaiswal, D.K.; Krishna, R.; Chouhan, G.K.; de Araujo Pereira, A.P.; Ade, A.B.; Prakash, S.; Verma, S.K.; Prasad, R.; Yadav, J.; Verma, J.P. Bio-fortification of minerals in crops: Current scenario and future prospects for sustainable agriculture and human health. Plant Growth Regul. 2022, 98, 5–22. [Google Scholar] [CrossRef]
- Liu, Z.; Huang, Y.; Tan, F.; Chen, W.; Ou, L. Effects of soil type on trace element absorption and fruit quality of pepper. Front. Plant Sci. 2021, 12, 698796. [Google Scholar] [CrossRef] [PubMed]
- Neina, D. The role of soil pH in plant nutrition and soil remediation. Appl. Environ. Soil Sci. 2019, 2019, 5794869. [Google Scholar] [CrossRef]
- Singhal, R.K.; Fahad, S.; Kumar, P.; Choyal, P.; Javed, T.; Jinger, D.; Singh, P.; Saha, D.; Prathibha, M.; Bose, B.; et al. Beneficial elements: New Players in improving nutrient use efficiency and abiotic stress tolerance. Plant Growth Regul. 2023, 100, 237–265. [Google Scholar] [CrossRef]
- Xu, G.Q.; Cao, X.Q.; Bai, L.P.; Qi, H.T.; Lu, H.B. Absorption, accumulation and distribution of metals and nutrient elements in poplars planted in land amended with composted sewage sludge: A field trial. Ecotoxicol. Environ. Saf. 2019, 182, 109360. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Kaur, H.; Kaur, H.; Srivastava, S. The beneficial roles of trace and ultratrace elements in plants. Plant Growth Regul. 2023, 100, 219–236. [Google Scholar] [CrossRef]
- Gondal, A.H.; Farooq, Q.; Sohail, S.; Kumar, S.S.; Toor, M.D.; Zafar, A.; Rehman, B. Adaptability of soil pH through innovative microbial approach. Curr. Res. Agric. Sci. 2021, 8, 71–79. [Google Scholar] [CrossRef]
- Gunnarsen, K.C.; Schjoerring, J.K.; Gómez-Muñoz, B.; de Neergaard, A.; Jensen, L.S. Can silicon in glacial rock flour enhance phosphorus availability in acidic tropical soil? Plant Soil. 2022, 477, 241–258. [Google Scholar] [CrossRef]
- Islam, M.R.; Jahan, R.; Uddin, S.; Harine, I.J.; Hoque, M.A.; Hassan, S.; Hassan, M.M.; Hossain, M. Lime and organic manure amendment enhances crop productivity of wheat–mungbean–T. aman cropping pattern in acidic piedmont soils. Agronomy 2021, 11, 1595. [Google Scholar] [CrossRef]
- Yang, X.; Ni, K.; Shi, Y.; Yi, X.; Ji, L.; Ma, L.; Ruan, J. Heavy nitrogen application increases soil nitrification through ammonia-oxidizing bacteria rather than archaea in acidic tea (Camellia sinensis L.) plantation soil. Sci. Total Environ. 2020, 717, 137248. [Google Scholar] [CrossRef] [PubMed]
- de Bang, T.C.; Husted, S.; Laursen, K.H.; Persson, D.P.; Schjoerring, J.K. The molecular–physiological functions of mineral macronutrients and their consequences for deficiency symptoms in plants. New Phytol. 2021, 229, 2446–2469. [Google Scholar] [CrossRef] [PubMed]
- Kang, E.; Li, Y.; Zhang, X.; Yan, Z.; Wu, H.; Li, M.; Yan, L.; Zhang, K.; Wang, J.; Kang, X. Soil pH and nutrients shape the vertical distribution of microbial communities in an alpine wetland. Sci. Total Environ. 2021, 774, 145780. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, T.; Wang, S.; Wang, Z. Soil pH and C/N ratio determines spatial variations in soil microbial communities and enzymatic activities of the agricultural ecosystems in Northeast China: Jilin Province case. Appl. Soil Ecol. 2020, 155, 103629. [Google Scholar] [CrossRef]
- Ye, J.; Wang, Y.; Lin, S.; Wang, Y.; Cheng, P.; Hong, L.; Jia, X.; Kang, J.; Wu, Z.; Wang, H. Metabolomics analysis of the effect of acidification on rhizosphere soil microecosystem of tea tree. Front. Plant Sci. 2023, 14, 1137465. [Google Scholar] [CrossRef] [PubMed]
- Arafat, Y.; Ud Din, I.; Tayyab, M.; Jiang, Y.; Chen, T.; Cai, Z.; Zhao, H.; Lin, X.; Lin, W.; Lin, S. Soil sickness in aged tea plantation is associated with a shift in microbial communities as a result of plant polyphenol accumulation in the tea gardens. Front. Plant Sci. 2020, 11, 601. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Yang, F.; Feng, H.; Yu, Z.; Liu, C.; Wei, C.; Liang, T. Organic fertilizer reduced carbon and nitrogen in runoff and buffered soil acidification in tea plantations: Evidence in nutrient contents and isotope fractionations. Sci. Total Environ. 2021, 762, 143059. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Zhao, M.; Zhang, H.; Rahman, M.A.; Luo, C.; Rahman, M.M. Distribution, contamination status and source of trace elements in the soil around brick kilns. Chemosphere 2021, 263, 127882. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.; Niazi, N.K.; Rinklebe, J.; Bundschuh, J.; Dumat, C.; Pinelli, E. Trace elements-induced phytohormesis: A critical review and mechanistic interpretation. Crit. Rev. Environ. Sci. Tec. 2020, 50, 1984–2015. [Google Scholar] [CrossRef]
- Wang, H.B.; Lin, L.W.; Wang, Y.H. Technical Specification for Tea Production, Processing and Safety Inspection; Xiamen University Press: Xiamen, China, 2020. [Google Scholar]
- Sun, L.L.; Zhang, M.S.; Liu, X.M.; Mao, Q.Z.; Shi, C.V.; Kochian, L.; Liao, H. Aluminium is essential for root growth and development of tea plants (Camellia sinensis). J. Integr. Plant Biol. 2020, 62, 984–997. [Google Scholar] [CrossRef] [PubMed]
- Gogoi, B.B.; Borgohain, A.; Konwar, K.; Handique, J.G.; Paul, R.K.; Khare, P.; Malakar, H.; Saikia, J.; Karak, T. National highway induced selected chemical properties of soils across tea bowl of India: Scale and assessment. Int. J. Environ. Sci. Technol. 2022, 19, 12019–12038. [Google Scholar] [CrossRef]
- Yang, W.L. Remedy for soil acidification at tea plantations in Anxi, Fujian. Acta Tea Sin. 2021, 62, 89–93. [Google Scholar] [CrossRef]
- Mleczek, P.; Borowiak, K.; Budka, A.; Szostek, M.; Niedzielski, P. Possible sources of rare earth elements near different classes of road in Poland and their phytoextraction to herbaceous plant species. Environ. Res. 2021, 193, 110580. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Ali, S.; Rehman, M.Z.; Riaz, M.; Adrees, M.; Hussain, A.; Zahir, Z.A.; Rinklebe, J. Effects of nanoparticles on trace element uptake and toxicity in plants: A review. Ecotoxicol. Environ. Saf. 2021, 221, 112437. [Google Scholar] [CrossRef] [PubMed]
- Mao, Q.; Cheng, F.; Chen, M. Experimental study on the chemical characterization of atmospheric aerosols in Wuhan, China. Atmosphere 2021, 12, 1393. [Google Scholar] [CrossRef]
- Nazzal, Y.; Bărbulescu, A.; Howari, F.; Al-Taani, A.A.; Iqbal, J.; Xavier, C.M.; Sharma, M.; Dumitriu, C.Ș. Assessment of metals concentrations in soils of Abu Dhabi Emirate using pollution indices and multivariate statistics. Toxics 2021, 9, 95. [Google Scholar] [CrossRef] [PubMed]
- Kleczkowski, L.A.; Igamberdiev, A.U. Magnesium and cell energetics: At the junction of metabolism of adenylate and non-adenylate nucleotides. J. Plant Physiol. 2023, 280, 153901. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Chai, X.; Zhong, M.; Zhang, L.; Zhao, P.; Kang, Y.; Guo, J.; Yang, X. Effects of nitrogen and magnesium nutrient on the plant growth, quality, photosynthetic characteristics, antioxidant metabolism, and endogenous hormone of Chinese kale (Brassica albograbra Bailey). Sci. Hortic. 2022, 303, 111243. [Google Scholar] [CrossRef]
- Islam, W.; Tauqeer, A.; Waheed, A.; Zeng, F. MicroRNA mediated plant responses to nutrient stress. Int. J. Mol. Sci. 2022, 23, 2562. [Google Scholar] [CrossRef]
- Nam, H.I.; Shahzad, Z.; Dorone, Y.; Clowez, S.; Zhao, K.; Bouain, N.; Lay-Pruitt, K.S.; Cho, H.; Rhee, S.Y.; Rouached, H. Interdependent iron and phosphorus availability controls photosynthesis through retrograde signaling. Nat. Commun. 2021, 12, 7211. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Wang, Y.; Kang, J.; Chen, Y.; Hong, L.; Li, M.; Jia, Y.; Wang, Y.; Jia, X.; Wu, Z.; et al. Effects of long-term use of organic fertilizer with different dosages on soil improvement, nitrogen transformation, tea yield and quality in acidified tea plantations. Plants 2022, 12, 122. [Google Scholar] [CrossRef] [PubMed]
- Chao, E.; Wu, M.; Yue, D.; Yuan, Y.; Qiu, N.; Zhou, F. Promoting effect of low concentration strontium on photosynthetic performance of Chinese cabbage seedlings: Combined leaf characteristics, photosynthetic carbon assimilation and chlorophyll fluorescence. Ecotoxicol. Environ. Saf. 2024, 274, 116200. [Google Scholar] [CrossRef] [PubMed]
- Sleimi, N.; Kouki, R.; Hadj Ammar, M.; Ferreira, R.; Pérez-Clemente, R. Barium effect on germination, plant growth, and antioxidant enzymes in Cucumis sativus L. plants. Food Sci. Nutr. 2021, 9, 2086–2094. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, M.; Wang, Y.; Zhang, Q.; Lin, S.; Zhang, Q.; Chen, Y.; Hong, L.; Jia, X.; Ye, J.; Wang, H. Effect of Soil pH on the Uptake of Essential Elements by Tea Plant and Subsequent Impact on Growth and Leaf Quality. Agronomy 2024, 14, 1338. https://doi.org/10.3390/agronomy14061338
Jia M, Wang Y, Zhang Q, Lin S, Zhang Q, Chen Y, Hong L, Jia X, Ye J, Wang H. Effect of Soil pH on the Uptake of Essential Elements by Tea Plant and Subsequent Impact on Growth and Leaf Quality. Agronomy. 2024; 14(6):1338. https://doi.org/10.3390/agronomy14061338
Chicago/Turabian StyleJia, Miao, Yuhua Wang, Qingxu Zhang, Shaoxiong Lin, Qi Zhang, Yiling Chen, Lei Hong, Xiaoli Jia, Jianghua Ye, and Haibin Wang. 2024. "Effect of Soil pH on the Uptake of Essential Elements by Tea Plant and Subsequent Impact on Growth and Leaf Quality" Agronomy 14, no. 6: 1338. https://doi.org/10.3390/agronomy14061338
APA StyleJia, M., Wang, Y., Zhang, Q., Lin, S., Zhang, Q., Chen, Y., Hong, L., Jia, X., Ye, J., & Wang, H. (2024). Effect of Soil pH on the Uptake of Essential Elements by Tea Plant and Subsequent Impact on Growth and Leaf Quality. Agronomy, 14(6), 1338. https://doi.org/10.3390/agronomy14061338







