Abstract
In recent years, skin blemish diseases of potato (including black dot (BD) caused by Colletotricum coccodes) have begun to be important for global potato marketing, since consumers often reject tubers with an imperfect appearance, which results in financial losses caused by the disposal of unwanted potatoes. Like for many non-fatal plant diseases, BD severity may depend on the immune status of plants influenced by other infectious agents. Using a set of 98 potato cultivars differing in their late blight (LB) resistance, we examined the correlation between the intensity of their infection with LB determined by their LB resistance and the occurrence of the BD disease under field conditions with a high background level of both diseases. Using LB-susceptible (Arizona) and moderately susceptible (Sante) cultivars, we also evaluated the effect of a crop protection against LB on BD development under the same field conditions. A strong negative correlation (r = −0.81, p < 0.05) between the LB resistance and the BD occurrence has been revealed. An experiment using the two cultivars, chemically protected against LB, showed a significant reduction in BD occurrence of 30% (cv. Arizona) and 20% (cv. Sante) compared to the untreated controls; the total yield and marketability of potatoes increased by 103.6 and 62.5% for cv. Arizona and by 65.9 and 43.8% for cv. Sante. The reduction in the LB affection of potato is one of the key factors improving the immune status of potato cultivars in relation to BD infection, so methods of LB protection should be included in a complex approach to BD control.
1. Introduction
Being one of the most common crops for processing and fresh markets, potato is an important crop for agriculture worldwide. In 2021, the total economic contribution of the US agricultural and industry sectors associated with this crop was estimated as USD 100.9 billion [1]. Along with high yields, the prosperity of potato growers is also strongly dependent on the quality of produced tubers and their marketability, and especially tuber appearance, which can be worsened by skin blemish diseases, such as black dot, black scurf, or silver scurf, caused by Colletotrichum coccodes, Rhizoctonia solani, and Helminthosporium solani, respectively. Tubers with an imperfect appearance are often rejected by retailers, which results in significant losses from their disposal [2]. Therefore, there is a need to protect this crop not only from diseases able to reduce its yield (such as late and early blights), but also from diseases affecting the tuber skin.
Potato is one of the basic agricultural crops in Russia, which belongs to the top three global potato producers. In 2022, the total area of potato crops in Russia reached 1.1 mln. hectares, while the gross potato yield made up 18.8 mln. tons, among which 7.2 mln. tons were produced by farmers and large companies and 11.6 mln. tons were grown on private plots [3]. In 2023, the gross potato yield increased to 20.2 mln. Tons, which corresponded to 363 kg per capita [4]; the maximum volumes of potato production fell to the share of the Central (Bryansk, Tula, and Moscow regions) and Volga (Astrakhan and Nizhni Novgorod regions) Federal districts. The total number of potato cultivars registered for use in Russia in 2023 reached almost 500 [5].
According to a number of publications [6,7,8,9,10], as well as the results of regular monitoring performed by our team at different potato-growing regions of Russia [11,12,13], the loss in quality of harvested tubers was significantly contributed to by the active development of diseases such as black scurf, black dot, and silver scurf. Changes in the sales of fresh potatoes, especially those associated with an increasing demand for washed potato (see, for example, [14]), resulted in an increased economical impact of black dot and silver scurf diseases, which were earlier considered rather insignificant problems. Along with skin defects, these diseases are also responsible for the turgor loss in stored potatoes.
The development of skin blemish diseases can be controlled via a complex approach, which includes the use of healthy seed material, correct land treatment, and modern chemical fungicides [12,15,16]. However, black-dot-resistant potato cultivars for large-scale production are almost absent, while most of those used in potato production are susceptible to this disease [17]. The number and efficiency of commercial fungicides suitable for the control of this disease is very low. In recent years, several new fungicides against R. solani were registered in the global market, while no new one was proposed against C. coccodes; recently, guava wood vinegar was reported as a quite efficient tool to control C. coccodes on potato in greenhouse experiments: both stem and root colonization with sclerotia were reduced by 40-50% compared to the control [18]. However, the effect of this biopreparation still should be confirmed in field trials.
At the same time, black dot has today become a common potato and tomato disease in many countries in Europe and Asia, as well as America, Australia, and Russia [6,7,9,13]. Unfortunately, since the symptoms of this disease manifesting on the aboveground parts of potato plants are often similar to those of other diseases affecting aboveground plant parts (early blight, bacterial infections) and tubers (silver scurf), many potato producers underestimate the significance of this disease (Figure 1).
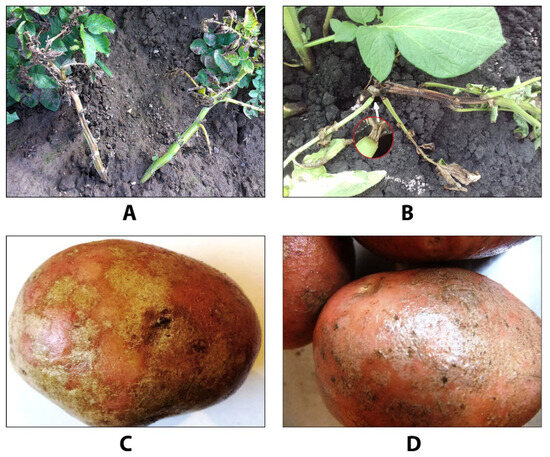
Figure 1.
Similarity of black dot symptoms with other potato diseases. (A) Potato plant with bacterial infection (stem and leaf wilting); (B) stem and leaf wilting in a plant affected with black dot disease; (C) potato tuber infected with silver scurf; (D), potato tuber infected with black dot.
A soil population of C. coccodes represents the main source of infection of potato plants [19]. In Russia, this infection was shown to be gradually accumulated in the soil and seed material in many potato-growing regions, which can be explained by a number of reasons, including the lack of crop rotation, the use of a low-quality infection-containing seed material, the post-harvest plowing of culled tubers into the soil, and the presence of weeds affected with the same pathogens as the potato (such as black nightshade (Solanum nigrum L.)). In addition, potato growers often leave piles of culled potato near their fields [11], so infection can spread over adjacent and neighboring fields. The development and severity of black dot disease also depends on crop cultivation conditions (temperature, irrigation, length of the cultivation period, cultivar susceptibility, fungicide application, crop rotation, etc.), the virulence of the pathogen population, and the predominant inoculum potential [16]. At the same time, like many diseases, which are not accompanied by a significant plant infection or even death, the severity of black dot may depend on the immune status of a plant, i.e., weak immunity or stress conditions may provide more severe affections.
Over the course of phytosanitary monitoring, performed by our team in recent years in the Northeastern, Central, Siberian, and Volga Federal districts of Russia, we registered black dot symptoms on various domestic and foreign potato cultivars (data not published). The maximum levels of disease development were observed on potato plants, which were weakened by abiotic stresses as well as by the late blight caused by Phytophthora infestans and bacterial infections. Therefore, it seemed to be very important to reveal key factors providing improvements to the immune status of potato cultivars in relation to the black dot infection.
The study presented in this paper included two main tasks. First, using a large set of potato cultivars differing in their late blight resistance, we examined a correlation between the intensity of the late blight infection of potato plants determined by the cultivar resistance to this disease and the occurrence of black dot infection. Second, the effect of crop protection against late blight on the development of black dot disease was evaluated. This study was arranged at the infection polygon, characterized by the very high infection load of both pathogens.
2. Materials and Methods
2.1. Field Study Arrangement
This study was arranged in 2023 at the experimental field of the All-Russian Research Institute of Phytopathology (ARRIP, Moscow region, Russia, edge points: 55.631607 N, 36.952211 E; 55.631094 N, 36.951921 E; 55.631355 N, 36.950537 E; 55.631899 N, and 36.950838 E), characterized by a high background of P. infestans and C. coccodes. To provide such conditions, we artificially infected it with P. infestans every year for 3 years: during planting, each 10th tuber was inoculated by the pathogen. No crop rotation was used. Starting from the 4th year, the infection background on this field became high enough to provide epiphytotic conditions for our annual trials; usually, to the end of the vegetation season, among all potato cultivars planted on this field, only resistant cultivars survived in the absence of any protective treatments; susceptible and moderately susceptible plants died. The same situation was generated for C. coccodes: we regularly used infected tubers for planting on this field to provide an accumulation of this infection in the soil.
The creation of such a highly self-maintaining infection polygon located far from other fields used for potato cultivation was required for the arrangement of registration trials of new potato cultivars for their disease resistance, performed on an annual basis for the State Commission of Russian Federation for Selection Achievements Test and Protection.
The land treatment included under-winter ploughing (27 October 2022), disking (15 April 2023), deep ground treatment (27 April 2023), pre-planting furrow formation (1 May 2023), and hilling with a rotary cultivation (26 May 2023). Organic compost (70 ton/ha) was introduced into the soil in autumn, while inorganic NPK 18/18/18 fertilizer was applied prior to potato planting at a dosage of 60 kg/ha. A weed control was provided by the field treatment with the metribuzin-based Zenkor (0.6 kg/ha, 29 May 2023) and prosulfocarb-based Boxer (2 L/ha, 26 June 2023) herbicides. In addition, one field treatment with the Aktara insecticide (a.i. thiamethoxam, 0.06 kg/ha) was carried out with an allowance for the economic threshold of the Colorado beet population magnitude.
The planting date was 5 May 2023. The date of manual harvesting was 20 August 2023.
2.2. Weather Conditions
During the whole vegetation season, meteorological data (temperature, atmospheric pressure, air humidity, precipitation, soil temperature and humidity, wind force and direction, etc.) were obtained using a CR10X weather station (Campbell Scientific Inc., Logan, UT, USA), located at ARRIP fields.
The weather conditions in the Moscow region in 2023 were favorable for the development of both late blight and black dot diseases of potato. The middle and the end of April were characterized by dry and warm weather, which made it possible to plant potato tubers in the beginning of May, when the soil temperature reached 8–9 °C. May was characterized by swings in the air temperature and humidity. Compared to the average annual data, the beginning of May was characterized by an average air temperature drop of 4.5 °C, while for the rest of the month, this index increased by 1.7 and 0.4 °C, respectively; the air humidity in the beginning and middle of May was decreased by 11 and 10%, respectively, while it increased by 5% in the end of the month. Under such weather conditions, the first shoots appeared in the period of May 15–20, while full shoots were observed within the first 10 days of June. Abundant precipitation was recorded in the third week of June, as well as in the second and third weeks of July (34.7, 22.6, and 26.4 mm above the average annual values, respectively; Table S1). Therefore, the total water content in the soil in this period was sufficient for the active formation and the further development of potato tubers. At the same time, such weather conditions were favorable for the quite early and epiphytotic development of the late blight infection, followed by the black dot development (Figure 2).

Figure 2.
Severe infection of the field with Phytophthora infestans. (Left): 12 July 2023; no visible disease symptoms. (Right): 31 July 2023; a complete death of susceptible cultivars caused by the late blight epiphytoty.
2.3. Evaluation of the Potato Resistance to Late Blight and Black Dot Occurrence
A set of 98 domestic and foreign potato cultivars was included in the study (see the Section 3). Due to a large number of cultivars and the restricted area of the field, each cultivar was presented by 10 plants (five plants in a two replications) that corresponded to the international protocol of field trials for potato resistance to diseases approved by the Euroblight network (potato late blight network in Europe) [20]. According to this protocol, in the case of trials including a large amount of cultivars, the minimal acceptable number of replications is 2, while the minimal plot size includes 3 plants. The size of each plot was 1.5 sq. m, so the total size of plots for each cultivar was 3 sq. m.
The late blight resistance of potato cultivars was evaluated under a natural high-infection background corresponding to the epiphytotic conditions. As a rule, predominant P. infestans races in the Moscow region are complex; in this season, they were represented by two races, 1.2.3.4.5.6.7.8.9.10.11 and 1.2.3.4.5.6.7.8.10.11. Field inspections were performed every 7 days in the period from 29 June to 15 August. Late blight and black dot infections were registered by a visual examination of plants, followed by the collection of infected samples, the isolation of pathogens into pure cultures, and their morphological identification (see Figures S1 and S2). The late blight development level was evaluated using the British Mycological Society scale (Table 1).

Table 1.
Scale for the assessment of the late blight infection level on potato [21].
Based on the obtained data, the corresponding AUDPC (area under the disease progress curve) values and estimated yield losses were calculated using a special program designed at the ARRIP Department of Potato & Vegetable Diseases [22]. Briefly, this program is based on the van der Plank hypothesis [23], which assumes a direct ratio between the AUDPC calculated for the potato foliage and the yield losses. According to our long-term field studies, this dependency can be expressed by the following equation:
where ω is the estimated yield loss (%) caused by an early leaf decay and q is the number of days between the bud formation phase and the dying-off of healthy leaves. The average q value for the early, intermediate, and mid-late potato cultivars is 46, 52, and 84 days, respectively. If the foliage is killed by the frost or a desiccant, or the harvesting is carried out before the natural dying-off of the foliage, then q is considered to be the number of days passed between the bud formation phase and the moment of foliage death [24].
Then, the data on yield losses were converted into a 9-point scoring system (Table 2). This evaluation scale was developed specially for potato breeders and potato growers to give them an understanding of the expected yield loss risks associated with late blight development in the field, depending on the resistance score of a cultivar. Taking into account this scale, potato producers can understand which level of yield losses they can expect using a certain potato cultivar without protective treatments.

Table 2.
Nine-point scale used for evaluation of late blight resistance of potato cultivars [24].
The occurrence of black dot infection on potato cultivars was evaluated during harvesting. The bottom parts of dug plants were examined for the presence of sclerotia. The plant was considered infected if an abundant production of black sclerotia was observed on the surface of the bottom parts of its stems. Such plants were also characterized by infected stolons and roots.
The calculation was performed using the following formula [25]:
where D is the disease occurrence (%), n is the number of infected plants, and N is the total number of plants examined for a certain plot area.
2.4. Evaluation of the Black Dot Occurrence on Potato Protected against Late Blight
This study was arranged on two potato cultivars differing in their late blight resistance: Arizona (susceptible) and Sante (moderately susceptible). For each cultivar, three experimental variants were arranged:
- (1)
- No treatment (control);
- (2)
- In total, 6× treatment with a Shirlan fungicide (a.i. fluazinam, 0.4 L/ha) during a vegetation season;
- (3)
- The in-furrow application of a Quadris fungicide (a.i. azoxystrobin, 3 L/ha) during planting, plus a 6× treatment with a Shirlan fungicide (0.4 L/ha) during a vegetation season.
Shirlan registered as a contact fungicide for potato protection against the late blight caused by P. infestans was chosen due to its inefficiency in relation to C. coccodes. At the same time, Quadris represented a wide-range fungicide efficient not only against late blight, but also against other fungal infections.
The area of each plot was 42 m2. Each plot included 6 rows and 198 plants in total. The late blight development was evaluated for 4 central rows (132 plants); the black dot occurrence was evaluated using 30 plants randomly dug out from 4 central rows. The experiment was arranged in four replications (random design). The evaluation of the late blight development, the calculation of AUDPC values and estimated yield losses, and the assessment of the black dot occurrence were carried out as described in Section 2.3. In addition, the total yield of harvested tubers and its marketable fraction (>35 mm in size) were evaluated by weighting.
2.5. Statistical Data Treatment
A correlation between the level of the late blight resistance of potato cultivars and the severity of their infection with C. coccodes was evaluated using a Pearson correlation coefficient (r) to measure the relationship between two variables from the same sample. The calculation of the Pearson coefficient at p < 0.05 (n = 98) was performed using the MS Excel 2003 program package.
The statistical treatment of the yield data was carried out by a one-factor ANOVA at the 95% confidence level [10] using the MS Excel 2003 program package. All data are presented with the corresponding least significant difference (LSD0.95) values.
3. Results
3.1. Correlation between the Late Blight Resistance Level and Black Dot Occurrence
For the whole set of the studied cultivars, the level of black dot infection varied from 0 to 88% (Table 3 and Table 4). In the case of late blight-susceptible cultivars strongly infected with P. infestans, black dot occurrence varied between 60 and 100%; the average and median values were 88.4 and 90.0%, respectively. During harvesting, a severe infection of plants with C. coccodes, and the presence of fungal sclerotia on the bottom parts of stems, roots, and stolons, were observed (Figure 3). The black dot occurrence on moderately susceptible cultivars varied from 20 to 80%; the average and median values were 43.4 and 40.0%, respectively. Finally, in the case of moderately resistant and resistant cultivars, the disease occurrence was 0–20%, with the mean and median values of 6.0 and 5.0%, respectively.

Table 3.
Black dot occurrence on potato cultivars differing in their late blight resistance.

Table 4.
Occurrence of the black dot infection on potato cultivars differing in their late blight resistance (generalized data).

Figure 3.
Sclerotia of Colletotrichum coccodes on potato stolons.
The analysis of the obtained data revealed a strong negative correlation between their late blight resistance level and the occurrence of black dot disease (r = −0.81, p < 0.05). The confidence interval limits at p < 0.05 were the following: rL = −0.87 and rU = −0.73.
3.2. Effect of Plant Protection against Late Blight on the Black Dot Occurrence and Marketable Yield Fraction
According to the obtained results, the chemical protection of potato plants against late blight using a Shirlan fungicide resulted in a significant reduction in black dot occurrence by 30% (cv. Arizona) and 20% (cv. Sante) compared to the untreated controls (Table 5). Obviously, the reduction in the late blight infection of potato plants represented one of the key factors improving plant immunity in relation to black dot infection. An additional protection with the Quadris fungicide efficient against C. coccodes resulted in the further efficient reduction in the disease occurrence.

Table 5.
Effect of potato protection against late blight on black dot occurrence.
In relation to the total yield and marketability of potatoes, potato protection against the late blight resulted in a significant improvement in both indices. Compared to the untreated control, Shirlan application increased the total yield and the fraction of marketable tubers by 103.6 and 62.5% for cv. Arizona and by 65.9 and 43.8% for cv. Sante (Figure 4). The addition of a C. coccodes-suppressing Quadris fungicide into the protection scheme provided the further improvement in both indices of up to 136.8 and 69.6% for the cv. Arizona and 98.6 and 50% for the cv. Sante (compared to the untreated control).
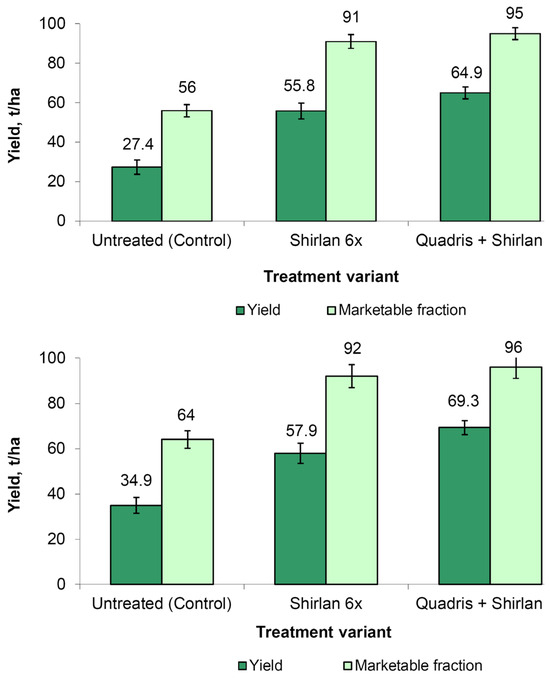
Figure 4.
Yield and marketable fraction of potatoes in different variants of chemical protection. (Top): cv. Arizona (LSD0.95 = 3.9 and 3.0 for yield and marketable fraction, respectively). (Bottom): cv. Sante (LSD0.95 = 3.1 and 5.0 for yield and marketable fraction, respectively).
4. Discussion
The first detailed reports on black dot disease on potato date back to 1920s [26]. Until the next several decades, this disease was not considered a serious problem of economical importance, but was described rather as a mild disease [27]. However, the number of reports on the occurrence of this disease in different regions of the world, as well as on the yield losses and stored crop damage associated with this pathogen, significantly increased in the last two decades. For example, the annual losses of ware potato associated with black dot disease in UK reached up to GBP 3 million [6]. Potato yield losses caused by soil infection with the pathogen may reach 22–30%, which can be determined by the revealed association of potato early dying syndrome with the pathogenic complex of Verticillium dahliae and C. coccodes [28]. Therefore, the attitude toward this disease has changed, so its management is now considered a required part of the potato protection strategy.
In spite of the fact that black dot disease was first revealed in Russia in 1965 [29], and that its occurrence gradually increased in the following years [13], many potato growers consider it to have appeared only recently, arriving with imported seed potato. This opinion can be explained by several facts about Russia’s past, including sales of unwashed table potato, low volumes of potato processing, and tuber storage at rather low (3–4 °C) temperatures vs. the current requirements for the storage of processed potato (7–12 °C), promoting black dot manifestation. Now the situation has changed. Moreover, the black dot problem has become even more aggravated; according to recent data, C. coccodes has been detected in 100% of lots of seed potato in Russia [17]. This situation is caused by a number of factors. The Russian State Standard for seed potatoes does not restrict the level of their infection with C. coccodes, so seed potato sellers do not need to test them for the presence of this pathogen thus, limiting the circulation of infected seed material across the country. The weed coenosis has changed and now includes more species infected by C. coccodes, which serve as the source of infection and promote the spreading of the pathogen [30]. Due to the similarity of the black dot manifestations with other potato diseases, farmers often do not recognize this disease and, therefore, do not provide correct protective treatments able to suppress C. coccodes. Last but not least, potato-growing companies often do not provide a long-term (5–9 years) crop rotation cycle for economical reasons. At the same time, C. coccodes sclerotia are able to survive in the soil for 5–13 years [31]; under such conditions, a short crop rotation cycle (3–4 years) results in a gradual accumulation of infection.
Therefore, potato growers in Russia and similar countries should understand the basis of this situation and focus their attention on the development of effective complex strategies to manage black dot.
Since potato breeders do not take into account black dot disease in their breeding programs, no black-dot-resistant cultivars were bred; the only known information is that potato cultivars with white skin are more susceptible to this disease than those with the purple or red skin [32]. Authors of some other studies have suggested the thick-skin and early-maturing cultivars may be less susceptible to black dot (see [33]). A recent study using an untargeted metabolomics approach revealed some possible biomarkers of the potato resistance to black dot, which included the glycoalkaloid alpha-chaconine and hydroxycinnamic acid amides [33]. The authors concluded that the metabolite composition may represent the main determinant of potato resistance to the black dot, and that the above-mentioned compounds may probably be used for the marker-assisted selection of potato for this type of resistance.
Being a strong tool to control fungal diseases, fungicides do not provide absolute protection against black dot. A pre-planting tuber treatment and in-furrow fungicide application are not able to sufficiently reduce disease incidence; moreover, they will not be efficient against infection surviving on plant debris in the soil [17,34]. Early (prior raw closure) foliar applications of strobilurin-based fungicides reduce black dot incidence in stems and daughter tubers [35,36]; however, if fungicide application occurs after inoculation, it is not able to significantly reduce infection [34]. In addition, some common fungicides (chlorothalonil and mancozeb) are ineffective against C. coccodes [37].
To improve the effect of protective chemical treatments on black dot suppression, one should take into account some other factors influencing the development of this disease. These factors include the immune status of plants able to significantly influence their ability to resist diseases. Plant stress (including that caused by biotic factors) was shown to promote sclerotia production on stems and stolons [38], thus allowing for the more intensive spreading of the pathogen across the field.
In this way, it was interesting for us to evaluate the contribution of the intensity of the late blight infection of potato plants in black dot occurrence. Late blight is very common in Russia, but the proper protection (including multiple fungicidal treatments) is usually performed only in large potato-growing companies. Small farmers and owners of private plots, who produce a significant part of the total potato yield in Russia, often try to save money and treat potato plots only 2–3 times per season. As a result, they cannot prevent late blight development on their plants and its further spreading over neighboring territories, thus worsening the immune status of potato plants. It is also important that 80% of the area under potato planting in Russia is located in zones of high epiphytolic risk (late blight epiphytoties occur every 3–5 years) [39,40], so the black dot problem in these regions can be rather serious and require some activity to control it.
As far as we know, no studies involving the representative sampling of potato cultivars differing in their late blight resistance have been arranged under either field, or laboratory conditions, to evaluate the correlation between the level of the late blight affection of potato cultivars and the occurrence of the black dot infection. Our team had the chance to work at the specially created infection polygon with a very high infection background of both diseases. We also had the chance to examine a large number of domestic and foreign potato cultivars with a known level of late blight resistance. These two unique possibilities, combined with the weather conditions of the vegetation season in 2023 (multiple “swings” between the dry and wet conditions), which were favorable for the intensive development of both infections, allowed us to first reveal a strong negative correlation between the two studied factors. The obtained experimental data confirmed our hypothesis based on the earlier non-documented observations that the C. coccodes infection of potato plants occurs more actively in the case of late blight-infected plants, characterized by a lower immune status. Taking into account that most of the top-20 commercial potato cultivars grown in Russia are susceptible or moderately susceptible to late blight (Table 6), which causes them to die under epiphytotic conditions if unprotected, this correlation demonstrates the importance of understanding the necessity of a serious complex approach for the control of both diseases to provide a good-quality potato yield.

Table 6.
Top-20 commercial potato cultivars grown in Russia.
We also showed that potato protection against late blight also provided a significant (20–30%) reduction in black dot severity, i.e., this kind of protection was a significant factor improving plant resistance against black dot. In addition, consecutive protective treatments against black dot (the in-furrow application of a fungicide) and late blight (foliar treatments during a vegetation season) resulted in the highest yield and tuber marketability. The revealed positive effect of plant protection against late blight was associated with the better development and immune status of protected plants, which reduced the chance of C. coccodes penetration into plant tissues; in addition, in the case of severe late blight infection, the corresponding death of leaves and stems causes a reduction in canopy density, which, in turn, facilitates the spreading of C. coccodes spores by air from the soil and the infected bottom parts of the plants to other plants.
Thus, our study showed that the reduction in black dot severity on potato fields requires a complex approach, which, along with the use of specific agrotechnic methods and fungicides intended to suppress the development of C. coccodes, should include an obligatory protection of cultivars, susceptible or moderately susceptible to late blight, against this disease, since the reduction in the late blight affection of potato plants is one of the key factors providing an improvement in the immune status of potato cultivars in relation to black dot infection.
Since this experiment was labor-consuming, included a large number of cultivars, and required a lot of area for our infection polygon, used also for some other purposes, it would be difficult to repeat it with the same set of cultivars and in the same volume. Nevertheless, we plan to perform further monitoring of late blight and black dot development on different potato cultivars to obtain data for other epiphytotic seasons, but using a smaller number of cultivars and (probably) with different protection schemes. At the same time, many potato growers in Russia, who consult with our department on a constant basis, asked us about this experiment and are waiting for the publication of its results, so even the one-year results will be relevant for them, for the correction of their potato protection strategies; since the experiment included many commercial cultivars used on a global scale, we also think these results will be relevant for foreign potato growers.
5. Conclusions
An experiment conducted on a large sample of domestic and foreign potato cultivars demonstrated a strong negative correlation between the occurrence of black dot disease and late blight resistance (and the corresponding late blight affection) of the tested cultivars. Black dot occurrence on late blight-susceptible cultivars, strongly infected with P. infestans, varied between 60 and 100%; in the case of moderately susceptible cultivars, this index varied between 20 and 80%, whereas moderately resistant and resistant cultivars demonstrated only 0–10% of the black dot occurrence. An experiment, which included potato cultivars susceptible (cv. Arizona) and moderately susceptible (cv. Sante) to late blight, showed that the protection of these cultivars against late blight resulted in a reduction in black dot severity by 30 and 20%, respectively, compared to the unprotected controls. Obviously, potato protection against late blight is one of the key factors ensuring an improved immune status in potato in relation to black dot infection, so it should be used as a part of a complex approach for black dot management.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/agronomy14071373/s1, Figure S1: Phytophthora infestance on the experimental potato field and stages of its isolation and morphological identification. Figure S2: Colletotrichum coccodes on the experimental potato field and stages of its isolation and morphological identification. Table S1: Weather data for the vegetation season of 2023 (weather station at the All-Russian Research Institute of Phytopathology, Moscow region, Russia).
Author Contributions
Conceptualization, M.A.K.; methodology, M.A.K.; validation, V.N.D.; formal analysis, N.V.S.; investigation, V.N.D., A.A.V., T.I.S., A.Y.U. and I.N.S.; data curation, M.A.K.; writing—original draft preparation, M.A.K. and N.V.S.; writing—review and editing, M.A.K.; visualization, M.A.K. and N.V.S.; supervision, M.A.K.; project administration, M.A.K.; funding acquisition, M.A.K. All authors have read and agreed to the published version of the manuscript.
Funding
This study was carried out within the framework of the State Assignment from the Ministry of Science and Higher Education of Russia “Population studies of the most harmful pathogens and pests of potato and vegetable crops in Russia; optimization of the crop protection management” (theme no. FGGU-2022-0007).
Data Availability Statement
The authors declare that the data supporting the findings of this study are available within the main text of the manuscript and the Supplementary Materials. The raw data are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Knudson, W.; Miller, S.R. Measuring the Economic Significance of the U.S. Potato Industry. Report for the National Potato Council. Available online: https://www.nationalpotatocouncil.org/wp-content/uploads/2023/02/NPCSpudNationReport.pdf (accessed on 12 March 2024).
- Afrianto, W.F.; Budioko, B. Do not judge these potatoes by its peel: Urban consumers’ perceptions of imperfect produce. Hexagro J. 2022, 6, 1–11. [Google Scholar] [CrossRef]
- Russian Statistical Yearbook 2023; Rosstat: Moscow, Russia, 2023. Available online: https://rosstat.gov.ru/storage/mediabank/Ejegodnik_2023.pdf (accessed on 15 June 2024).
- Sibiryaev, A.S. Results of the functioning of agriculture of RF in 2023: Challenges of the future. Bull. NGIEI 2024, 4, 99–110. [Google Scholar] [CrossRef]
- Potato Union: The Most Popular Potato Cultivars in Russia Are of the Foreign Breeding. Available online: https://www.agroinvestor.ru/markets/news/39995-kartofelnyy-soyuz-samye-populyarnye-sorta-kartofelya-v-rossii-importnoy-selektsii/ (accessed on 15 June 2024).
- Sanzo-Miró, M.; Simms, D.M.; Rezwan, F.I.; Terry, L.A.; Carmen Alamar, M. An integrated approach to control and manage potato black dot disease: A review. Am. J. Potato Res. 2023, 100, 362–370. [Google Scholar] [CrossRef]
- Çakır, E.; Karahan, A.; Kurbetli, İ. Involvement of Colletotrichum coccodes causing atypical symptoms of potato tubers in Turkey. J. Plant Dis. Prot. 2019, 126, 173–176. [Google Scholar] [CrossRef]
- Tiwari, R.K.; Kumar, R.; Sharma, S.; Naga, K.C.; Subhash, S.; Sagar, V. Continuous and emerging challenges of silver scurf disease in potato. Int. J. Pest Manag. 2022, 68, 89–101. [Google Scholar] [CrossRef]
- Lan, Q.; Liu, Y.; Mu, R.; Wang, X.; Zhou, Q.; Islam, R.; Su, X.; Tian, Y. Biological control effect of antagonistic bacteria on potato black scurf disease caused by Rhizoctonia solani. Agronomy 2024, 14, 351. [Google Scholar] [CrossRef]
- Belov, G.L.; Zeiruk, V.N.; Kokaeva, L.Y.; Kutuzova, I.A.; Elansky, S.N. Anthracnose or black dot of potato tubers. Zashchita Karantin Rastenii 2018, 10, 36–38. (In Russian) [Google Scholar]
- Kuznetsova, M.A.; Erokhova, M.D.; Demidova, V.N. Potato tuber blemish diseases and the methods for controlling. Zashchita Karantin Rastenii 2024, 1, 35–40. (In Russian) [Google Scholar]
- Kuznetsova, M.A.; Rogozhin, A.N.; Smetanina, T.I.; Denisenkov, I.A. Protection of potatoes from rhizoctonia, anthracnose, and silvery scab. Potato Veg. 2017, 4, 27–29. (In Russian) [Google Scholar]
- Kuznetsova, M.A.; Denisenkov, I.A.; Rogozhin, A.N.; Smetanina, T.I. Anthracnosis is a harmful disease of potato. Potato Veg. 2020, 6, 20–23. (In Russian) [Google Scholar]
- Jansegers, H. The Demand for Unwashed Potatoes is Declining. Available online: https://www.freshplaza.com/north-america/article/9197485/the-demand-for-unwashed-potatoes-is-declining/ (accessed on 13 March 2024).
- Denisenkov, I.A. Efficient protection of potato from diseases of various etiology under conditions of Bryansk region. Achiev. Sci. Technol. AIC 2018, 32, 76–78. (In Russian) [Google Scholar] [CrossRef]
- Massana-Codina, J.; Schnee, S.; Lecoultre, N.; Droz, E.; Dupuis, B.; Keiser, A.; de Werra, P.; Wolfender, J.-L.; Gindro, K.; Schürch, S. Influence of abiotic factors, inoculum source and cultivar susceptibility on the potato tuber blemish diseases black dot (Colletotrichum coccodes) and silver scurf (Helminthosporium solani). Plant Pathol. 2021, 70, 885–897. [Google Scholar] [CrossRef]
- Belov, D.A.; Khiutti, A.V. Modern phytopathogenic complex of potato diseases and measures to prevent their spread in Russia. Potato Veg. 2022, 5, 18–24. (In Russian) [Google Scholar] [CrossRef]
- El-Fawy, M.M.; Abo-Elyousr, K.A.M.; Sallam, N.M.A.; El-Sharkawy, R.M.I.; Ibrahim, Y.E. Fungicidal effect of guava wood vinegar against Colletotrichum coccodes causing black dot disease of potatoes. Horticulturae 2023, 9, 710. [Google Scholar] [CrossRef]
- Lees, A.K.; Hilton, A.J. Black dot (Colletotrichum coccodes): An increasingly important disease of potato. Plant Pathol. 2003, 52, 3–12. [Google Scholar] [CrossRef]
- Colon, L.; Nielsen, B.; Darsow, U. Field Test for Foliage Blight Resistance. EUROBLIGHT Protocol ver.1.2. Available online: https://agro.au.dk/fileadmin/Field_Test_Foliar_Blight_revised.pdf (accessed on 15 June 2024).
- James, W.C.; Shih, C.S.; Hodgson, W.A.; Callbeck, L.C. The quantitative relationship between late blight of potato and loss in tuber yield. Phytopathology 1972, 62, 92–96. [Google Scholar] [CrossRef]
- Filippov, A.; Kuznetsova, M.; Rogozhin, A.; Iakusheva, O.; Demidova, V.; Statsyuk, N. Development and testing of a weather-based model to determine potential yield losses caused by potato late blight and optimize fungicide application. Front. Agr. Sci. Eng. 2018, 5, 462–468. [Google Scholar]
- van der Plank, J.E. Disease Resistance in Plants; Academic Press Inc.: New York, NY, USA; London, UK, 1968. [Google Scholar]
- Kuznetsova, M.A.; Spiglazova, S.Y.; Rogozhin, A.N.; Smetanina, T.I.; Filippov, A.V. Late blight assessment of potato cultivars using a new express method. In Agrosym 2013: Books of Proceedings; Kovacevic, D., Ed.; Poljoprivredni fakultet: Istočno Sarajevo, Bosnia and Herzegovina, 2013; pp. 601–606. Available online: http://www2.agrosym.rs.ba/agrosym/agrosym_2013/documents/2pfs/pfs13.pdf (accessed on 15 June 2024).
- Polyakov, I.Y.; Persov, M.P.; Smirnov, V.A. Forecasting of the Development of Pests and Diseases of Agricultural Crops; Textbook for a Practical Training; Kolos: Leningrad, Russia, 1984. [Google Scholar]
- Dickson, B.T. The black dot disease of potato. Phytopathology 1926, 16, 23–40. [Google Scholar]
- Chesters, C.G.C.; Hornby, D. Studies on Colletotrichum coccodes 1. The taxonomic significance of variation in isolates from tomato roots. Trans. Brit. Mycol. Soc. 1965, 48, 573–581. [Google Scholar] [CrossRef]
- Tsror (Lahkim), L.; Hazanovsky, M. Effect of coinoculation by Verticillium dahliae and Colletotrichum coccodes on disease symptoms and fungal colonization in four potato cultivars. Plant Pathol. 2001, 50, 483–488. [Google Scholar] [CrossRef]
- Nikitina, E.V. Potato black dot. Kartof. Ovoshchi 1972, 3, 40–41. (In Russian) [Google Scholar]
- Kazartsev, I.A.; Gomzhina, M.M.; Gasich, E.L.; Khlopunova, L.D.; Gannibal, P.B. Biodiversity of Colletotrichum spp. on several wild and cultivated plants. Mikol. Fitopatol. 2022, 56, 127–139. [Google Scholar] [CrossRef]
- Cullen, D.W.; Lees, A.K.; Toth, I.K.; Duncan, J.M. Detection of Colletotrichum coccodes from soil and potato tubers by conventional and quantitative real-time PCR. Plant Pathol. 2002, 51, 281–292. [Google Scholar] [CrossRef]
- Read, P.J. The susceptibility of tubers of potato cultivars to black dot (Colletotrichum coccodes (Wallrs.) Hughes). Ann. Appl. Biol. 1991, 119, 475–482. [Google Scholar] [CrossRef]
- Massana-Codina, J.; Schnee, S.; Allard, P.-M.; Rutz, A.; Boccard, J.; Michellod, E.; Cléroux, M.; Schürch, S.; Gindro, K.; Wolfender, J.-L. Insights on the structural and metabolic resistance of potato (Solanum tuberosum) cultivars to tuber black dot (Colletotrichum coccodes). Front. Plant Sci. 2020, 11, 1287. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.A.; Geary, B.; Tsror, L. Potato black dot—The elusive pathogen, disease development and management. Am. J. Potato Res. 2018, 95, 340–350. [Google Scholar] [CrossRef]
- Brierley, J.L.; Hilton, A.J.; Wale, S.J.; Peters, J.C.; Gladders, P.; Bradshaw, N.J.; Ritchie, F.; MacKenzie, K.; Lees, A.K. Factors affecting the development and control of black dot on potato tubers. Plant Pathol. 2015, 64, 167–177. [Google Scholar] [CrossRef]
- Cummings, T.F.; Johnson, D.A. Effectiveness of early-season, single applications of azoxystrobin for the control of potato black dot as evaluated by three assessment methods. Am. J. Potato Res. 2008, 85, 422–431. [Google Scholar] [CrossRef]
- Ingram, J.; Cummings, T.F.; Johnson, D.A. Response of Colletotrichum coccodes to selected fungicides using a plant inoculation assay and efficacy of azoxystrobin applied by chemigation. Am. J. Potato Res. 2011, 88, 309–317. [Google Scholar] [CrossRef]
- Johnson, D.A.; Miliczky, E.R. Effects of wounding and wetting duration on infection of potato foliage by Colletotrichum coccodes. Plant Dis. 1993, 77, 13–17. [Google Scholar] [CrossRef]
- Schepers, H.; Hausladen, H.; Hansen, J.; Kwesi Abuley, I.; Andersson, B.; Liljeroth, E.; Edin, E.; Bain, R.; Kennedy, C.; Ritchie, F.; et al. Epidemics and control of early & late blight, 2017 & 2018, in Europe. PPO-Spec. Rep. 2019, 19, 11–34. Available online: https://agro.au.dk/fileadmin/euroblight/Workshops/York/Presentations_and_posters/Proceedings/1._Huub_Schepers-p11-34.pdf (accessed on 16 June 2024).
- Kuznetsova, M.A.; Iakusheva, O.I.; Rogozhin, A.N.; Statsyuk, N.V.; Borovsky, K.V.; Demidova, V.N. Risk of development of epiphytoties of potato late blight in the regions of the Russian Federation: Assessment for the period 2019–2023. Dostizheniya Nauk. Tech. APK 2024, 38, 4–10. Available online: https://www.elibrary.ru/item.asp?id=65637065 (accessed on 15 June 2024). (In Russian).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).