Assessment of Soil Enzyme Activities in Plant Root Zone of Saline Soil Reclaimed by Drip Irrigation with Saline Groundwater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experiment Site
2.2. Agronomic Practice
2.3. Experiment Design
2.4. Measurements
2.5. Data Analysis
3. Results
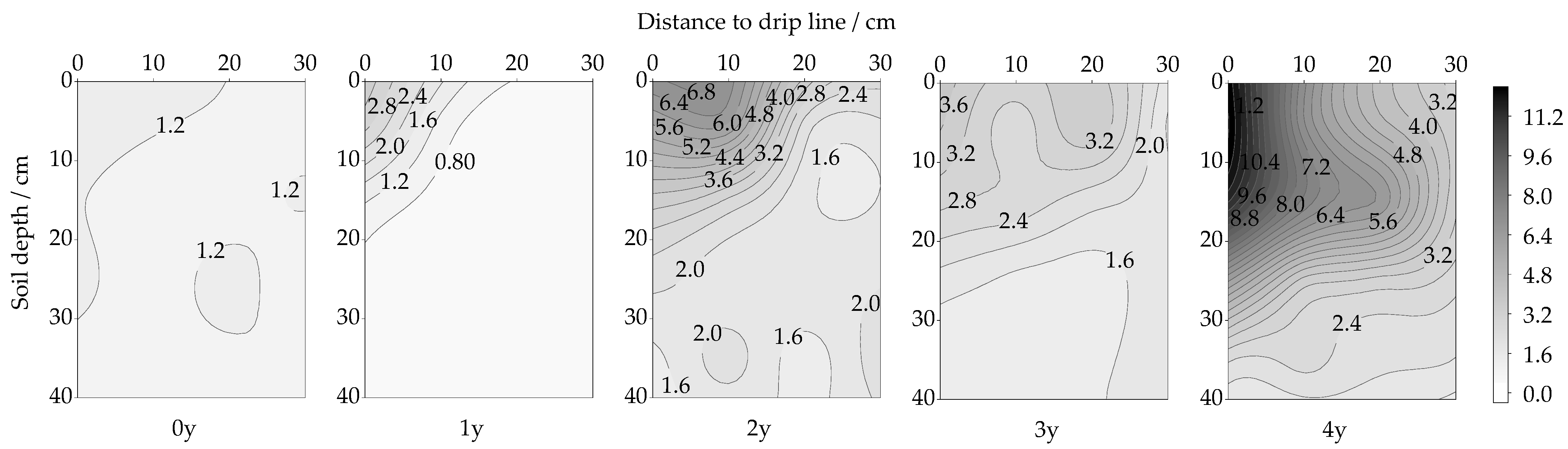
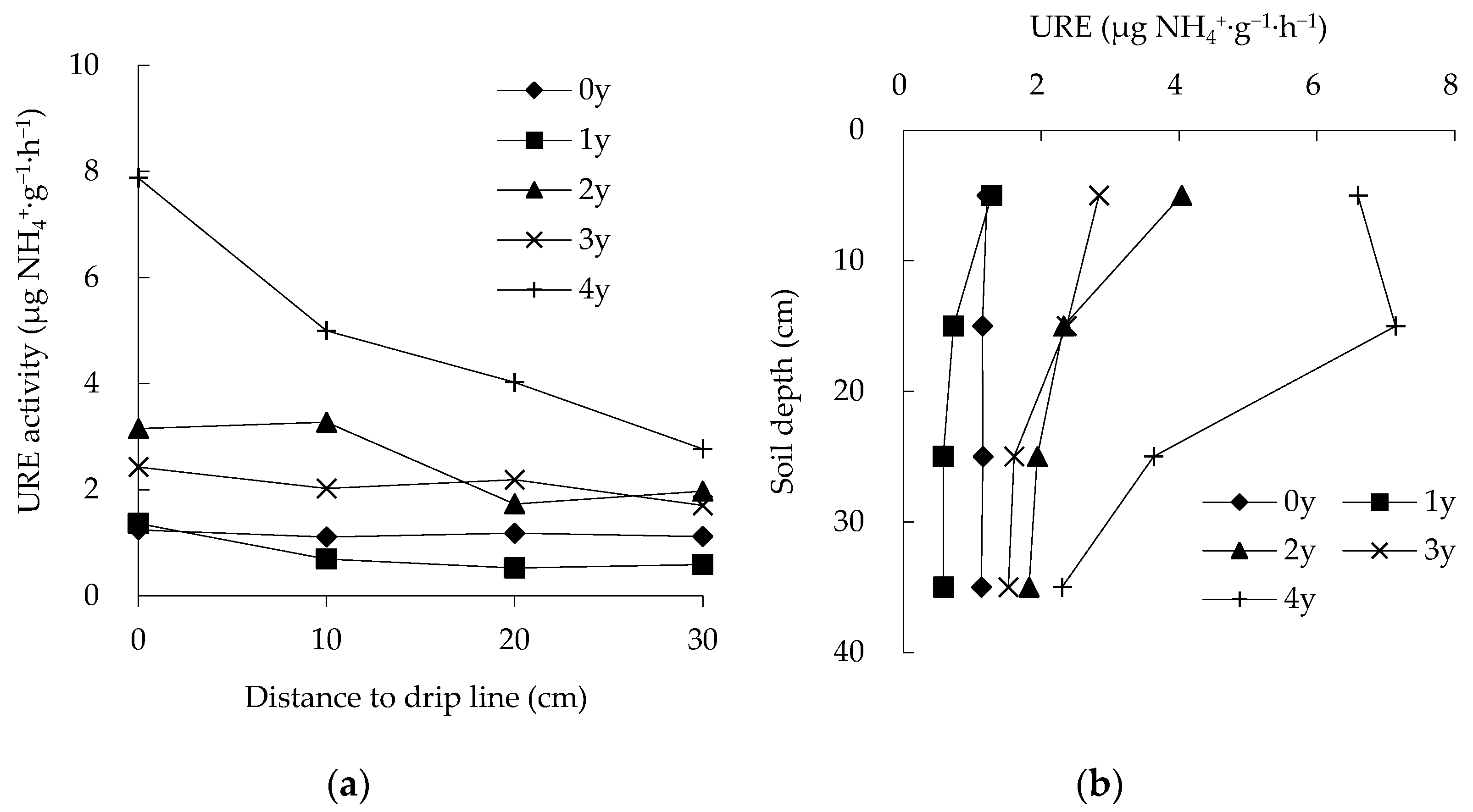
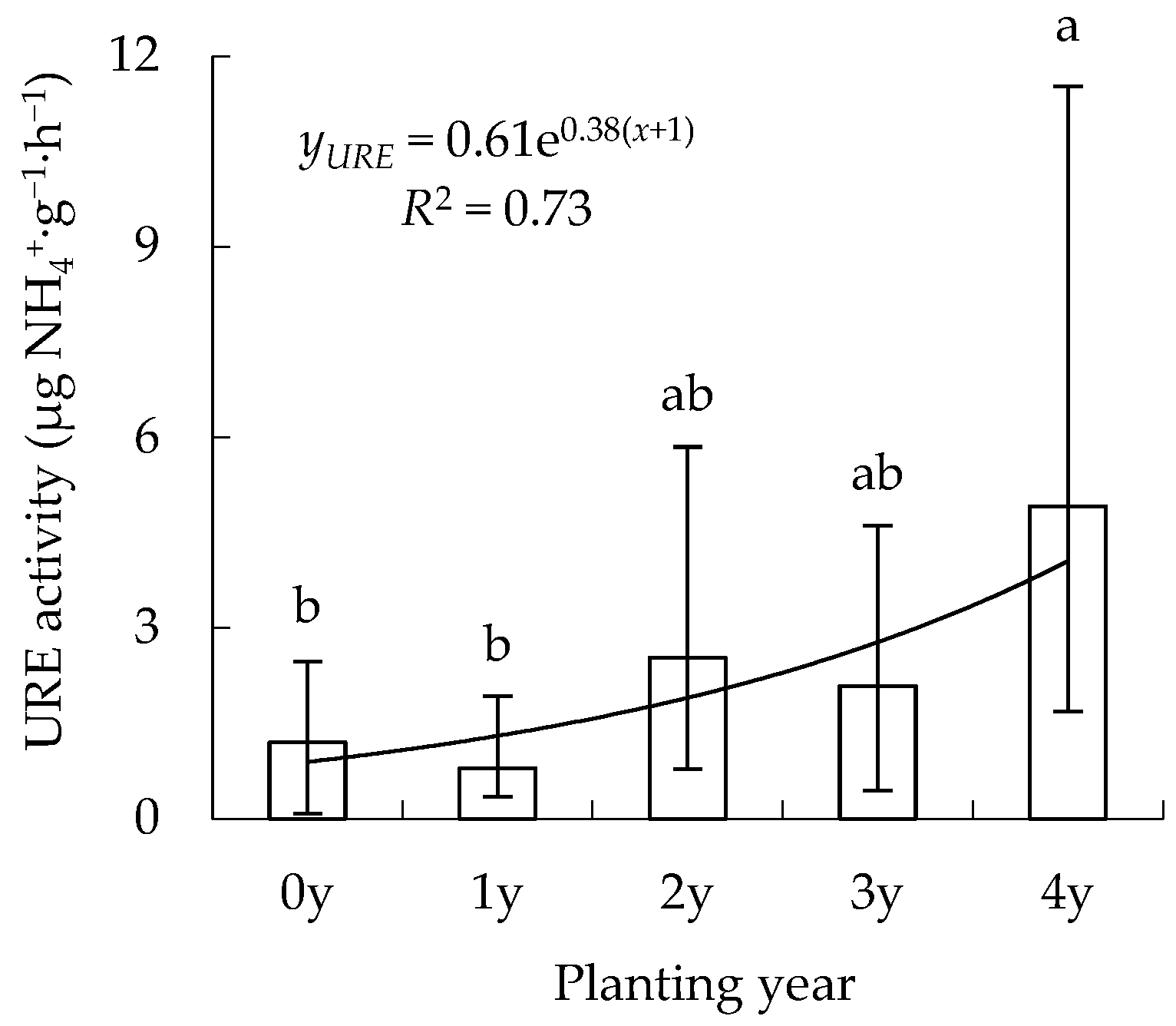
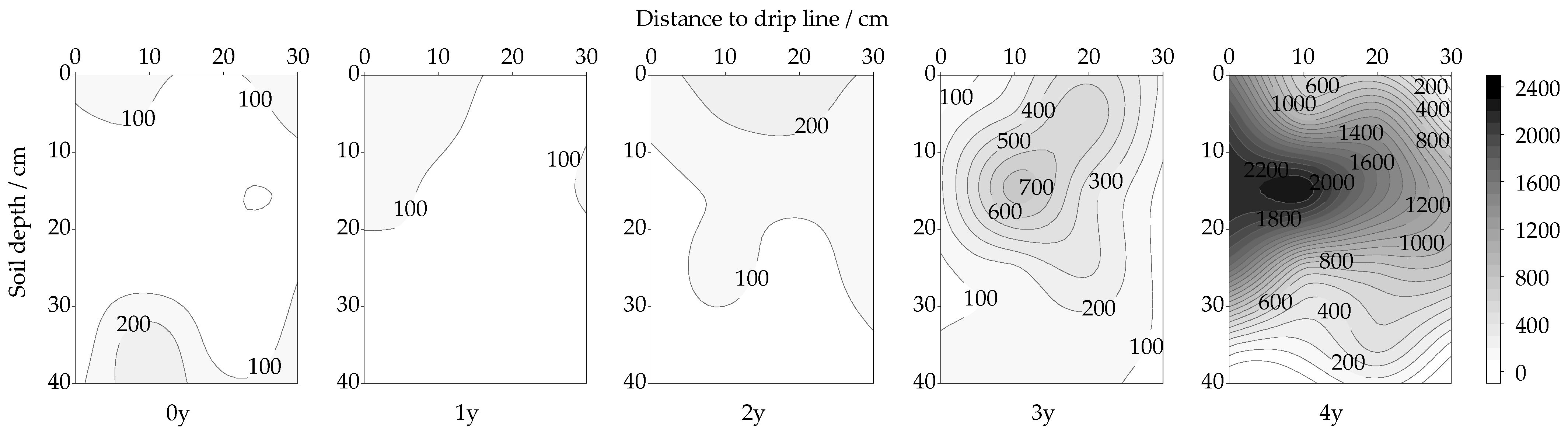
3.1. Spatiotemporal Changes in URE Activity
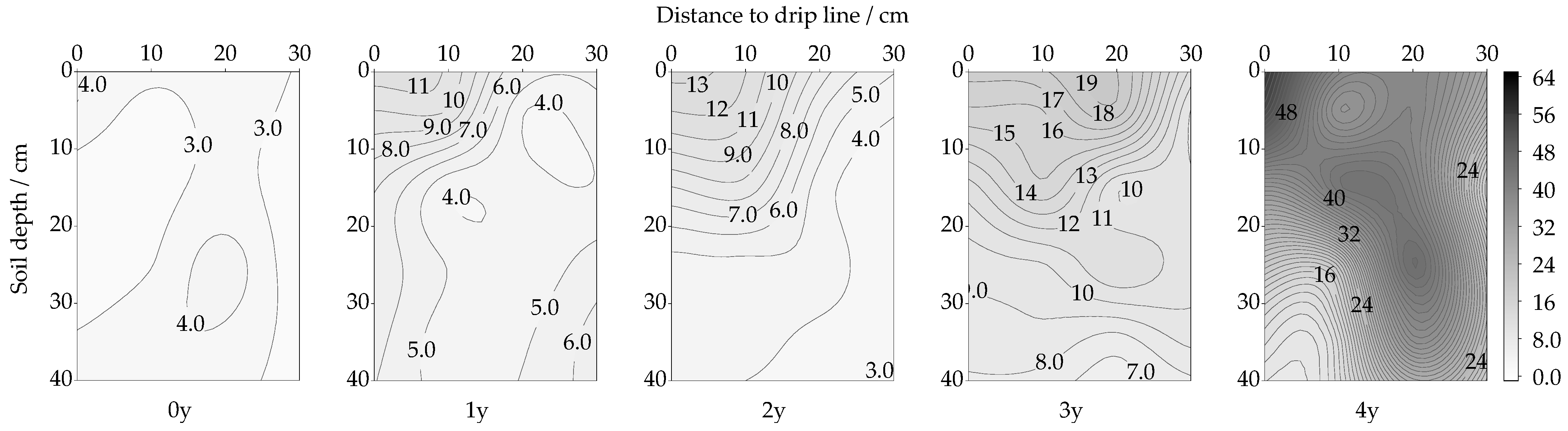
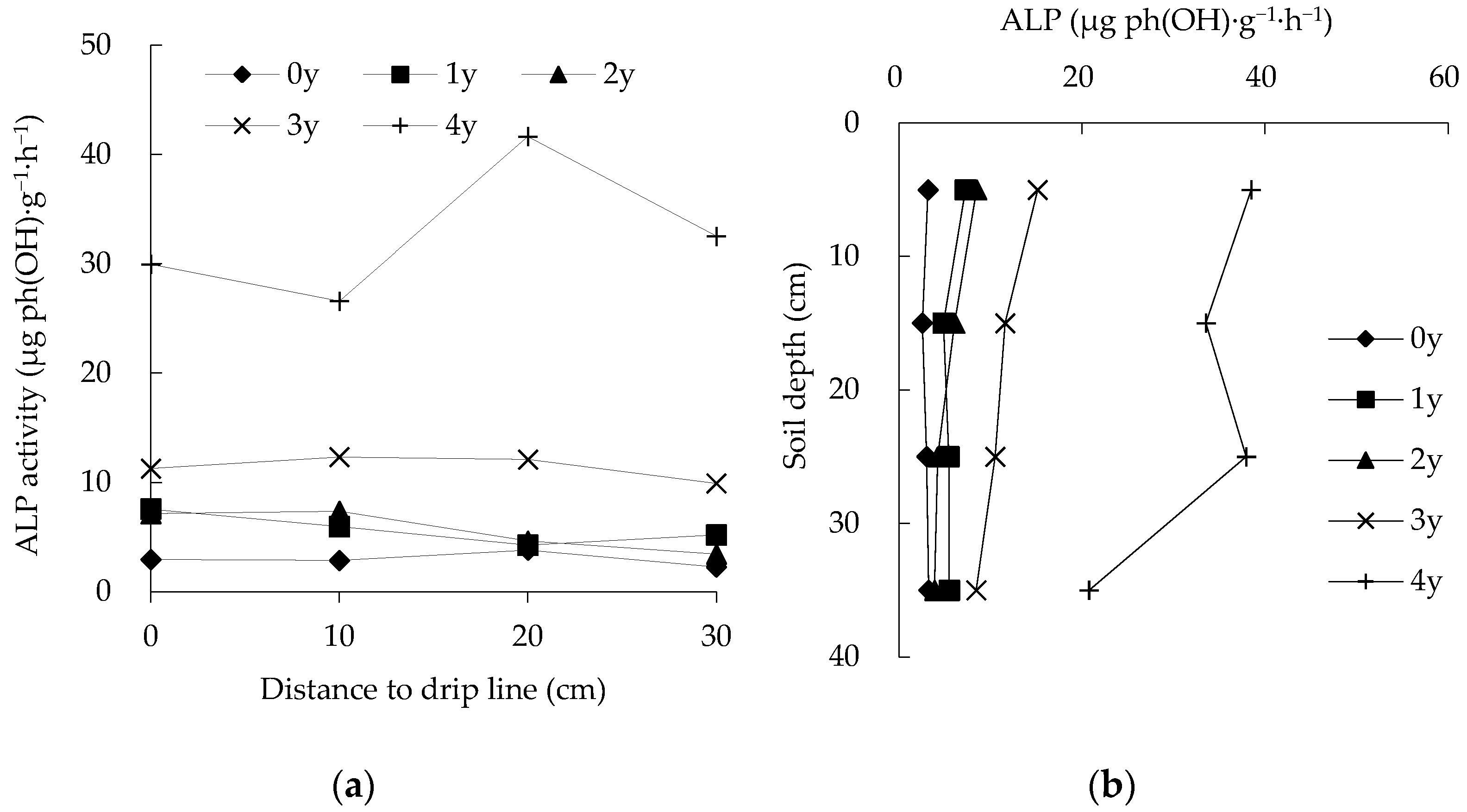
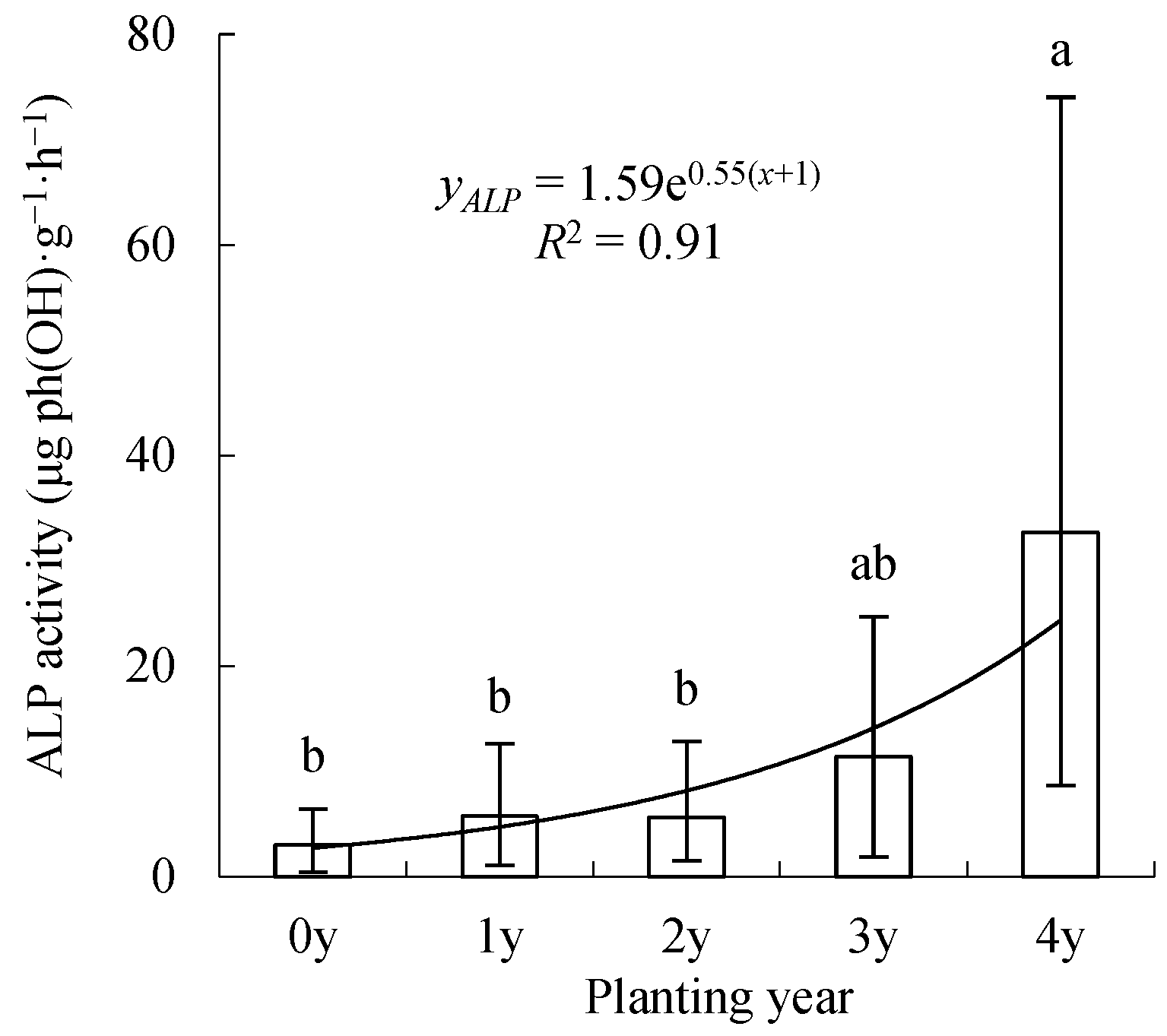
3.2. Spatiotemporal Changes in ALP Activity
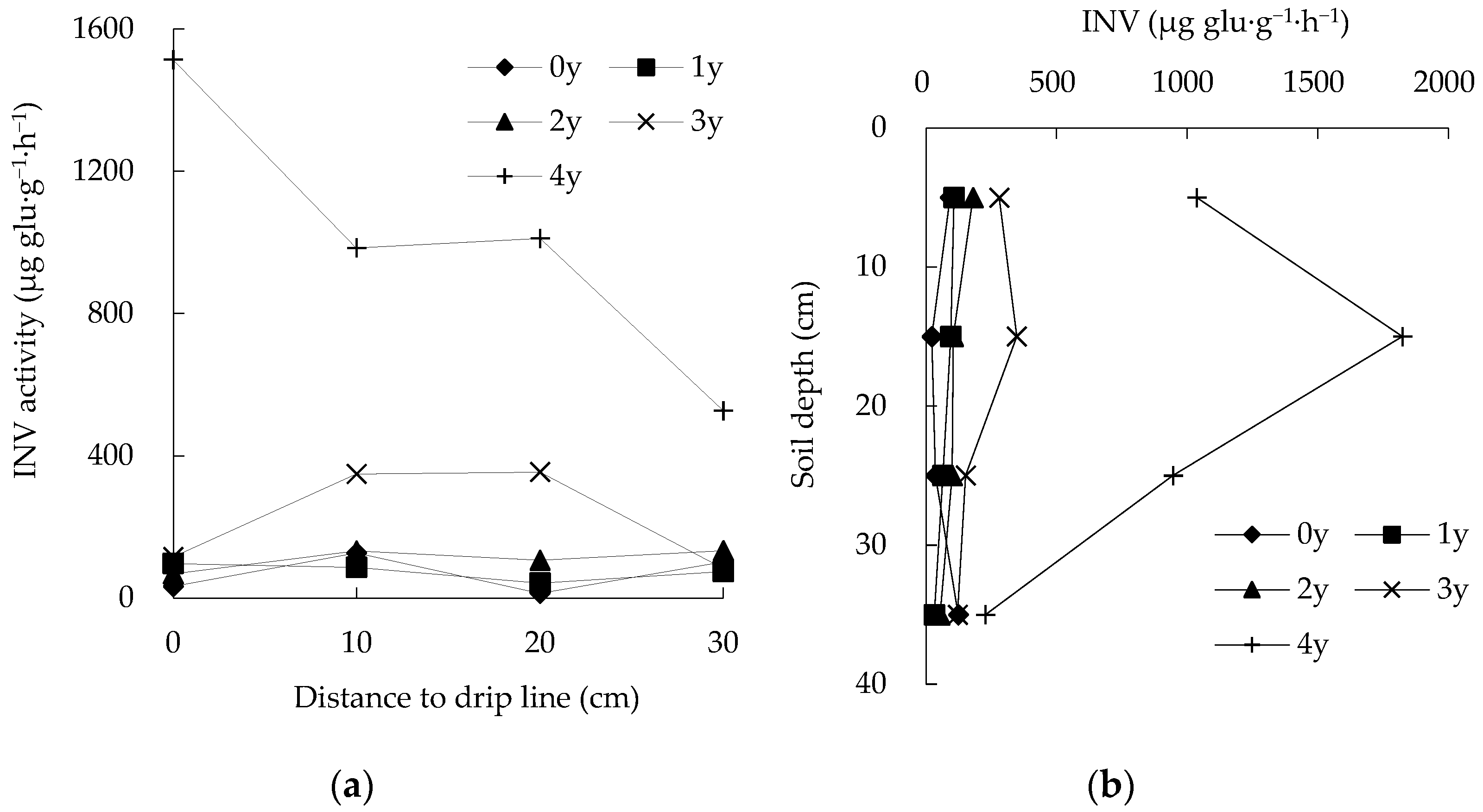
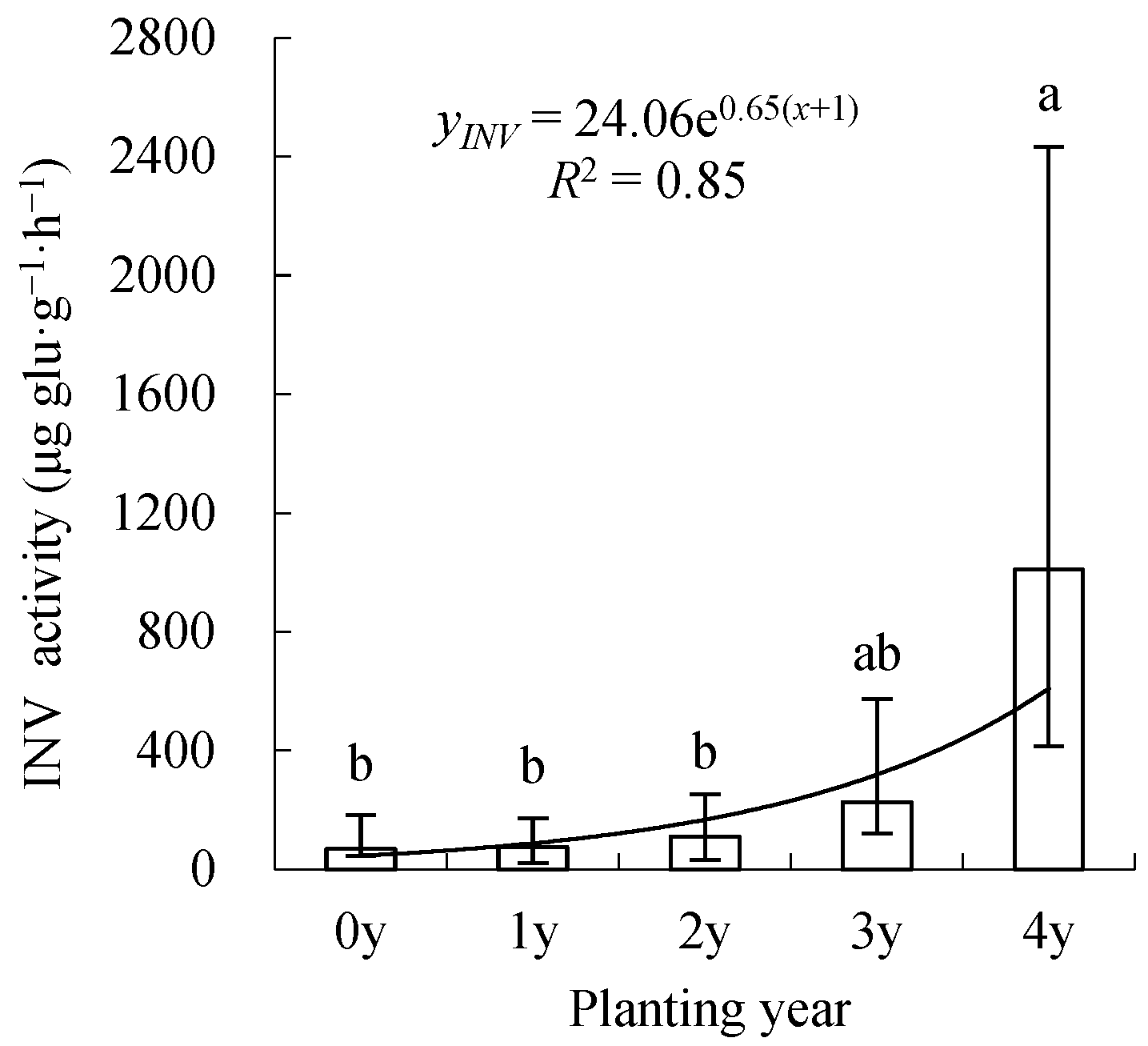
3.3. Spatiotemporal Changes in INV Activity
4. Discussion
4.1. Soil Enzyme Activities
4.2. Urease Enzyme (URE)
4.3. Alkaline Phosphatase (ALP)
4.4. Invertase (INV)
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fan, Z.L.; Ma, Y.J.; Ma, Y.J. Salinized soils and their improvement and utilization in west China. Arid. Zone Res. 2001, 18, 1–6, (In Chinese, with English Abstract). [Google Scholar]
- Fu, H.Q.; Yang, Y.Q. How Plants Tolerate Salt Stress. Curr. Issues Mol. Biol. 2023, 45, 5914–5934. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.P.; Shi, H.F.; Yang, Y.Q.; Feng, X.X.; Chen, X.; Xiao, F.; Lin, H.H.; Guo, Y. Insights into plant salt stress signaling and tolerance. J. Genet. Genom. 2024, 51, 16–34. [Google Scholar] [CrossRef] [PubMed]
- Sidike, A.; Zhao, S.H.; Wen, Y.M. Estimating soil salinity in Pingluo County of China using QuickBird data and soil reflectance spectra. Int. J. Appl. Earth Obs. 2014, 26, 156–175. [Google Scholar] [CrossRef]
- Abhilash, J.E. Rice cultivation in saline tracts of Kerala: An overview. Int. J. Fish. Aquat. Stud. 2016, 4, 355–358. [Google Scholar]
- Wang, J.; Lin, C.Y.; Han, Z.M.; Fu, C.B.; Huang, D.; Cheng, H.G. Dissolved nitrogen in salt-affected soils reclaimed by planting rice: How is it influenced by soil physicochemical properties? Sci. Total Environ. 2022, 824, 153863. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.K.; Shao, T.Y.; Lv, Z.X.; Yue, Y.; Liu, A.H.; Long, X.H.; Zhou, Z.S.; Gao, X.M.; Rengel, Z. The mechanisms of improving coastal saline soils by planting rice. Sci. Total Environ. 2020, 703, 135529. [Google Scholar] [CrossRef] [PubMed]
- Singh, A. Soil salinization management for sustainable development: A review. J. Environ. Manag. 2021, 277, 111383. [Google Scholar] [CrossRef] [PubMed]
- Shokri-Kuehni, S.M.S.; Bernadette, R.; Theresa, K.; Dani, O.; Rainer, H.; Nima, S. Water table depth and soil salinization: From pore-scale processes to field-scale responses. Water Resour. Res. 2020, 56, e2019WR026707. [Google Scholar] [CrossRef]
- Greene, R.; Timms, W.; Rengasamy, P.; Arshad, M.; Cresswell, R. Soil and aquifer salinization: Toward an integrated approach for salinity management of groundwater. In Integrated Groundwater Management: Concepts, Approaches and Challenges; Springer: Cham, Switzerland, 2016; pp. 377–412. [Google Scholar] [CrossRef]
- Chhabra, R. Management and Reclamation of Saline Soils. In Salt-Affected Soils and Marginal Waters; Springer: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Shankar, V.; Evelin, H. Strategies for Reclamation of Saline Soils. In Microorganisms in Saline Environments: Strategies and Functions; Soil Biology; Giri, B., Varma, A., Eds.; Springer: Cham, Switzerland, 2019; Volume 56. [Google Scholar] [CrossRef]
- Rajput, T.B.S.; Patel, N. Water and nitrate movement in drip-irrigated onion under fertigation and irrigation treatments. Agr. Water Manag. 2006, 79, 293–311. [Google Scholar] [CrossRef]
- Li, J.S.; Zhang, J.J.; Ren, L. Water and nitrogen distribution as affected by fertigation of ammonium nitrate from a point source. Irrig. Sci. 2003, 22, 19–30. [Google Scholar] [CrossRef]
- Kahlon, M.S.; Josan, A.S.; Khera, K.L. A comparative study on soil and plant parameters under furrow and drip irrigated tomatoes with poor quality water. Res. Crops. 2008, 9, 61–72. [Google Scholar]
- Li, X.B.; Kang, Y.H.; Wan, S.Q.; Chen, X.L.; Chu, L.L.; Xu, J.C. First and second-year assessments of the rapid reconstruction and re-vegetation method for reclaiming two saline–sodic, coastal soils with drip-irrigation. Ecol. Eng. 2015, 84, 496–505. [Google Scholar] [CrossRef]
- Hussain, M.I.; Farooq, M.; Muscolo, A.; Rehman, A. Crop diversification and saline water irrigation as potential strategies to save freshwater resources and reclamation of marginal soils—A review. Environ. Sci. Pollut. Res. 2020, 27, 28695–28729. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.H.; Chen, M.; Wan, S.Q. Effects of drip irrigation with saline water on waxy maize (Zea mays L. var. ceratina Kulesh) in North China Plain. Agric. Water Manag. 2010, 97, 1303–1309. [Google Scholar] [CrossRef]
- Dou, C.Y.; Kang, Y.H.; Wan, S.Q.; Hu, W. Soil salinity changes under cropping with Lycium barbarum L. and irrigation with saline-sodic water. Pedosphere 2011, 21, 539–548. [Google Scholar] [CrossRef]
- Dong, S.D.; Wan, S.Q.; Kang, Y.H.; Sun, J.X. Effect of different soil matric potentials on distribution of soil salt and growth of Poplar (Populus) by drip irrigation in low-lying saline area. J. Soil Water Conserv. 2017, 31, 236–247. [Google Scholar] [CrossRef]
- Dong, S.D.; Wan, S.; Kang, Y.; Li, X.B. Prospects of using drip irrigation for ecological conservation and reclaiming highly saline soils at the edge of Yinchuan Plain. Agric. Water Manag. 2020, 239, 106255. [Google Scholar] [CrossRef]
- Li, D.; Wan, S.; Li, X.; Kang, Y.; Han, X. Effect of water-salt regulation drip irrigation with saline water on tomato quality in an arid region. Agric. Water Manag. 2022, 261, 107347. [Google Scholar] [CrossRef]
- Dong, S.D.; Wang, G.M.; Kang, Y.H.; Ma, Q.; Wan, S.Q. Soil water and salinity dynamics under the improved drip-irrigation scheduling for ecological restoration in the saline area of Yellow River basin. Agric. Water Manag. 2022, 264, 107255. [Google Scholar] [CrossRef]
- Ghosh, A.; Singh, A.B.; Kumar, R.V.; Manna, M.C.; Bhattacharyya, R.; Rahman, M.M.; Sharma, P.; Rajput, P.S.; Misra, S. Soil enzymes and microbial elemental stoichiometry as bio-indicators of soil quality in diverse cropping systems and nutrient management practices of Indian Vertisols. Appl. Soil Ecol. 2020, 145, 103304. [Google Scholar] [CrossRef]
- Karaca, A.; Cetin, S.C.; Turgay, O.C.; Kizilkaya, R. Soil Enzymes as Indication of Soil Quality. In Soil Enzymology; Soil Biology; Shukla, G., Varma, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; Volume 22. [Google Scholar] [CrossRef]
- Nannipieri, P.; Trasar-Cepeda, C.; Dick, R.P. Soil enzyme activity: A brief history and biochemistry as a basis for appropriate interpretations and meta-analysis. Biol. Fertil. Soils. 2018, 54, 11–19. [Google Scholar] [CrossRef]
- Bowles, T.M.; Acosta-Martínez, V.; Calderón, F.; Jackson, L.E. Soil enzyme activities, microbial communities, and carbon and nitrogen availability in organic agroecosystems across an intensively-managed agricultural landscape. Soil Boil. Biochem. 2014, 68, 252–262. [Google Scholar] [CrossRef]
- Zagal, E.; Munoz, C.; Quiroz, M.; Cordova, C. Sensitivity of early indicators for evaluating quality changes in soil organic matter. Geoderma. 2009, 151, 191–198. [Google Scholar] [CrossRef]
- Burke, D.J.; Weintraub, M.N.; Hewins, C.R.; Kalisz, S. Relationship between soil enzyme activities, nutrient cycling and soil fungal communities in a northern hardwood forest. Soil Boil. Biochem. 2011, 43, 795–803. [Google Scholar] [CrossRef]
- Neemisha; Sharma, S. Soil Enzymes and Their Role in Nutrient Cycling. In Structure and Functions of Pedosphere; Giri, B., Kapoor, R., Wu, Q.S., Varma, A., Eds.; Springer: Singapore, 2022. [Google Scholar] [CrossRef]
- Rahul, R.; Sharma, P.; Singh, A.; Singh, J.; Kumar, M. Soil Enzymes and Their Role in Soil Health Improvement. In Advances in Agricultural and Industrial Microbiology; Nayak, S.K., Baliyarsingh, B., Mannazzu, I., Singh, A., Mishra, B.B., Eds.; Springer: Singapore, 2022. [Google Scholar] [CrossRef]
- Chrost, R.J.; Siuda, W.; Halemejko, G.Z. Long term studies on alkaline phosphatase activity(APA) in a lake with fish-aquaculture in relation to lake eutrophication and phosphorus cycle. Arch. Hydrobiol. Suppl. 1984, 70, 1–32. [Google Scholar]
- Sun, X.L.; Li, Y.Z.; Heinen, M.; Ritzema, H.; Hellegers, P.; Dam, J.V. Fertigation strategies to improve water and nitrogen use efficiency in surface irrigation system in the North China Plain. Agriculture 2023, 13, 17. [Google Scholar] [CrossRef]
- Kumar, C.; Verma, S.B.; Singh, A.K. Sustainable management of soil salinity with special reference to smart fertigation systems. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2023. [Google Scholar] [CrossRef]
- Li, Z.G. Soil and Environmental Microbiology; Science Press: Beijing, China, 2008. [Google Scholar]
- Ji, L.D.; Sun, Q.; Ma, X.Q.; Chu, Y.N.; Shen, R.Q. Soil enzyme activities and spatial variances in Yellow River irrigated area of Ningxia. Ecol. Environ. 2007, 16, 1737–1741, (In Chinese, with English Abstract). [Google Scholar]
- Qiu, L.P.; Liu, J.; Wang, Y.Q.; Sun, H.M.; He, W.X. Research on relationship between soil enzyme activities and soil fertility. Plant Nutr. Fert. Sci. 2004, 10, 277–280, (In Chinese, with English Abstract). [Google Scholar]
- Paz-Ferreiro, J.; Fu, S. Biological indices for soil quality evaluation: Perspectives and limitations. Land Degrad. Dev. 2016, 27, 14–25. [Google Scholar] [CrossRef]
- Waring, B.G.; Weintraub, S.R.; Sinsabaugh, R.L. Ecoenzymatic stoichiometry of microbial nutrient acquisition in tropical soils. Biogeochemistry 2014, 117, 101–113. [Google Scholar] [CrossRef]
- Lange, M.; Eisenhauer, N.; Sierra, C.A.; Bessler, H.; Engels, C.; Griffiths, R.I.; Mellado-Vázquez, P.G.; Malik, A.A.; Roy, J.; Scheu, S.; et al. Plant diversity increases soil microbial activity and soil carbon storage. Nat. Commun. 2015, 6, 6707. [Google Scholar] [CrossRef] [PubMed]
- Spohn, M.; Bagchi, S.; Biederman, L.A.; Borer, E.T.; Bråthen, K.A.; Bugalho, M.N.; Caldeira, M.C.; Catford, J.A.; Collins, S.L.; Eisenhauer, N.; et al. The positive effect of plant diversity on soil carbon depends on climate. Nat. Commun. 2023, 14, 6624. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.P.; Marín-Spiotta, E.; Graaff, M.A.D.; Balser, T.C. Microbial community structure varies across soil organic matter aggregate pools during tropical land cover change. Soil Biol. Biochem. 2014, 77, 292–303. [Google Scholar] [CrossRef]
- Shedeed, S.I.; Zaghloul, S.M.; Yassen, A.A. Effect of method and rate of fertilizer application under drip irrigation on yield and nutrient uptake by tomato. Ozean. J. Appl. Sci. 2009, 2, 139–147. [Google Scholar]
- Tiwari, K.N.; Kumar, M.; Santosh, D.T.; Singh, V.K.; Maji, M.K.; Karan, A.K. Influence of drip irrigation and plastic mulch on yield of sapota (achraszapota) and soil nutrients. Irrig. Drain. Syst. Eng. 2014, 3, 116. [Google Scholar] [CrossRef]
- Wang, L.; Hamel, C.; Lu, P.; Wang, J.; Sun, D.; Wang, Y.; Lee, S.J.; Gan, G.Y. Using enzyme activities as an indicator of soil fertility in grassland -an academic dilemma. Front. Plant Sci. 2023, 14, 1175946. [Google Scholar] [CrossRef]
- Davies, B.; Coulter, J.A.; Pagliari, P.H. Soil enzyme activity behavior after urea nitrogen application. Plants 2022, 11, 2247. [Google Scholar] [CrossRef]
- Wallenstein, M.D.; Mcmahon, S.K.; Schimel, J.P. Seasonal variation in enzyme activities and temperature sensitivities in Arctic tundra soils. Glob. Chang. Biol. 2009, 15, 1631–1639. [Google Scholar] [CrossRef]
- Krajewska, B.; Ureases, I. Functional, catalytic and kinetic properties: A review. J Mol Catal B-Enzym. 2009, 59, 9–21. [Google Scholar] [CrossRef]
- Chen, Y.X.; Wei, T.X.; Sha, G.L.; Zhu, Q.K.; Liu, Z.; Ren, K.; Yang, C. Soil enzyme activities of typical plant communities after vegetation restoration on the Loess Plateau, China. Appl. Soil Ecol. 2022, 170, 104292. [Google Scholar] [CrossRef]
- Mungai, N.W.; Motavalli, P.P.; Kremer, R.J.; Nelson, K.A. Spatial variation of soil enzyme activities and microbial functional diversity in temperate alley cropping systems. Biol. Fert. Soils 2005, 42, 129–136. [Google Scholar] [CrossRef]
- Aon, M.; Colaneri, A., II. Temporal and spatial evolution of enzymatic activities and physico-chemical properties in an agricultural soil. Appl. Soil Ecol. 2001, 18, 255–270. [Google Scholar] [CrossRef]
- Azam, H.M.; Alam, S.T.; Hasan, M.; Yameogo, D.D.S.; Kannan, A.D.; Rahman, A.; Kwon, M.J. Phosphorous in the environment: Characteristics with distribution and effects, removal mechanisms, treatment technologies, and factors affecting recovery as minerals in natural and engineered systems. Environ. Sci. Pollut. Res. 2019, 26, 20183–20207. [Google Scholar] [CrossRef]
- Chen, X.D.; Jiang, N.; Condronc, L.M.; Dunfieldd, K.E.; Chen, Z.H.; Wang, J.K.; Chen, L.J. Soil alkaline phosphatase activity and bacterial phoD gene abundance and diversity under long-term nitrogen and manure inputs. Geoderma 2019, 349, 36–44. [Google Scholar] [CrossRef]
- Zheng, M.M.; Wang, C.; Li, W.X.; Guo, L.; Cai, Z.J.; Wang, B.R.; Chen, J.; Shen, R.F. Changes of acid and alkaline phosphatase activities in long-term chemical fertilization are driven by the similar soil properties and associated microbial community composition in acidic soil. Eur. J. Soil Biol. 2021, 104, 103312. [Google Scholar] [CrossRef]
- Chhabra, S.; Brazil, D.; Morrissey, J.; Burke, J.; O’Gara, F.; Dowling, D.N. Fertilization management affects the alkaline phosphatase bacterial community in barley rhizosphere soil. Biol. Fertil. Soils. 2013, 49, 31–39. [Google Scholar] [CrossRef]
- Lemanowicz, J.; Krzyżaniak, M. Vertical distribution of phosphorus concentrations, phosphatase activity and further soil chemical properties in salt-affected Mollic Gleysols in Poland. Environ. Earth Sci. 2015, 74, 2719–2728. [Google Scholar] [CrossRef]
- Frankenberger, W.T.; Johanson, J.B. Factors affecting invertase activity in soils. Plant Soil 1983, 74, 313–323. [Google Scholar] [CrossRef]
- Pancholy, S.K.; Rice, E.L. Carbohydrases in soil as affected by successional stages of revegetation. Soil Sci. Soc. Am. Proc. 1973, 37, 227–229. [Google Scholar] [CrossRef]
- Sun, H.; Jiang, S.; Jiang, C.; Wu, C.F.; Gao, M.; Wang, Q.H. A review of root exudates and rhizosphere microbiome for crop production. Environ. Sci. Pollut. Res. 2021, 28, 54497–54510. [Google Scholar] [CrossRef] [PubMed]
- Kulshrestha, S.; Tyagi, P.; Sindhi, V.; Yadavilli, K.S. Invertase and its applications—A brief review. J. Pharm. Res. 2013, 7, 792–797. [Google Scholar] [CrossRef]
| Soil Layer cm | pH | ECe dS·m−1 | Major Anions, mmol·L−1 | Major Cations, mmol·L−1 | |||||
|---|---|---|---|---|---|---|---|---|---|
| HCO3− | SO42− | Cl− | K+ | Na+ | Ca2+ | Mg2+ | |||
| 0–10 | 7.5 | 62.3 | 6.0 | 24.5 | 260.6 | 0.5 | 219.0 | 10.0 | 20.3 |
| 10–20 | 7.8 | 13.5 | 8.0 | 1.0 | 22.5 | 0.1 | 21.8 | 1.5 | 5.1 |
| 20–30 | 7.8 | 9.0 | 8.0 | 0.5 | 13.5 | 0.1 | 8.2 | 1.5 | 4.1 |
| 30–40 | 7.8 | 10.2 | 7.5 | 2.0 | 13.5 | 0.0 | 8.2 | 2.0 | 4.1 |
| pH | TDS g·L−1 | Major Anions, mmol·L−1 | Major Cations/mmol·L−1 | |||||
|---|---|---|---|---|---|---|---|---|
| HCO3− | SO42− | Cl− | K+ | Na+ | Ca2+ | Mg2+ | ||
| 7.6 | 7.27 | 7.4 | 15.3 | 79.4 | 0.3 | 96.1 | 3.3 | 7.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dou, C.; Lv, Y.; Sun, Y.; Chen, X.; Li, Y. Assessment of Soil Enzyme Activities in Plant Root Zone of Saline Soil Reclaimed by Drip Irrigation with Saline Groundwater. Agronomy 2024, 14, 1416. https://doi.org/10.3390/agronomy14071416
Dou C, Lv Y, Sun Y, Chen X, Li Y. Assessment of Soil Enzyme Activities in Plant Root Zone of Saline Soil Reclaimed by Drip Irrigation with Saline Groundwater. Agronomy. 2024; 14(7):1416. https://doi.org/10.3390/agronomy14071416
Chicago/Turabian StyleDou, Chaoyin, Yuping Lv, Yidi Sun, Xiaoping Chen, and Yan Li. 2024. "Assessment of Soil Enzyme Activities in Plant Root Zone of Saline Soil Reclaimed by Drip Irrigation with Saline Groundwater" Agronomy 14, no. 7: 1416. https://doi.org/10.3390/agronomy14071416




