Influence of Weather Conditions and the Aphid Population on the Potato Virus Y Infection of Tobacco in the Field
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Meteorological Data
2.3. Collection and Identification of Aphid Flight
2.4. Serological Tests
2.5. Statistical Analysis
3. Results
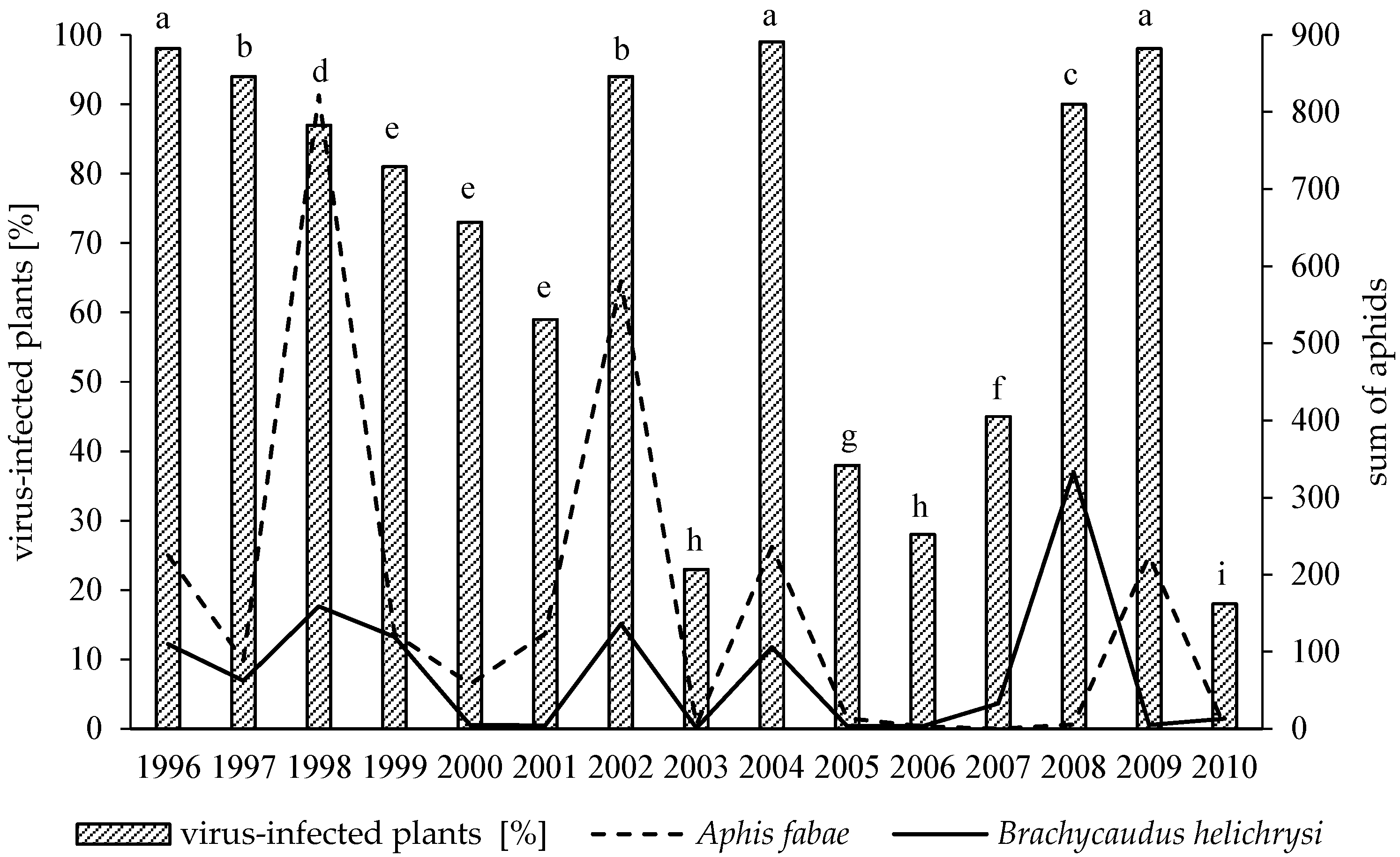
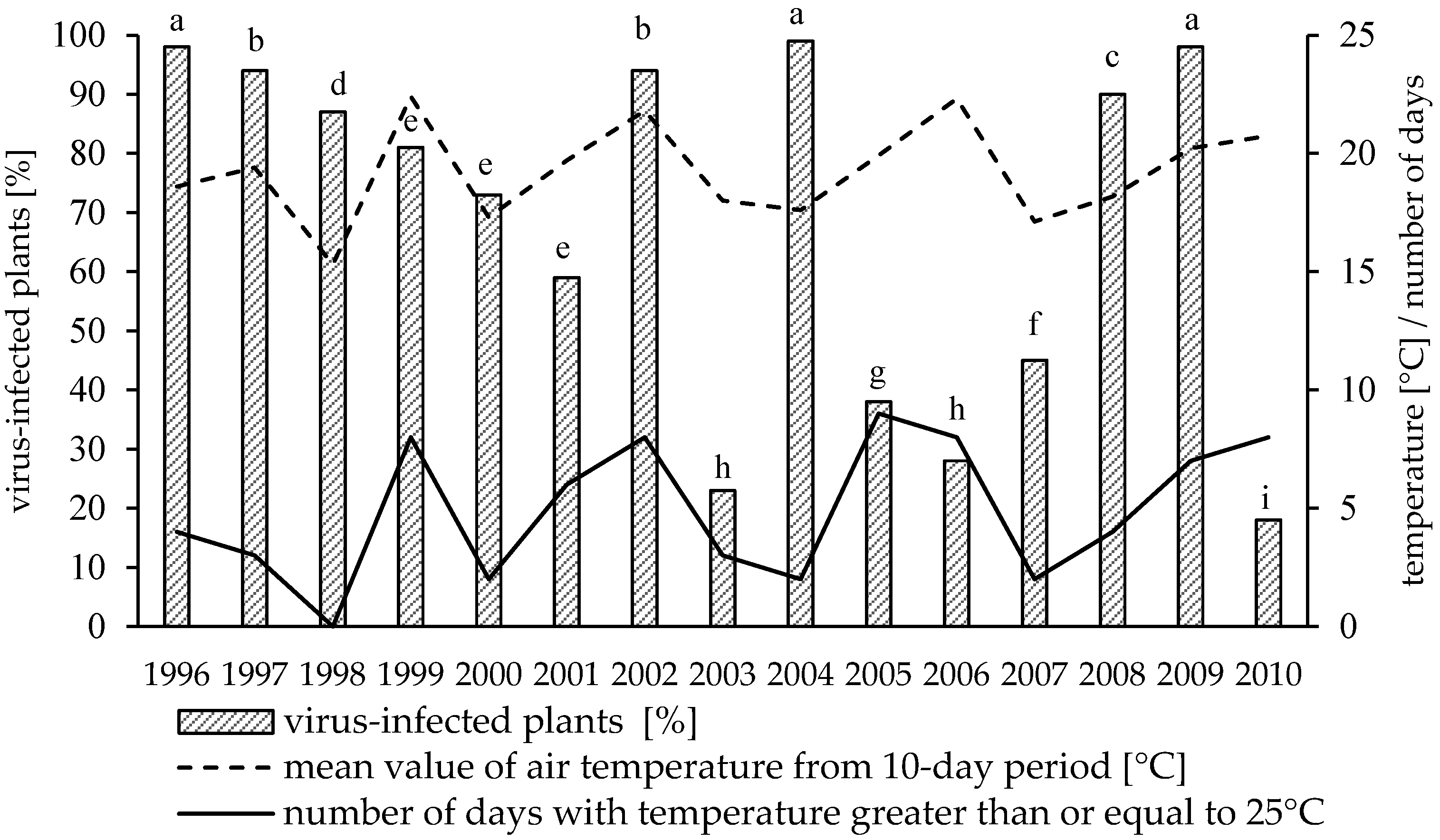
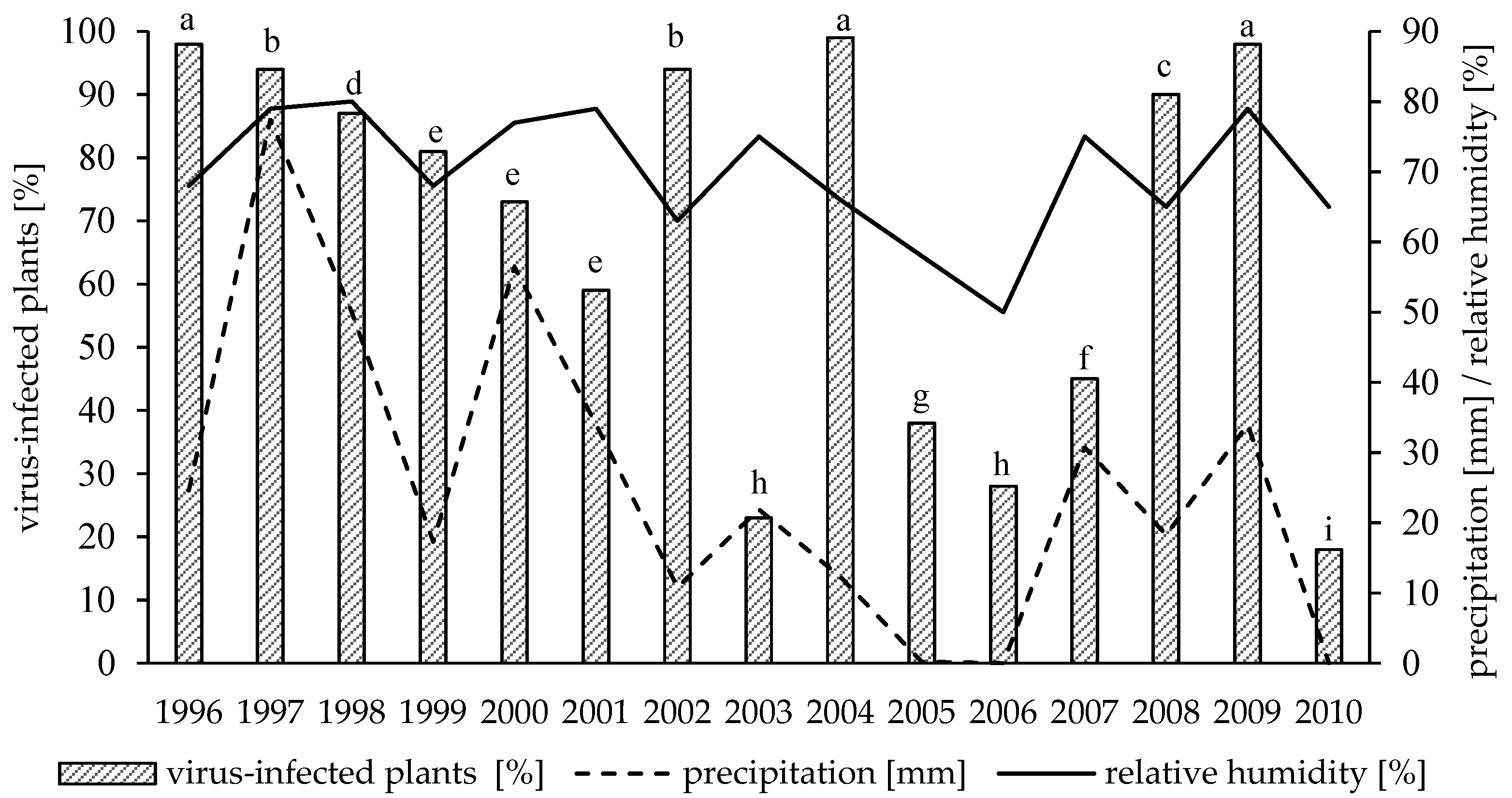
3.1. Variation in Virus Incidence among Years
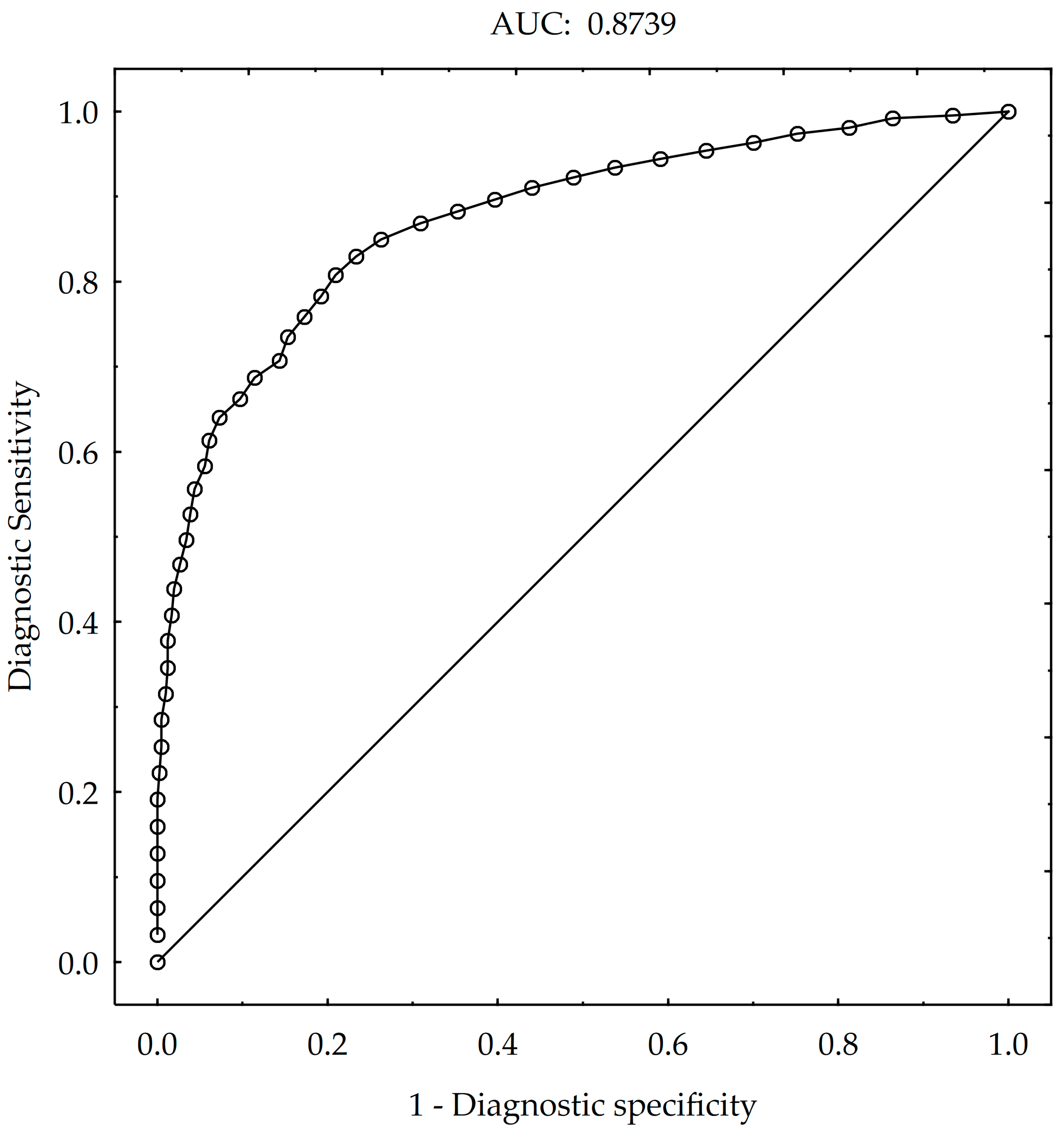
3.2. Effect of Weather Conditions and Aphid Population on Virus Infections Caused by PVY
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Aramburu, J.; Galipienso, L.; Matas, M. Characterization of potato virus Y isolates from tomato crops in northeast Spain. Eur. J. Plant Pathol. 2006, 115, 247–258. [Google Scholar] [CrossRef]
- Nikolaeva, O.V.; Roop, D.J.; Galvino-Costa, S.B.F.; Figueira, A.R.; Gray, S.M.; Karasev, A.V. Epitope Mapping for Monoclonal Antibodies Recognizing Tuber Necrotic Isolates of Potato Virus Y. Am. J. Potato Res. 2012, 89, 121–128. [Google Scholar] [CrossRef]
- DeBokx, J.A.; Huttinga, H. Potato virus Y. CMI/AAB Descriptions of Plant Viruses No.242; Commonwealth Microbiology Institute and Association of Applied Biology: Kew, UK, 1981. [Google Scholar]
- Kaliciak, A.; Syller, J. New hosts of Potato virus Y (PVY) among common wild plants in Europe. Eur. J. Plant Pathol. 2009, 124, 707–713. [Google Scholar] [CrossRef]
- Fuentes, S.; Jones, R.A.C.; Matsuoka, H.; Ohshima, K.; Kreuze, J.; Gibbs, A.J. Potato virus Y; the Andean connection. Virus Evol. 2019, 5, vez037. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.M. Composite nature of certain potato viruses of the mosaic group. Nature 1931, 127, 702. [Google Scholar] [CrossRef]
- Smith, K.M.; Dennis, R.W.G. Some notes on a suspected variant of solanum virus 2 (potato virus Y). Ann. Appl. Biol. 1940, 27, 65–70. [Google Scholar] [CrossRef]
- Shukla, D.D.; Ward, C.W.; Brunt, A.A. The Potyviridae; CAB International: Wallingford, UK, 1994; 516p. [Google Scholar]
- Choi, H.-S.; Bhat, H.-I.; Park, J.-W.; Cheon, J.-U.; Kim, J.-S.; Pappu, H.-R.; Choi, J.-K.; Takanami, Y. Studies on Potato virus Y isolates infecting potato and tobacco in Korea. J. Fac. Agric. Kyushu Univ. 2004, 49, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Jacquot, E.; Tribodet, M.; Croizat, F.; Balme-Sinibaldi, V.; Kerlan, C. A single nucleotide polymorphism-based technique for specific characterization of YO and YN isolates of Potato virus Y (PVY). J. Virol. Methods 2005, 125, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Van der Zaag, D.E. Yield reduction in relation to virus infection. In Viruses of Potatoes and Seed-Potato Production, 2nd ed.; De Bokx, J.A., van des Want, J.P.H., Eds.; Pudoc: Wageningen, The Netherlands, 1987; pp. 149–150. [Google Scholar]
- Latore, B.A.; Flores, V.; Marholz, G. Effect of Potato virus Y on growth, yield and chemical composition of flue-cured tobacco in Chile. Plant Dis. 1984, 68, 884–886. [Google Scholar] [CrossRef]
- Valkonen, J.P.T. Viruses: Economical losses and biotechnological potential. In Potato Biology and Biotechnology: Advances and Perspectives; Elsevier BV: Amsterdam, The Netherlands, 2007; pp. 619–641. [Google Scholar]
- Dupuis, B.; Nkuriyingoma, P.; Ballmer, T. Economic Impact of Potato Virus Y (PVY) in Europe. Potato Res. 2024, 67, 55–72. [Google Scholar] [CrossRef]
- McIntosh, C. The economics of PVY. In Proceedings of the Idaho Potato Conferences, Pocatello, ID, USA, 22–24 January 2014. [Google Scholar]
- Powell, A.P.; Nelson, R.S.; Barun de Hoffmann, N.; Rogers, S.G.; Fraley, R.T.; Beachy, R.N. Delay of disease development in transgenic plants that express the tobacco mosaic virus coat protein gene. Science 1986, 232, 738–743. [Google Scholar]
- Carrington, J.C.; Freed, D.D. Cap-independent enhancement of translation by a plant potyvirus 5’ non translated region. J. Virol. 1990, 64, 1590–1597. [Google Scholar] [CrossRef] [PubMed]
- Verchot, J.M.; Koonin, E.V.; Carrington, J.C. The 35-kDa protein from the N-terminus of a potyviral polyprotein functions as a third virus encoded proteinase. Virology 1991, 185, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Oh, C.S.; Carrington, J.C. Identification of essential residues in potyvirus proteinase HC-Pro by site-directed mutagenesis. Virology 1989, 173, 692–724. [Google Scholar] [CrossRef] [PubMed]
- Atreya, C.D.; Atreya, P.L.; Thornbury, D.W.; Pirone, T.P. Site-directed mutations in the potyvirus HC-Pro gene affect helper component activity, virus accumulation and symptom expression in infected tobacco plants. Virology 1992, 191, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Atreya, C.D.; Pirone, T.P. Mutational analysis of the helper component-proteinase gene of a potyvirus: Effects of amino acid substitutions, deletions and gene replacement on virulence and aphid transmissibility. Proc. Natl. Acad. Sci. USA 1993, 90, 11919–11923. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.Y.; Ammar, E.D.; Thornbury, D.W.; Lopez-Moya, J.J.; Pirone, T.P. Loss off potyvirus transmissibility and helper-component activity correlate with non-retention of virions in aphid stylets. J. Gen. Virol. 1996, 77, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Blanc, S.; Lopez-Moya, J.J.; Pirone, T.P. La Transmission par Pucerons du Tobacco Vein Mottling Virus Necessite une Interaction Entre HC-Pro et la Protéine de Capside; Résumés, 48; Sixiemes Rencontres de Virologie Végétale: Aussois, France, 1997. [Google Scholar]
- Rojas, M.R.; Zerbini, F.M.; Allison, R.F.; Gilbertson, R.L.; Lucas, W.J. Capsid protein and helper component-proteinase function as potyvirus cell-to-cell movement proteins. Virology 1997, 237, 283–295. [Google Scholar] [CrossRef]
- Cronin, S.; Verchot, J.; Haldeman-Cahill, R.; Schaad, M.C.; Carrington, J.C. Long-distance movement factor: A transport function of the potyvirus helper component proteinase. Plant Cell 1995, 7, 549–559. [Google Scholar]
- Saenz, P.; Salvador, B.; Simon-Mateo, C.; Kasschau, K.D.; Carrington, J.C.; Garcia, J.A. Host-specific involvement of the HC protein in the long-distance movement of potyviruses. J. Virol. 2002, 76, 1922–1931. [Google Scholar] [CrossRef]
- Dolja, V.V.; Haldemann-Cahill, R.; Montgomery, A.E.; Vandenbosch, K.A.; Carrington, J.C. Capsid proteins determinants involved in cell-to-cell and long distance movement of tobacco etch potyvirus. Virology 1995, 206, 1007–1016. [Google Scholar] [CrossRef] [PubMed]
- Roudet-Tavert, G.; Grerman-Retana, S.; Delaunay, T.; Delecolle, B.; Candresse, T.; Le Gall, O. Interaction between potyvirus helper component-proteinase and capsid protein in infected plants. J. Gen. Virol. 2002, 83, 1765–1770. [Google Scholar] [CrossRef] [PubMed]
- Flasinski, S.; Cassidy, B.G. Potyvirus aphid transmission requires helper component and homologous coat protein for maximal efficiency. Arch. Virol. 1998, 143, 2159–2172. [Google Scholar] [CrossRef]
- Shand, K.; Theodoropoulos, C.; Stenzel, D.; Dale, J.L.; Harrison, M.D. Expression of potato virus Y cytoplasmic inclusion protein in tobacco results in disorganization of parenchyma cells, distortion of epidermal cells and induces mitochondrial and chloroplast abnormalities, formation of membrane whorls and atypical lipid accumulation. Micron 2009, 40, 730–736. [Google Scholar] [PubMed]
- Shahabuddin, M.; Shaw, J.G.; Rhoads, R.E. Mapping of the tobacco vein mottling virus VPg cistron. Virology 1988, 173, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Barrett, A.J.; Rawlings, N.D. Families and clans of cysteine peptidases. Perspect. Drug Discov. Des. 1996, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Koonin, E.V. The phylogeny of RNA-dependent RNA polymerases of positive-strand RNA viruses. J. Gen. Virol. 1991, 72, 2197–2206. [Google Scholar] [CrossRef]
- Chai, M.; Li, L.; Li, Y.; Yang, Y.; Wang, Y.; Jiang, X.; Luan, Y.; Li, F.; Cui, H.; Wang, A.; et al. The 6-kilodalton peptide 1 in plant viruses of the family Potyviridae is a viroporin. Proc. Natl. Acad. Sci. USA 2024, 121, 21. [Google Scholar] [CrossRef]
- Tomimura, K.; Špak, J.; Katis, N.; Jenner, C.E.; Walsh, J.A.; Gibbs, A.; Ohshima, K. Comparisons of the genetic structure of populations of turnip mosaic virus in west and east Eurasia. Virology 2004, 330, 408–423. [Google Scholar] [CrossRef]
- Merits, A.; Rajamäki, M.L.; Lindholm, P.; Runeberg-Roos, P.; Kekarainen, T.; Puustinen, P.; Mäkeläinen, K.; Valkonen, J.P.T.; Saarma, M. Proteolytic processing of potyviral proteins and polyprotein processing intermediates in insect and plant cells. J. Gen. Virol. 2002, 83, 1211–1221. [Google Scholar] [CrossRef]
- Verchot, J.; Carrington, J.C. Debilitation of plant potyvirus infectivity by P1 proteinase-inactivating mutations and restoration by second-site modifications. J. Virol. 1995, 69, 1582–1590. [Google Scholar] [CrossRef] [PubMed]
- Brantley, J.D.; Hunt, A.G. The N-terminal protein of the polyprotein encoded by by the potyvirus tobacco vein mottling virus is an RNA-binding protein. J. Gen. Virol. 1993, 74, 1157–1162. [Google Scholar] [CrossRef] [PubMed]
- Klein, P.G.; Klein, R.R.; Rodriguez-Cerezo, E.; Hunt, A.G.; Shaw, J.G. Mutational analysis of the tobacco vein mottling virus genome. Virology 1994, 204, 759–769. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.T.; Cervera, M.T.; García, J.A.; Bonay, P. Properties of the active plum pox potyvirus RNA polymerase complex in defined glycerol gradient fractions. Virus Res. 1995, 37, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Coutts, B.A.; Jones, R.A. Potato virus Y: Contact transmission, stability, inactivation, and infection sources. Plant Dis. 2015, 100, 387–394. [Google Scholar] [CrossRef]
- Singh, R.P.; Valkonen, J.P.; Gray, S.M.; Boonham, N.; Jones, R.A.; Kerlan, C.; Schubert, J. Discussion paper: The naming of Potato virus Y strains infecting potato. Arch. Virol. 2008, 153, 1–13. [Google Scholar] [CrossRef]
- Kehoe, M.A.; Jones, R.A.C. Improving Potato virus Y strain nomenclature: Lessons from comparing isolates obtained over a 73-year period. Plant Path 2016, 65, 322–333. [Google Scholar] [CrossRef]
- Jones, R.A.C.; Vincent, S.J. Strain-specific hypersensitive and extreme resistance phenotypes elicited by Potato virus Y among 39 potato cultivars released in three world regions over a 11-year period. Plant Dis. 2018, 102, 185–196. [Google Scholar] [CrossRef]
- Przybyś, M.; Doroszewska, T.; Berbeć, A. Point mutation in the viral genome-linked protein (VPg) of Potato virus Y probably correspond with ability to overcome resistance of tobacco. J. Food Agric. Environ. 2013, 11, 132–135. [Google Scholar]
- Verrier, J.L.; Marchand, V.; Cailleteau, B.; Delon, R. Chemical change and cigarette smoke mutagenicity increase associated with CMV-DTL and PVY-N infection in burley tobacco. In Proceedings of the Cooperation Centre for Scientific Research Relative to Tobacco Meeting Agro-Phyto Groups, Cape Town, South Africa, 30 September–4 October 2001. [Google Scholar]
- Manasseh, R.; Berim, A.; Kappagantu, M.; Moyo, L.; Gang, D.R.; Pappu, H.R. Pathogen-triggered metabolic adjustments to potato virus Y infection in potato. Front. Plant Sci. 2023, 13, 1031629. [Google Scholar] [CrossRef]
- Close, R. Some effects of other viruses and of temperature on the multiplication of potato virus X. Ann. Appl. Biol. 1964, 53, 151–164. [Google Scholar] [CrossRef]
- DeBokx, J.A.; Piron, P.G.M. Effect of temperature on symptom expression and relative virus concentration in potato plants infected with potato virus YN and YO. Potato Res. 1977, 20, 207–213. [Google Scholar] [CrossRef]
- Del Torro, F.J.; Aguilar, E.; Hernandez-Wallas, F.J.; Tenellado, F.; Chung, B.-N.; Canto, T. High temperature, high ambient CO2, affect the interactions between three positive-sense RNA viruses and a compatible host differentially, but not their silencing suppression efficiencies. PLoS ONE 2015, 10, e0136062. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.N.; Khurana, S.M.P.; Nagaich, B.B.; Agrawal, H.O. Environmental factors influencing aphid transmission of potato virus Y and potato leafroll virus. Potato Res. 1988, 31, 501–509. [Google Scholar] [CrossRef]
- Glasa, M.; Labonne, G.; Quiot, J.B. Effect of temperature on plum pox virus infection. Acta Virol. 2003, 47, 49–52. [Google Scholar] [PubMed]
- Chung, B.N.; Canto, T.; Tenllado, F.; Choi, K.S.; Joa, J.H.; Ahn, J.J.; Kim, C.H.; Do, K.S. The effects of high temperature on infection by Potato virus Y, Potato virus A and Potato leafroll virus. Plant Pathol. J. 2016, 32, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Matthews, R.E.F. Plant Virology, 3rd ed.; Elsevier BV: Amsterdam, The Netherlands, 1991; 864p. [Google Scholar]
- Doroszewski, A. Warunki termiczne i opadowe w Puławach w latach z ekstremalnymi suszami rolniczymi w okresie 2006–2022. Studia i Raporty IUNG-PIB 2023, 71, 9–25. [Google Scholar]
- IPCC. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013; 1535p. [Google Scholar]
- Jones, R.A.C. Plant virus emergence and evolution: Origins, new encounter scenarios, factors driving emergence, effects of changing world conditions, and prospects for control. Virus Res. 2009, 141, 113–130. [Google Scholar] [CrossRef]
- Jones, R.A.C. Future Scenarios for Plant Virus Pathogens as Climate Change Progresses. Adv. Virus Res. 2016, 95, 87–147. [Google Scholar]
- Van Harten, A. The relation between aphid flights and the spread of Potato virus YN(PVYN) in the Netherlands. Potato Res. 1983, 26, 1–15. [Google Scholar] [CrossRef]
- Verbeek, M.; Piron, P.; Dullemans, A.; Cuperus, C.; van derVlugt, R. Determination of aphid transmission efficiencies for N, NTN and Wilga strains of Potato virus Y. Ann. Appl. Biol. 2010, 156, 39–49. [Google Scholar] [CrossRef]
- Basky, Z. The relationship between aphid dynamics and two prominent potato viruses (PVY and PLRV) in seed potatoes in Hungary. Crop Prot. 2002, 21, 823–827. [Google Scholar] [CrossRef]
- Basky, Z. Cumulative vector intensity and seed potato virus infection in Hungary. Int. J. Hortic. Sci. 2006, 12, 61–64. [Google Scholar] [CrossRef]
- Northing, P. Extensive field based aphid monitoring asan information tool for the UK seed potato industry. Asp. Appl. Biol. 2009, 94, 31–34. [Google Scholar]
- Kirchner, S.M.; Döring, T.F.; Hiltunen, L.H.; Virtanen, E.; Valkonen, J.P.T. Information-theory-based model selection for determining the main vector and period of transmission of Potato virus Y. Ann. Appl. Biol. 2011, 159, 414–427. [Google Scholar] [CrossRef]
- Steinger, T.; Goy, G.; Gilliand, H.; Hebeisen, T.; Derron, J. Forecasting virus disease in seed potatoes using flight activity data of aphid vectors. Ann. Appl. Biol. 2015, 166, 410–419. [Google Scholar] [CrossRef]
- Nemecek, T. The Role of Aphid Behaviour in the Epidemiology of Potato Virus Y: A Simulation Study. Ph.D. Thesis, ETH Zurich, Zurich, Switzerland, 1993. [Google Scholar]
- Robert, Y.; Woodford, J.A.T.; Ducray-Bourdin, D.G. Some epidemiological approaches to the control of aphid-borne virus diseases in seed potato crops in northern Europe. Virus Res. 2000, 71, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Boquel, S.; Delayen, C.; Couty, A.; Giordanengo, P.; Ameline, A. Modulation of aphid vector activity by potato virus Y on in vitro potato plants. Plant Dis. 2012, 96, 82–86. [Google Scholar] [CrossRef]
- Cambra, M.; Vidal, E. Sharka, a vector-borne disease caused by plum pox virus: Vector species, transmission mechanism, epidemiology and mitigation strategies to reduce its natural spread. Acta Hortic. 2017, 1163, 57–68. [Google Scholar] [CrossRef]
- Dupuis, B. The movement of potato virus Y (PVY) in the vascular system of potato plants. Eur. J. Plant Pathol. 2017, 147, 365–373. [Google Scholar] [CrossRef]
- Gadhave, K.R.; Gautam, S.; Rasmussen, D.A.; Srinivasan, R. Aphid Transmission of Potyvirus: The Largest Plant-Infecting RNA Virus Genus. Viruses 2020, 12, 773. [Google Scholar] [CrossRef]
- Jeger, M.J. The Epidemiology of Plant Virus Disease: Towards a New Synthesis. Plants 2020, 9, 1768. [Google Scholar] [CrossRef] [PubMed]
- Ruffel, S.; Gallois, J.L.; Lesage, M.L.; Caranta, C. The recessive potyvirus resistance gene pot-1 is the tomato orthologue of the pepper pvr2-eIF4E gene. Mol. Genet. Genom. 2005, 274, 346–353. [Google Scholar] [CrossRef]
- Hashimoto, M.; Neriya, Y.; Yamaji, Y.; Namba, S. Recessive Resistance to Plant Viruses: Potential Resistance Genes Beyond Translation Initiation Factors. Front. Microbiol. 2016, 7, 1695. [Google Scholar] [CrossRef]
- Michel, V.; Julio, E.; Candresse, T.; Cotucheau, J.; Decorps, C.; Volpatti, R.; Moury, B.; Glais, L.; Jacquot, E.; de Borne, F.D.; et al. A complex eIF4E locus impacts the durability of va resistance to Potato virus Y in tobacco. Mol. Plant Pathol. 2019, 20, 1051–1066. [Google Scholar] [CrossRef]
- Doroszewska, T. Transfer of tolerance to different Potato virus Y (PVY) isolates from Nicotiana africana Merxm. to Nicotiana tabacum L. Plant Breed. 2009, 129, 76–81. [Google Scholar] [CrossRef]
- Korbecka-Glinka, G.; Czubacka, A.; Depta, A.; Doroszewska, T. Inheritance of Potato virus Y tolerance introgressed from Nicotiana africana to cultivated tobacco. Pol. J. Agron. 2017, 31, 39–44. [Google Scholar]
- Le, N.T.; Tran, H.T.; Bui, T.P.; Nguyen, G.T.; Van Nguyen, D.; Ta, D.T.; Trinh, D.D.; Molnar, A.; Pham, N.B.; Chu, H.H.; et al. Simultaneously induced mutations in eIF4E genes by CRISPR/Cas9 enhance PVY resistance in tobacco. Sci. Rep. 2022, 12, 14627. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.X.; Tian, Y.P.; Ren, L.L. A natural substitution of a conserved amino acid in eIF4E confers resistance against multiple potyviruses. Mol. Plant Pathol. 2024, 25, e13418. [Google Scholar] [CrossRef]
- Thieme, T.; Steiner, H.; Busch, T. Vergleich der Blattlausfänge in verschiedenen Gelbschalen. Nachrichtenblatt Dtsch. Pflanzenschutzd. 1994, 46, 65–68. [Google Scholar]
- Chittka, L.; Döring, T.F. Are autumn foliage colors red signals to aphids? PLoS Biol. 2007, 5, e187. [Google Scholar] [CrossRef]
- Döring, T.F.; Archetti, M.; Hardie, J. Autumn leaves seen through herbivore eyes. Proc. R. Soc. Lond. B Biol. 2009, 276, 121–127. [Google Scholar] [CrossRef]
- Heie, O.E. The Aphidoidea (Hemiptera) of Fennoscandia and Denmark. I. General Part. The Families Mindaridae, Hormaphididae, Thelaxidae, Anoeciidae, and Pemphigidae; Scandinavian Science Press: Klampenborg, Denmark, 1980; 236p. [Google Scholar]
- Heie, O.E. The Aphidoidea (Hemiptera) of Fennoscandia and Denmark. II. Family Drepanosiphidae; Scandinavian Science Press: Klampenborg, Denmark, 1982; 176p. [Google Scholar]
- Heie, O.E. The Aphidoidea (Hemiptera) of Fennoscandia and Denmark. III. Family Aphididae: Pterocommatinae and Tribe Aphidini of Subfamily Aphidinae; Brill: Leiden, The Netherlands, 1986; 314p. [Google Scholar]
- Heie, O.E. The Aphidoidea (Hemiptera) of Fennoscandia and Denmark. IV. Family Aphididae: Part 1 of Tribe Macrosiphini of Subfamily Aphidinae; Brill: Leiden, The Netherlands, 1992; 189p. [Google Scholar]
- Heie, O.E. The Aphidoidea (Hemiptera) of Fennoscandia and Denmark. V. Family Aphididae: Part 2 of Tribe Macrosiphini of Subfamily Aphidinae; Brill: Leiden, The Netherlands, 1994; 242p. [Google Scholar]
- Heie, O.E. The Aphidoidea (Hemiptera) of Fennoscandia and Denmark. VI. Family Aphididae: Part 3 of Tribe Macrosiphini of Subfamily Aphidinae and Family Lachnidae; Brill: Leiden, The Netherlands, 1995; 222p. [Google Scholar]
- Taylor, L.R. A Handbook for Aphid Identification; Rothamsted Experimental Station: Harpenden, UK, 1984; 171p. [Google Scholar]
- Clark, M.F.; Adams, A.N. Characteristic of the microplate of enzyme-linked immunosorbent assay for the detection of plant viruses. J. Gen. Virol. 1977, 34, 475–483. [Google Scholar] [CrossRef]
- Hosmer, D.W.; Lemeshow, S. Applied Logistic Regression, 2nd ed.; John Wiley & Sons Inc.: New York, NY, USA, 2000. [Google Scholar]
- Bewick, V.; Cheek, L.; Ball, J. Statistics review 14: Logistic regression. Crit. Care 2005, 9, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.J.; So, T.H. Logistic regression analysis and reporting: A primer. Underst. Stat. 2002, 1, 31–70. [Google Scholar] [CrossRef]
- Song, S.W. Using the receiver operating characteristic curve to measure sensitivity and specificity. Korean J. Fam. Med. 2009, 30, 841–842. [Google Scholar] [CrossRef]
- Bewick, V.; Cheek, L.; Ball, J. Statistics review 13: Receiver operating characteristic curves. Crit. Care 2004, 8, 508–512. [Google Scholar] [CrossRef]
- Peel, M.C.; Finlayson, B.L.; McMahon, T.A. Updated world map of the Köppen-Geiger climate classification. Hydrol. Earth Syst. Sci. 2007, 11, 1633–1644. [Google Scholar] [CrossRef]
- Arnfield, A.J. Köppen Climate Classification. Encyclopedia Britannica. 28 May 2024. Available online: https://www.britannica.com/science/Koppen-climate-classification (accessed on 16 July 2024).
- Chellappan, P.; Vanitharani, R.; Ogbe, F.; Fauquet, C.M. Effect of temperature on geminivirus-induced RNA silencing in plants. Plant Physiol. 2005, 138, 1828–1841. [Google Scholar] [CrossRef]
- Szittya, G.; Silhavy, D.; Molnár, A.; Havelda, Z.; Lovas, Á.; Lakatos, L.; Bánfalvi, Z.; Burgyán, J. Low temperature inhibits RNA silencing-mediated defence by the control of siRNA generation. EMBO J. 2003, 22, 633–640. [Google Scholar] [CrossRef]
- Choi, K.S.; Del Toro, F.; Tenllado, F.; Canto, T.; Nam Chung, B. A Model to Explain Temperature Dependent Systemic Infection of Potato Plants by Potato virus Y. Plant Pathol. J. 2017, 33, 206–211. [Google Scholar] [CrossRef]
- Tsai, W.-A.; Brosnan, C.A.; Mitter, N.; Dietzgen, R.G. Perspectives on plant virus diseases in a climate change scenario of elevated temperatures. Stress Biol. 2022, 2, 37. [Google Scholar] [CrossRef] [PubMed]
- Hunjan, M.S.; Lore, J.S. Climate Change: Impact on Plant Pathogens, Diseases and Their Management. In Crop Protection under Changing Climate; Jabran, K., Florentine, S., Chauhan, B.S., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 85–100. [Google Scholar]
- Jeger, M.J.; Madden, L.V.; van den Bosch, F. Plant virus epidemiology: Applications and prospects for mathematical modeling and analysis to improve understanding and disease control. Plant Dis. 2018, 102, 837–854. [Google Scholar] [CrossRef] [PubMed]
- Korbecka-Glinka, G.; Przybyś, M.; Feledyn-Szewczyk, B.A. Survey of Five Plant Viruses in Weeds and Tobacco in Poland. Agronomy 2021, 11, 1667. [Google Scholar] [CrossRef]
- Chappell, T.M.; Beaudoin, A.L.P.; Kennedy, G.G. Interacting virus abundance and transmission intensity underlie tomato spotted wilt virus incidence: An example weather-based model for cultivated tobacco. PLoS ONE 2013, 8, e73321. [Google Scholar] [CrossRef] [PubMed]
- Steinger, T.; Gilliand, H.; Hebeisen, T. Epidemiological analysis of risk factors for the spread of potato viruses in Switzerland. Ann. Appl. Biol. 2014, 164, 200–207. [Google Scholar] [CrossRef]
- Beemster, A.B.R. Translocation of the potato viruses YN and YO in some potato varieties. Potato Res. 1976, 19, 169–172. [Google Scholar] [CrossRef]
- Lindner, K.; Trautwein, F.; Kellermann, A.; Bauch, G. Potato virus Y (PVY) in seed potato certification. J. Plant Dis. Prot. 2015, 122, 109–119. [Google Scholar] [CrossRef]
- Przybyś, M. Characterization and the Occurrence of PVY in the Main Tobacco Growing Areas in Poland. Ph.D. Thesis, Institute of Soil Science and Plant Cultivation—State Research Institute, Puławy, Poland, 2011. [Google Scholar]
- Doroszewska, T.; Depta, A. Resistance of wild Nicotiana species to different PVY isolates. Phytopathologia 2011, 59, 9–24. [Google Scholar]
- Rigotti, S.; Balmelli, C.; Gugerli, P. Census Report of the Potato Virus Y (PVY) Population in Swiss Seed Potato Production in 2003 and 2008. Potato Res 2011, 54, 105–117. [Google Scholar] [CrossRef]
- Draper, M.D.; Pasche, J.S.; Gudmestad, N.C. Factors influencing PVY development and disease expression in three potato cultivars. Am. J. Potato Res. 2002, 79, 155–165. [Google Scholar] [CrossRef]
- Valkonen, J.P.T.; Rokka, V.M. Combination and expression of two virus resistance mechanisms in interspecific somatic hybrids of potato. Plant Sci. 1998, 131, 85–94. [Google Scholar] [CrossRef]
- Amari, K.; Huang, C.; Heinlein, M. Potential impact of global warming on virus propagation in infected plants and agricultural productivity. Front. Plant Sci. 2021, 12, 478. [Google Scholar] [CrossRef] [PubMed]
- Radcliffe, E.B.; Ragsdale, D.W. Aphid-transmitted potato viruses: The importance of understanding vector biology. Am. J. Potato Res. 2002, 79, 353–386. [Google Scholar] [CrossRef]
- Van Hoof, H.A. Determination of the infection pressure of potato virus YN. Eur. J. Plant Pathol. 1977, 83, 123–127. [Google Scholar]
- Rydén, K.; Brishammar, S.; Sigvald, R. The infection pressure of potato virus YO and the occurrence of winged aphids in potato fields in Sweden. Potato Res. 1983, 26, 229–235. [Google Scholar] [CrossRef]
- Sigvald, R. Relationship between aphid occurrence and spread of potato virus Yo (PVY) in field experiments in southern Sweden. J. Appl. Entomol. 1989, 108, 35–43. [Google Scholar] [CrossRef]
- Kirchner, S.M.; Hiltunen, L.; Döring, T.F.; Virtanen, E.; Palohuhta, J.P.; Valkonen, J.P.T. Seasonal phenology and species composition of the aphid fauna in a northern crop production area. PLoS ONE 2013, 8, e71030. [Google Scholar] [CrossRef]
- DiFonzo, C.D.; Ragsdale, D.W.; Radcliffe, E.B.; Gudmestad, N.C.; Secor, G.A. Seasonal abundance of aphid vectors of potato virus Y in the Red River Valley of Minnesota and North Dakota. J. Econ. Entomol. 1997, 90, 824–831. [Google Scholar] [CrossRef]
- Heimbach, U.; Thieme, T.; Weidemann, H.L.; Thieme, R. Transmission of potato virus Y by aphid species which do not colonise potatoes. In Aphids in Natural and Managed Ecosystems; Dixon., A.F.G., Ed.; Universidad de León: León, Spain, 1998; pp. 555–559. [Google Scholar]
- Woodford, J.A.T. Virus transmission by aphids in potato crops. Neth. J. Plant Pathol. 1992, 98, 47–54. [Google Scholar] [CrossRef]
- MacKenzie, T.D.B.; Fageria, M.S.; Nie, X. Effects of crop management practices on current-season spread of potato virus Y. Plant Dis. 2014, 98, 213–222. [Google Scholar] [CrossRef] [PubMed]
| χ2 | df | p | |
|---|---|---|---|
| Goodness-of-fit test | 3.325 | 9 | 0.853 |
| Predictor | β | Wald Statistic | df | p | OR | 95% CI for OR | |
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| intercept | 1850.27 | 23.08 | 1 | <0.001 | |||
| mean temperature (t) | −6.20 | 12.82 | 1 | <0.001 | 0.002 | 0.000 | 0.004 |
| precipitation (p) | −60.90 | 2.20 | 1 | 0.138 | 0.000 | 0.000 | 315.661 |
| relative humidity (h) | −0.63 | 29.15 | 1 | <0.001 | 0.535 | 0.426 | 1.000 |
| max. temperature (max.t) | 1.38 | 0.50 | 1 | 0.480 | 3.956 | 0.087 | 179 |
| number of days t ≥ 20 (t20) | −2.13 | 0.08 | 1 | 0.774 | 0.119 | 0.000 | 242.013 |
| number of days t ≥ 25 (t25) | −1.42 | 12.67 | 1 | <0.001 | 0.241 | 0.110 | 1.000 |
| interaction temp. x prec. (tp) | 0.21 | 2.24 | 1 | 0.134 | 1.233 | 0.937 | 2.000 |
| Myzus persicae (Mp) | −0.02 | 0.96 | 1 | 0.327 | 0.976 | 0.931 | 1.000 |
| Aphis nasturtii An | −0.06 | 0.09 | 1 | 0.763 | 0.943 | 0.644 | 1.000 |
| Aphis frangulae Afr | 0.03 | 0.22 | 1 | 0.984 | 1.030 | 0.055 | 19.000 |
| Aphis fabae Af | 0.20 | 7.69 | 1 | <0.001 | 1.224 | 1.220 | 1.228 |
| Brachycaudus helichrysi Bh | 0.57 | 6.54 | 1 | <0.001 | 1.773 | 1.708 | 1.841 |
| Hyperomyzus lactucae Hl | −0.18 | 1.32 | 1 | 0.148 | 0.832 | 0.629 | 1.101 |
| Cryptomyzus galeopsidis Cg | −0.01 | 0.39 | 1 | 0.516 | 0.799 | 0.745 | 1.313 |
| Cavariella aegopodii Ca | 0.03 | 2.84 | 1 | 0.251 | 0.192 | 0.187 | 0.196 |
| Brevicoryne brassicae Bb | −0.23 | 0.76 | 1 | 0.087 | 0.794 | 0.580 | 1.087 |
| Cavariella theobaldi Ct | 0.01 | 2.91 | 1 | 0.362 | 1.011 | 0.737 | 1.388 |
| Rhopalosiphum padi Rp | −0.12 | 3.56 | 1 | 0.060 | 0.886 | 0.781 | 1.005 |
| Acyrthosiphon pisum Ap | −0.01 | 1.74 | 1 | 0.862 | 0.989 | 0.879 | 1.114 |
| Observed | Predicted | % Correct | |
|---|---|---|---|
| Yes | No | ||
| Yes | 852 | 151 | 84.95 |
| No | 108 | 303 | 73.72 |
| Overall % correct | 81.68 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Przybyś, M.; Doroszewska, T.; Doroszewski, A.; Erlichowski, T. Influence of Weather Conditions and the Aphid Population on the Potato Virus Y Infection of Tobacco in the Field. Agronomy 2024, 14, 1725. https://doi.org/10.3390/agronomy14081725
Przybyś M, Doroszewska T, Doroszewski A, Erlichowski T. Influence of Weather Conditions and the Aphid Population on the Potato Virus Y Infection of Tobacco in the Field. Agronomy. 2024; 14(8):1725. https://doi.org/10.3390/agronomy14081725
Chicago/Turabian StylePrzybyś, Marcin, Teresa Doroszewska, Andrzej Doroszewski, and Tomasz Erlichowski. 2024. "Influence of Weather Conditions and the Aphid Population on the Potato Virus Y Infection of Tobacco in the Field" Agronomy 14, no. 8: 1725. https://doi.org/10.3390/agronomy14081725






