The Impact of the Growth Regulators and Cultivation Conditions of Temporary Immersion Systems (TISs) on the Morphological Characteristics of Potato Explants and Microtubers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Apical Meristem Cultivation
2.3. Cultivation of Single-Node Potato Explants in SETISTM
2.4. Potato MTs in SETISTM
2.5. Statistical Analysis
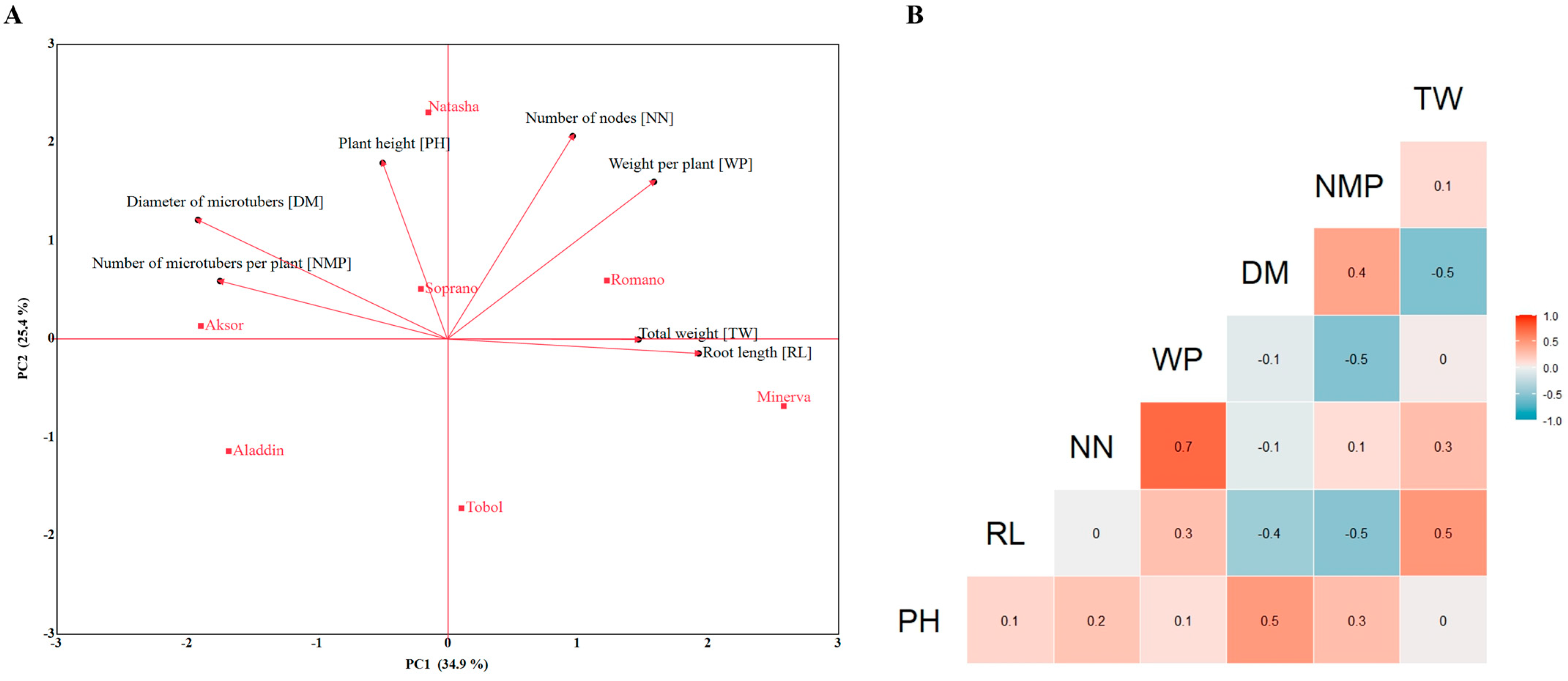
3. Results
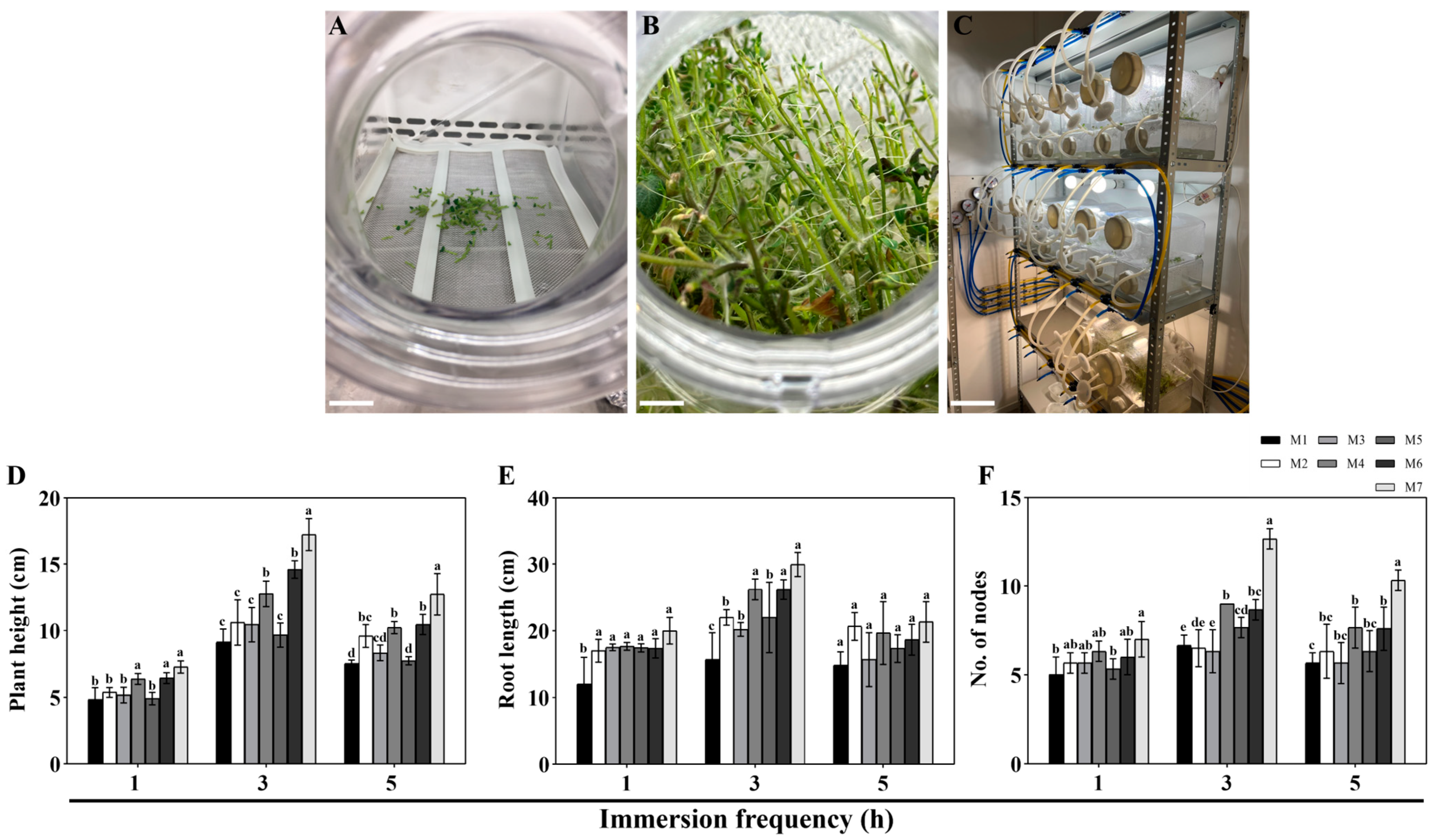
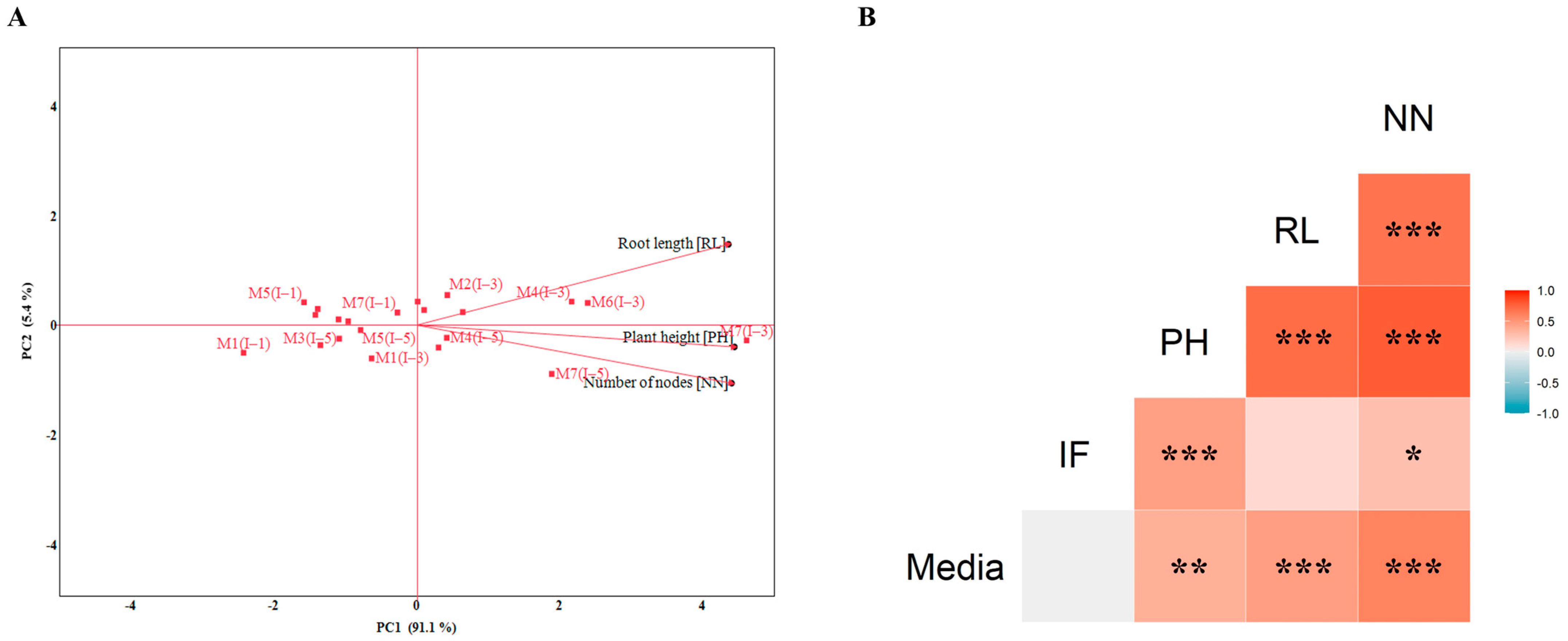
3.1. Selection of the Optimal Nutrient Medium for Cultivating Single-Node Potato Explants in SETISTM
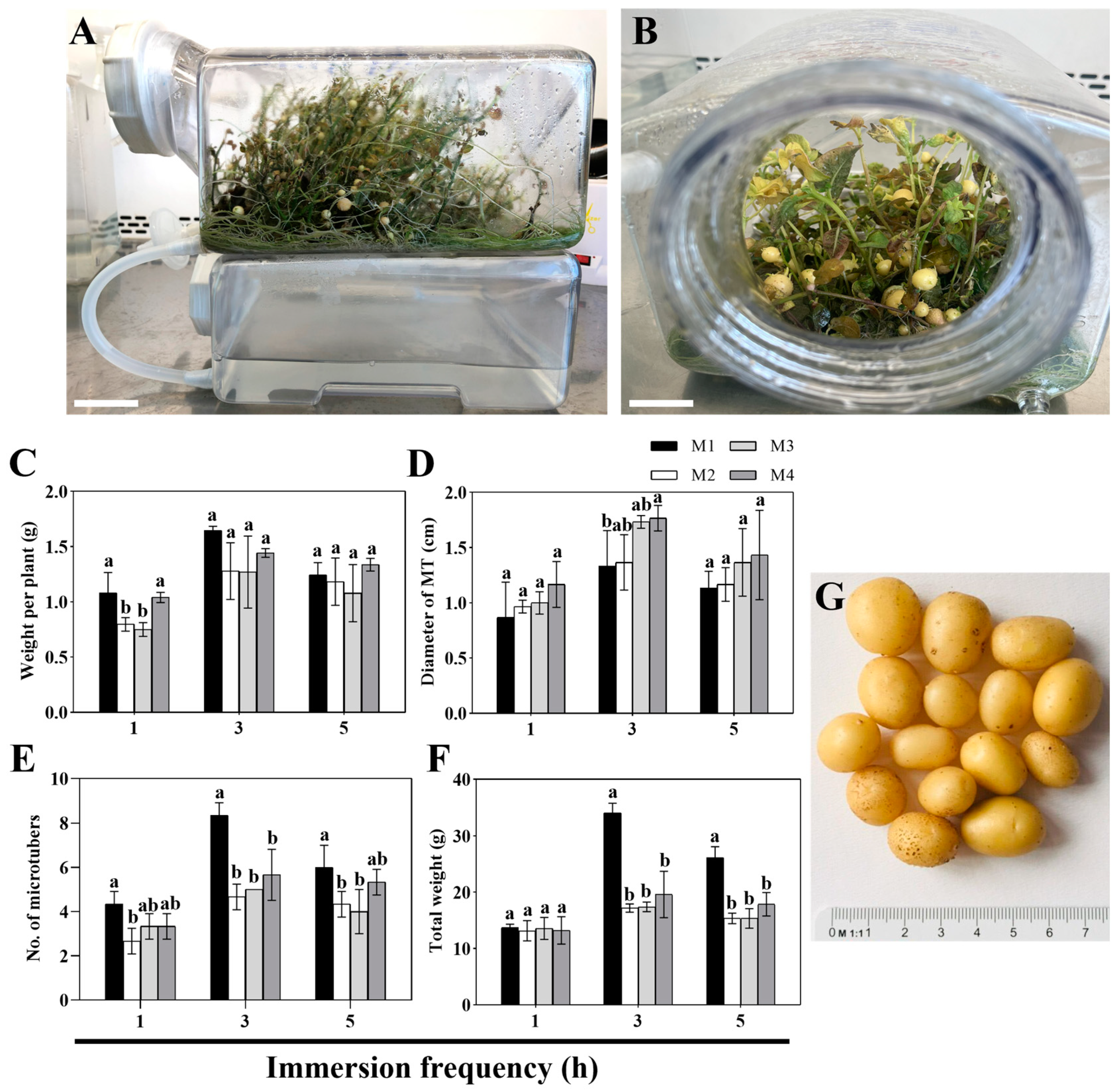
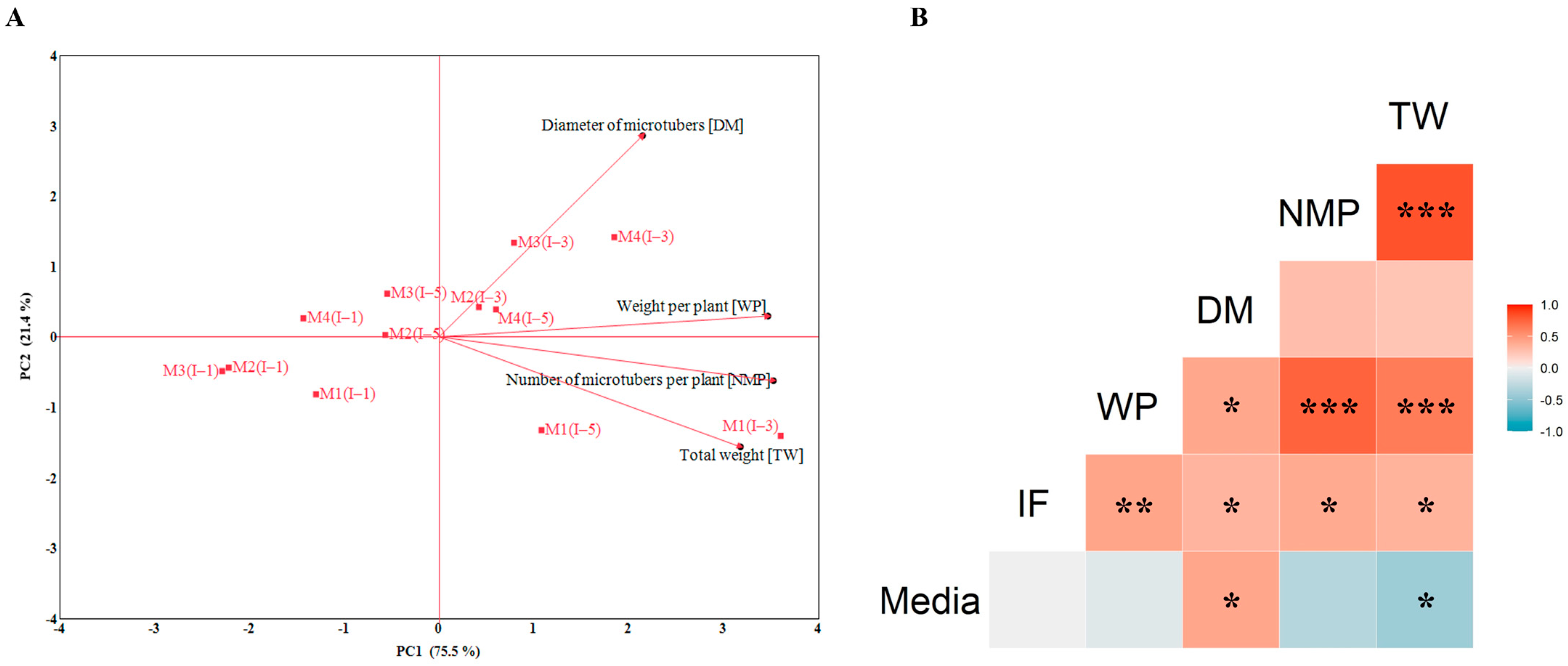
3.2. Selection of the Optimal Nutrient Medium for Potato MT Formation in SETISTM
3.3. Introduction of Potato Varieties into SETISTM
4. Discussion
4.1. Selection of the Optimal Nutrient Medium for Cultivating Single-Node Potato Explants in SETISTM
4.2. Selection of Optimal Nutrient Medium for Potato MT Formation in SETISTM
4.3. Introduction of Potato Varieties into SETISTM
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org (accessed on 3 July 2024).
- Bureau of National Statistics. Agency for Strategic Planning and Reforms of the Republic of Kazakhstan. Available online: https://stat.gov.kz (accessed on 3 July 2024).
- Im, J.S.; Seo, S.G.; Kim, M.O.; Cheon, C.G.; Park, Y.E.; Cho, J.H.; Cho, K.S.; Chang, D.C.; Choi, J.K.; Lee, J.N.; et al. Recent trend and prospects of potato industry in Kazakhstan. J. Korean Soc. Int. Agric. 2018, 30, 177–183. [Google Scholar] [CrossRef]
- Jones, R.A.C. Global Plant Virus Disease Pandemics and Epidemics. Plants 2021, 10, 233. [Google Scholar] [CrossRef] [PubMed]
- Lal, P.; Tiwari, R.K.; Behera, B.; Yadav, M.R.; Sharma, E.; Altaf, M.A.; Jena, R.; Ahmad, A.; Dey, A.; Kumar, A.; et al. Exploring potato seed research: A bibliometric approach towards sustainable food security. Front. Sustain. Food Syst. 2023, 7, 1229272. [Google Scholar] [CrossRef]
- Bettoni, J.C.; Mathew, L.; Pathirana, R.; Wiedow, C.; Hunter, D.A.; McLachlan, A.; Khan, S.; Tang, J.; Nadarajan, J. Eradication of Potato Virus S, Potato Virus A, and Potato Virus M from Infected in vitro-Grown Potato Shoots Using In vitro Therapies. Front. Plant Sci. 2022, 13, 878733. [Google Scholar] [CrossRef] [PubMed]
- Silva Filho, J.B.; Fontes, P.C.R.; Ferreira, J.F.d.S.; Cecon, P.R.; Santos, M.F.S.d. Best Morpho-Physiological Parameters to Characterize Seed-Potato Plant Growth under Aeroponics: A Pilot Study. Agronomy 2024, 14, 517. [Google Scholar] [CrossRef]
- Abu Zeid, I.M.; Soliman, H.I.A.; Metwali, E.M.R. In vitro evaluation of some high yield potato (Solanum tuberosum L.) cultivars under imposition of salinity at the cellular and organ levels. Saudi J. Biol. Sci. 2022, 29, 2541–2551. [Google Scholar] [CrossRef] [PubMed]
- Mamiya, K.; Tanabe, K.; Onishi, N. Production of potato (Solanum tuberosum, L.) microtubers using plastic culture bags. Plant Biotechnol. 2020, 37, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.Z.; Islam, S.S.; Chowdhury, A.N.; Subramaniam, S. Efficient microtuber production of potato in modified nutrient spray bioreactor system. Sci. Hortic. 2015, 192, 369–374. [Google Scholar] [CrossRef]
- Mohamed, A.E.S.; Girgis, N.D. Factors affecting in vitro tuberization of potato. Bull. Natl. Res. Cent. 2023, 47, 80. [Google Scholar] [CrossRef]
- Wojtania, A.; Mieszczakowska-Frąc, M. In Vitro Propagation Method for Production of Phenolic-Rich Planting Material of Culinary Rhubarb ‘Malinowy’. Plants 2021, 10, 1768. [Google Scholar] [CrossRef]
- Engels, J.M.M.; Ebert, A.W. A Critical Review of the Current Global Ex Situ Conservation System for Plant Agrobiodiversity. I. History of the Development of the Global System in the Context of the Political/Legal Framework and Its Major Conservation Components. Plants 2021, 10, 1557. [Google Scholar] [CrossRef] [PubMed]
- Mirzabe, A.H.; Hajiahmad, A.; Fadavi, A.; Rafiee, S. Temporary immersion systems (TISs): A comprehensive review. J. Biotechnol. 2022, 357, 56–83. [Google Scholar] [CrossRef]
- Uma, S.; Karthic, R.; Kalpana, S.; Backiyarani, S.; Saraswathi, M.S. A novel temporary immersion bioreactor system for large scale multiplication of banana (Rasthali AAB-Silk). Sci. Rep. 2021, 11, 20371. [Google Scholar] [CrossRef]
- Kikowska, M.; Danek, K.; Gornowicz-Porowska, J.; Thiem, B. Application of temporary immersion system RITA® for efficient biomass multiplication and production of artificial seeds for ex situ conservation of Linnaea borealis L. Plant Cell Tissue Organ Cult. 2022, 151, 673–680. [Google Scholar] [CrossRef]
- Monja-Mio, K.M.; Ojeda, G.; Herrera-Alamillo, M.Á.; Sánchez-Teyer, L.F.; Rescalvo-Morales, A. BioMINT: A Temporary Immersion System for Agave Micropropagation. Methods Mol. Biol. 2024, 2759, 77–88. [Google Scholar] [CrossRef]
- Robert, M.L.; Herrera-Herrera, J.L.; Herrera-Herrera, G.; Herrera-Alamillo, M.A.; Fuentes-Carrillo, P. A new temporary immersion bioreactor system for micropropagation. Methods Mol. Biol. 2006, 318, 121–129. [Google Scholar] [CrossRef]
- De Carlo, A.; Tarraf, W.; Lambardi, M.; Benelli, C. Temporary Immersion System for Production of Biomass and Bioactive Compounds from Medicinal Plants. Agronomy 2021, 11, 2414. [Google Scholar] [CrossRef]
- Murthy, H.N.; Joseph, K.S.; Paek, K.Y.; Park, S.Y. Bioreactor systems for micropropagation of plants: Present scenario and future prospects. Front. Plant Sci. 2023, 14, 1159588. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Hernández, H.A.; Galaz-Ávalos, R.M.; Quintana-Escobar, A.O.; Pech-Hoil, R.; Collí-Rodríguez, A.M.; Salas-Peraza, I.Q.; Loyola-Vargas, V.M. In Vitro Conversion of Coffea spp. Somatic Embryos in SETIS™ Bioreactor System. Plants 2023, 12, 3055. [Google Scholar] [CrossRef]
- SETIS. Available online: https://setis-systems.be (accessed on 3 July 2024).
- Gautam, S.; Solis-Gracia, N.; Teale, M.K.; Mandadi, K.; da Silva, J.A.; Vales, M.I. Development of an in vitro Microtuberization and Temporary Immersion Bioreactor System to Evaluate Heat Stress Tolerance in Potatoes (Solanum tuberosum L.). Front. Plant Sci. 2021, 12, 700328. [Google Scholar] [CrossRef]
- Steingroewer, J.; Bley, T.; Georgiev, V.; Ivanov, I.; Lenk, F.; Marchev, A.; Pavlov, A. Bioprocessing of differentiated plant in vitro systems. Eng. Life Sci. 2013, 13, 26–38. [Google Scholar] [CrossRef]
- Werner, S.; Maschke, R.; Eibl, D.; Eibl, R. Bioreactor Technology for Sustainable Production of Plant Cell-Derived Products. In Bioprocessing of Plant In Vitro Systems; Reference Series in Phytochemistry; Pavlov, A., Bley, T., Eds.; Springer: Cham, Switzerland, 2017; pp. 413–432. [Google Scholar]
- Escalona, M.; Samson, G.; Borroto, C.; Desjardins, Y. Physiology of Effects of Temporary Immersion Bioreactors on Micropropagated Pineapple Plantlets. In Vitro Cell. Dev. Biol. Plant. 2003, 39, 651–656. [Google Scholar] [CrossRef]
- Pożoga, M.; Olewnicki, D.; Latocha, P. A Temporary Immer-sion System as a Tool for Lowering Planting Material Production Costs Using the Example of Pennisetum × advena ‘Rubrum’. Agriculture 2024, 14, 1177. [Google Scholar] [CrossRef]
- Volkov, D.V.; Daurov, D.L.; Daurova, A.K.; Abay, Z.S.; Zhapar, K.K.; Zhambakin, K.Z.; Shamekova, M.K. Obtaining potato microtubers in a liquid nutrient medium. Proc. Natl. Acad. Sci. Belarus 2020, 58, 432–442. [Google Scholar] [CrossRef]
- Hajare, S.T.; Chauhan, N.M.; Kassa, G. Effect of Growth Regulators on In Vitro Micropropagation of Potato (Solanum tuberosum L.) Gudiene and Belete Varieties from Ethiopia. Sci. World J. 2021, 2021, 5928769. [Google Scholar] [CrossRef] [PubMed]
- Abeuova, L.S.; Kali, B.R.; Rakhimzhanova, A.O.; Bekkuzhina, S.S.; Manabayeva, S.A. High frequency direct shoot regeneration from Kazakh commercial potato cultivars. PeerJ 2020, 8, e9447. [Google Scholar] [CrossRef]
- Daurov, D.; Argynbayeva, A.; Daurova, A.; Zhapar, K.; Sapakhova, Z.; Zhambakin, K.; Shamekova, M. Monitoring the Spread of Potato Virus Diseases in Kazakhstan. Am. J. Potato Res. 2023, 100, 63–70. [Google Scholar] [CrossRef]
- Daurov, D.; Daurova, A.; Karimov, A.; Tolegenova, D.; Volkov, D.; Raimbek, D.; Zhambakin, K.; Shamekova, M. Determining Effective Methods of Obtaining Virus-Free Potato for Cultivation in Kazakhstan. Am. J. Potato Res. 2020, 97, 367–375. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Duncan, O.D.; Duncan, B. A methodological analysis of segregation indexes. Am. Sociol. Rev. 1955, 20, 210. [Google Scholar] [CrossRef]
- RStudio Team. RStudio: Integrated Development for R. RStudio, PBC: Boston, MA, USA. 2020. Available online: http://www.rstudio.com/ (accessed on 13 August 2024).
- Sharipova, D.S.; Aitbayev, T.E.; Tazhibayev, T.S.; Nacheva, E.K. The impact of new and improved elements of agricultural technologies on potato productivity in the south-east of Kazakhstan. Biosci. Biotechnol. Res. Asia 2016, 13, 1031–1036. [Google Scholar] [CrossRef]
- Vollmer, R.; Villagaray, R.; Cárdenas, J.; Castro, M.; Chávez, O.; Anglin, N.L.; Ellis, D. A large-scale viability assessment of the potato cryobank at the International Potato Center (CIP). In Vitro Cell. Dev. Biol.-Plant 2017, 53, 309–317. [Google Scholar] [CrossRef]
- Kushnarenko, S.; Romadanova, N.; Aralbayeva, M.; Zholamanova, S.; Alexandrova, A.; Karpova, O. Combined ribavirin treatment and cryotherapy for efficient Potato virus M and Potato virus S eradication in potato (Solanum tuberosum L.) in vitro shoots. In Vitro Cell. Dev. Biol.-Plant 2017, 53, 425–432. [Google Scholar] [CrossRef]
- Yuorieva, N.; Sinetova, M.; Messineva, E.; Kulichenko, I.; Fomenkov, A.; Vysotskaya, O.; Osipova, E.; Baikalova, A.; Prudnikova, O.; Titova, M.; et al. Plants, Cells, Algae, and Cyanobacteria In Vitro and Cryobank Collections at the Institute of Plant Physiology, Russian Academy of Sciences—A Platform for Research and Production Center. Biology 2023, 12, 838. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Leal, C.A.; Puente-Garza, C.A.; García-Lara, S. In vitro plant tissue culture: Means for production of biological active compounds. Planta 2018, 248, 1–18. [Google Scholar] [CrossRef]
- Pandey, S.K.; Singh, S.V.; Sarkar, D. Potato (Solanum tuberosum) for sustaining food and nutrition security in developing world. Indian J. Agric. Sci. 2005, 75, 9043. [Google Scholar]
- Purohit, S.D.; Teixeira da Silva, J.A.; Habibi, N. Current approaches for cheaper and better micropropagation technologies. Int. J. Plant Dev. Biol. 2011, 5, 1–36. [Google Scholar]
- Andriani, S.; Siregar, L.A.M.; Safni, I. Microtubers production by using Temporary Immersion System (TIS) bioreactor to potato varieties. IOP Conf. Ser. Earth Environ. Sci. 2021, 886, 012005. [Google Scholar] [CrossRef]
- Kämäräinen-Karppinen, T.; Virtanen, E.A.; Rokka, V.; Pirttilä, A.M. Novel bioreactor technology for mass propagation of potato microtubers. Plant Cell Tissue Organ Cult. (PCTOC) 2010, 101, 245–249. [Google Scholar] [CrossRef]
- Erol, M.H.; Dönmez, D.; Biçen, B.; Şimşek, Ö.; Kaçar, Y.A. Modern Approaches to In Vitro Clonal Banana Production: Next-Generation Tissue Culture Systems. Horticulturae 2023, 9, 1154. [Google Scholar] [CrossRef]
- Shukla, M.R.; Piunno, K.; Saxena, P.K.; Jones, A.M.P. Improved in vitro rooting in liquid culture using a two piece scaffold system. Eng. Life Sci. 2019, 20, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Pati, P.K.; Kaur, J.; Singh, P. A liquid culture system for shoot proliferation and analysis of pharmaceutically active constituents of Catharanthus roseus (L.) G. Don. Plant Cell Tissue Organ Cult. 2011, 105, 299–307. [Google Scholar] [CrossRef]
- Latawa, J.; Shukla, M.R.; Saxena, P.K. An efficient temporary immersion system for micropropagation of hybrid hazelnut. Botany 2016, 94, 1–8. [Google Scholar] [CrossRef]
- Pérez-Alonso, N.; Wilken, D.; Gerth, A.; Jähn, A.; Nitzsche, H.M.; Kerns, G.; Capote-Perez, A.; Jiménez, E. Cardiotonic glycosides from biomass of Digitalis purpurea L. cultured in temporary immersion systems. Plant Cell Tissue Organ Cult. 2009, 99, 151–156. [Google Scholar] [CrossRef]
- Coetser, E.; du Toit, E.S.; Prinsloo, G. An Investigation into Using Temporary Immer-sion Bioreactors to Micropropagate Moringa oleifera Lam. Callus, Roots, and Shoots. Agronomy 2022, 12, 2672. [Google Scholar] [CrossRef]
- Hwang, H.-D.; Kwon, S.-H.; Murthy, H.N.; Yun, S.-W.; Pyo, S.-S.; Park, S.-Y. Temporary Immersion Bioreactor System as an Efficient Method for Mass Production of In Vitro Plants in Horticulture and Medicinal Plants. Agronomy 2022, 12, 346. [Google Scholar] [CrossRef]
- Frick, E.M.; Strader, L.C. Roles for IBA-derived auxin in plant development. J. Exp. Bot. 2018, 69, 169–177. [Google Scholar] [CrossRef]
- Kazan, K. Auxin and the integration of environmental signals into plant root development. Ann. Bot. 2013, 112, 1655–1665. [Google Scholar] [CrossRef] [PubMed]
- Saidi, A.; Hajibarat, Z. Phytohormones: Plant switchers in developmental and growth stages in potato. J. Genet. Eng. Biotechnol. 2021, 19, 89. [Google Scholar] [CrossRef]
- Šimko, I. Effects of kinetin, paclobutrazol and their interactions on the microtuberization of potato stem segments cultured in vitro in the light. Plant Growth Regul. 1993, 12, 23–27. [Google Scholar] [CrossRef]
- Rahman, M.H.; Islam, M.J.; Mumu, U.H.; Ryu, B.R.; Lim, J.D.; Azad, M.O.K.; Cheong, E.J.; Lim, Y.S. Effect of Light Quality on Seed Potato (Solanum tuberose L.) Tuberization When Aeroponically Grown in a Controlled Greenhouse. Plants 2024, 13, 737. [Google Scholar] [CrossRef]
- Sakha, B.M.; Bhatia, A.K.; Batra, V.K.; Chaudhary, V.K.; Batra, P.; Khurana, S.C. In vitro microtuberization in potato (Solanum tuberosum L.) cultivars. Indian J. Exp. Biol. 2004, 42, 1245–1247. [Google Scholar]
- Kaur, A.; Reddy, M.S.; Kumar, A. Efficient, one step and cultivar independent shoot organogenesis of potato. Physiol. Mol. Biol. Plants 2017, 23, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Kolachevskaya, O.O.; Myakushina, Y.A.; Getman, I.A.; Lomin, S.N.; Deyneko, I.V.; Deigraf, S.V.; Romanov, G.A. Hormonal Regulation and Crosstalk of Auxin/Cytokinin Signaling Pathways in Potatoes In Vitro and in Relation to Vegetation or Tuberization Stages. Int. J. Mol. Sci. 2021, 22, 8207. [Google Scholar] [CrossRef] [PubMed]
- Lomin, S.N.; Myakushina, Y.A.; Kolachevskaya, O.O.; Getman, I.A.; Savelieva, E.M.; Arkhipov, D.V.; Deigraf, S.V.; Romanov, G.A. Global View on the Cytokinin Regulatory System in Potato. Front. Plant Sci. 2020, 11, 613624. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Mosqueda, M.A.; Cruz-Cruz, C.A.; Cano-Ricárdez, A.; Bello-Bello, J.J. Assessment of different temporary immersion systems in the micropropagation of anthurium (Anthurium andreanum). 3 Biotech 2019, 9, 307. [Google Scholar] [CrossRef] [PubMed]
- Bello-Bello, J.J.; Schettino-Salomón, S.; Ortega-Espinoza, J.; Spinoso-Castillo, J.L. A temporary immersion system for mass micropropagation of pitahaya (Hylocereus undatus). 3 Biotech 2021, 11, 437. [Google Scholar] [CrossRef]
- Polivanova, O.B.; Bedarev, V.A. Hyperhydricity in Plant Tissue Culture. Plants 2022, 11, 3313. [Google Scholar] [CrossRef]
- Sonnewald, S.; Sonnewald, U. Regulation of potato tuber sprouting. Planta 2014, 239, 27–38. [Google Scholar] [CrossRef]
- Suttle, J.C. Physiological regulation of potato tuber dormancy. Am. J. Potato Res. 2004, 81, 253–262. [Google Scholar] [CrossRef]
- Mashhad, S.; Moeini, M. The effect of cytokinin and coumarin on in vitro micrrotuberization of potato (Solanum tuberosum L.) cv. Marfona. Ludus Vitalis 2015, 11, 165–170. [Google Scholar]
- Siregar, L.A.M.; Turnip, L.; Damanik, I.R. Immersion in 6-benzylaminopurine for dormancy release and initiation of potato sprouts at various tuber weight and storage duration. J. Agron. Indones. 2021, 49, 60–67. [Google Scholar] [CrossRef]
- Peng, M.; Wang, X.; Li, L. The effect of plant growth regulator and active charcoal on the development of microtubers of potatoes. Am. J. Plant Sci. 2012, 3, 1535–1540. [Google Scholar] [CrossRef]
- Dhital, S.P.; Lim, H.T. Microtuberization of potato (Solanum tuberosum L.) as influenced by supplementary nutrients, plant growth regulators, and in vitro culture conditions. Potato Res. 2012, 55, 97–108. [Google Scholar] [CrossRef]
- Suttle, J.C. Involvement of endogenous gibberellins in potato tuber dormancy and early sprout growth: A critical assessment. J. Plant Physiol. 2004, 161, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.F.; Abdalla, M.M.A.; Damarany, A.A.M. Differential axillary-bud proliferation responses of two sweet potato cultivars to benzyl adenine and thidiazuron. Ass. Univ. Bull. Environ. Res. 2007, 10, 21–30. [Google Scholar]
- Kumlay, A.M.; Ercisli, S. Callus induction, shoot proliferation and root regeneration of potato (Solanum tuberosum L.) stem node and leaf explants under long-day conditions. Biotechnol. Equip. 2015, 29, 1075–1084. [Google Scholar] [CrossRef]
- Naqvi, B.; Abbas, H.; Ali, H. Evaluation of in vitro tuber induction ability of two potato genotypes. Pak. J. Agric. Sci. 2019, 56, 77–81. [Google Scholar]
- Shukla, S.R.; Zala, H.N.; Solanki, S.D.; Ant, H.M. Optimizing Microtubers Production for Sustainable Potato Cultivation in Gujarat, India. Biol. Life Sci. Forum 2023, 27, 2. [Google Scholar] [CrossRef]
- Fufa, M.; Diro, M. The effects of sucrose on in vitro tuberization of potato cultivars. Adv. Crop Sci. Tech. 2013, 1, 2. [Google Scholar]
- Etienne, H.; Berthouly, M. Temporary immersion systems in plant micropropagation. Plant Cell Tissue Organ Cult. 2002, 69, 215–231. [Google Scholar] [CrossRef]
- Tapia, M.D.L.; Arbizu, C.; Beraún, F.; Lorenzo, J.; Escalona, M. Pre-basic seed potato (Solanum tuberosum L.) production using temporary immersion bioreactors. Peruv. J. Agron. 2018, 2, 9. [Google Scholar] [CrossRef]
- Jova, M.C.; Kosky, R.G.; Cabrera, R.; Feria, M.D.; Perez, M.B.; Vega, V.M.; Torres, J.L. Performance of yam microtubers from temporary immersion system in field conditions. Afr. J. Biotechnol. 2011, 10, 9268–9271. [Google Scholar]
- Ashraf, M.F.; Abd Aziz, M.; Stanslas, J.; Kadir, M.A. Optimization of immersion frequency and medium substitution on microtuberization of Chlorophytum borivilianum in RITA system on production of saponins. Process Biochem. 2013, 48, 73–77. [Google Scholar] [CrossRef]
- Nongdam, P.; Beleski, D.G.; Tikendra, L.; Dey, A.; Varte, V.; El Merzougui, S.; Pereira, V.M.; Barros, P.R.; Vendrame, W.A. Orchid Micropropagation Using Conventional Semi-Solid and Temporary Immersion Systems: A Review. Plants 2023, 12, 1136. [Google Scholar] [CrossRef] [PubMed]
- Novikov, O.O.; Romanova, M.S.; Leonov, N.I.; Kosinova, E.I. Influence of various phytohormones on the growth and development of the Solnechny potato variety in vitro. BIO Web Conf. 2021, 36, 05008. [Google Scholar] [CrossRef]
- Guade, Y.F. The effect of plant growth hormones (auxins and cytokinins) on in-vitro shooting and rooting ability of potato nodal culture. Eur. J. Biomed. 2017, 4, 489–493. [Google Scholar]
- Miri, M.; Janakirama, P.; Held, M.; Ross, L.; Szczyglowski, K. Into the root: How cytokinin controls rhizobial infection. Trends Plant Sci. 2016, 21, 178–186. [Google Scholar] [CrossRef]
- Gong, H.L.; Dusengemungu, L.; Igiraneza, C.; Rukundo, P. Molecular Regulation of Potato Tuber Dormancy and Sprouting: A Mini-Review. Plant Biotechnol. Rep. 2021, 15, 417–434. [Google Scholar] [CrossRef]
- Viola, R.; Roberts, A.G.; Haupt, S.; Gazzani, S.; Hancock, R.D.; Marmiroli, N.; Machray, G.C.; Oparka, K.J. Tuberization in Potato Involves a Switch from Apoplastic to Sym-plastic Phloem Unloading. Plant Cell 2001, 13, 385–398. [Google Scholar] [CrossRef]
- Boubaker, H.; Saadaoui, W.; Dasgan, H.Y.; Tarchoun, N.; Gruda, N.S. Enhancing Seed Potato Production from In Vitro Plantlets and Microtubers through Biofertilizer Application: Investigating Effects on Plant Growth, Tuber Yield, Size, and Quality. Agronomy 2023, 13, 2541. [Google Scholar] [CrossRef]
- Diémé, A.; Ba, O.; Sagna, M.; Sy, M. Influence of the Size of Potato Microtubers (Solanum tuberosum L.) on the Yield of Plants under Semi-Axenic Conditions. Adv. Biosci. Biotechnol. 2021, 12, 65–77. [Google Scholar] [CrossRef]
- Herrera-Isidron, L.; Valencia-Lozano, E.; Rosiles-Loeza, P.Y.; Robles-Hernández, M.G.; Napsuciale-Heredia, A.; Cabrera-Ponce, J.L. Gene Expression Analysis of Microtubers of Potato Solanum tuberosum L. Induced in Cytokinin Containing Medium and Osmotic Stress. Plants 2021, 10, 876. [Google Scholar] [CrossRef]
- Otroshy, M. Utilization of Tissue Culture Techniques in a Seed Potato Tuber Production Scheme. Ph.D. Thesis, Wageningen University and Research, Wageningen, The Netherlands, 2006. [Google Scholar]
- Pour, M.S.; Omidi, M.; Majidi, I.; Davoodi, D.; Tehrani, P.A. In-vitro plantlet propagation and microtuberization of meristem culture in some of wild and commercial potato cultivars as affected by NaCl. Afr. J. Agric. Res. 2010, 5, 268–274. [Google Scholar]
- Long, J.; Yu, F.; Wu, Y.; Xu, Z.; Liu, X. Regulation of Different Lights on Energy Acquisitions, Microtuber Formation, and Growth of In Vitro-Grown Solanum tuberosum L. Agronomy 2024, 14, 1232. [Google Scholar] [CrossRef]


| Media | Hormones (mg/L) | TIS—Immersion Frequency (2 min Immersion) | ||
|---|---|---|---|---|
| Kinetin | GA | IBA | ||
| M1 | 0 | 0 | 0 | 1 h |
| 3 h | ||||
| 5 h | ||||
| M2 | 0 | 0.5 | 0 | 1 h |
| 3 h | ||||
| 5 h | ||||
| M3 | 2 | 0 | 0 | 1 h |
| 3 h | ||||
| 5 h | ||||
| M4 | 2 | 0.5 | 0 | 1 h |
| 3 h | ||||
| 5 h | ||||
| M5 | 0 | 0 | 0.5 | 1 h |
| 3 h | ||||
| 5 h | ||||
| M6 | 0 | 0.5 | 0.5 | 1 h |
| 3 h | ||||
| 5 h | ||||
| M7 | 2 | 0.5 | 0.5 | 1 h |
| 3 h | ||||
| 5 h | ||||
| Media | Hormones (mg/L) | TIS—Immersion Frequency (2 min Immersion) | |
|---|---|---|---|
| Kinetin | IBA | ||
| M1 | 0 | 0 | 1 h |
| 3 h | |||
| 5 h | |||
| M2 | 0 | 2 | 1 h |
| 3 h | |||
| 5 h | |||
| M3 | 2 | 0 | 1 h |
| 3 h | |||
| 5 h | |||
| M4 | 2 | 2 | 1 h |
| 3 h | |||
| 5 h | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daurov, D.; Daurova, A.; Sapakhova, Z.; Kanat, R.; Akhmetzhanova, D.; Abilda, Z.; Toishimanov, M.; Raissova, N.; Otynshiyev, M.; Zhambakin, K.; et al. The Impact of the Growth Regulators and Cultivation Conditions of Temporary Immersion Systems (TISs) on the Morphological Characteristics of Potato Explants and Microtubers. Agronomy 2024, 14, 1782. https://doi.org/10.3390/agronomy14081782
Daurov D, Daurova A, Sapakhova Z, Kanat R, Akhmetzhanova D, Abilda Z, Toishimanov M, Raissova N, Otynshiyev M, Zhambakin K, et al. The Impact of the Growth Regulators and Cultivation Conditions of Temporary Immersion Systems (TISs) on the Morphological Characteristics of Potato Explants and Microtubers. Agronomy. 2024; 14(8):1782. https://doi.org/10.3390/agronomy14081782
Chicago/Turabian StyleDaurov, Dias, Ainash Daurova, Zagipa Sapakhova, Rakhim Kanat, Dana Akhmetzhanova, Zhanar Abilda, Maxat Toishimanov, Nurgul Raissova, Murat Otynshiyev, Kabyl Zhambakin, and et al. 2024. "The Impact of the Growth Regulators and Cultivation Conditions of Temporary Immersion Systems (TISs) on the Morphological Characteristics of Potato Explants and Microtubers" Agronomy 14, no. 8: 1782. https://doi.org/10.3390/agronomy14081782






