Systematic Analysis and Expression Profiling of the Ginger FWL Gene Family Reveal Its Potential Functions in Rhizome Development and Response to Abiotic Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material Growth and Treatment Conditions
2.2. Identification and Physicochemical Properties Analysis of Ginger FWL Gene-Family Members
2.3. Phylogenetic Analysis of ZoFWL Gene Family
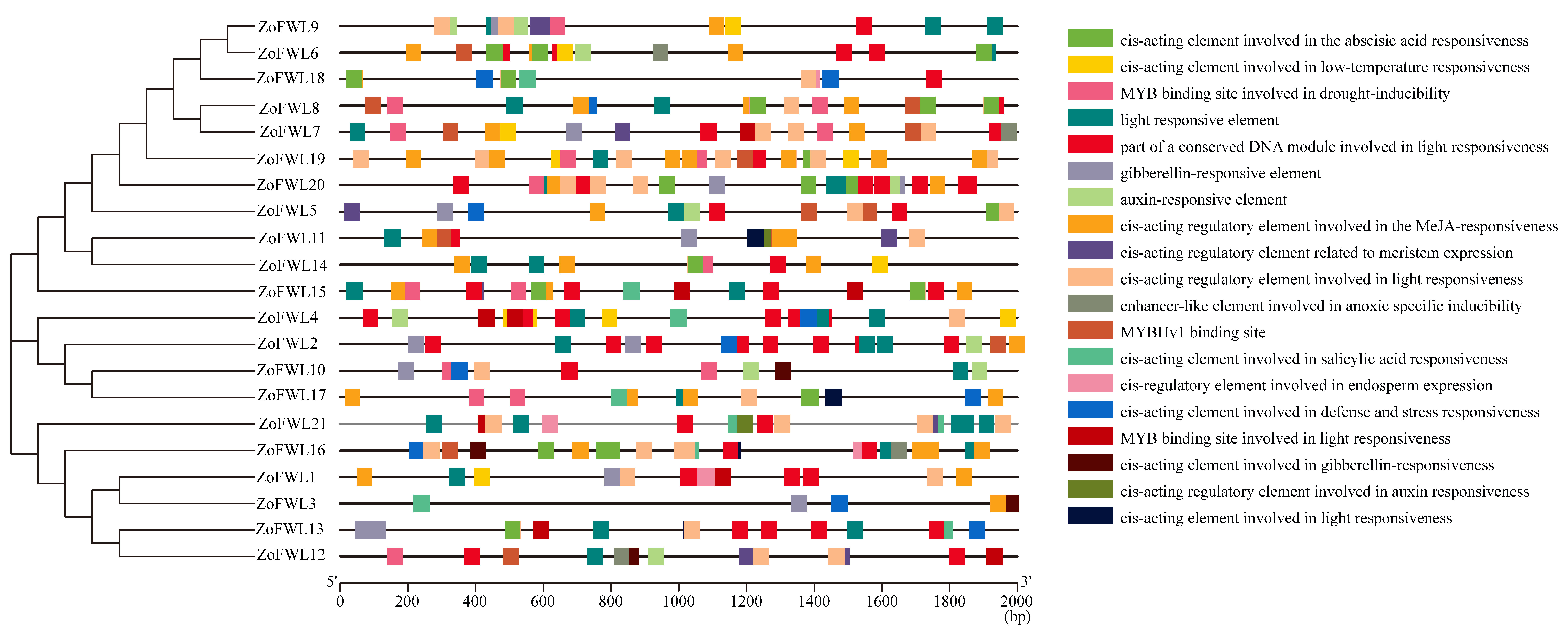
2.4. Analysis of Factorial Structure, Conserved Motifs, and Cis-Acting Elements
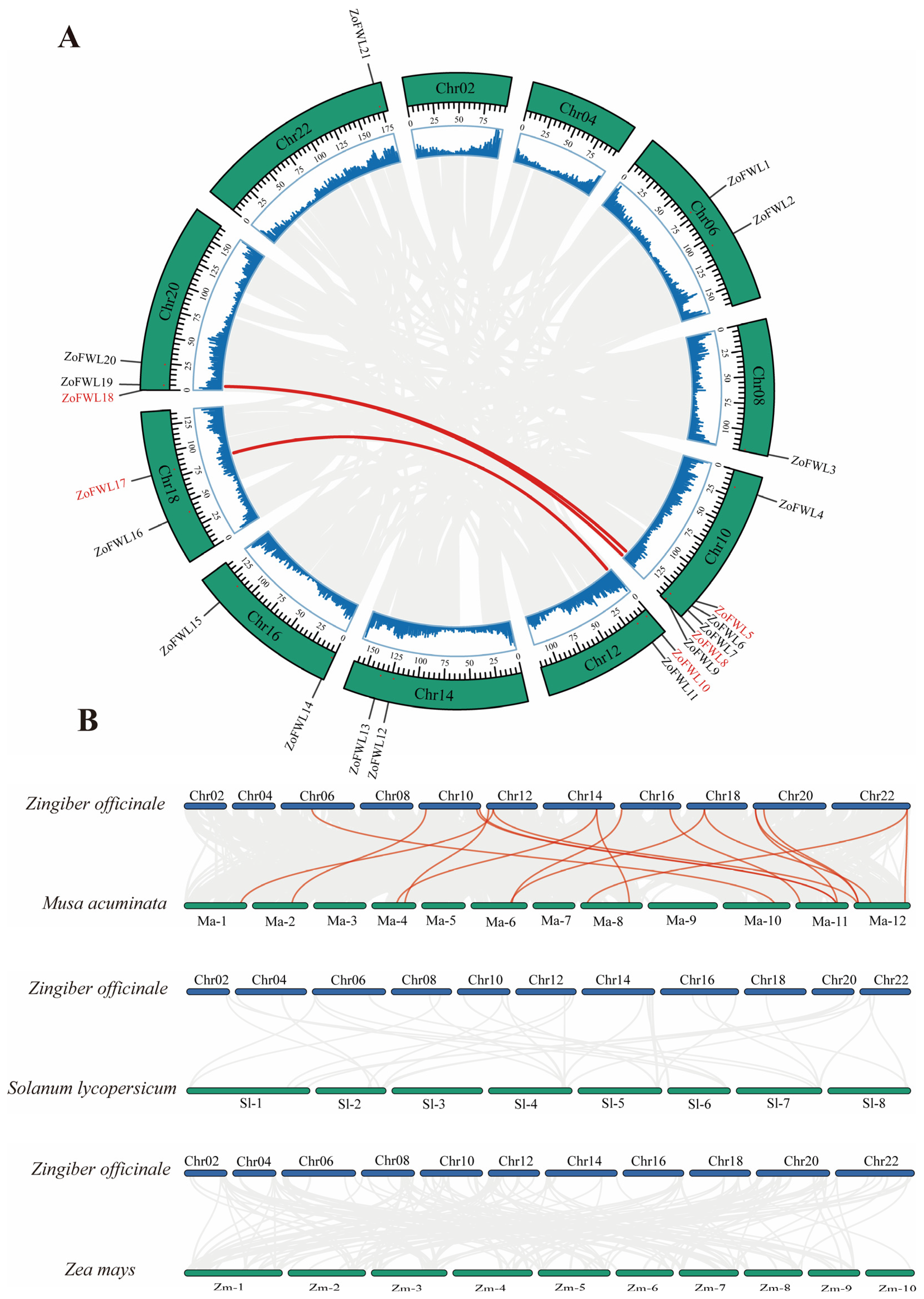
2.5. Gene Replication, Collinearity Analysis, and Protein Interaction Network Prediction
2.6. Expression Pattern and qRT-PCR Analysis of ZoFWL
2.7. Statistical Analyses
3. Results
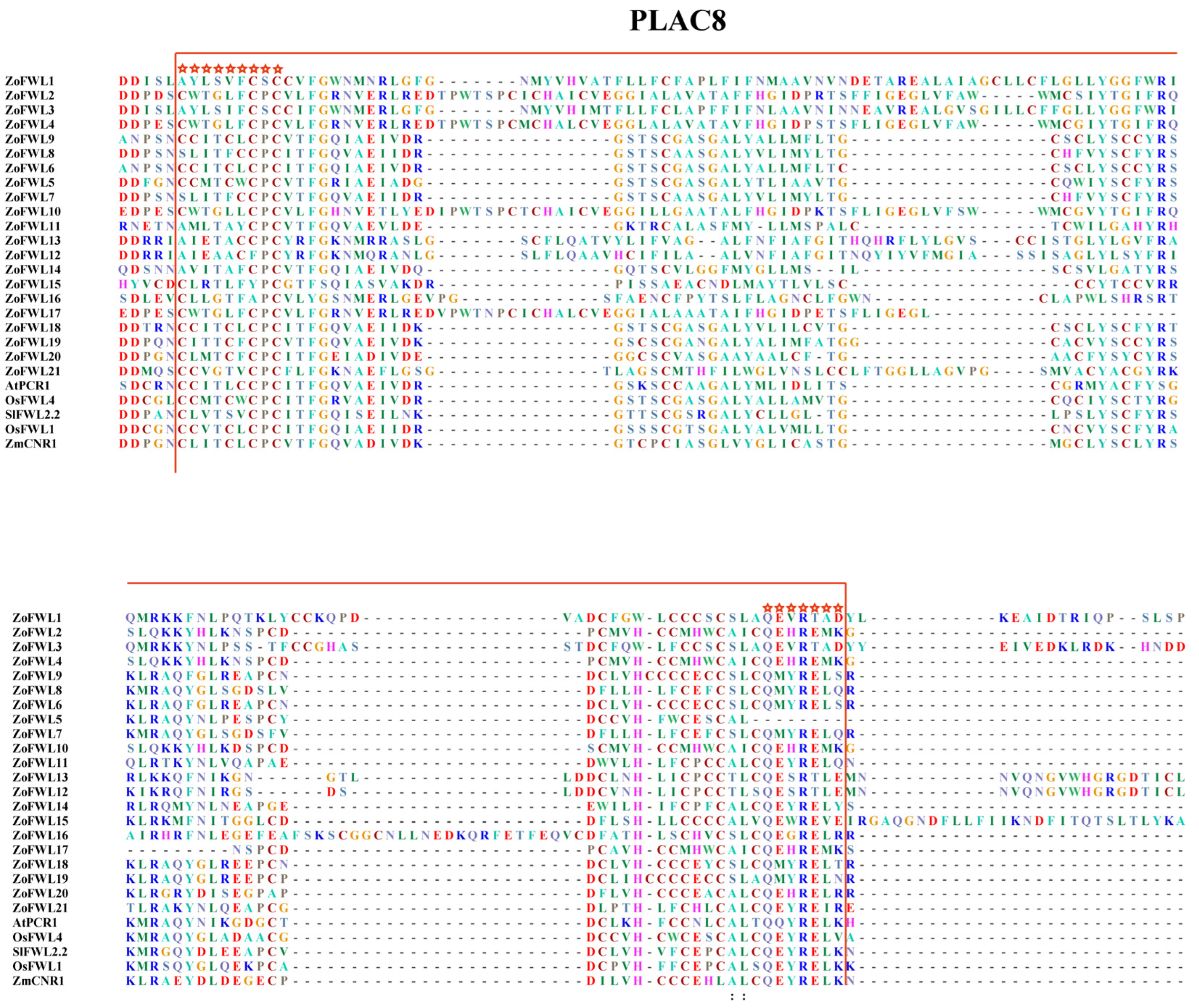
3.1. Identification and Sequence Analysis of the Ginger FWL Gene Family
3.2. Chromosome Localization Analysis of ZoFWL Genes
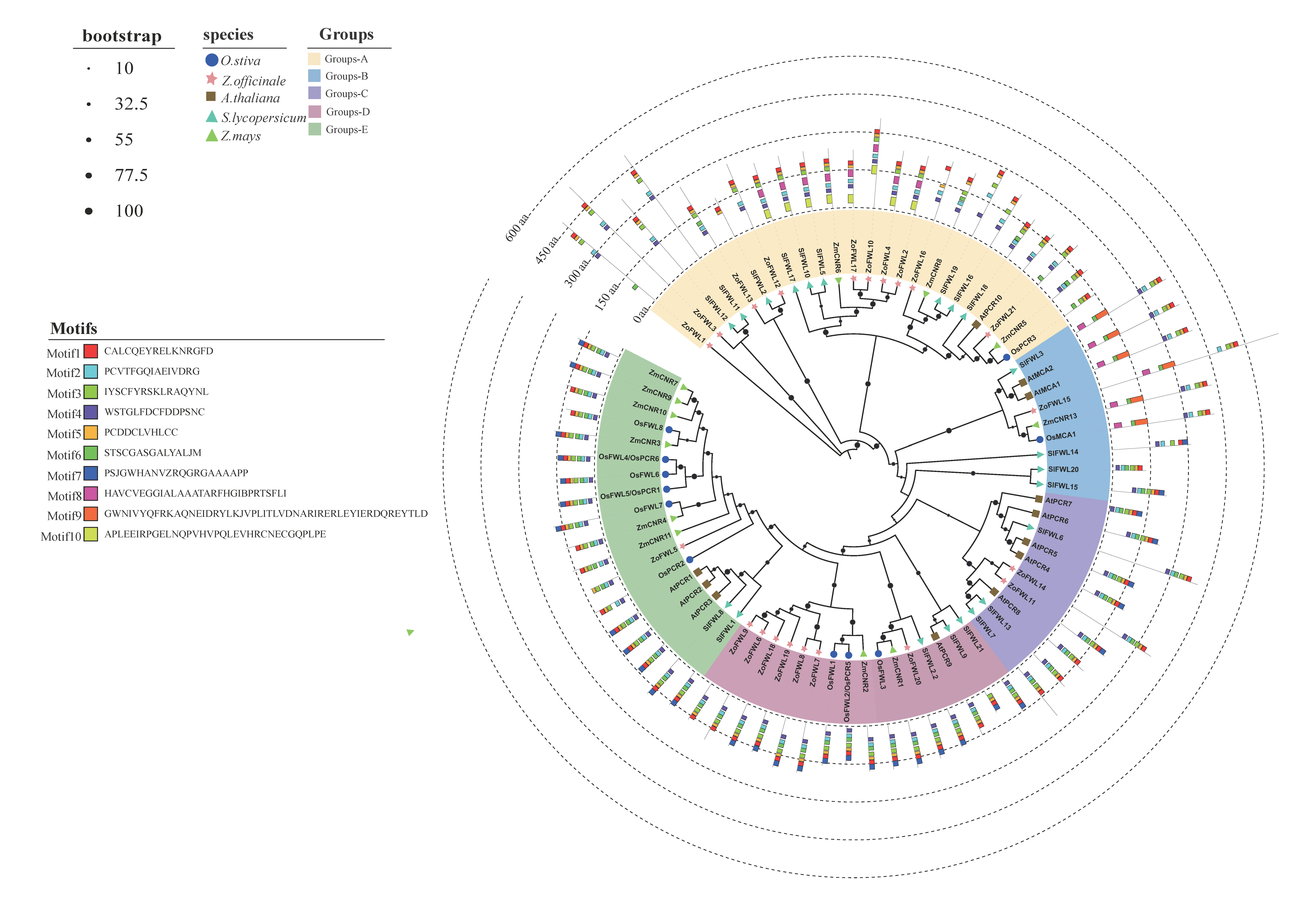
3.3. Phylogenetic Analysis of the ZoFWL Gene Family
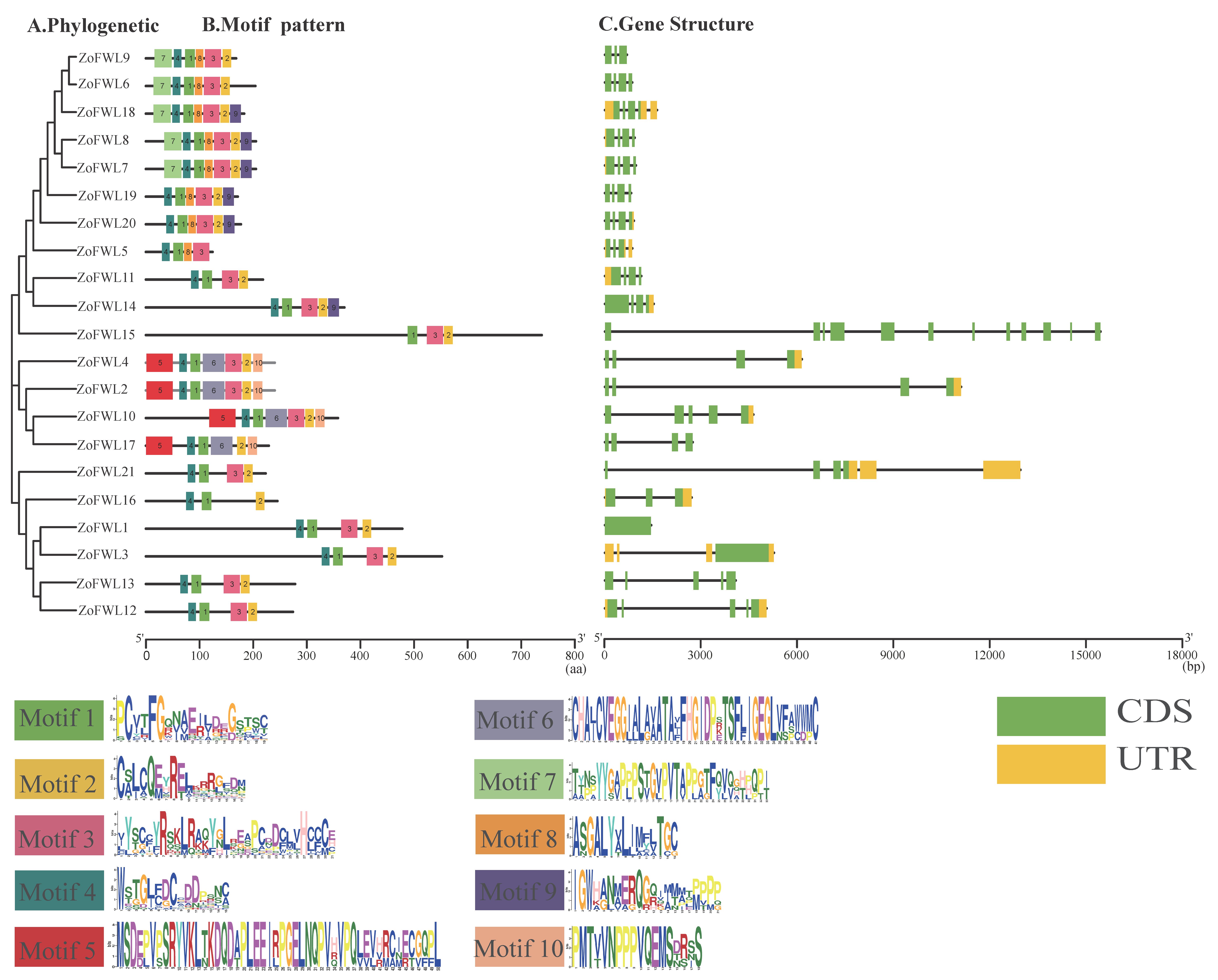
3.4. Phylogenetic Evolution, Gene Structure, and Conserved Motif Analysis of ZoFWL Gene
3.5. Analysis of Cis-Acting Elements of the ZoFWL Gene Family
3.6. Genome-Wide Duplication Events and Collinearity Analysis of ZoFWLs
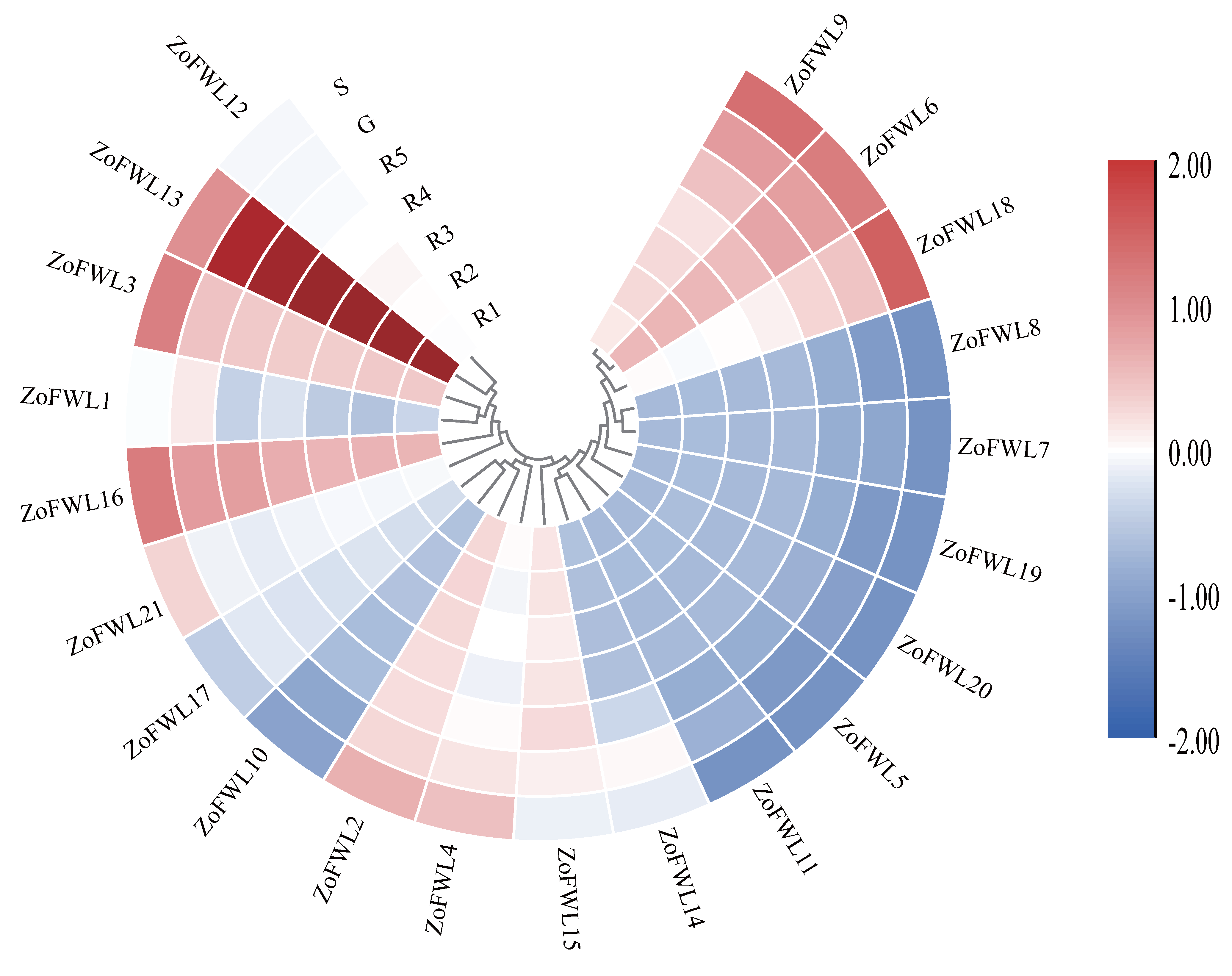
3.7. Expression Patterns of ZoFWL Genes in Different Ginger Organs
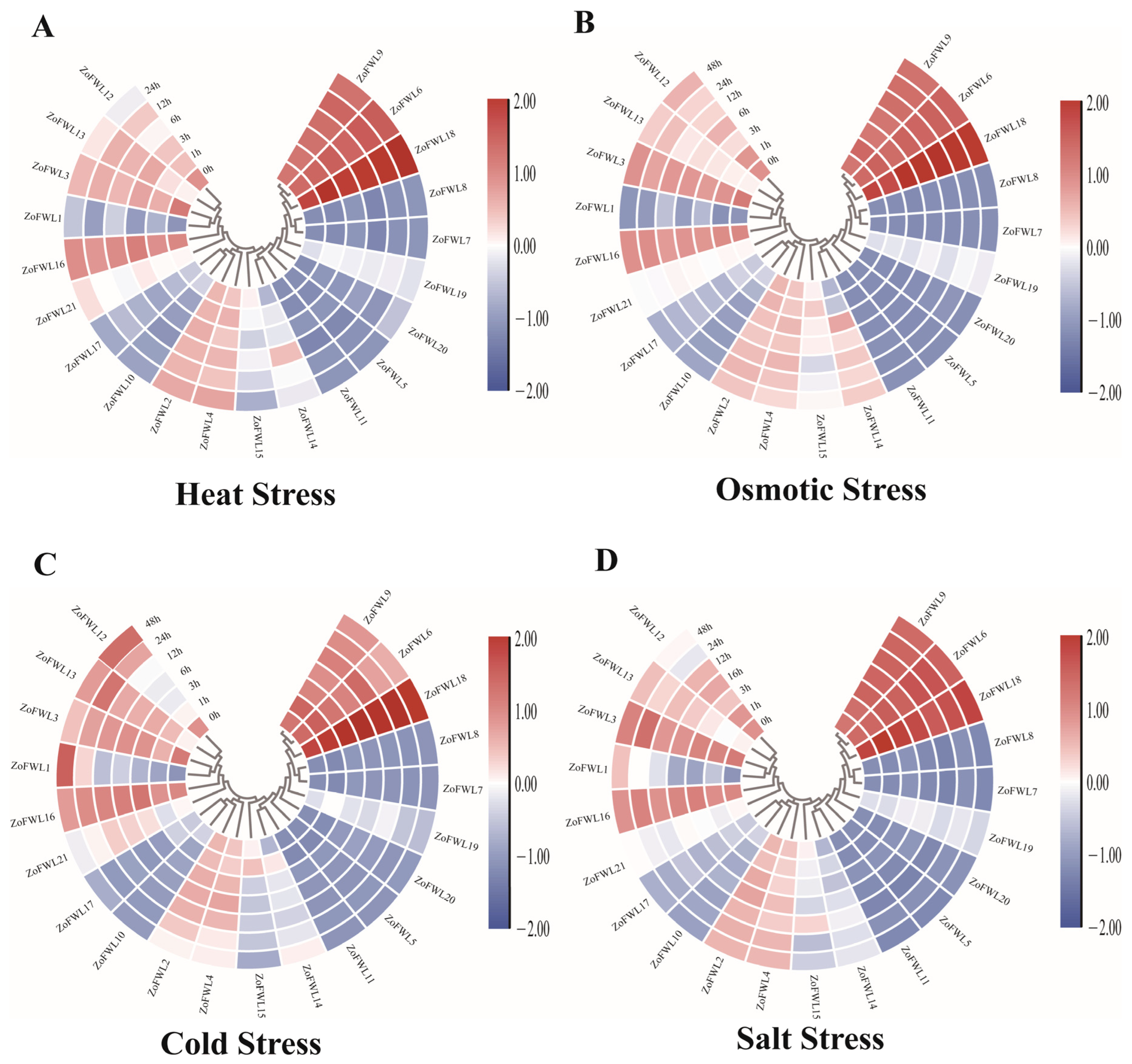
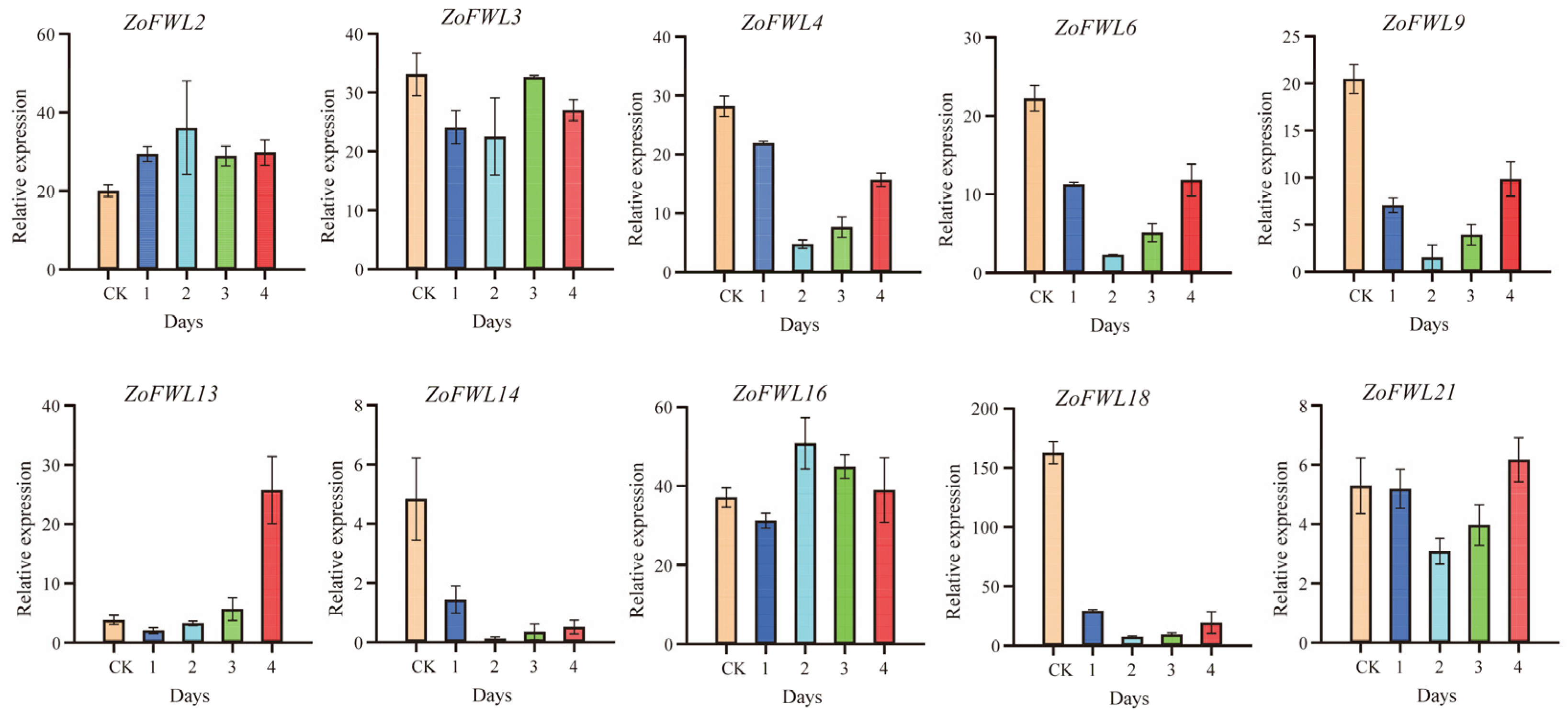
3.8. Expression Patterns of ZoFWL Genes under Abiotic Stress
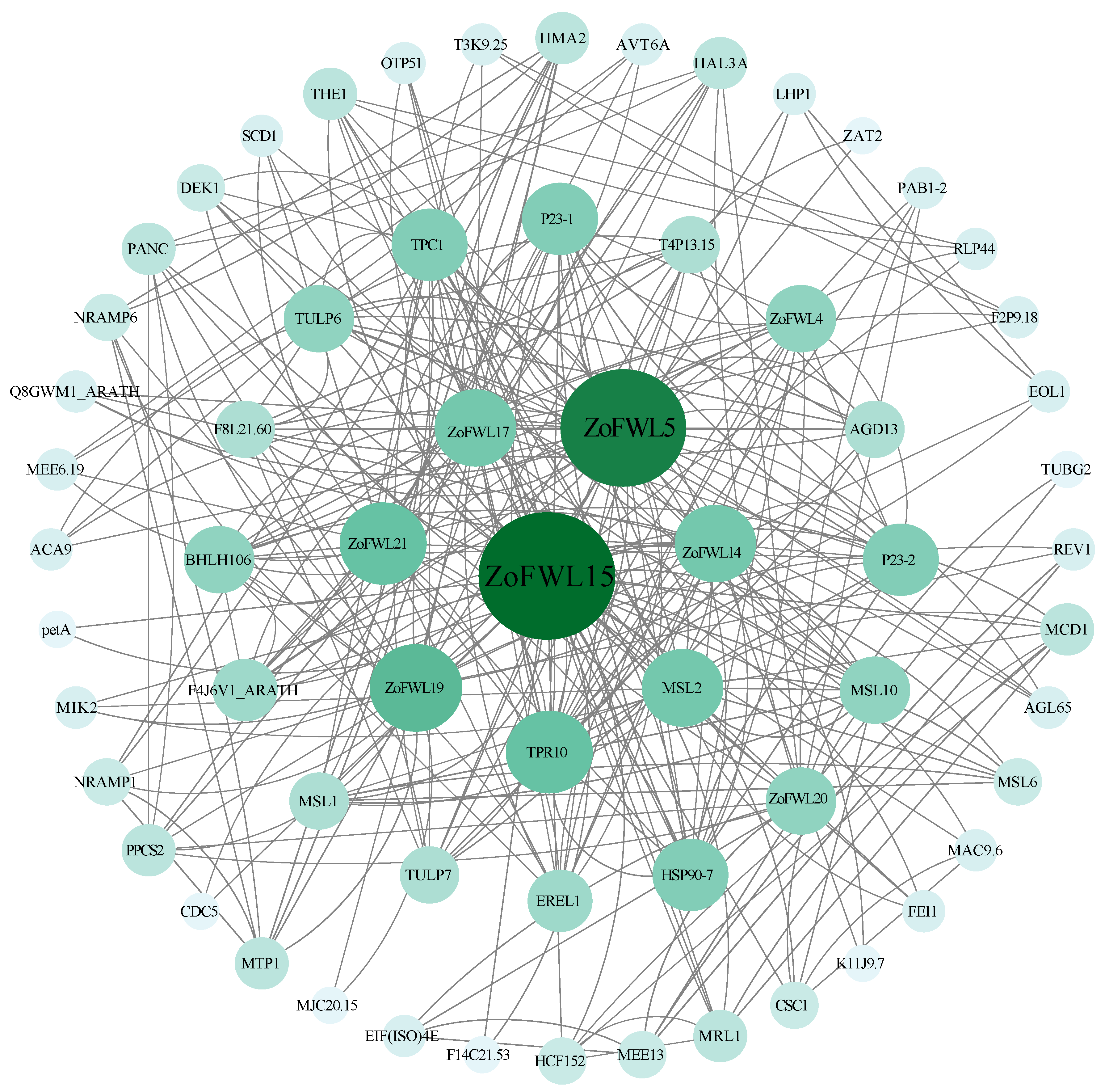
3.9. Prediction of ZoFWL Protein Interaction Network
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guo, M.; Rupe, M.A.; Dieter, J.A.; Zou, J.; Spielbauer, D.; Duncan, K.E.; Howard, R.J.; Hou, Z.; Simmons, C.R. Cell Number Regulator1 Affects Plant and Organ Size in Maize: Implications for Crop Yield Enhancement and Heterosis. Plant Cell 2010, 22, 1057–1073. [Google Scholar] [CrossRef] [PubMed]
- Libault, M.; Stacey, G. Evolution of FW2.2-like (FWL) and PLAC8 genes in eukaryotes. Plant Signal. Behav. 2010, 5, 1226–1228. [Google Scholar] [CrossRef] [PubMed]
- Frary, A.; Nesbitt, T.C.; Frary, A.; Grandillo, S.; Knaap, E.v.d.; Cong, B.; Liu, J.; Meller, J.; Elber, R.; Alpert, K.B.; et al. fw2.2: A Quantitative Trait Locus Key to the Evolution of Tomato Fruit Size. Science 2000, 289, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Galaviz-Hernandez, C.; Stagg, C.; de Ridder, G.; Tanaka, T.S.; Ko, M.S.; Schlessinger, D.; Nagaraja, R. Plac8 and Plac9, novel placental-enriched genes identified through microarray analysis. Gene 2003, 309, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Nesbitt, T.C.; Tanksley, S.D. fw2.2 Directly Affects the Size of Developing Tomato Fruit, with Secondary Effects on Fruit Number and Photosynthate Distribution. Plant Physiol. 2001, 127, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Alpert, K.B.; Grandillo, S.; Tanksley, S.D. fw 2.2:a major QTL controlling fruit weight is common to both red- and green-fruited tomato species. Theor. Appl. Genet. 1995, 91, 994–1000. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Cong, B.; Tanksley, S.D. Generation and Analysis of an Artificial Gene Dosage Series in Tomato to Study the Mechanisms by Which the Cloned Quantitative Trait Locus fw2.2 Controls Fruit Size. Plant Physiol. 2003, 132, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Cong, B.; Tanksley, S.D. FW2.2 and cell cycle control in developing tomato fruit: A possible example of gene co-option in the evolution of a novel organ. Plant Mol. Biol. 2006, 62, 867–880. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, S.L.; Ruderman, J.V. CK2 beta, which inhibits Mos function, binds to a discrete domain in the N-terminus of Mos. Dev. Biol. 2004, 268, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Romero, J.; Espunya, M.C.; Platara, M.; Ariño, J.; Martínez, M.C. A role for protein kinase CK2 in plant development: Evidence obtained using a dominant-negative mutant. Plant J. 2010, 55, 118–130. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Z.; Brechenmacher, L.; Smith, B.; Strout, G.W.; Mangin, W.; Taylor, C.; Russell, S.D.; Stacey, G.; Libault, M. The GmFWL1 (FW2-2-like) nodulation gene encodes a plasma membrane microdomain-associated protein. Plant Cell Environ. 2017, 40, 1442–1455. [Google Scholar] [CrossRef] [PubMed]
- Libault, M.; Zhang, X.C.; Govindarajulu, M.; Qiu, J.; Ong, Y.T.; Brechenmacher, L.; Berg, R.H.; Hurley-Sommer, A.; Taylor, C.G.; Stacey, G. A member of the highly conserved FWL (tomato FW2.2-like) gene family is essential for soybean nodule organogenesis. Plant J. 2010, 62, 852–864. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Xiong, W.; Cao, B.; Yan, T.; Luo, T.; Fan, T.; Luo, M. Molecular characterization and functional analysis of “fruit-weight2.2-like” gene family in rice. Planta 2013, 238, 643–655. [Google Scholar] [CrossRef] [PubMed]
- De Franceschi, P.; Stegmeir, T.; Cabrera, A.; van der Knaap, E.; Rosyara, U.R.; Sebolt, A.M.; Dondini, L.; Dirlewanger, E.; Quero-Garcia, J.; Campoy, J.A.; et al. Cell number regulator genes in Prunus provide candidate genes for the control of fruit size in sweet and sour cherry. Mol. Breed. 2013, 32, 311–326. [Google Scholar] [CrossRef] [PubMed]
- Dahan, Y.; Rosenfeld, R.; Zadiranov, V.; Irihimovitch, V. A proposed conserved role for an avocado fw2.2-like gene as a negative regulator of fruit cell division. Planta 2010, 232, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Song, W.-Y.; Martinoia, E.; Lee, J.; Kim, D.; Kim, D.-Y.; Vogt, E.; Shim, D.; Choi, K.S.; Hwang, I.; Lee, Y. A Novel Family of Cys-Rich Membrane Proteins Mediates Cadmium Resistance in Arabidopsis. Plant Physiol. 2004, 135, 1027–1039. [Google Scholar] [CrossRef] [PubMed]
- Song, W.-Y.; Hörtensteiner, S.; Tomioka, R.; Lee, Y.; Martinoia, E. Common Functions or Only Phylogenetically Related? The Large Family of PLAC8 Motif-Containing/PCR Genes. Mol. Cells 2011, 31, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Wang, P.; Yan, T.; Cao, B.; Xu, J.; Liu, D.; Luo, M. The rice “fruit-weight 2.2-like” gene family member OsFWL4 is involved in the translocation of cadmium from roots to shoots. Planta 2018, 247, 1247–1260. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.J.; Tan, H.F.; Han, J.H.; Zhang, Y.T.; He, X.D.L.; Ding, Y.F.; Chen, Z.X.; Zhu, C. A novel family of PLAC8 motif-containing/PCR genes mediates Cd tolerance and Cd accumulation in rice. Environ. Sci. Eur. 2019, 31, 82. [Google Scholar] [CrossRef]
- Song, W.-Y.; Lee, H.-S.; Jin, S.-R.; Ko, D.; Martinoia, E.; Lee, Y.; An, G.; Ahn, S.-N. Rice PCR1 influences grain weight and Zn accumulation in grains. Plant Cell Environ. 2015, 38, 2327–2339. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Sun, S.; Gao, X.; Wu, M.; Deng, Y.; Zhang, Q.; Li, X.; Xiao, J.; Ke, Y.; Wang, S. Overexpression a “fruit-weight 2.2-like” gene OsFWL5 improves rice resistance. Rice 2019, 12, 51. [Google Scholar] [CrossRef] [PubMed]
- Song, W.-Y.; Choi, K.S.; Kim, D.Y.; Geisler, M.; Park, J.; Vincenzetti, V.; Schellenberg, M.; Kim, S.H.; Lim, Y.P.; Noh, E.W.; et al. Arabidopsis PCR2 Is a Zinc Exporter Involved in Both Zinc Extrusion and Long-Distance Zinc Transport. Plant Cell 2010, 22, 2237–2252. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, Y.; Katagiri, T.; Shinozaki, K.; Qi, Z.; Tatsumi, H.; Furuichi, T.; Kishigami, A.; Sokabe, M.; Kojima, I.; Sato, S.; et al. Arabidopsis plasma membrane protein crucial for Ca2+ influx and touch sensing in roots. Proc. Natl. Acad. Sci. USA 2007, 104, 3639–3644. [Google Scholar] [CrossRef] [PubMed]
- Iida, H. Determination of Structural Regions Important for Ca2+ Uptake Activity in Arabidopsis MCA1 and MCA2 Expressed in Yeast. Plant Cell Physiol. 2011, 52, 1915–1930. [Google Scholar]
- Mori, K.; Renhu, N.; Naito, M.; Nakamura, A.; Shiba, H.; Yamamoto, T.; Suzaki, T.; Iida, H.; Miura, K. Ca2+-permeable mechanosensitive channels MCA1 and MCA2 mediate cold-induced cytosolic Ca2+ increase and cold tolerance in Arabidopsis. Sci. Rep. 2018, 8, 550. [Google Scholar] [CrossRef] [PubMed]
- Ran, C.; Zhang, Y.; Chang, F.; Yang, X.; Liu, Y.; Wang, Q.; Zhu, W. Genome-Wide Analyses of SlFWL Family Genes and Their Expression Profiles under Cold, Heat, Salt and Drought Stress in Tomato. Int. J. Mol. Sci. 2023, 24, 11783. [Google Scholar] [CrossRef] [PubMed]
- Yeh, H.Y.; Chuang, C.H.; Chen, H.C.; Wan, C.J.; Chen, T.L.; Lin, L.Y. Bioactive components analysis of two various gingers Zingiber officinale Roscoe) and antioxidant effect of ginger extracts. LWT-Food Sci. Technol. 2014, 55, 329–334. [Google Scholar] [CrossRef]
- Bartley, J.P.; Jacobs, A.L. Effects of drying on flavour compounds in Australian-grown ginger (Zingiber officinale). J. Sci. Food Agric. 2000, 80, 209–215. [Google Scholar] [CrossRef]
- Zhu, Y.X.; Xi, K.Y.; Ma, H.H.; Yang, P.H.; Wang, Y.H.; Li, H.L.; Yin, J.L.; Qin, M.L.; Liu, Y.Q. Exogenous silica nanoparticles improve drought tolerance in ginger by modulating the water relationship. Environ. Sci. Nano 2024, 11, 1259–1270. [Google Scholar] [CrossRef]
- Liu, M.; Lv, Y.; Cao, B.; Chen, Z.; Xu, K. Physiological and molecular mechanism of ginger (Zingiber officinale Roscoe) seedling response to salt stress. Front. Plant Sci. 2023, 14, 1073434. [Google Scholar] [CrossRef] [PubMed]
- García, M.N.M.; Stritzler, M.; Capiati, D.A. Heterologous expression of Arabidopsis ABF4 gene in potato enhances tuberization through ABA-GA crosstalk regulation. Planta 2014, 239, 615–631. [Google Scholar] [CrossRef]
- Finn, R.D.; Tate, J.; Mistry, J.; Coggill, P.C.; Sammut, S.J.; Hotz, H.-R.; Ceric, G.; Forslund, K.; Eddy, S.R.; Sonnhammer, E.L.L.; et al. The Pfam protein families database. Nucleic Acids Res. 2007, 36, D281–D288. [Google Scholar] [CrossRef]
- Prakash, A.; Jeffryes, M.; Bateman, A.; Finn, R.D. The HMMER Web Server for Protein Sequence Similarity Search. Curr. Protoc. Bioinform. 2017, 60, 3–15. [Google Scholar] [CrossRef]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef]
- Lu, S.; Wang, J.; Chitsaz, F.; Derbyshire, M.K.; Geer, R.C.; Gonzales, N.R.; Gwadz, M.; Hurwitz, D.I.; Marchler, G.H.; Song, J.S.; et al. CDD/SPARCLE: The conserved domain database in 2020. Nucleic Acids Res. 2019, 48, D265–D268. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, M.R.; Gasteiger, E.; Bairoch, A.; Sanchez, J.-C.; Williams, K.L.; Appel, R.D.; Hochstrasser, D.F. Protein Identification and Analysis Tools in the ExPASy Server. In 2-D Proteome Analysis Protocols; Link, A.J., Ed.; Humana Press: Totowa, NJ, USA, 1999; pp. 531–552. [Google Scholar]
- Alzohairy, A. BioEdit: An important software for molecular biology. GERF Bull. Biosci. 2011, 2, 60–61. [Google Scholar]
- Chou, K.-C.; Shen, H.-B. Plant-mPLoc: A Top-Down Strategy to Augment the Power for Predicting Plant Protein Subcellular Localization. PLoS ONE 2010, 5, e11335. [Google Scholar] [CrossRef] [PubMed]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; von Haeseler, A.; Lanfear, R. IQ-TREE 2: New Models and Efficient Methods for Phylogenetic Inference in the Genomic Era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.; Li, W.W.; Noble, W.S. MEME Suite: Tools for motif discovery and searching. Nucleic Acids Res. 2009, 37, W202–W208. [Google Scholar] [CrossRef]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING database in 2023: Protein-protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Kuang, C.; Li, J.; Liu, H.F.; Liu, J.; Sun, X.C.; Zhu, X.Y.; Hua, W. Genome-Wide Identification and Evolutionary Analysis of the Fruit-Weight 2.2-Like Gene Family in Polyploid Oilseed Rape (Brassica napus L.). DNA Cell Biol. 2020, 39, 766–782. [Google Scholar] [CrossRef]
- He, C.; Wang, L.; Yan, L.; Li, Q.; Yong, B.; Zhu, W. Evolutionary developmental mechanisms underlying the origin and diversification of the fruits. Sci. Sin. Vitae 2019, 49, 301–319. [Google Scholar] [CrossRef]
- Arnoys, E.J.; Wang, J.L. Dual localization: Proteins in extracellular and intracellular compartments. Acta Histochem. 2007, 109, 89–110. [Google Scholar] [CrossRef]
- Espunya, M.C.; Combettes, B.; Dot, J.; Chaubet-Gigot, N.; Martinez, M.C. Cell-cycle modulation of CK2 activity in tobacco BY-2 cells. Plant J. 2010, 19, 655–666. [Google Scholar] [CrossRef]
- Titapiwatanakun, B.; Blakeslee, J.J.; Bandyopadhyay, A.; Yang, H.; Mravec, J.; Sauer, M.; Cheng, Y.; Adamec, J.; Nagashima, A.; Geisler, M.; et al. ABCB19/PGP19 stabilises PIN1 in membrane microdomains in Arabidopsis. Plant J. 2009, 57, 27–44. [Google Scholar] [CrossRef]
- Zheng, C.; Yang, Y.-Z.; Luo, X.-F.; Dai, Y.-J.; Liu, W.-G.; Yang, W.-Y.; Shu, K. Current understanding of the roles of phytohormone abscisic acid in the regulation of plant root growth. Plant Sci. 2019, 37, 690–698. [Google Scholar]
- Azzi, L. Study of the FW2.2 Role during Tomato Fruit Development Etude du Rôle de FW2.2 Dans le Développement du Fruit de Tomate; Université Sciences et Technologies—Bordeaux I: Talence, France, 2013. [Google Scholar]
- Gao, Q.; Li, G.; Sun, H.; Xu, M.; Wang, H.; Ji, J.; Wang, D.; Yuan, C.; Zhao, X. Targeted Mutagenesis of the Rice FW 2.2-Like Gene Family Using the CRISPR/Cas9 System Reveals OsFWL4 as a Regulator of Tiller Number and Plant Yield in Rice. Int. J. Mol. Sci. 2020, 21, 809. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Simmons, C.R. Cell number counts—The fw2.2 and CNR genes and implications for controlling plant fruit and organ size. Plant Sci. 2011, 181, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Li, W.L.S.; Rodrigo, A.G. Covariation of Branch Lengths in Phylogenies of Functionally Related Genes. PLoS ONE 2009, 4, e8487. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.C.; He, C.Y. Physalis floridana Cell Number Regulator1 encodes a cell membrane-anchored modulator of cell cycle and negatively controls fruit size. J. Exp. Bot. 2015, 66, 257–270. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Guo, Q.; Pang, X.; Zhang, P.; Kong, D.; Liu, J. New Insights into Evolution of Plant Heat Shock Factors (Hsfs) and Expression Analysis of Tea Genes in Response to Abiotic Stresses. Plants 2020, 9, 311. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.M.; Song, W.J.; Xie, X.M.; Wang, Z.H.; Guan, P.F.; Peng, H.R.; Jiao, Y.N.; Ni, Z.F.; Sun, Q.X.; Guo, W.L. A Collinearity-Incorporating Homology Inference Strategy for Connecting Emerging Assemblies in the Triticeae Tribe as a Pilot Practice in the Plant Pangenomic Era. Mol. Plant 2020, 13, 1694–1708. [Google Scholar] [CrossRef] [PubMed]
- Song, R.; Messing, J. Gene expression of a gene family in maize based on noncollinear haplotypes. Proc. Natl. Acad. Sci. USA 2003, 100, 9055–9060. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Zhang, J. Gene Complexity and Gene Duplicability. Curr. Biol. 2005, 15, 1016–1021. [Google Scholar] [CrossRef] [PubMed]
- Freeling, M. Bias in Plant Gene Content Following Different Sorts of Duplication: Tandem, Whole-Genome, Segmental, or by Transposition. Annu. Rev. Plant Biol. 2009, 60, 433–453. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.H.; Shangguan, L.F.; Ma, R.J.; Sun, X.; Tao, R.; Guo, L.; Korir, N.K.; Yu, M.L. Genome-wide analysis of the AP2/ERF superfamily in peach (Prunus persica). Genet. Mol. Res. 2012, 11, 4789–4809. [Google Scholar] [CrossRef] [PubMed]
- Pei, M.; Xie, X.; Peng, B.; Chen, X.; Chen, Y.; Li, Y.; Wang, Z.; Lu, G. Identification and Expression Analysis of Phosphatidylinositol Transfer Proteins Genes in Rice. Plants 2023, 12, 2122. [Google Scholar] [CrossRef]
- Wolf, Y.I.; Carmel, L.; Koonin, E.V. Unifying measures of gene function and evolution. Proc. Biol. Sci. 2006, 273, 1507–1515. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Lyu, H.-M.; Zhu, K.; Van de Peer, Y.; Cheng, Z.-M. The emergence and evolution of intron-poor and intronless genes in intron-rich plant gene families. Plant J. 2021, 105, 1072–1082. [Google Scholar] [CrossRef]
- Marand, A.P.; Eveland, A.L.; Kaufmann, K.; Springer, N.M. cis-Regulatory Elements in Plant Development, Adaptation, and Evolution. Annu. Rev. Plant Biol. 2023, 74, 111–137. [Google Scholar] [CrossRef]
- Fujita, Y.; Fujita, M.; Satoh, R.; Maruyama, K.; Parvez, M.M.; Seki, M.; Hiratsu, K.; Ohme-Takagi, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. AREB1 Is a Transcription Activator of Novel ABRE-Dependent ABA Signaling That Enhances Drought Stress Tolerance in Arabidopsis. Plant Cell 2005, 17, 3470–3488. [Google Scholar] [CrossRef]
- Robert-Seilaniantz, A.; Grant, M.; Jones, J.D.G. Hormone Crosstalk in Plant Disease and Defense: More Than Just JASMONATE-SALICYLATE Antagonism. Annu. Rev. Phytopathol. 2011, 49, 317–343. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-Y.; Suzuki, A.; Washida, H.; Takaiwa, F. The GCN4 motif in a rice glutelin gene is essential for endosperm-specific gene expression and is activated by Opaque-2 in transgenic rice plants. Plant J. 1998, 14, 673–683. [Google Scholar] [CrossRef]
- Khuman, A.; Yadav, V.; Chaudhary, B. Evolutionary dynamics of the cytoskeletal profilin gene family in Brassica juncea L. reveal its roles in silique development and stress resilience. Int. J. Biol. Macromol. 2024, 266, 131247. [Google Scholar] [CrossRef]
- Wei, K.; Han, P. Comparative functional genomics of the TPR gene family in Arabidopsis, rice and maize. Mol. Breed. 2017, 37, 152. [Google Scholar] [CrossRef]
- Prasad, B.D.; Goel, S.; Krishna, P. In Silico Identification of Carboxylate Clamp Type Tetratricopeptide Repeat Proteins in Arabidopsis and Rice As Putative Co-Chaperones of Hsp90/Hsp70. PLoS ONE 2010, 5, e12761. [Google Scholar] [CrossRef] [PubMed]
- Paeng, S.K.; Kang, C.H.; Chi, Y.H.; Chae, H.B.; Lee, E.S.; Park, J.H.; Wi, S.D.; Bae, S.B.; Phan, K.A.; Lee, S.Y. AtTPR10 Containing Multiple ANK and TPR Domains Exhibits Chaperone Activity and Heat-Shock Dependent Structural Switching. Appl. Sci. 2020, 10, 1265. [Google Scholar] [CrossRef]
- Lee, J.S.; Wilson, M.E.; Richardson, R.A.; Haswell, E.S. Genetic and physical interactions between the organellar mechanosensitive ion channel homologs MSL1, MSL2, and MSL3 reveal a role for inter-organellar communication in plant development. Plant Direct 2019, 3, e00124. [Google Scholar] [CrossRef] [PubMed]
- Haswell, E.S.; Meyerowitz, E.M. MscS-like Proteins Control Plastid Size and Shape in Arabidopsis thaliana. Curr. Biol. 2006, 16, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, T.; Nakagawa, Y.; Mori, K.; Nakano, M.; Imamura, T.; Kataoka, H.; Terashima, A.; Iida, K.; Kojima, I.; Katagiri, T.; et al. MCA1 and MCA2 That Mediate Ca2+ Uptake Have Distinct and Overlapping Roles in Arabidopsis. Plant Physiol. 2010, 152, 1284–1296. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhu, J.; Gong, Z.; Zhu, J.-K. Abiotic stress responses in plants. Nat. Rev. Genet. 2022, 23, 104–119. [Google Scholar] [CrossRef] [PubMed]
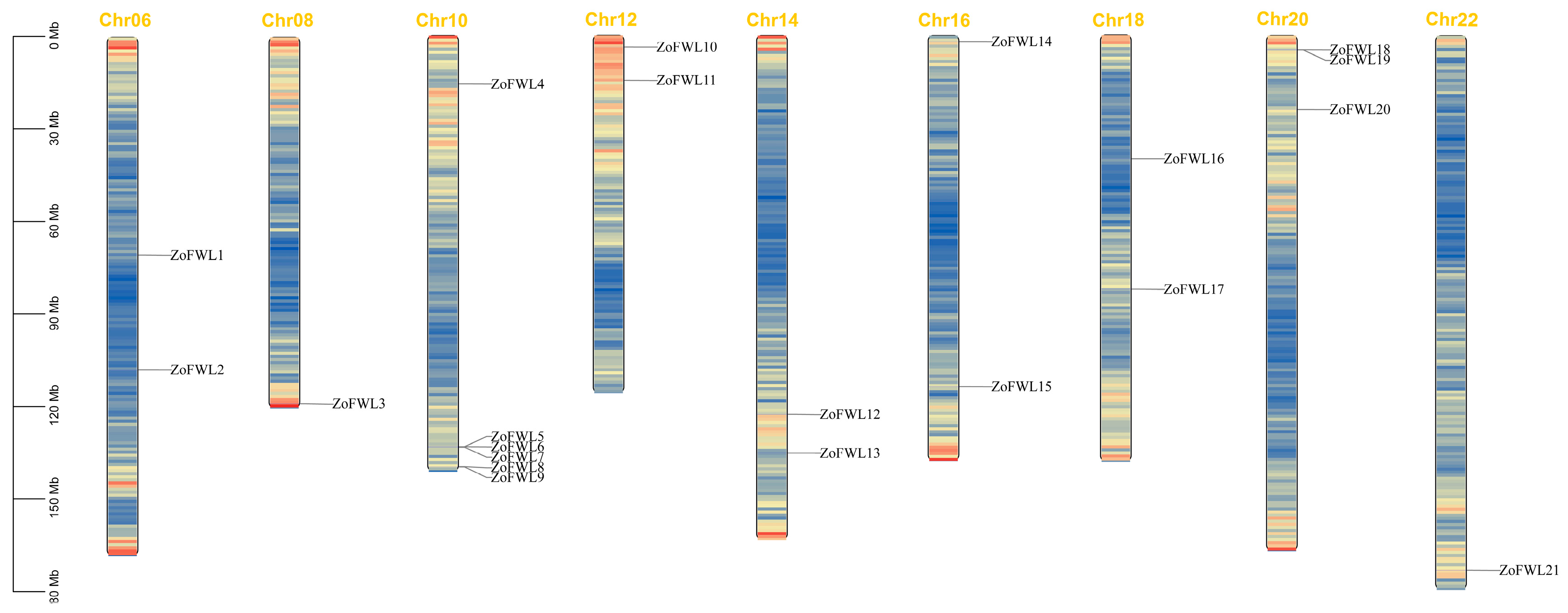
| Gene Name | Gene ID | Chromosome | Localization (bp) | Animo Acid Number | Molecular Weight (kDa) | PI | Subcellular Localization |
|---|---|---|---|---|---|---|---|
| ZoFWL1 | Maker00025051 | Chr06 | 70661613–70663046(+) | 477 | 53.43 | 8.46 | Cell membrane Nucleus |
| ZoFWL2 | Maker00026518 | Chr06 | 107790889–107801973(+) | 239 | 26.76 | 5.03 | Cell membrane Chloroplast |
| ZoFWL3 | Maker00016179 | Chr08 | 118773164–118778419(−) | 551 | 62.51 | 8.02 | Cell membrane |
| ZoFWL4 | Maker00009473 | Chr10 | 15788317–15794441(+) | 239 | 26.58 | 4.88 | Cell membrane |
| ZoFWL5 | Maker00032137 | Chr10 | 133341584–133342428(−) | 123 | 13.24 | 4.46 | Cell membrane |
| ZoFWL6 | Maker00032080 | Chr10 | 133355752–133356594(+) | 203 | 21.90 | 6.34 | Cell membrane |
| ZoFWL7 | Maker00032144 | Chr10 | 133496230–133497188(+) | 204 | 22.30 | 5.69 | Cell membrane |
| ZoFWL8 | Maker00030347 | Chr10 | 139765351–139766271(−) | 204 | 22.36 | 5.87 | Cell membrane |
| ZoFWL9 | Maker00030271 | Chr10 | 139875528–139876205(−) | 167 | 18.08 | 6.37 | Cell membrane |
| ZoFWL10 | Maker00020626 | Chr12 | 3849932–3854545(−) | 357 | 40.03 | 6.92 | Cell membrane Nucleus |
| ZoFWL11 | Maker00042686 | Chr12 | 14606275–14607401(+) | 217 | 23.71 | 4.87 | Cell membrane |
| ZoFWL12 | Maker00035127 | Chr14 | 122859462–122864489(−) | 273 | 30.00 | 4.93 | Cell membrane |
| ZoFWL13 | Maker00014483 | Chr14 | 135425628–135429693(−) | 277 | 30.37 | 5.43 | Cell membrane |
| ZoFWL14 | Maker00004172 | Chr16 | 2000297–2001809(+) | 369 | 40.34 | 4.92 | Cell membrane Nucleus |
| ZoFWL15 | Maker00009037 | Chr16 | 113827519–113842946(−) | 737 | 83.68 | 6.11 | Cell membrane Nucleus |
| ZoFWL16 | Maker00012794 | Chr18 | 40089638–40092331(+) | 244 | 26.93 | 5.36 | Cell membrane |
| ZoFWL17 | Maker00028616 | Chr18 | 82462280–82465007(+) | 228 | 25.25 | 4.52 | Cell membrane |
| ZoFWL18 | Maker00022350 | Chr20 | 4671208–4672823(−) | 182 | 19.83 | 6.83 | Cell membrane |
| ZoFWL19 | Maker00022475 | Chr20 | 4717902–4718711(−) | 170 | 18.76 | 6.75 | Cell membrane |
| ZoFWL20 | Maker00026904 | Chr20 | 24062166–24063058(+) | 176 | 19.06 | 7.47 | Cell membrane |
| ZoFWL21 | Maker00001158 | Chr22 | 173270323–173283255(+) | 222 | 24.11 | 4.97 | Cell membrane |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Y.; Tang, S.; Xia, M.; Li, H.; Xiao, D.; Li, X.; Xing, H.; Wang, B.; Huang, H.; Zhou, S.; et al. Systematic Analysis and Expression Profiling of the Ginger FWL Gene Family Reveal Its Potential Functions in Rhizome Development and Response to Abiotic Stress. Agronomy 2024, 14, 1805. https://doi.org/10.3390/agronomy14081805
Jiang Y, Tang S, Xia M, Li H, Xiao D, Li X, Xing H, Wang B, Huang H, Zhou S, et al. Systematic Analysis and Expression Profiling of the Ginger FWL Gene Family Reveal Its Potential Functions in Rhizome Development and Response to Abiotic Stress. Agronomy. 2024; 14(8):1805. https://doi.org/10.3390/agronomy14081805
Chicago/Turabian StyleJiang, Yajun, Shihao Tang, Maoqin Xia, Hui Li, Daoyan Xiao, Xingyue Li, Haitao Xing, Biao Wang, Hao Huang, Shengmao Zhou, and et al. 2024. "Systematic Analysis and Expression Profiling of the Ginger FWL Gene Family Reveal Its Potential Functions in Rhizome Development and Response to Abiotic Stress" Agronomy 14, no. 8: 1805. https://doi.org/10.3390/agronomy14081805




