Nanopriming-Induced Enhancement of Cucumber Seedling Development: Exploring Biochemical and Physiological Effects of Silver Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Green Synthesis of Silver Nanoparticles (AgNPs) and Reaction Mixture Preparation
2.2. Characterization of Silver Nanoparticles
- UV-Visible Spectrophotometry: The UV-visible absorption spectra of the Ag nanoparticle solutions were recorded between 300 and 500 nm using a UV-visible spectrophotometer.
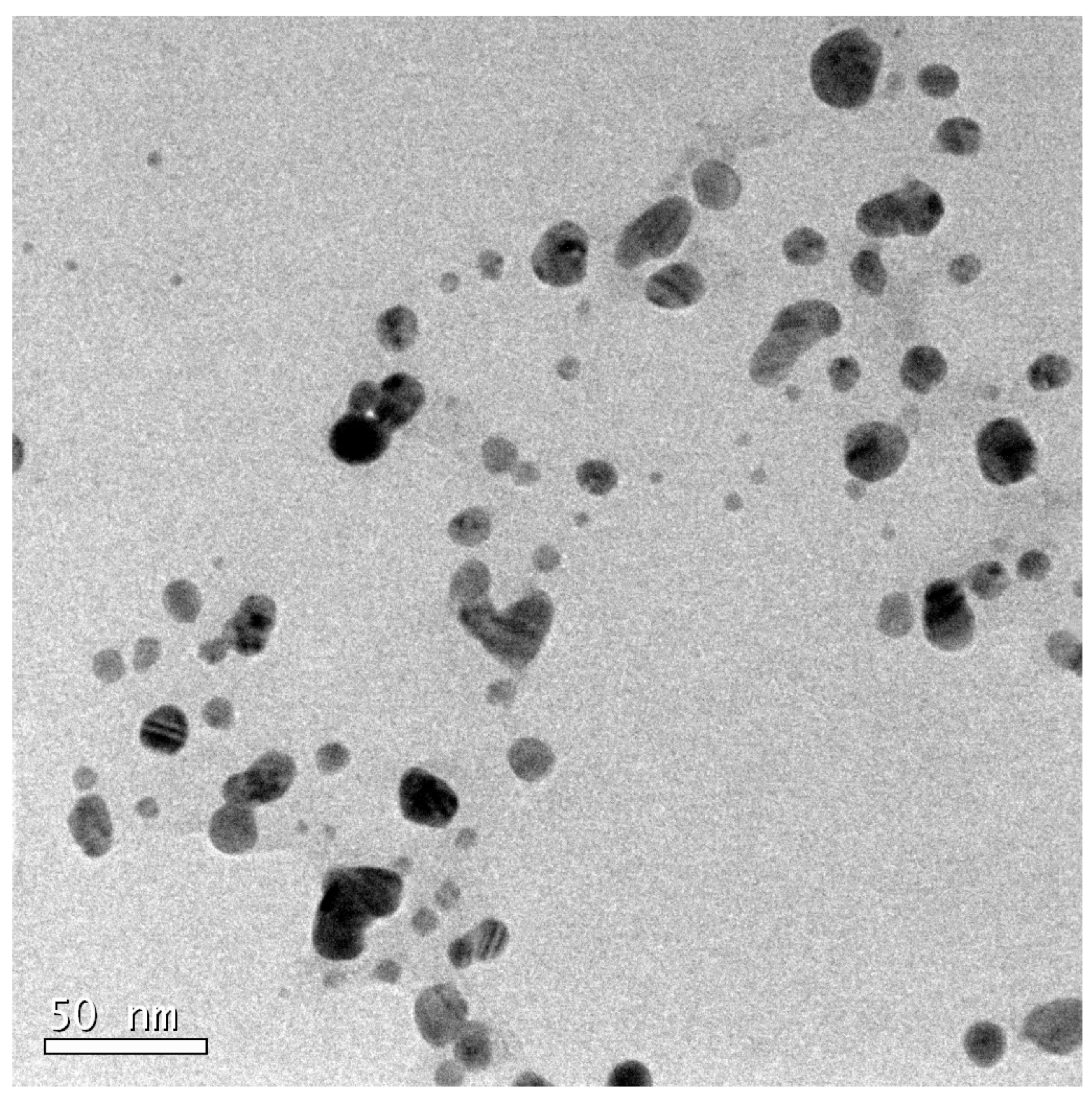
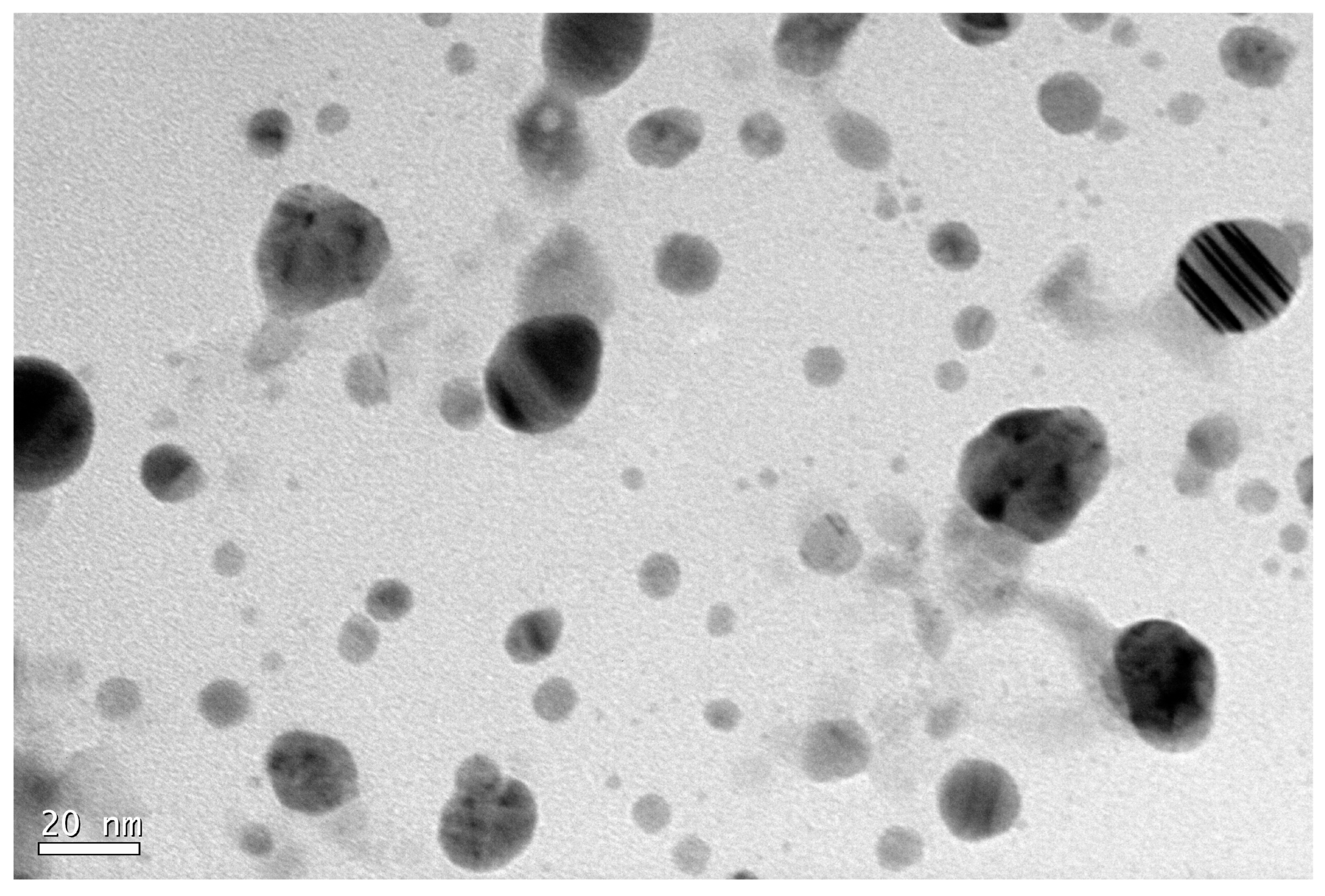
- Transmission Electron Microscopy (TEM): Nanoparticles were suspended in 1 mL of water. A drop of this solution was placed on a carbon-coated copper grid and air-dried. TEM analysis was performed using a JEOL2100 microscope at the Centro Nacional de Microscopía Electrónica, Madrid.
- X-ray Powder Diffraction (XRD): XRD profiles were obtained using an Empyrean Cu LFF X-ray diffractometer within a 2θ range from 2° to 80°. The analyses were conducted at the Research Support Centre (CAI) for X-Ray Diffraction, Universidad Complutense de Madrid. The resulting diffraction patterns were compared with reference patterns from the International Centre for Diffraction Data (ICDD) database.
- Fourier-Transform Infrared Spectroscopy (FTIR): FTIR measurements were carried out using a JASCO (FT/IR-6200) spectrophotometer at the CAI of Physical and Chemical Techniques, Complutense University of Madrid. The interpretation of results followed guidelines provided in infrared spectroscopy tables [14].
2.3. Nanopriming of Cucumber Seeds with AgNPs and In Vitro Cultivation
2.4. In Vitro Cultivation of Cucumber Seedlings
2.5. Measurement of Morphological Parameters during Germination
2.6. Measurement of Photosynthetic Parameters
2.7. Measurement of Biochemical Parameters
2.8. Statistical Analysis
3. Results
3.1. Synthesis and Characterization of Silver Nanoparticles Using Reducing Agents
3.2. Morphological Parameters
3.2.1. No Adverse Effects on Germination Observed with Nanopriming Treatments
3.2.2. Silver Nanoparticle Treatments Enhanced Both Shoot and Root Lengths in Cucumber Seedlings
3.3. Photosynthetic Parameters
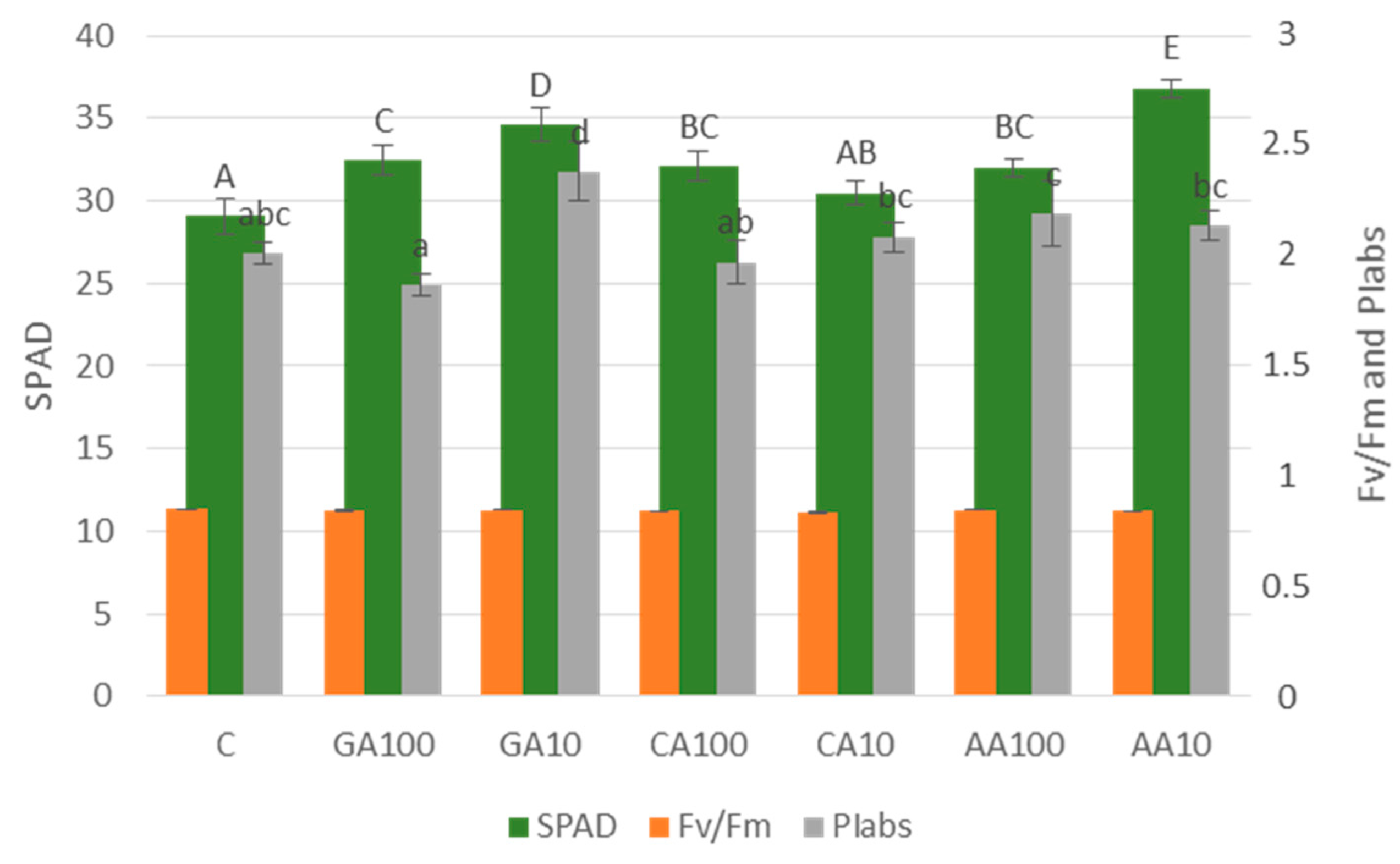
3.3.1. Nano-Priming Treatments Significantly Improved the Chlorophyll Index of Cucumber Seedlings
3.3.2. Chlorophyll Fluorescence
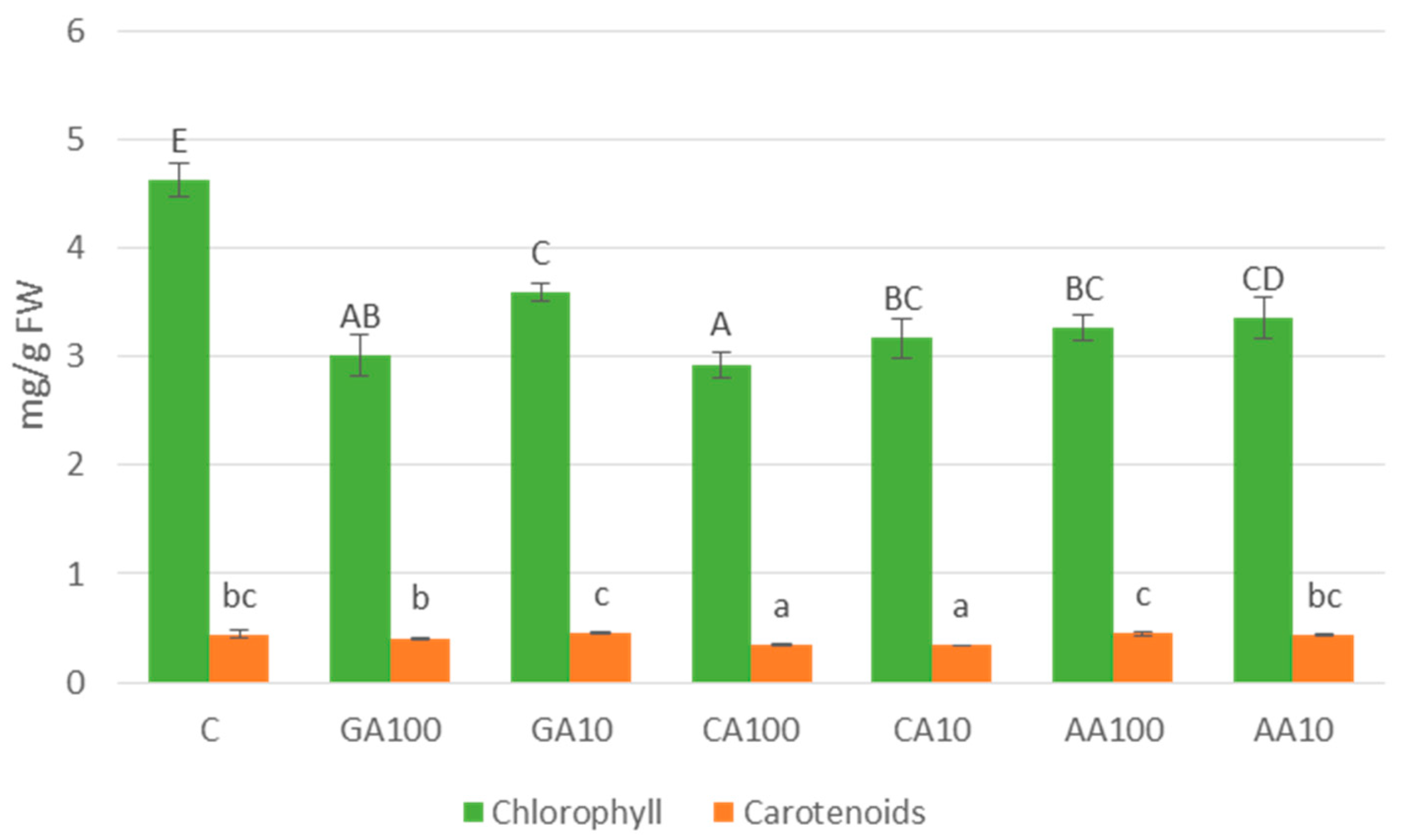
3.3.3. Photosynthetic Pigment Content: Chlorophyll and Carotenoids
3.4. Biochemical Parameters
3.4.1. Malondialdehyde Content Varied with Different Nano-Priming Treatments, Indicating Changes in Oxidative Stress Levels
3.4.2. Silver Nanoparticles Induced Significant Changes in Free Amino Acids Content in Cucumber Seedlings
3.4.3. Variation in Total Phenolic Compounds and Flavonoid Contents Observed across Different Nano-Priming Treatments
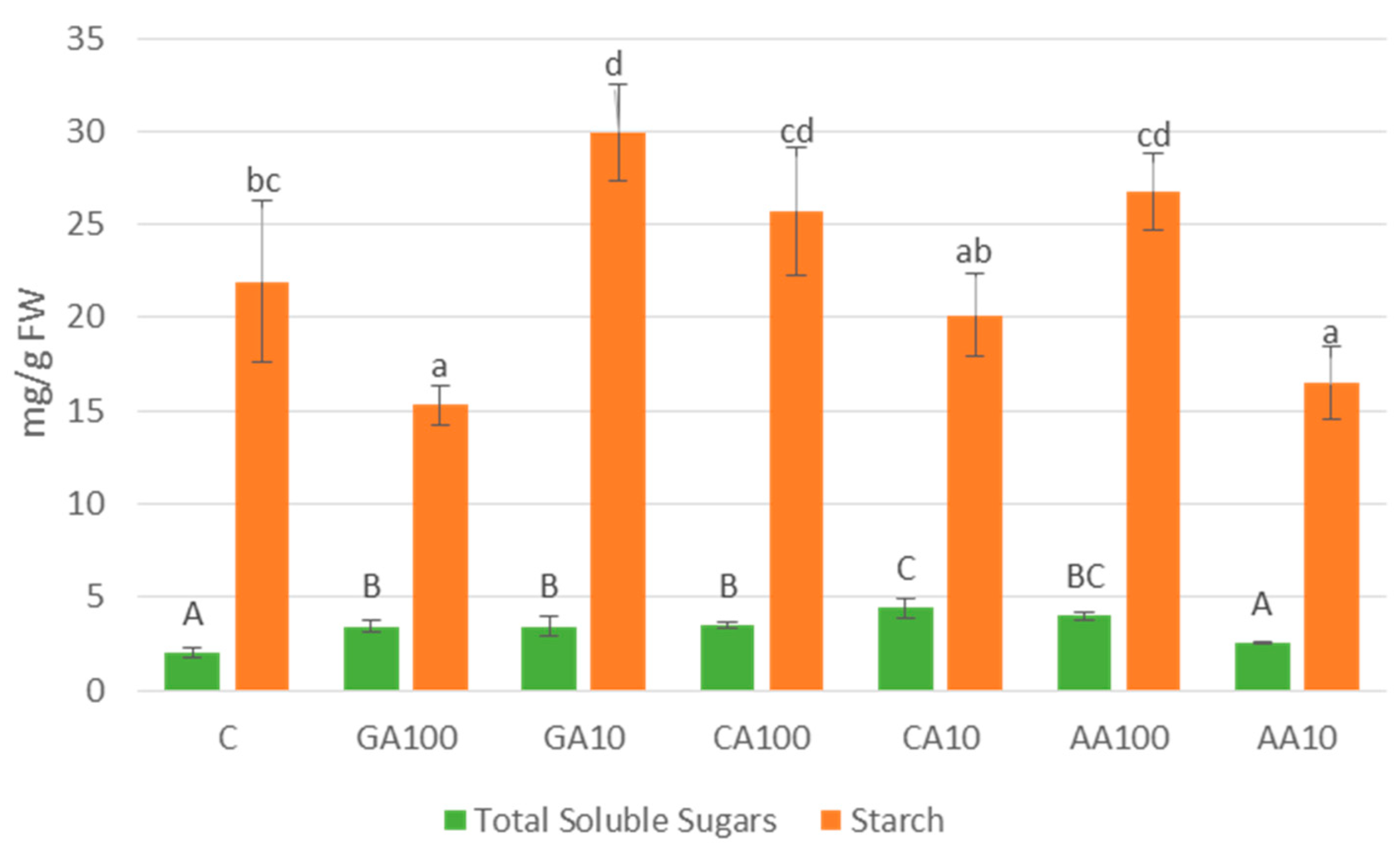
3.4.4. Nano-Priming Treatments Altered Total Soluble Sugars and Starch Contents in Cucumber Seedlings
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Batool, S.U.; Javed, B.; Sohail; Zehra, S.S.; Mashwani, Z.-u.-R.; Raja, N.I.; Khan, T.; ALHaithloul, H.A.S.; Alghanem, S.M.; Al-Mushhin, A.A.M.; et al. Exogenous Applications of Bio-fabricated Silver Nanoparticles to Improve Biochemical, Antioxidant, Fatty Acid and Secondary Metabolite Contents of Sunflower. Nanomaterials 2021, 11, 1750. [Google Scholar] [CrossRef]
- León-Silva, S.; Fernández-Luqueño, F.; López-Valdez, F. Silver Nanoparticles (AgNP) in the Environment: A Review of Potential Risks on Human and Environmental Health. Water Air Soil Pollut. 2016, 227, 306. [Google Scholar] [CrossRef]
- Elgorban, A.M.; El-Samawaty, A.E.-R.M.; Yassin, M.A.; Sayed, S.R.; Adil, S.F.; Elhindi, K.M.; Bakri, M.; Khan, M. Antifungal silver nanoparticles: Synthesis, characterization and biological evaluation. Biotechnol. Biotechnol. Equip. 2016, 30, 56–62. [Google Scholar] [CrossRef]
- Kumar, V.K.; Muthukrishnan, S.; Rajalakshmi, R. Phytostimulatory effect of phytochemical fabricated nanosilver (AgNPs) on Psophocarpus tetragonolobus (L.) DC. seed germination: An insight from antioxidative enzyme activities and genetic similarity studies. Curr. Plant Biol. 2020, 23, 100158. [Google Scholar] [CrossRef]
- Mishra, S.; Singh, H.B. Biosynthesized silver nanoparticles as a nanoweapon against phytopathogens: Exploring their scope and potential in agriculture. Appl. Microbiol. Biotechnol. 2015, 99, 1097–1107. [Google Scholar] [CrossRef]
- Shelar, A.; Singh, A.V.; Maharjan, R.S.; Laux, P.; Luch, A.; Gemmati, D.; Tisato, V.; Singh, S.P.; Santilli, M.F.; Shelar, A.; et al. Sustainable agriculture through multi-disciplinary seed nanopriming: Prospects of opportunities and challenges. Cells 2021, 10, 2428. [Google Scholar] [CrossRef]
- Abdelghany, T.M.; Al-Rajhi, A.M.H.; Al Abboud, M.A.; Alawlaqi, M.M.; Magdah, A.G.; Helmy, E.A.M.; Mabrouk, A.S. Recent Advances in Green Synthesis of Silver Nanoparticles and Their Applications: About Future Directions. A Review. BioNanoScience 2017, 8, 5–16. [Google Scholar] [CrossRef]
- Ovais, M.; Khalil, A.T.; Raza, A.; Khan, M.A.; Ahmad, I.; Islam, N.U.; Saravanan, M.; Ubaid, M.F.; Ali, M.; Shinwari, Z.K. Green synthesis of silver nanopar-ticles via plant extracts: Beginning a new era in cancer theranostics. Nanomedicine 2016, 12, 3157–3177. [Google Scholar] [CrossRef]
- Nejatzadeh, F. Effect of silver nanoparticles on salt tolerance of Satureja hortensis l. during in vitro and in vivo germination tests. Heliyon 2021, 7, e05981. [Google Scholar] [CrossRef]
- Oukarroum, A.; Barhoumi, L.; Pirastru, L.; Dewez, D. Silver nanoparticle toxicity effect on growth and cellular viability of the aquatic plant Lemna gibba. Environ. Toxicol. Chem. 2013, 32, 902–907. [Google Scholar] [CrossRef]
- Thuesombat, P.; Hannongbua, S.; Akasit, S.; Chadchawan, S. Effect of silver nanoparticles on rice (Oryza sativa L. cv. KDML 105) seed germination and seedling growth. Ecotoxicol. Environ. Saf. 2014, 104, 302–309. [Google Scholar] [CrossRef]
- Mallik, J.; Das, P.; Das, S. Pharmacological activity of Cucumis sativus L.—A complete overview. Asian J. Pharm. Res. Dev. 2013, 1, 1–6. [Google Scholar]
- Sharma, P.; Bhatt, D.; Zaidi, M.G.H.; Saradhi, P.P.; Khanna, P.K.; Arora, S. Silver Nanoparticle-Mediated Enhancement in Growth and Antioxidant Status of Brassica juncea. Appl. Biochem. Biotechnol. 2012, 167, 2225–2233. [Google Scholar] [CrossRef] [PubMed]
- Callejas, F.R. Tablas de Espectroscopía Infrarroja; Departamento de Física y Química, UNAM (Universidad Nacional Autónoma de México): Mexico City, Mexico, 2000. [Google Scholar]
- López-Hidalgo, C.; Meijón, M.; Lamelas, L.; Valledor, L. The rainbow protocol: A sequential method for quantifying pigments, sugars, free amino acids, phenolics, flavonoids and MDA from a small amount of sample. Plant Cell Environ. 2021, 44, 1977–1986. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, M.; Jin, Y.; Fan, X.; Xu, J.; Zhu, Y.; Fu, Z.; Pan, X.; Qian, H. The Effects of Low Concentrations of Silver Nanoparticles on Wheat Growth, Seed Quality, and Soil Microbial Communities. Water Air Soil Pollut. 2017, 228, 348. [Google Scholar] [CrossRef]
- Al-Zahrani, S.; Astudillo-Calderón, S.; Pintos, B.; Pérez-Urria, E.; Manzanera, J.A.; Martín, L.; Gomez-Garay, A. Role of Synthetic Plant Extracts on the Production of Silver-Derived Nanoparticles. Plants 2021, 10, 1671. [Google Scholar] [CrossRef]
- Xie, W.; Guo, Z.; Gao, F.; Gao, Q.; Wang, D.; Liaw, B.-S.; Cai, Q.; Sun, X.; Wang, X.; Zhao, L. Shape-, size- and structure-controlled synthesis and biocompatibility of iron oxide nanoparticles for magnetic theranostics. Theranostics 2018, 8, 3284–3307. [Google Scholar] [CrossRef]
- Ghodake, G.; Shinde, S.; Kadam, A.; Saratale, R.G.; Saratale, G.D.; Syed, A.; Shair, O.; Alsaedi, M.; Kim, D.-Y. Gallic acid-functionalized silver nanoparticles as colorimetric and spectrophotometric probe for detection of Al3+ in aqueous medium. J. Ind. Eng. Chem. 2019, 82, 243–253. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, Y.; Yang, X.; Zheng, X.; Wen, S.; Wang, F.; Vidal, X.; Zhao, J.; Liu, D.; Zhou, Z.; et al. Amplified stimulated emission in upconversion nanoparticles for super-resolution nanoscopy. Nature 2017, 543, 229–233. [Google Scholar] [CrossRef]
- Savithramma, N.; Ankanna, S.; Bhumi, G. Effect of nanoparticles on seed germination and seedling growth of Boswellia ovalifoliolata an endemic and endangered medicinal tree taxon. Nano Vis. 2012, 2, 2. [Google Scholar]
- Hojjat, S.S.; Hojjat, H. Effect of Nano Silver on Seed Germination and Seedling Growth in Fenugreek Seed. ETP Int. J. Food Eng. 2015, 1, 106–110. [Google Scholar] [CrossRef]
- Rezvani, N.; Sorooshzadeh, A.; Farhadi, N. Effect of nano-silver on growth of saffron in flooding stress. Int. J. Agric. Biosyst. Eng. 2012, 6, 11–16. [Google Scholar]
- Štefanić, P.P.; Jarnević, M.; Cvjetko, P.; Biba, R.; Šikić, S.; Tkalec, M.; Cindrić, M.; Letofsky-Papst, I.; Balen, B. Comparative proteomic study of phytotoxic effects of silver nanoparticles and silver ions on tobacco plants. Environ. Sci. Pollut. Res. 2019, 26, 22529–22550. [Google Scholar] [CrossRef]
- Yasur, J.; Rani, P.U. Environmental effects of nanosilver: Impact on castor seed germination, seedling growth, and plant physiology. Environ. Sci. Pollut. Res. 2013, 20, 8636–8648. [Google Scholar] [CrossRef]
- Gomez-Garay, A.; Pintos, B.; Manzanera, J.A.; Lobo, C.; Villalobos, N.; Martín, L. Uptake of CeO2 Nanoparticles and Its Effect on Growth of Medicago arborea In Vitro Plantlets. Biol. Trace Element Res. 2014, 161, 143–150. [Google Scholar] [CrossRef]
- Tripathi, D.; Singh, M.; Pandey-Rai, S. Crosstalk of nanoparticles and phytohormones regulate plant growth and metabolism under abiotic and biotic stress. Plant Stress 2022, 6, 100107. [Google Scholar] [CrossRef]
- Gomez-Garay, A.; Pintos, B.; Manzanera, J.A.; Prada, C.; Martin, L.; Galan, J.M.G.Y. Nanoceria and bulk cerium oxide effects on the germination of asplenium adiantum-nigrum spores. For. Syst. 2016, 25, e067. [Google Scholar] [CrossRef]
- El-Temsah, Y.S.; Joner, E.J. Impact of Fe and Ag nanoparticles on seed germination and differences in bioavailability during exposure in aqueous suspension and soil. Environ. Toxicol. 2011, 27, 42–49. [Google Scholar] [CrossRef]
- Dimkpa, C.O.; McLean, J.E.; Martineau, N.; Britt, D.W.; Haverkamp, R.; Anderson, A.J. Silver nanoparticles disrupt wheat (Triticum aestivum L.) growth in a sand matrix. Environ. Sci. Technol. 2013, 47, 1082–1090. [Google Scholar] [CrossRef]
- Mehmood, A. Brief overview of the application of silver nanoparticles to improve growth of crop plants. IET Nanobiotechnol. 2018, 12, 701–705. [Google Scholar] [CrossRef]
- Uddling, J.; Gelang-Alfredsson, J.; Piikki, K.; Pleijel, H. Evaluating the relationship between leaf chlorophyll concentration and SPAD-502 chlorophyll meter readings. Photosynth. Res. 2007, 91, 37–46. [Google Scholar] [CrossRef]
- Mourato, M.; Reis, R.; Martins, L.L. Characterization of plant antioxidative system in response to abiotic stresses: A focus on heavy metal toxicity. Adv. Sel. Plant Physiol. Asp. 2012, 12, 1–17. [Google Scholar]
- Yan, A.; Chen, Z. Impacts of Silver Nanoparticles on Plants: A Focus on the Phytotoxicity and Underlying Mechanism. Int. J. Mol. Sci. 2019, 20, 1003. [Google Scholar] [CrossRef]
- Cvjetko, P.; Zovko, M.; Štefanić, P.P.; Biba, R.; Tkalec, M.; Domijan, A.M.; Vrček, I.V.; Letofsky-Papst, I.; Šikić, S.; Balen, B. Phytotoxic effects of silver nanoparticles in tobacco plants. Environ. Sci. Pollut. Res. 2018, 25, 5590–5602. [Google Scholar] [CrossRef]
- Gupta, S.D.; Agarwal, A.; Pradhan, S. Phytostimulatory effect of silver nanoparticles (AgNPs) on rice seedling growth: An insight from antioxidative enzyme activities and gene expression patterns. Ecotoxicol. Environ. Saf. 2018, 161, 624–633. [Google Scholar] [CrossRef] [PubMed]
- De la Torre-Roche, R.; Hawthorne, J.; Musante, C.; Xing, B.; Newman, L.A.; Ma, X.; White, J.C. Impact of Ag nanoparticle exposure on p,p′-DDE bioaccumulation by Cucurbita pepo (Zucchini) and Glycine max (Soybean). Environ. Sci. Technol. 2013, 47, 718–725. [Google Scholar] [CrossRef] [PubMed]
- Nair, P.M.G.; Chung, I.M. Assessment of silver nanoparticle-induced physiological and molecular changes in Arabidopsis thaliana. Environ. Sci. Pollut. Res. 2014, 21, 8858–8869. [Google Scholar] [CrossRef]
- Karami Mehrian, S.; Heidari, R.; Rahmani, F. Effect of silver nanoparticles on free amino acids content and antioxidant defense system of tomato plants. Indian J. Plant Physiol. 2015, 20, 257–263. [Google Scholar] [CrossRef]
- Liu, Y.-S.; Chang, Y.-C.; Chen, H.-H. Silver nanoparticle biosynthesis by using phenolic acids in rice husk extract as reducing agents and dispersants. J. Food Drug Anal. 2018, 26, 649–656. [Google Scholar] [CrossRef]
- Zhang, H.; Du, W.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L.; White, J.C.; Keller, A.A.; Guo, H.; Ji, R.; Zhao, L. Metabolomics Reveals How Cucumber (Cucumis sativus) Reprograms Metabolites To Cope with Silver Ions and Silver Nanoparticle-Induced Oxidative Stress. Environ. Sci. Technol. 2018, 52, 8016–8026. [Google Scholar] [CrossRef]
- Acharya, P.; Jayaprakasha, G.K.; Crosby, K.M.; Jifon, J.L.; Patil, B.S. Nanoparticle-Mediated Seed Priming Improves Germination, Growth, Yield, and Quality of Watermelons (Citrullus lanatus) at multi-locations in Texas. Sci. Rep. 2020, 10, 5037. [Google Scholar] [CrossRef]
- Tymoszuk, A. Silver Nanoparticles Effects on In Vitro Germination, Growth, and Biochemical Activity of Tomato, Radish, and Kale Seedlings. Materials 2021, 14, 5340. [Google Scholar] [CrossRef] [PubMed]
- Younis, M.E.; Abdel-Aziz, H.M.M.; Heikal, Y.M. Nanopriming technology enhances vigor and mitotic index of aged Vicia faba seeds using chemically synthesized silver nanoparticles. S. Afr. J. Bot. 2019, 125, 393–401. [Google Scholar] [CrossRef]
- Salih, A.M.; Qahtan, A.A.; Al-Qurainy, F.; Al-Munqedhi, B.M. Impact of Biogenic Ag-Containing Nanoparticles on Ger-mination Rate, Growth, Physiological, Biochemical Parameters, and Antioxidants System of Tomato (Solanum tuberosum L.) In Vitro. Processes 2022, 10, 825. [Google Scholar] [CrossRef]
- Bagherzadeh Homaee, M.; Ehsanpour, A.A. Physiological and biochemical responses of potato (Solanum tuberosum) to silver nanoparticles and silver nitrate treatments under in vitro conditions. Indian J. Plant Physiol. 2015, 20, 353–359. [Google Scholar] [CrossRef]
- Azeez, L.; Lateef, A.; Adebisi, S.A. Silver nanoparticles (AgNPs) biosynthesized using pod extract of Cola nitida enhances antioxidant activity and phytochemical composition of Amaranthus caudatus Linn. Appl. Nanosci. 2017, 7, 59–66. [Google Scholar] [CrossRef]
- Mosa, W.F.; Mackled, M.I.; Abdelsalam, N.R.; Behiry, S.I.; Al-Askar, A.A.-A.; Basile, A.; Abdelkhalek, A.; Elsharkawy, M.M.; Salem, M.Z.M. Impact of Silver Na-noparticles on Lemon Growth Performance: Insecticidal and Antifungal Activities of Essential Oils From Peels and Leaves. Front. Plant Sci. 2022, 13, 898846. [Google Scholar] [CrossRef]
- Nair, P.M.G.; Chung, I.M. Physiological and molecular level effects of silver nanoparticles exposure in rice (Oryza sativa L.) seedlings. Chemosphere 2014, 112, 105–113. [Google Scholar] [CrossRef]
- Salama, H.M. Effects of silver nanoparticles in some crop plants, common bean (Phaseolus vulgaris L.) and corn (Zea mays L.). Int. Res. J. Biotechnol. 2012, 3, 190–197. [Google Scholar]



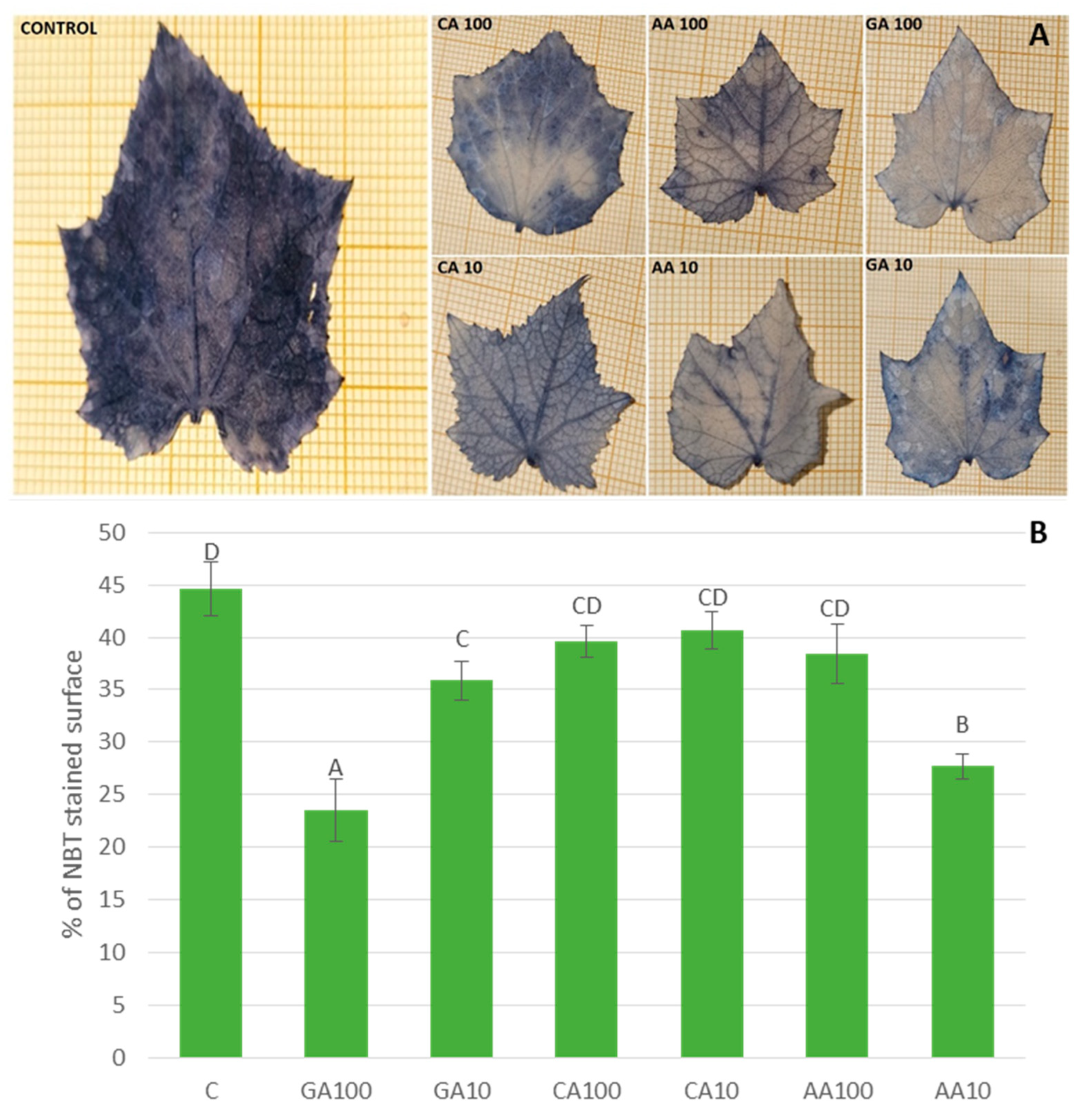
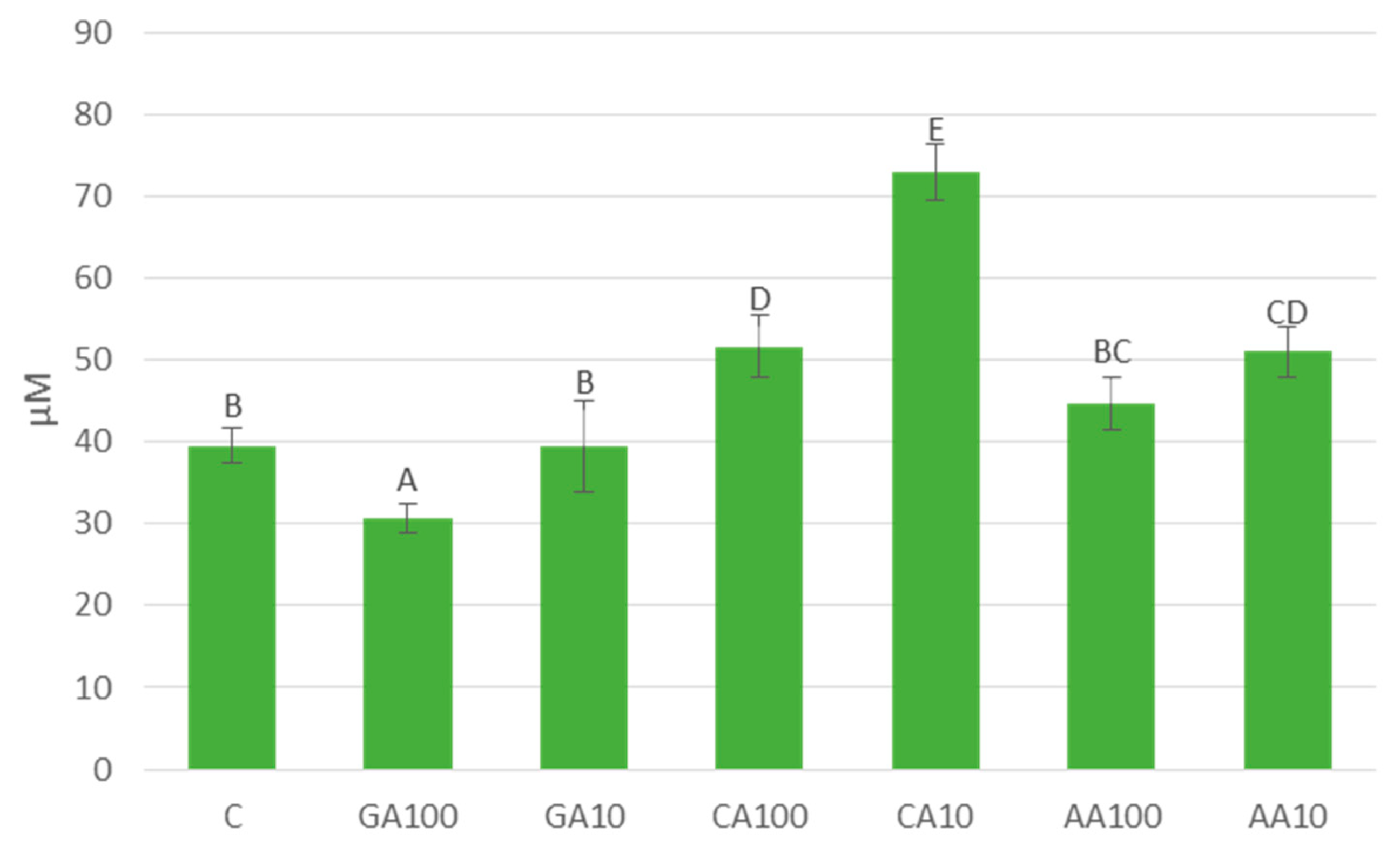
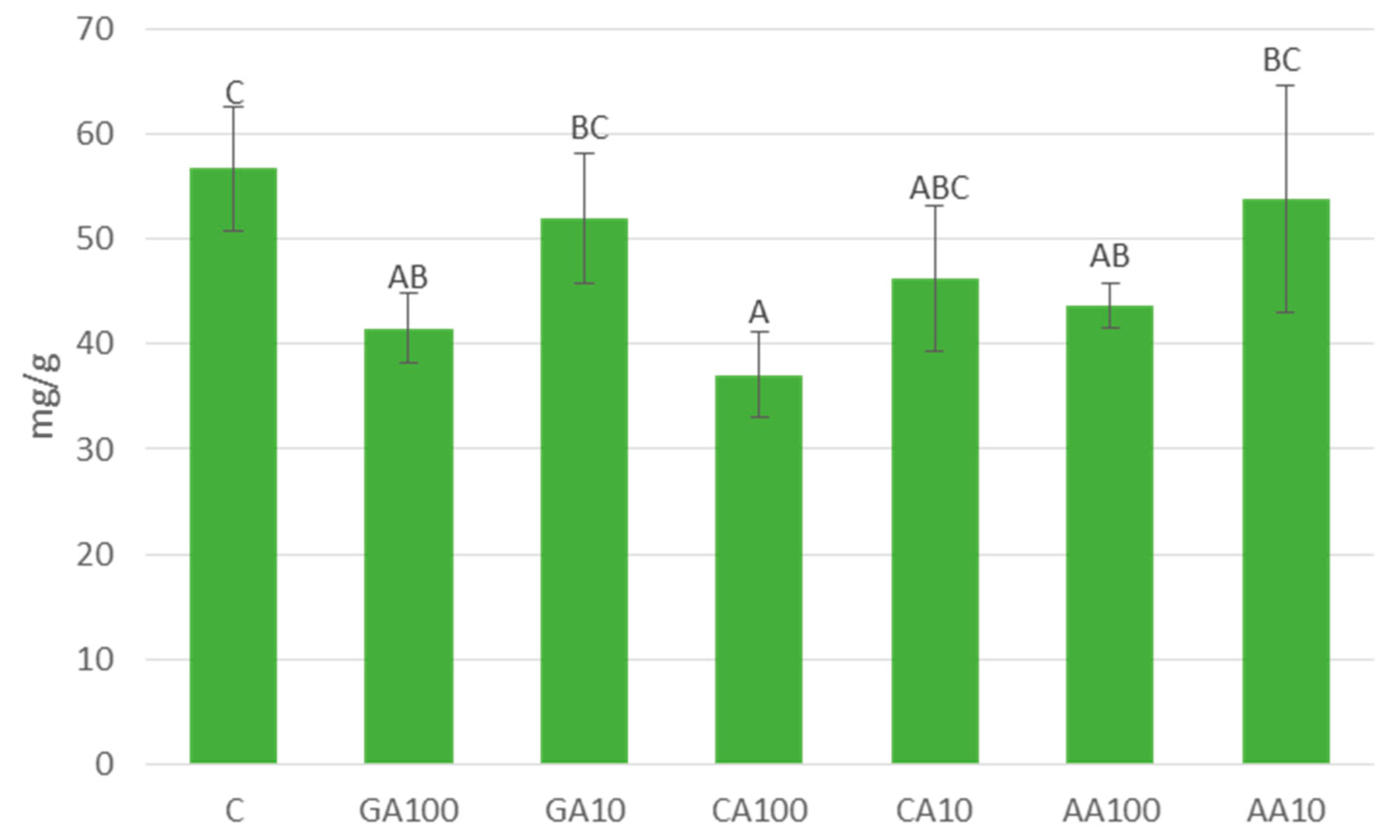
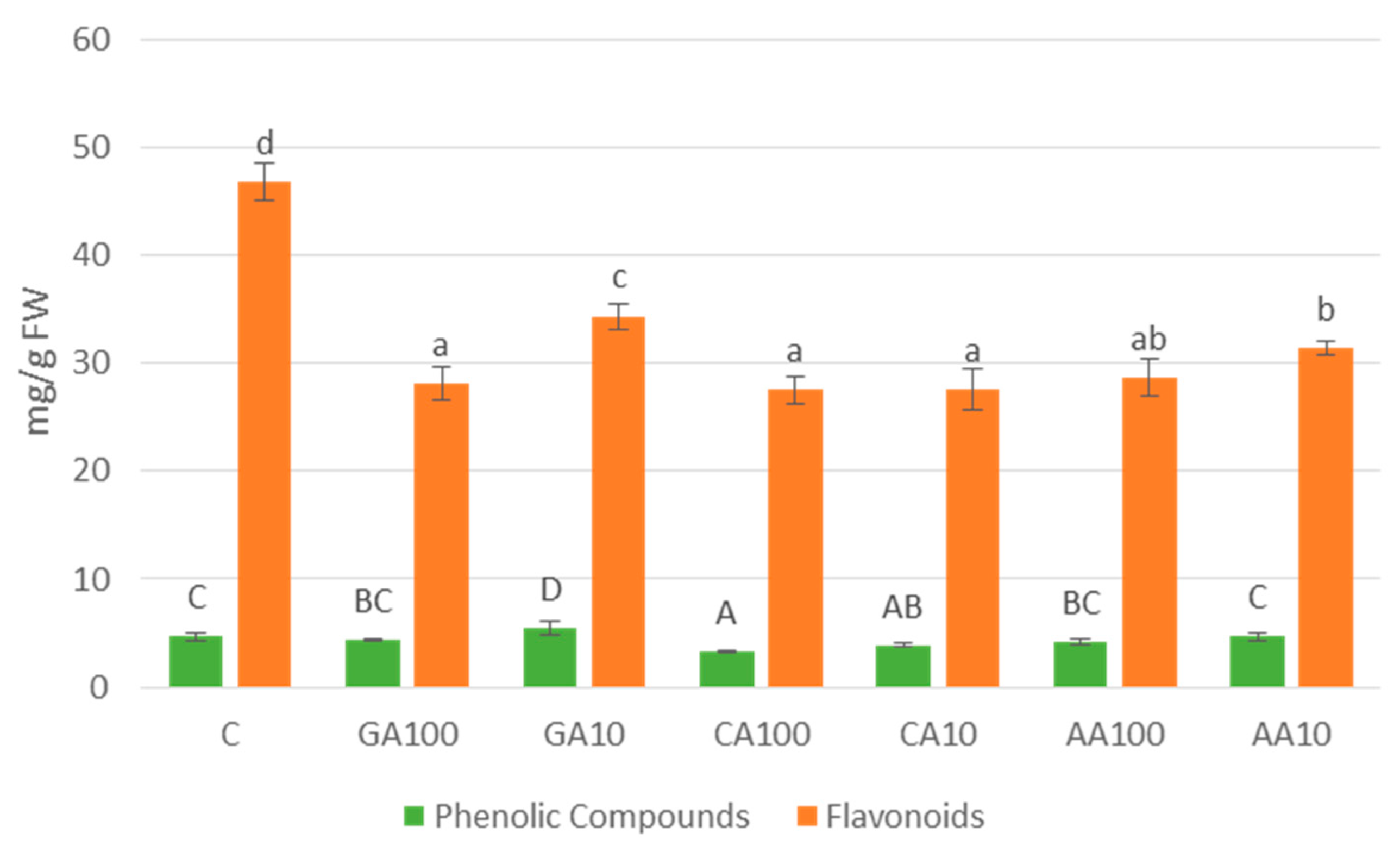
| Priming Treatment | Concentration | Germination (%) |
|---|---|---|
| Water-Control | - | 100 |
| Bulk AgNO3 | 100 μM | 0 |
| 10 mM | 0 | |
| GA-AgNPs | 100 μM | 100 |
| 10 mM | 100 | |
| CA-AgNPs | 100 μM | 88.8 ± 9.6 |
| 10 mM | 100 | |
| AA-AgNPs | 100 μM | 83.3 |
| 10 mM | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pintos, B.; de Diego, H.; Gomez-Garay, A. Nanopriming-Induced Enhancement of Cucumber Seedling Development: Exploring Biochemical and Physiological Effects of Silver Nanoparticles. Agronomy 2024, 14, 1866. https://doi.org/10.3390/agronomy14081866
Pintos B, de Diego H, Gomez-Garay A. Nanopriming-Induced Enhancement of Cucumber Seedling Development: Exploring Biochemical and Physiological Effects of Silver Nanoparticles. Agronomy. 2024; 14(8):1866. https://doi.org/10.3390/agronomy14081866
Chicago/Turabian StylePintos, Beatriz, Hugo de Diego, and Arancha Gomez-Garay. 2024. "Nanopriming-Induced Enhancement of Cucumber Seedling Development: Exploring Biochemical and Physiological Effects of Silver Nanoparticles" Agronomy 14, no. 8: 1866. https://doi.org/10.3390/agronomy14081866






