Marigold (Tagetes erecta) MADS-Box Genes: A Systematic Analysis and Their Implications for Floral Organ Development
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. RNA Preparation
2.3. Identification of MADS-Box Genes in Marigold
2.4. Maximum Likelihood Phylogeny of MADS-Box Genes
2.5. Gene Structure and Conserved Motif Analysis
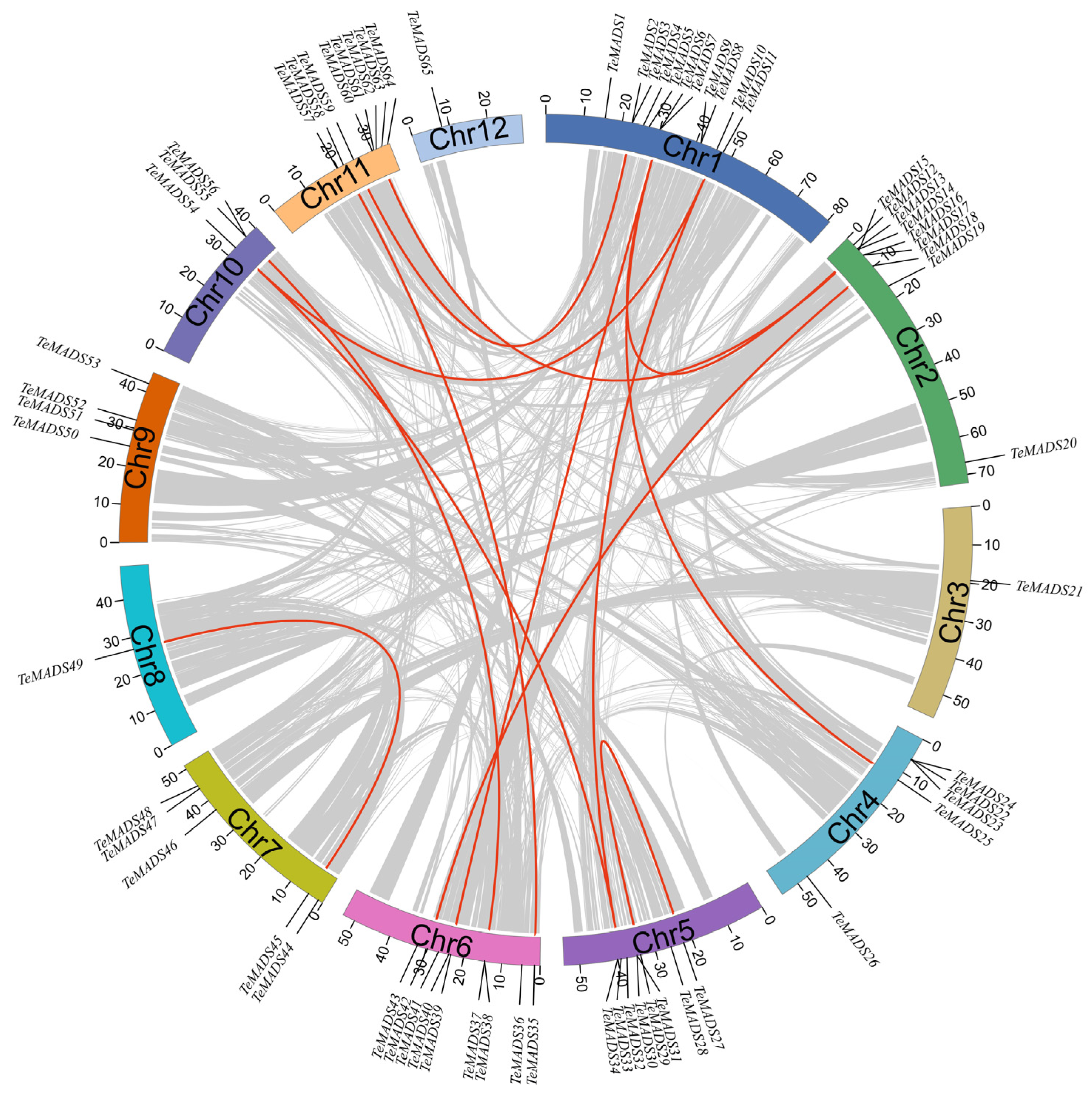
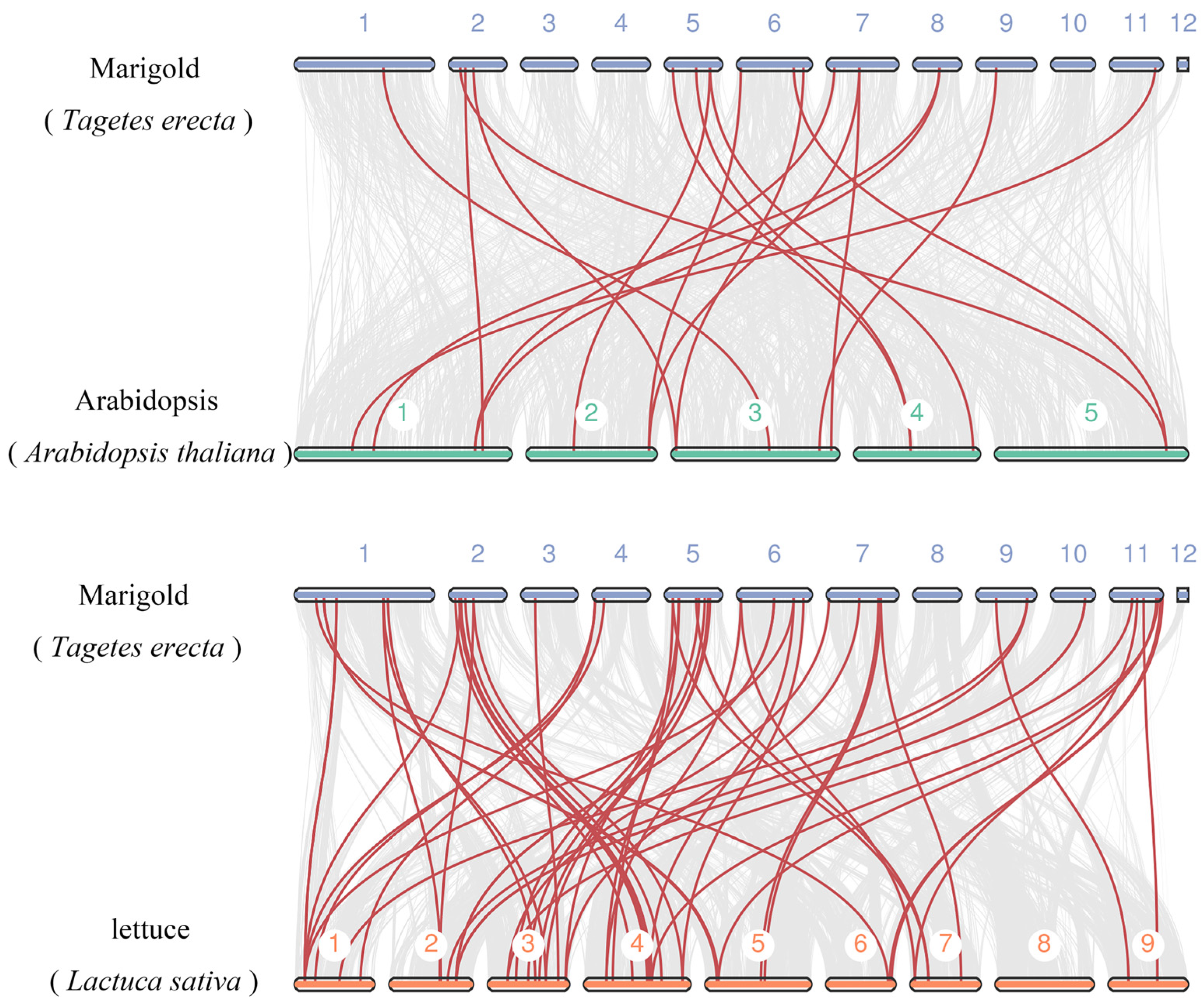
2.6. Chromosomal Locations, Gene Duplication and Synteny Analysis
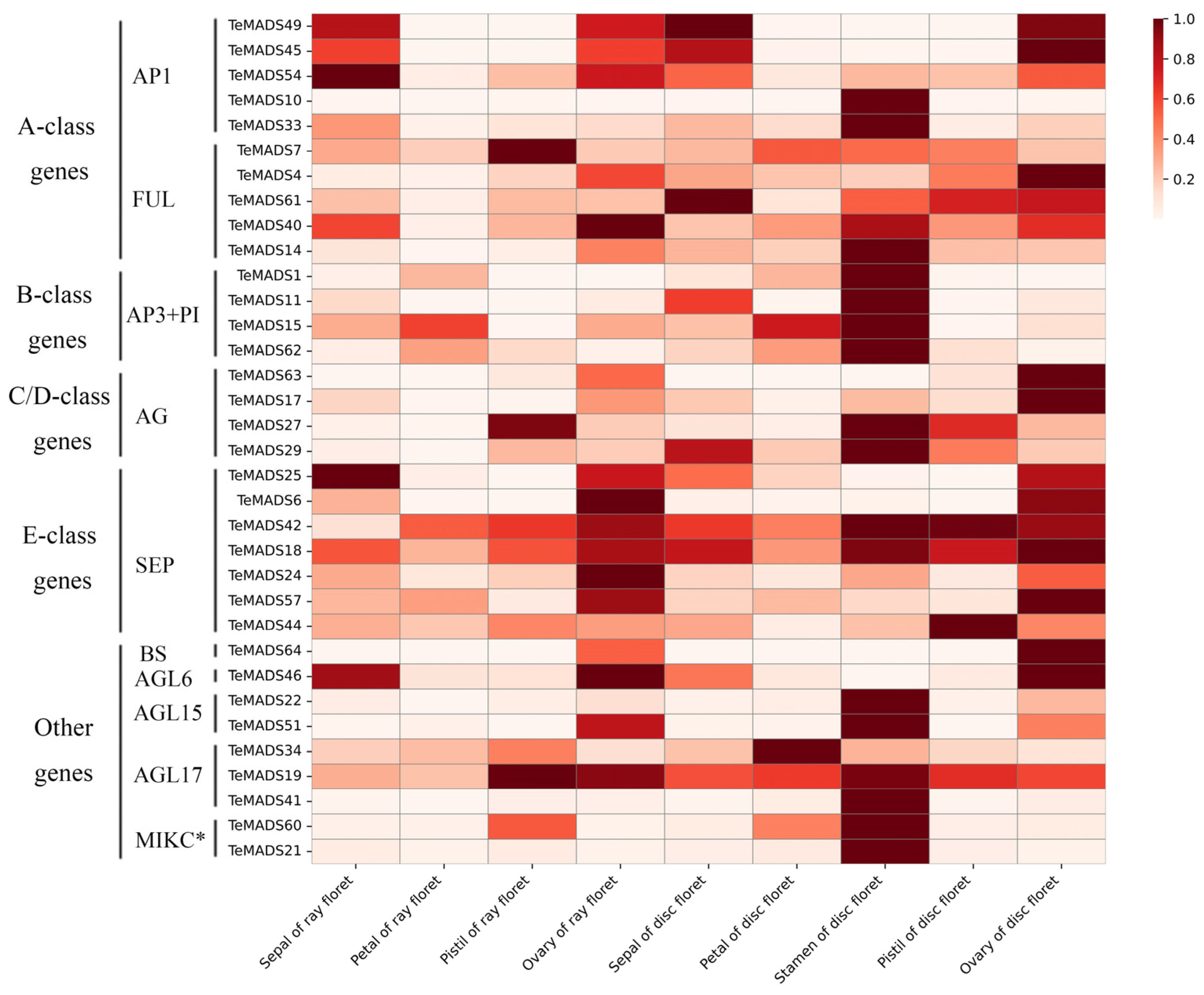
2.7. Expression Analysis of MADS-Box Genes in Marigold
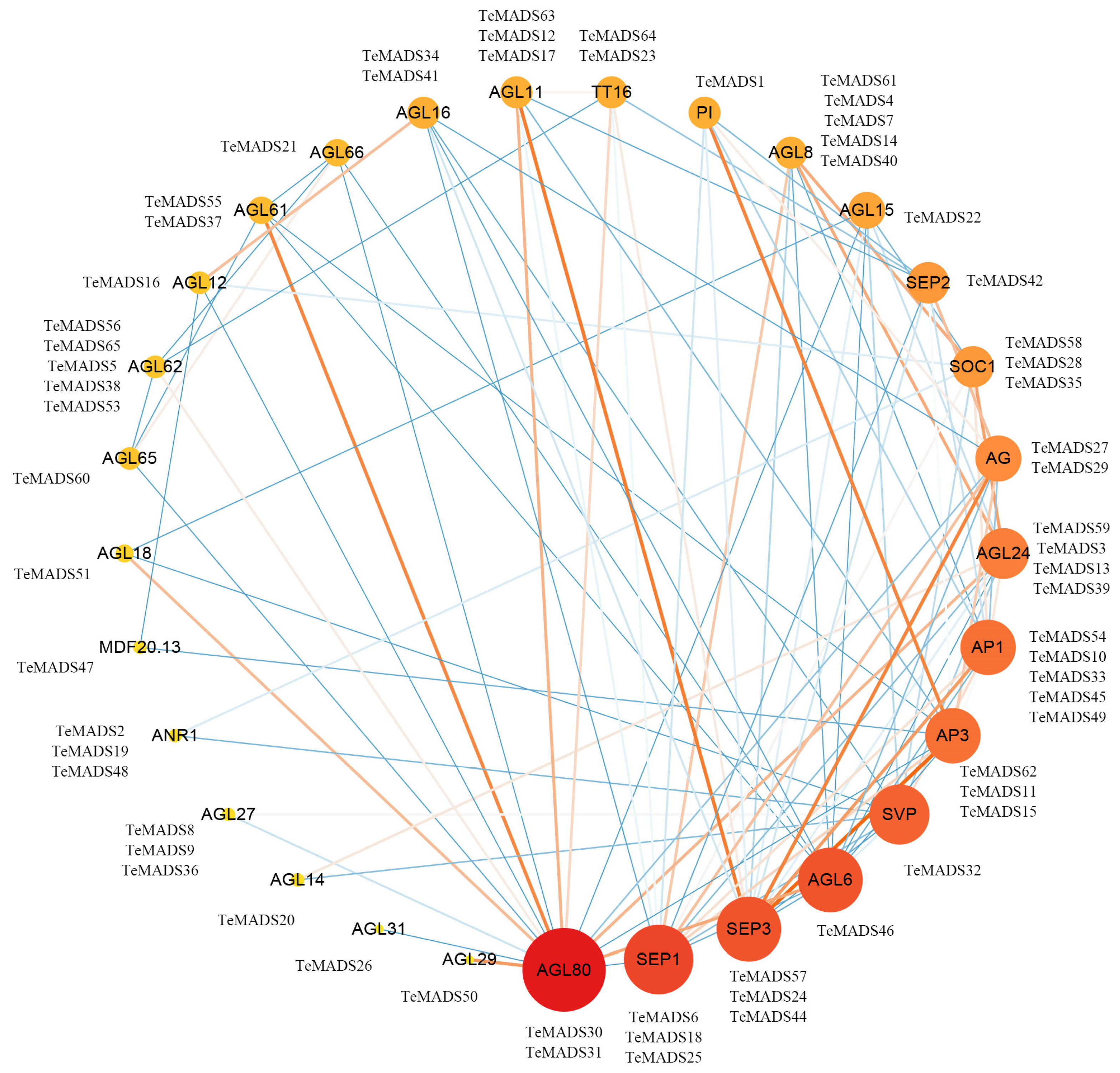
2.8. Analysis of MADS-Box Protein Interacting Network
3. Results
3.1. Identification and Characterization of MADS-Box Genes in Marigold
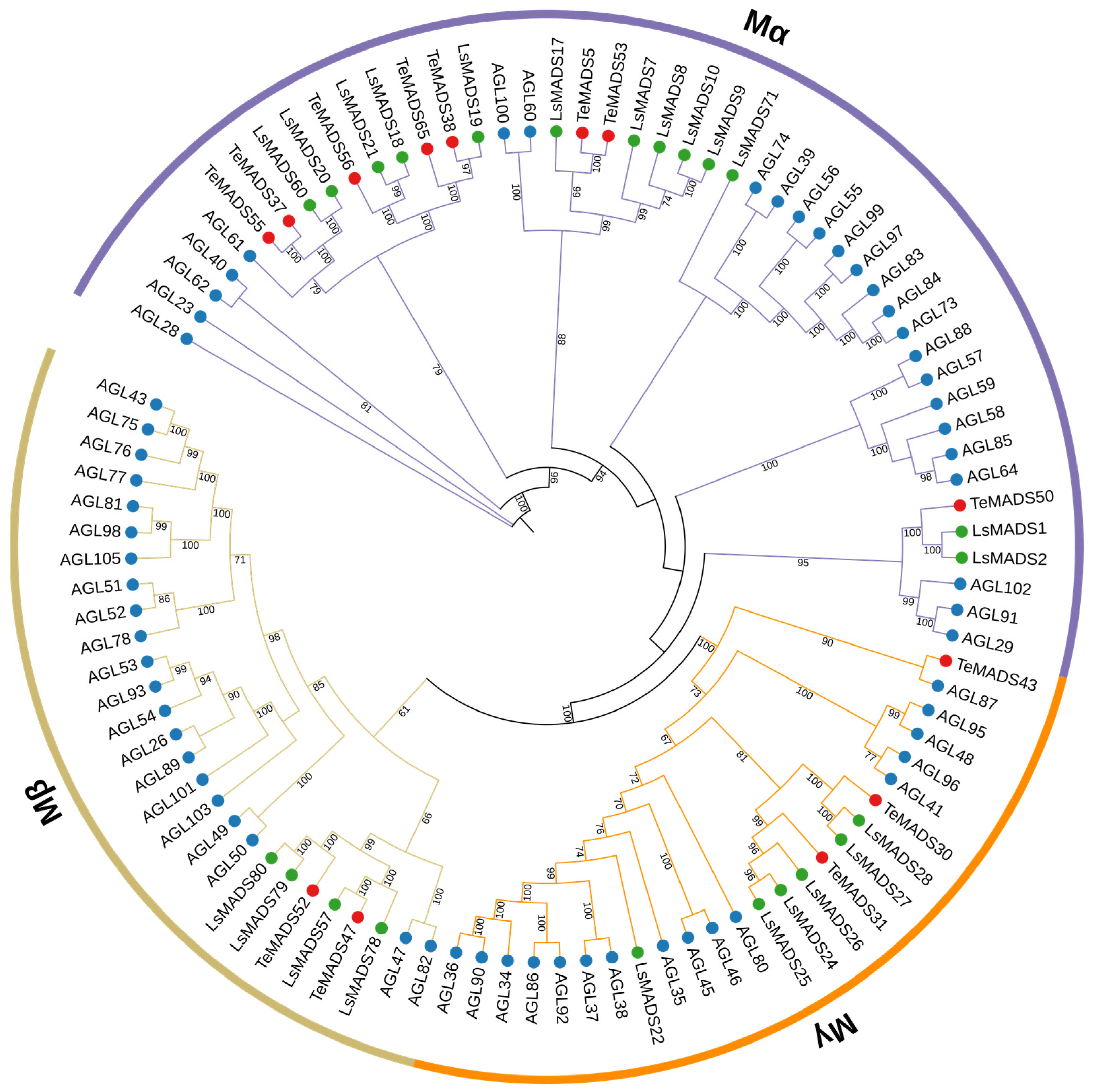
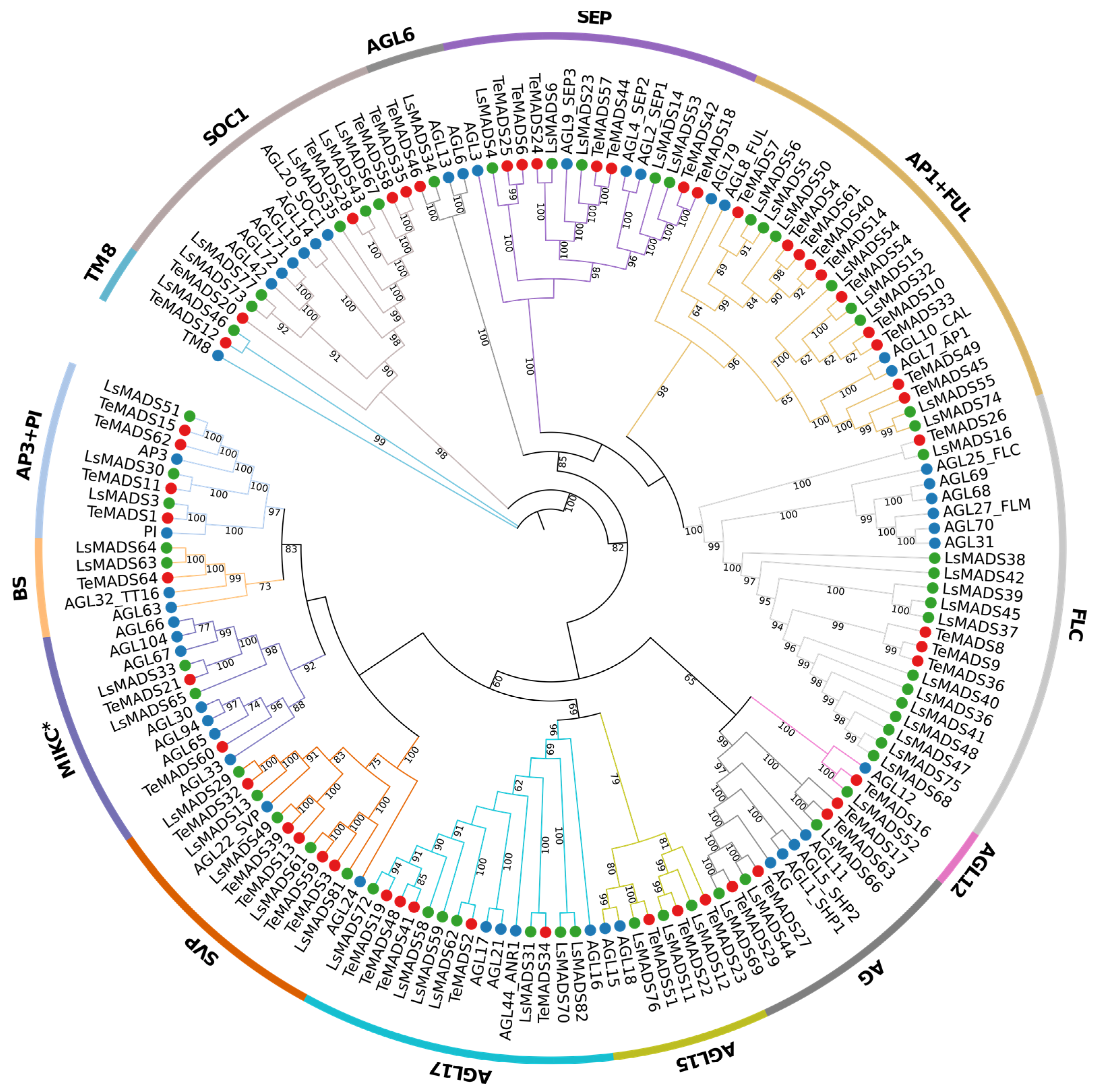
3.2. Phylogenetic Analysis of MADS-Box Genes in Marigold
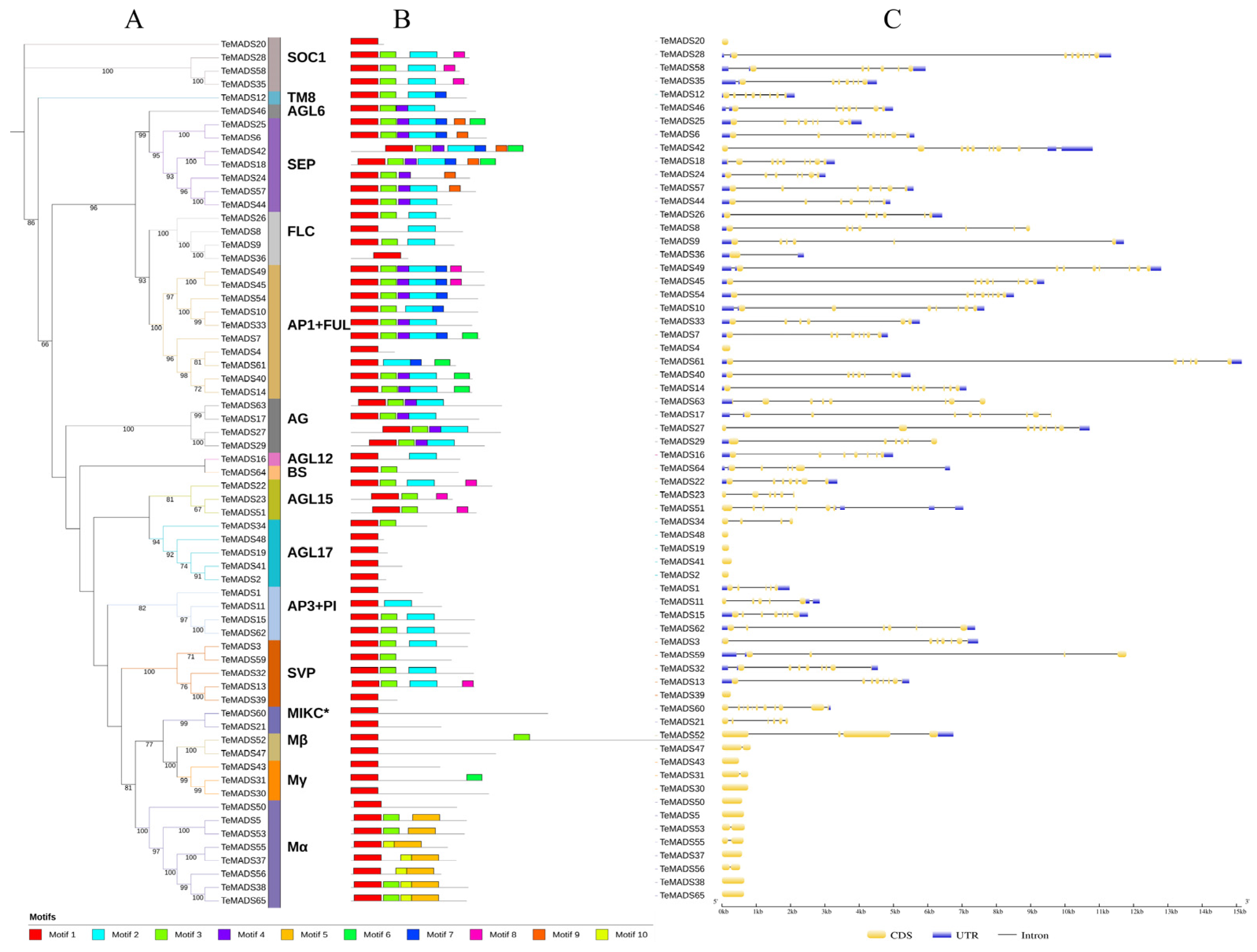
3.3. Gene Structure and Conserved Motif Analysis
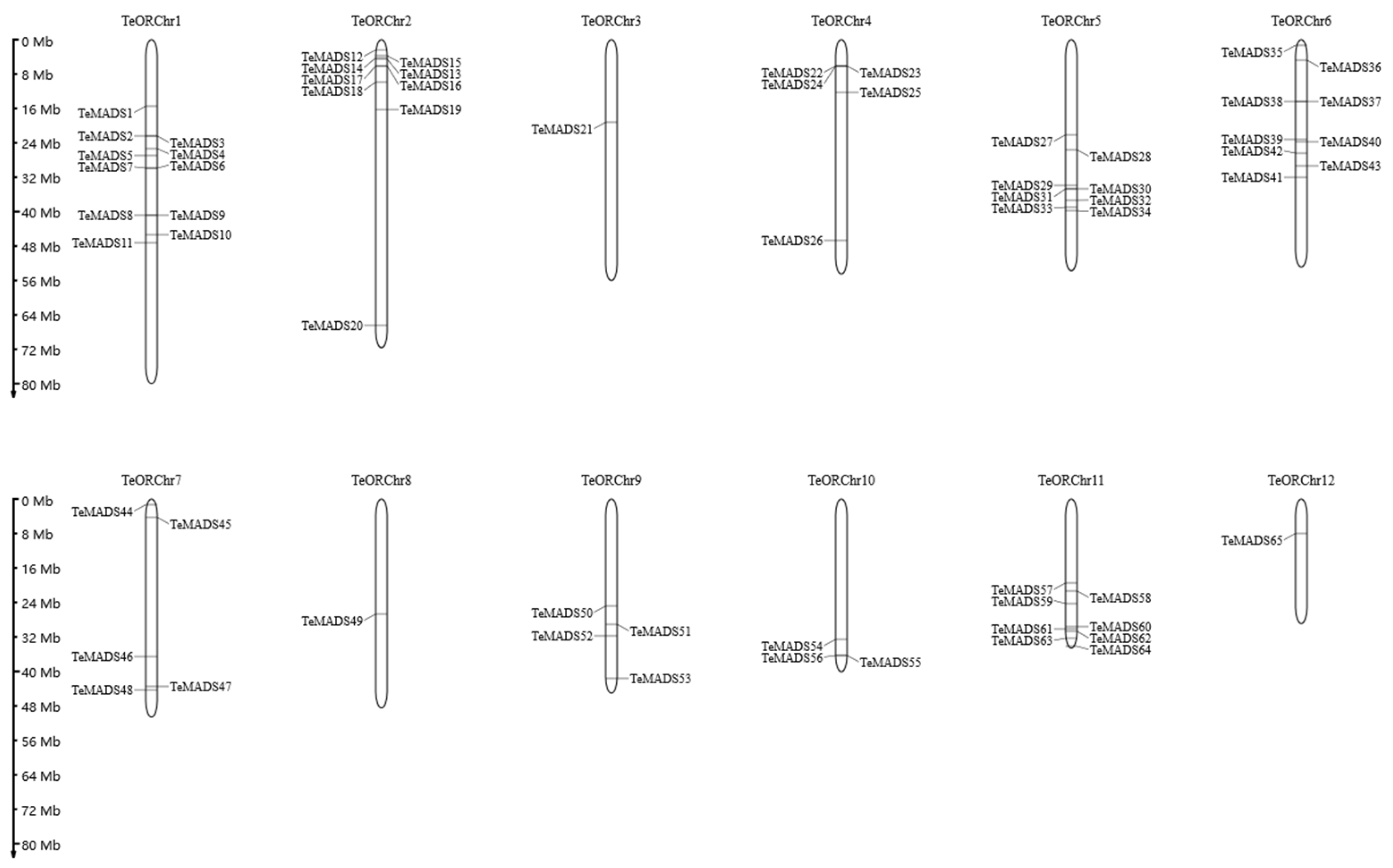
3.4. Chromosomal Locations, Gene Duplication, and Synteny Analysis
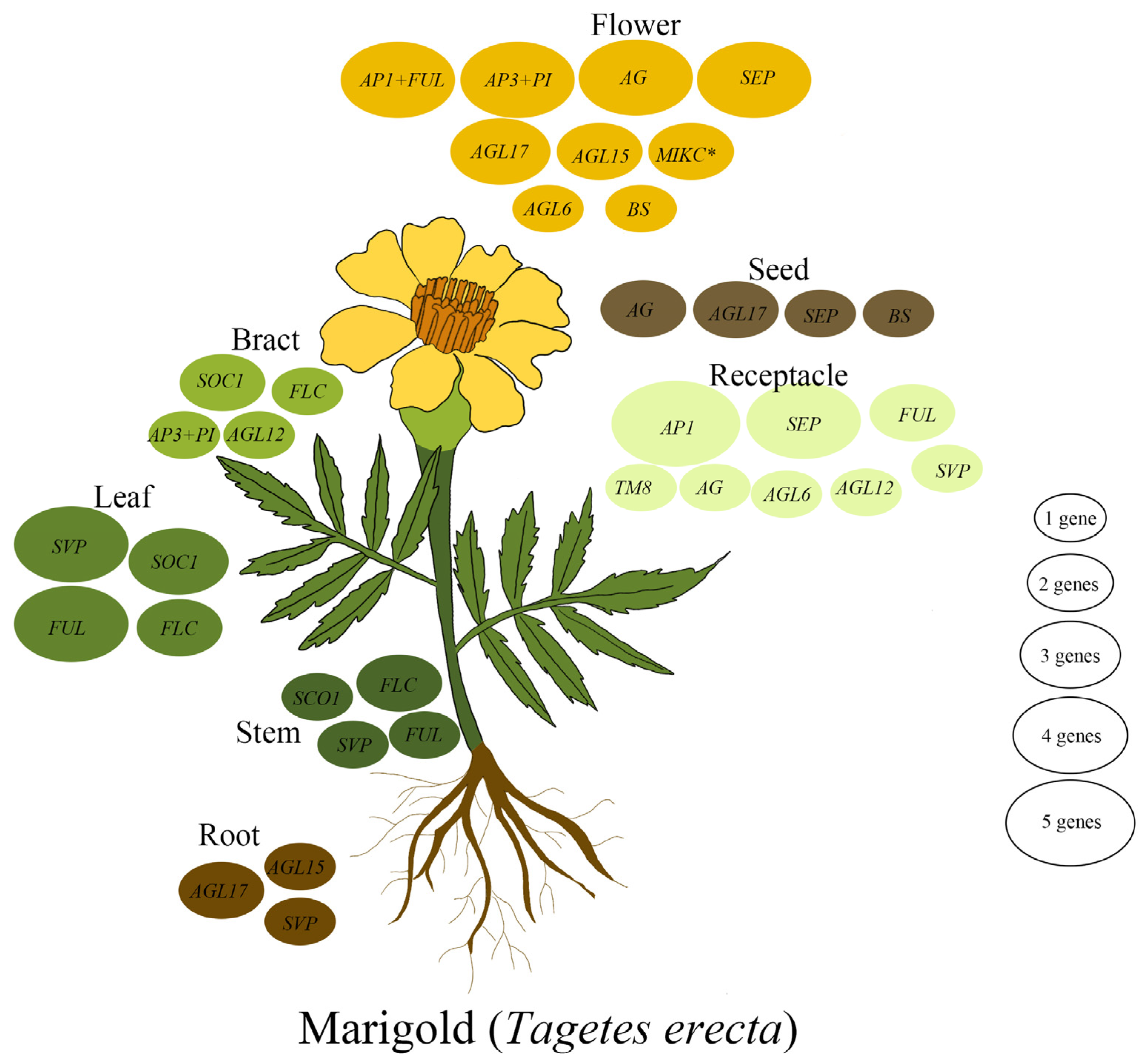
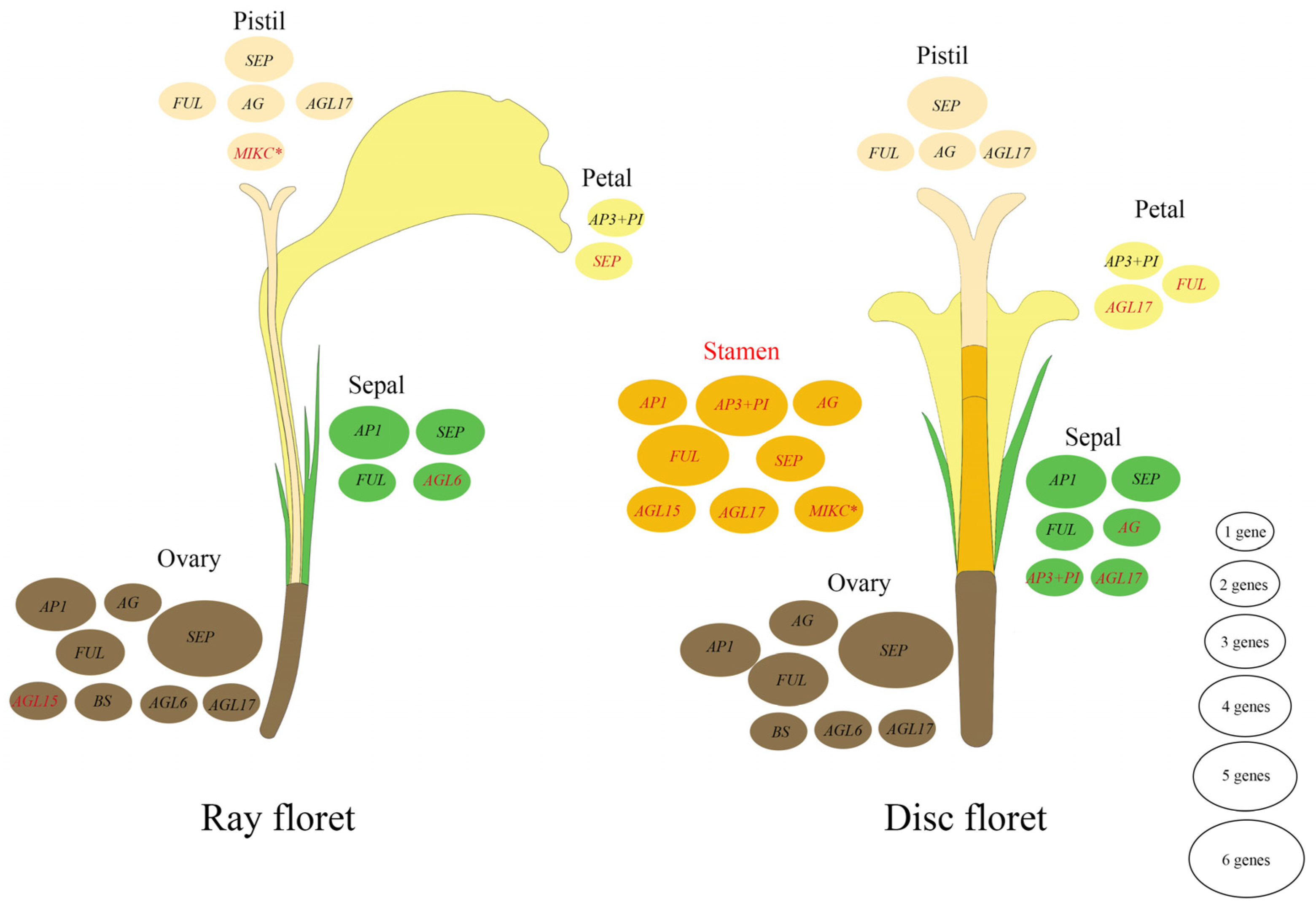
3.5. Expression Analysis of MADS-Box Genes in Marigold
3.6. Protein Interaction Network of MADS-Box Genes
4. Discussion
4.1. Genome-Wide Identification and Evolution of MADS-Box Gene Family in Marigold
4.2. Conservation of the Type II Subfamilies in Marigold
4.3. Expression Analysis and Functional Prediction of MADS-Box Genes in Marigold
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xiong, W.; Risse, J.; Berke, L.; Zhao, T.; van de Geest, H.; Oplaat, C.; Busscher, M.; Ferreira, D.C.J.; van der Meer, I.M.; Verhoeven, K.; et al. Phylogenomic analysis provides insights into MADS-box and TCP gene diversification and floral development of the Asteraceae, supported by de novo genome and transcriptome sequences from dandelion (Taraxacum officinale). Front. Plant Sci. 2023, 14, 1198909. [Google Scholar] [CrossRef]
- Zhang, T.; Elomaa, P. Development and evolution of the Asteraceae capitulum. New Phytol. 2024, 242, 33–48. [Google Scholar] [CrossRef]
- Elomaa, P.; Zhao, Y.; Zhang, T. Flower heads in Asteraceae-recruitment of conserved developmental regulators to control the flower-like inflorescence architecture. Hortic. Res. 2018, 5, 36. [Google Scholar] [CrossRef]
- Dezar, C.A.; Tioni, M.F.; Gonzalez, D.H.; Chan, R.L. Identification of three MADS-box genes expressed in sunflower capitulum. J. Exp. Bot. 2003, 54, 1637–1639. [Google Scholar] [CrossRef]
- Zhang, C.; Wei, L.; Yu, X.; Li, H.; Wang, W.; Wu, S.; Duan, F.; Bao, M.; Chan, Z.; He, Y. Functional conservation and divergence of SEPALLATA-like genes in the development of two-type florets in marigold. Plant Sci. 2021, 309, 110938. [Google Scholar] [CrossRef]
- Shore, P.; Sharrocks, A.D. The MADS-Box Family of Transcription Factors. Eur. J. Biochem. 1995, 229, 1–13. [Google Scholar] [CrossRef]
- Tao, Z.; Shen, L.; Liu, C.; Liu, L.; Yan, Y.; Yu, H. Genome-wide identification of SOC1 and SVP targets during the floral transition in Arabidopsis. Plant J. 2012, 70, 549–561. [Google Scholar] [CrossRef]
- He, Y. Chromatin regulation of flowering. Trends Plant Sci. 2012, 17, 556–562. [Google Scholar] [CrossRef]
- Treccarichi, S.; Ben, A.H.; Amari, M.; Cali, R.; Tribulato, A.; Branca, F. Molecular Markers for Detecting Inflorescence Size of Brassica oleracea L. Crops and B. oleracea Complex Species (n = 9) Useful for Breeding of Broccoli (B. oleracea var. italica) and Cauliflower (B. oleracea var. botrytis). Plants 2023, 12, 407. [Google Scholar] [CrossRef]
- Theissen, G. Development of floral organ identity: Stories from the MADS house. Curr. Opin. Plant Biol. 2001, 4, 75–85. [Google Scholar] [CrossRef]
- Ng, M.; Yanofsky, M.F. Function and evolution of the plant MADS-box gene family. Nat. Rev. Genet. 2001, 2, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Schwarz-Sommer, Z.; Huijser, P.; Nacken, W.; Saedler, H.; Sommer, H. Genetic Control of Flower Development by Homeotic Genes in Antirrhinum majus. Science 1990, 250, 931–936. [Google Scholar] [CrossRef]
- Becker, A.; Theissen, G. The major clades of MADS-box genes and their role in the development and evolution of flowering plants. Mol. Phylogenet Evol. 2003, 29, 464–489. [Google Scholar] [CrossRef]
- Ma, H.; Yanofsky, M.F.; Meyerowitz, E.M. AGL1-AGL6, an Arabidopsis gene family with similarity to floral homeotic and transcription factor genes. Gene Dev. 1991, 5, 484–495. [Google Scholar] [CrossRef] [PubMed]
- Parenicova, L.; de Folter, S.; Kieffer, M.; Horner, D.S.; Favalli, C.; Busscher, J.; Cook, H.E.; Ingram, R.M.; Kater, M.M.; Davies, B.; et al. Molecular and phylogenetic analyses of the complete MADS-box transcription factor family in Arabidopsis: New openings to the MADS world. Plant Cell 2003, 15, 1538–1551. [Google Scholar] [CrossRef] [PubMed]
- Munster, T.; Pahnke, J.; Di Rosa, A.; Kim, J.T.; Martin, W.; Saedler, H.; Theissen, G. Floral homeotic genes were recruited from homologous MADS-box genes preexisting in the common ancestor of ferns and seed plants. Proc. Natl. Acad. Sci. USA 1997, 94, 2415–2420. [Google Scholar] [CrossRef]
- Davies, B.; Egea-Cortines, M.; de Andrade, S.E.; Saedler, H.; Sommer, H. Multiple interactions amongst floral homeotic MADS box proteins. Embo J. 1996, 15, 4330–4343. [Google Scholar] [CrossRef]
- Honma, T.; Goto, K. Complexes of MADS-box proteins are sufficient to convert leaves into floral organs. Nature 2001, 409, 525–529. [Google Scholar] [CrossRef]
- van Dijk, A.D.; Morabito, G.; Fiers, M.; van Ham, R.C.; Angenent, G.C.; Immink, R.G. Sequence motifs in MADS transcription factors responsible for specificity and diversification of protein-protein interaction. PLoS Comput. Biol. 2010, 6, e1001017. [Google Scholar] [CrossRef]
- Gramzow, L.; Theissen, G. Phylogenomics reveals surprising sets of essential and dispensable clades of MIKC(c)-group MADS-box genes in flowering plants. J. Exp. Zool. Part B 2015, 324, 353–362. [Google Scholar] [CrossRef]
- Schilling, S.; Kennedy, A.; Pan, S.; Jermiin, L.S.; Melzer, R. Genome-wide analysis of MIKC-type MADS-box genes in wheat: Pervasive duplications, functional conservation and putative neofunctionalization. New Phytol. 2020, 225, 511–529. [Google Scholar] [CrossRef]
- Masiero, S.; Colombo, L.; Grini, P.E.; Schnittger, A.; Kater, M.M. The emerging importance of type I MADS box transcription factors for plant reproduction. Plant Cell 2011, 23, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Verelst, W.; Saedler, H.; Munster, T. MIKC* MADS-protein complexes bind motifs enriched in the proximal region of late pollen-specific Arabidopsis promoters. Plant Physiol. 2007, 143, 447–460. [Google Scholar] [CrossRef] [PubMed]
- Verelst, W.; Twell, D.; de Folter, S.; Immink, R.; Saedler, H.; Munster, T. MADS-complexes regulate transcriptome dynamics during pollen maturation. Genome Biol. 2007, 8, R249. [Google Scholar] [CrossRef] [PubMed]
- Bowman, J.L.; Smyth, D.R.; Meyerowitz, E.M. Genetic interactions among floral homeotic genes of Arabidopsis. Development 1991, 112, 1–20. [Google Scholar] [CrossRef]
- Carpenter, R.; Coen, E.S. Floral homeotic mutations produced by transposon-mutagenesis in Antirrhinum majus. Gene Dev. 1990, 4, 1483–1493. [Google Scholar] [CrossRef] [PubMed]
- Coen, E.S.; Meyerowitz, E.M. The war of the whorls: Genetic interactions controlling flower development. Nature 1991, 353, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Weigel, D.; Meyerowitz, E.M. The ABCs of floral homeotic genes. Cell 1994, 78, 203–209. [Google Scholar] [CrossRef]
- Pinyopich, A.; Ditta, G.S.; Savidge, B.; Liljegren, S.J.; Baumann, E.; Wisman, E.; Yanofsky, M.F. Assessing the redundancy of MADS-box genes during carpel and ovule development. Nature 2003, 424, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Pelaz, S.; Ditta, G.S.; Baumann, E.; Wisman, E.; Yanofsky, M.F. B and C floral organ identity functions require SEPALLATA MADS-box genes. Nature 2000, 405, 200–203. [Google Scholar] [CrossRef]
- Kim, S.; Koh, J.; Yoo, M.J.; Kong, H.; Hu, Y.; Ma, H.; Soltis, P.S.; Soltis, D.E. Expression of floral MADS-box genes in basal angiosperms: Implications for the evolution of floral regulators. Plant J. 2005, 43, 724–744. [Google Scholar] [CrossRef] [PubMed]
- Yant, L.; Mathieu, J.; Dinh, T.T.; Ott, F.; Lanz, C.; Wollmann, H.; Chen, X.; Schmid, M. Orchestration of the floral transition and floral development in Arabidopsis by the bifunctional transcription factor APETALA2. Plant Cell 2010, 22, 2156–2170. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; DePamphilis, C. The ABCs of floral evolution. Cell 2000, 101, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Irish, V.F.; Litt, A. Flower development and evolution: Gene duplication, diversification and redeployment. Curr. Opin. Genet. Dev. 2005, 15, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Ning, K.; Han, Y.; Chen, Z.; Luo, C.; Wang, S.; Zhang, W.; Li, L.; Zhang, X.; Fan, S.; Wang, Q. Genome-wide analysis of MADS-box family genes during flower development in lettuce. Plant Cell Environ. 2019, 42, 1868–1881. [Google Scholar] [CrossRef] [PubMed]
- Laitinen, R.A.; Broholm, S.; Albert, V.A.; Teeri, T.H.; Elomaa, P. Patterns of MADS-box gene expression mark flower-type development in Gerbera hybrida (Asteraceae). BMC Plant Biol. 2006, 6, 11. [Google Scholar] [CrossRef]
- Pu, Y.; Liao, M.; Li, J.; Tian, Y.; Wang, Z.; Song, X.; Dai, S. Floral Development Stage-Specific Transcriptomic Analysis Reveals the Formation Mechanism of Different Shapes of Ray Florets in Chrysanthemum. Genes 2023, 14, 766. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Liu, Y.; Wang, W.; Li, H.; Liu, C.; Dou, L.; Wei, L.; Cheng, W.; Bao, M.; Yi, Q.; et al. Identification and characterization of CYC2-like genes related to floral symmetric development in Tagetes erecta (Asteraceae). Gene 2023, 889, 147804. [Google Scholar] [CrossRef] [PubMed]
- Mandel, J.R.; Dikow, R.B.; Siniscalchi, C.M.; Thapa, R.; Watson, L.E.; Funk, V.A. A fully resolved backbone phylogeny reveals numerous dispersals and explosive diversifications throughout the history of Asteraceae. Proc. Natl. Acad. Sci. USA 2019, 116, 14083–14088. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.; Ji, F.; Wu, J.; Zhang, S.; Yi, C.; Zhao, S.; Cong, R.; Zhao, L.; Zhang, H.; Zhang, Z. Chromosome-scale genome assembly of marigold (Tagetes erecta L.): An ornamental plant and feedstock for industrial lutein production. Hortic. Plant J. 2023, 9, 1119–1130. [Google Scholar] [CrossRef]
- Ai, Y.; Zhang, Q.; Wang, W.; Zhang, C.; Cao, Z.; Bao, M.; He, Y. Transcriptomic Analysis of Differentially Expressed Genes during Flower Organ Development in Genetic Male Sterile and Male Fertile Tagetes erecta by Digital Gene-Expression Profiling. PLoS ONE 2016, 11, e150892. [Google Scholar] [CrossRef]
- Zhang, C.; Sun, Y.; Yu, X.; Li, H.; Bao, M.; He, Y. Functional Conservation and Divergence of Five AP1/FUL-like Genes in Marigold (Tagetes erecta L.). Genes. 2021, 12, 2011. [Google Scholar] [CrossRef] [PubMed]
- Ai, Y.; Zhang, C.; Sun, Y.; Wang, W.; He, Y.; Bao, M. Characterization and Functional Analysis of Five MADS-Box B Class Genes Related to Floral Organ Identification in Tagetes erecta. PLoS ONE 2017, 12, e169777. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wei, L.; Wang, W.; Qi, W.; Cao, Z.; Li, H.; Bao, M.; He, Y. Identification, characterization and functional analysis of AGAMOUS subfamily genes associated with floral organs and seed development in Marigold (Tagetes erecta). Bmc Plant Biol. 2020, 20, 439. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.D.; Clements, J.; Eddy, S.R. HMMER web server: Interactive sequence similarity searching. Nucleic Acids Res. 2011, 39, W29–W37. [Google Scholar] [CrossRef] [PubMed]
- Mistry, J.; Chuguransky, S.; Williams, L.; Qureshi, M.; Salazar, G.A.; Sonnhammer, E.; Tosatto, S.; Paladin, L.; Raj, S.; Richardson, L.J.; et al. Pfam: The protein families database in 2021. Nucleic Acids Res 2021, 49, D412–D419. [Google Scholar] [CrossRef] [PubMed]
- Marchler-Bauer, A.; Bryant, S.H. CD-Search: Protein domain annotations on the fly. Nucleic Acids Res. 2004, 32, W327–W331. [Google Scholar] [CrossRef]
- Wilkins, M.R.; Gasteiger, E.; Bairoch, A.; Sanchez, J.C.; Williams, K.L.; Appel, R.D.; Hochstrasser, D.F. Protein identification and analysis tools in the ExPASy server. Methods Mol. Biol. 1999, 112, 531–552. [Google Scholar] [CrossRef] [PubMed]
- Chou, K.C.; Shen, H.B. Large-scale plant protein subcellular location prediction. J. Cell Biochem. 2007, 100, 665–678. [Google Scholar] [CrossRef]
- Berardini, T.Z.; Reiser, L.; Li, D.; Mezheritsky, Y.; Muller, R.; Strait, E.; Huala, E. The arabidopsis information resource: Making and mining the “gold standard” annotated reference plant genome. Genesis 2015, 53, 474–485. [Google Scholar] [CrossRef]
- Goodstein, D.M.; Shu, S.; Howson, R.; Neupane, R.; Hayes, R.D.; Fazo, J.; Mitros, T.; Dirks, W.; Hellsten, U.; Putnam, N.; et al. Phytozome: A comparative platform for green plant genomics. Nucleic Acids Res. 2012, 40, D1178–D1186. [Google Scholar] [CrossRef] [PubMed]
- Daminato, M.; Masiero, S.; Resentini, F.; Lovisetto, A.; Casadoro, G. Characterization of TM8, a MADS-box gene expressed in tomato flowers. BMC Plant Biol. 2014, 14, 319. [Google Scholar] [CrossRef] [PubMed]
- Sayers, E.W.; Beck, J.; Bolton, E.E.; Brister, J.R.; Chan, J.; Comeau, D.C.; Connor, R.; DiCuccio, M.; Farrell, C.M.; Feldgarden, M.; et al. Database resources of the National Center for Biotechnology Information. Nucleic Acids Res. 2024, 52, D33–D43. [Google Scholar] [CrossRef]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform. 2019, 20, 1160–1166. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.; von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef] [PubMed]
- Guindon, S.; Dufayard, J.F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New algorithms and methods to estimate maximum-likelihood phylogenies: Assessing the performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Minh, B.Q.; Nguyen, M.A.; von Haeseler, A. Ultrafast approximation for phylogenetic bootstrap. Mol. Biol. Evol. 2013, 30, 1188–1195. [Google Scholar] [CrossRef] [PubMed]
- Hoang, D.T.; Chernomor, O.; von Haeseler, A.; Minh, B.Q.; Vinh, L.S. UFBoot2: Improving the Ultrafast Bootstrap Approximation. Mol. Biol. Evol. 2018, 35, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL) v4: Recent updates and new developments. Nucleic Acids Res. 2019, 47, W256–W259. [Google Scholar] [CrossRef]
- Hu, B.; Jin, J.; Guo, A.Y.; Zhang, H.; Luo, J.; Gao, G. GSDS 2.0: An upgraded gene feature visualization server. Bioinformatics 2015, 31, 1296–1297. [Google Scholar] [CrossRef]
- Bailey, T.L.; Elkan, C. Fitting a mixture model by expectation maximization to discover motifs in biopolymers. Proc. Int. Conf. Intell. Syst. Mol. Biol. 1994, 2, 28–36. [Google Scholar] [PubMed]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.; Li, W.W.; Noble, W.S. MEME SUITE: Tools for motif discovery and searching. Nucleic Acids Res. 2009, 37, W202–W208. [Google Scholar] [CrossRef]
- Chao, J.; Li, Z.; Sun, Y.; Aluko, O.O.; Wu, X.; Wang, Q.; Liu, G. MG2C: A user-friendly online tool for drawing genetic maps. Mol. Hortic. 2021, 1, 16. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tang, H.; Debarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.H.; Jin, H.; Marler, B.; Guo, H.; et al. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef] [PubMed]
- Tang, N.; Zhang, W.; Chen, L.; Wang, Y.; Tang, D. Reference Gene Selection for Real-time Quantitative Reverse-transcription Polymerase Chain Reaction in Flower Buds of Marigold. J. Am. Soc. Hortic. Sci. 2021, 146, 363. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING database in 2023: Protein-protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Tu, Y.; Cheng, X.; Zhang, L.; Meng, H.; Zhao, X.; Zhang, W.; He, B. Genome-wide identification and expression profile of the MADS-box gene family in Erigeron breviscapus. PLoS ONE 2019, 14, e226599. [Google Scholar] [CrossRef] [PubMed]
- Won, S.Y.; Jung, J.A.; Kim, J.S. Genome-wide analysis of the MADS-Box gene family in Chrysanthemum. Comput. Biol. Chem. 2021, 90, 107424. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ge, H.; Ahmad, N.; Li, J.; Wang, Y.; Liu, X.; Liu, W.; Li, X.; Wang, N.; Wang, F.; et al. Genome-Wide Identification of MADS-Box Family Genes in Safflower (Carthamus tinctorius L.) and Functional Analysis of CtMADS24 during Flowering. Int. J. Mol. Sci. 2023, 24, 1026. [Google Scholar] [CrossRef] [PubMed]
- Cittadino, G.M.; Andrews, J.; Purewal, H.; Estanislao, A.A.P.; Arnone, J.T. Functional Clustering of Metabolically Related Genes Is Conserved across Dikarya. J. Fungi 2023, 9, 523. [Google Scholar] [CrossRef] [PubMed]
- Laloum, T.; Martin, G.; Duque, P. Alternative Splicing Control of Abiotic Stress Responses. Trends Plant Sci. 2018, 23, 140–150. [Google Scholar] [CrossRef]
- Sims, D.; Sudbery, I.; Ilott, N.E.; Heger, A.; Ponting, C.P. Sequencing depth and coverage: Key considerations in genomic analyses. Nat. Rev. Genet. 2014, 15, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Zhou, W.; Steemers, F.; Trapnell, C.; Shendure, J. Sci-fate characterizes the dynamics of gene expression in single cells. Nat. Biotechnol. 2020, 38, 980–988. [Google Scholar] [CrossRef] [PubMed]
- Raz, T.; Kapranov, P.; Lipson, D.; Letovsky, S.; Milos, P.M.; Thompson, J.F. Protocol dependence of sequencing-based gene expression measurements. PLoS ONE 2011, 6, e19287. [Google Scholar] [CrossRef]
- Ng, P.C.; Kirkness, E.F. Whole genome sequencing. Methods Mol. Biol. 2010, 628, 215–226. [Google Scholar] [CrossRef]
- Liu, Y.; Cui, S.; Wu, F.; Yan, S.; Lin, X.; Du, X.; Chong, K.; Schilling, S.; Theissen, G.; Meng, Z. Functional conservation of MIKC*-Type MADS box genes in Arabidopsis and rice pollen maturation. Plant Cell 2013, 25, 1288–1303. [Google Scholar] [CrossRef]
- Broholm, S.K.; Pollanen, E.; Ruokolainen, S.; Tahtiharju, S.; Kotilainen, M.; Albert, V.A.; Elomaa, P.; Teeri, T.H. Functional characterization of B class MADS-box transcription factors in Gerbera hybrida. J. Exp. Bot. 2010, 61, 75–85. [Google Scholar] [CrossRef]
- Gioppato, H.A.; Dornelas, M.C. When Bs Are Better than As: The Relationship between B-Class MADS-Box Gene Duplications and the Diversification of Perianth Morphology. Trop. Plant Biol. 2019, 12, 1–11. [Google Scholar] [CrossRef]
- Schreiber, D.N.; Bantin, J.; Dresselhaus, T. The MADS box transcription factor ZmMADS2 is required for anther and pollen maturation in maize and accumulates in apoptotic bodies during anther dehiscence. Plant Physiol. 2004, 134, 1069–1079. [Google Scholar] [CrossRef] [PubMed]
- Puig, J.; Meynard, D.; Khong, G.N.; Pauluzzi, G.; Guiderdoni, E.; Gantet, P. Analysis of the expression of the AGL17-like clade of MADS-box transcription factors in rice. Gene Expr. Patterns 2013, 13, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Dreni, L.; Zhang, D. Flower development: The evolutionary history and functions of the AGL6 subfamily MADS-box genes. J. Exp. Bot. 2016, 67, 1625–1638. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wang, S.; Chen, Y.; Xu, X.; Guang, X.; Zhang, Y. Genome-wide Analysis of the MADS-Box Gene Family in Watermelon. Comput. Biol. Chem. 2019, 80, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, Y.; Xu, L.; Nie, S.; Chen, Y.; Liang, D.; Sun, X.; Karanja, B.K.; Luo, X.; Liu, L. Genome-Wide Characterization of the MADS-Box Gene Family in Radish (Raphanus sativus L.) and Assessment of Its Roles in Flowering and Floral Organogenesis. Front. Plant Sci. 2016, 7, 1390. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Wang, F.; Li, R.; Zhu, Y.; Zhang, C.; He, Y. Marigold (Tagetes erecta) MADS-Box Genes: A Systematic Analysis and Their Implications for Floral Organ Development. Agronomy 2024, 14, 1889. https://doi.org/10.3390/agronomy14091889
Liu C, Wang F, Li R, Zhu Y, Zhang C, He Y. Marigold (Tagetes erecta) MADS-Box Genes: A Systematic Analysis and Their Implications for Floral Organ Development. Agronomy. 2024; 14(9):1889. https://doi.org/10.3390/agronomy14091889
Chicago/Turabian StyleLiu, Cuicui, Feifan Wang, Runhui Li, Yu Zhu, Chunling Zhang, and Yanhong He. 2024. "Marigold (Tagetes erecta) MADS-Box Genes: A Systematic Analysis and Their Implications for Floral Organ Development" Agronomy 14, no. 9: 1889. https://doi.org/10.3390/agronomy14091889




