Oxidative Degradation Characteristics of Low-Temperature Pyrolysis Biochar and the Synergistic Effect on Released Nutrients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site Description
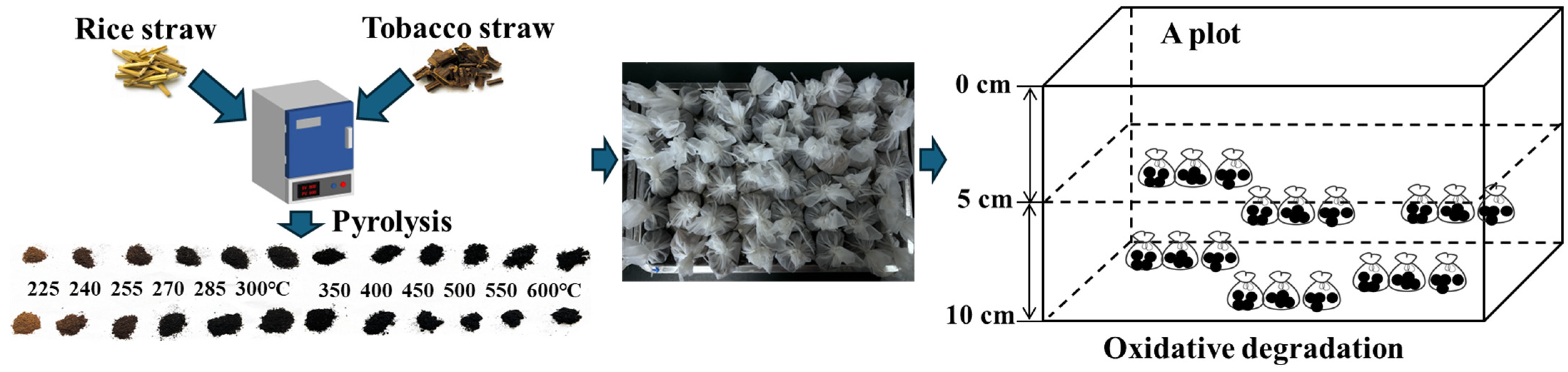
2.2. Straw Biochar Production
2.3. Experimental Setup
2.4. Indicator Measurement Method
2.5. Statistical Analysis
3. Results and Discussion
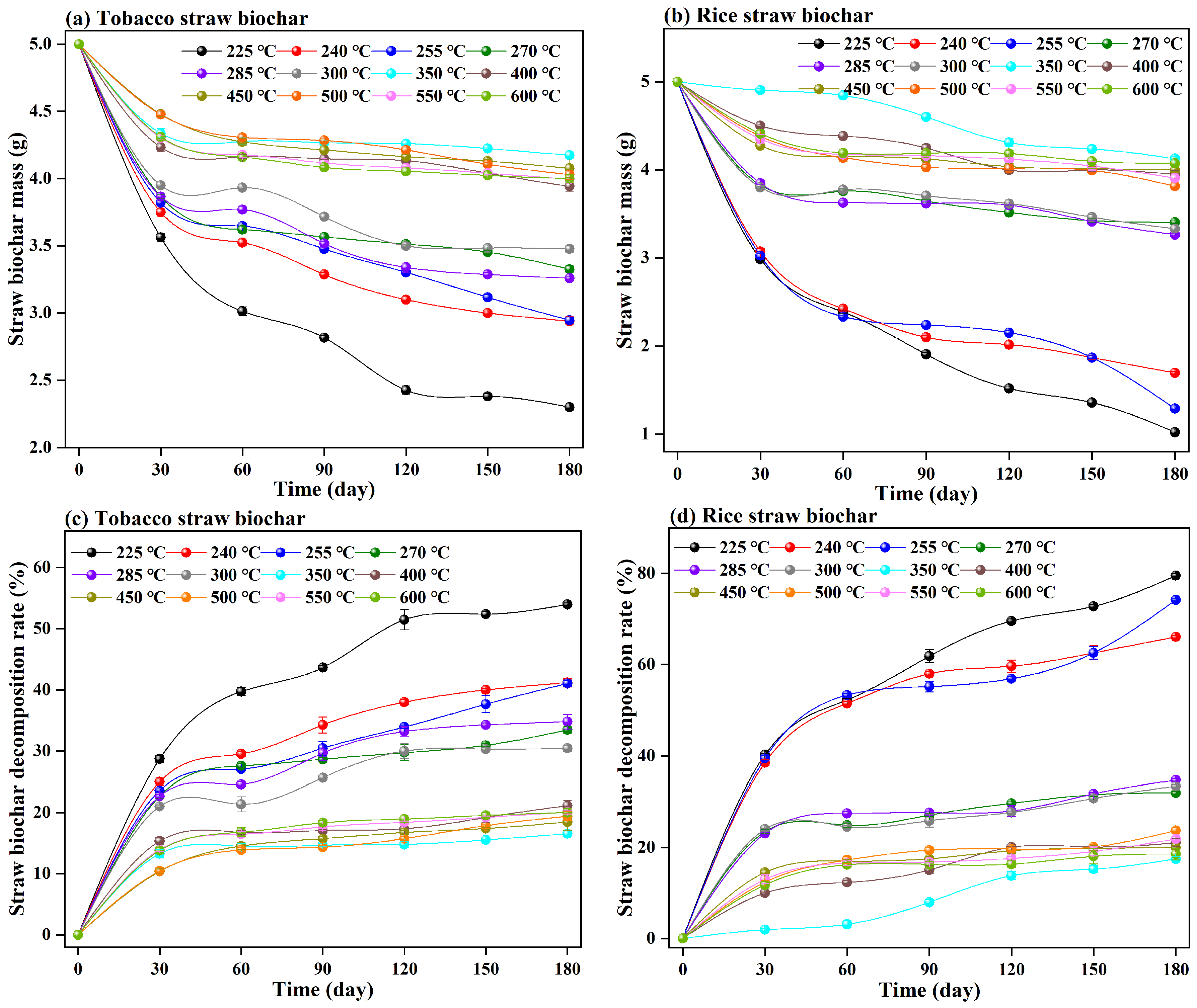
3.1. Changes in Straw Biochar Mass under Different Temperatures
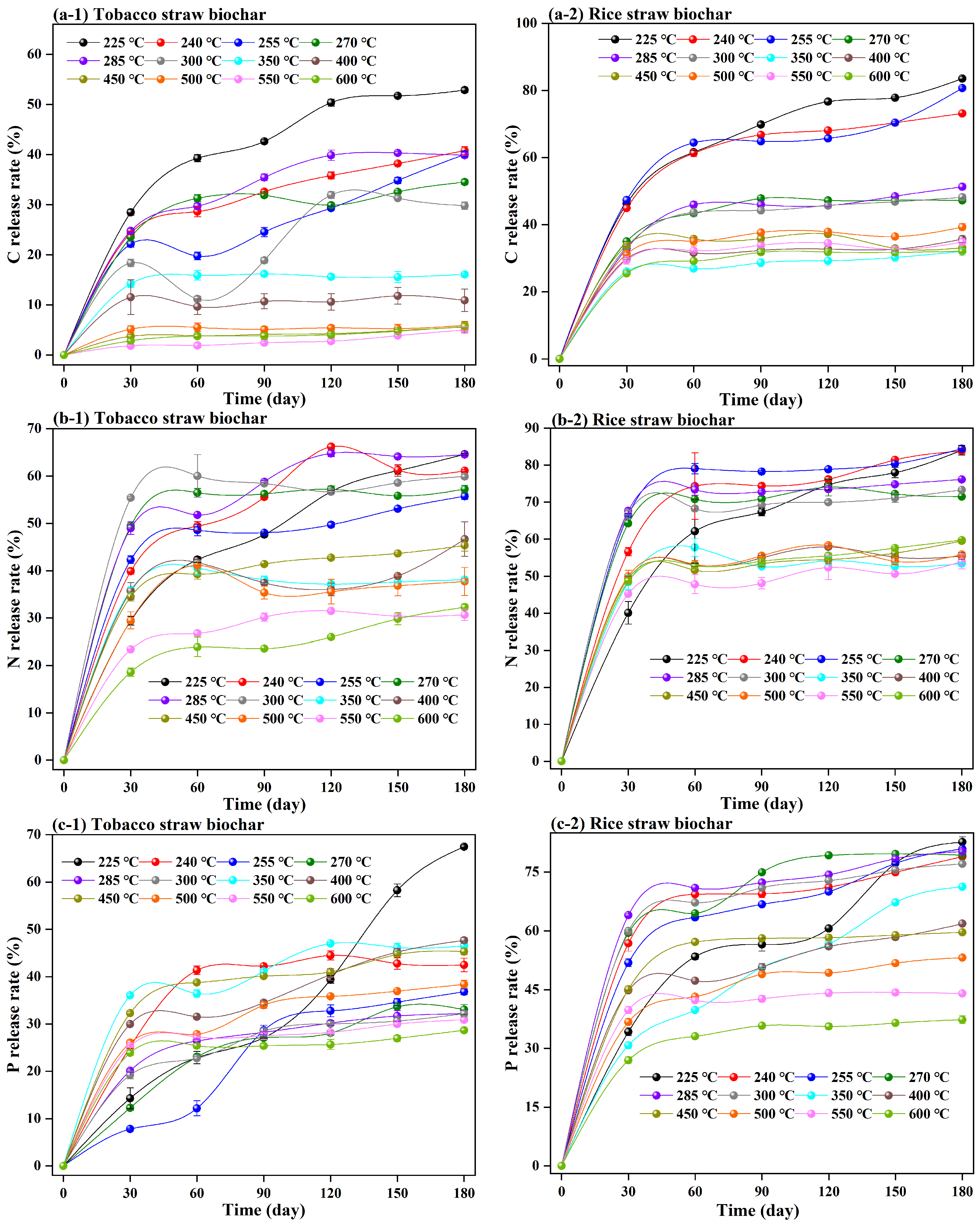
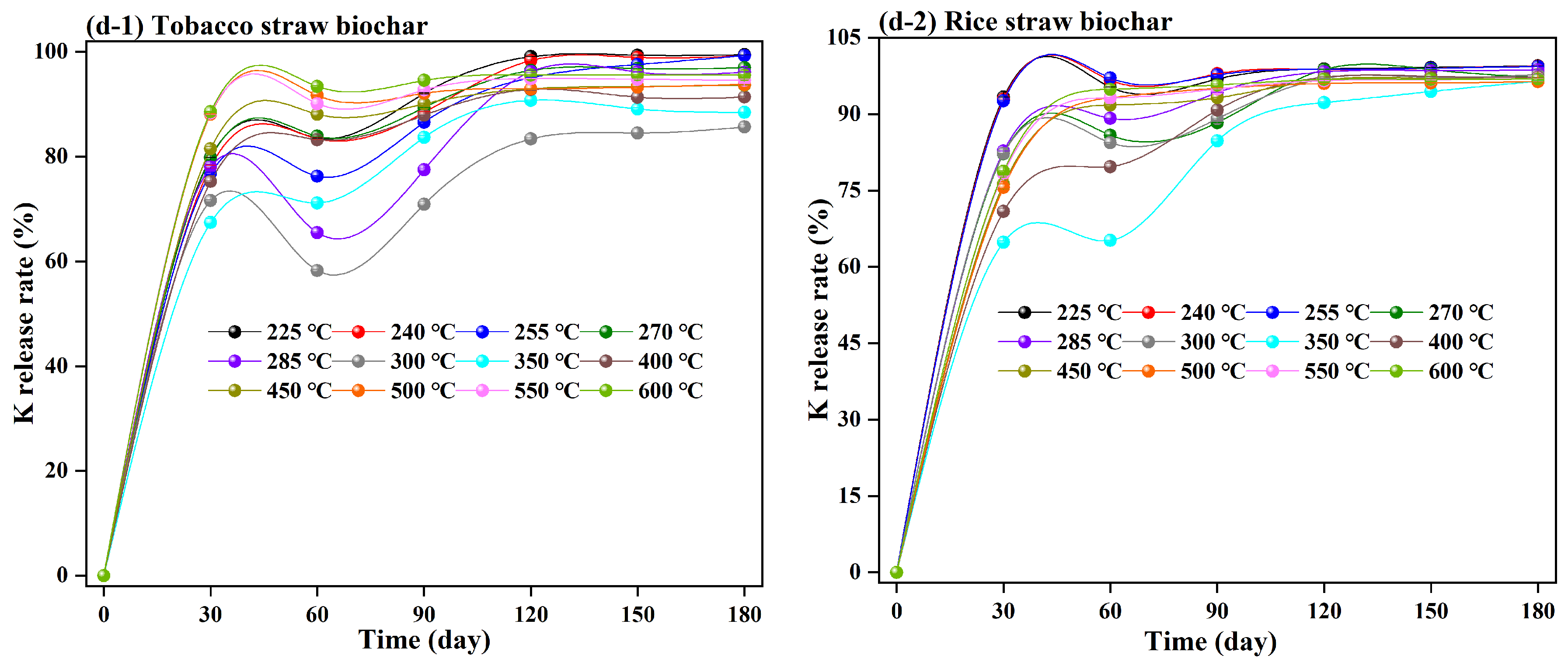
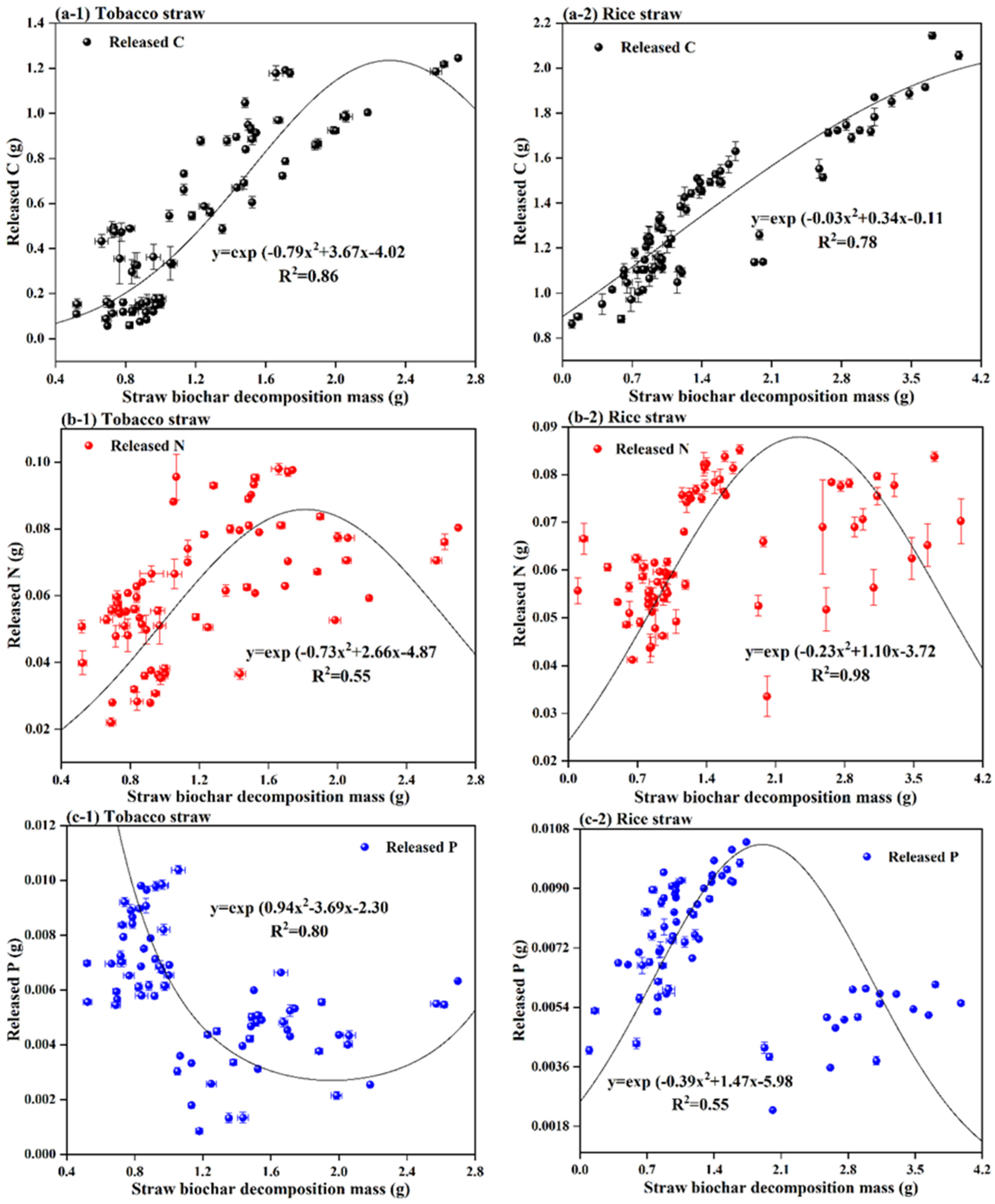
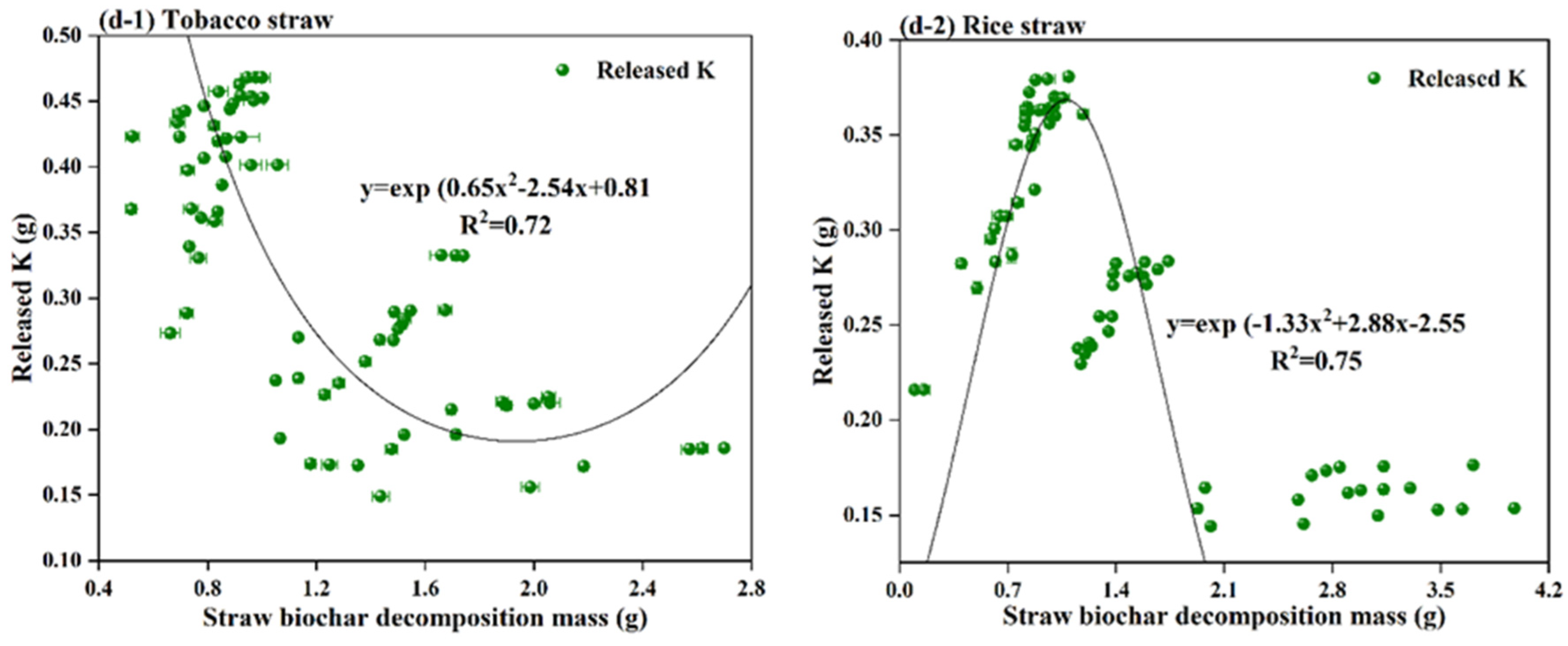
3.2. C, N, P, and K Release from Straw Biochar Decomposition
3.3. Relationship between Straw Biochar Decomposition Mass and Released Nutrients
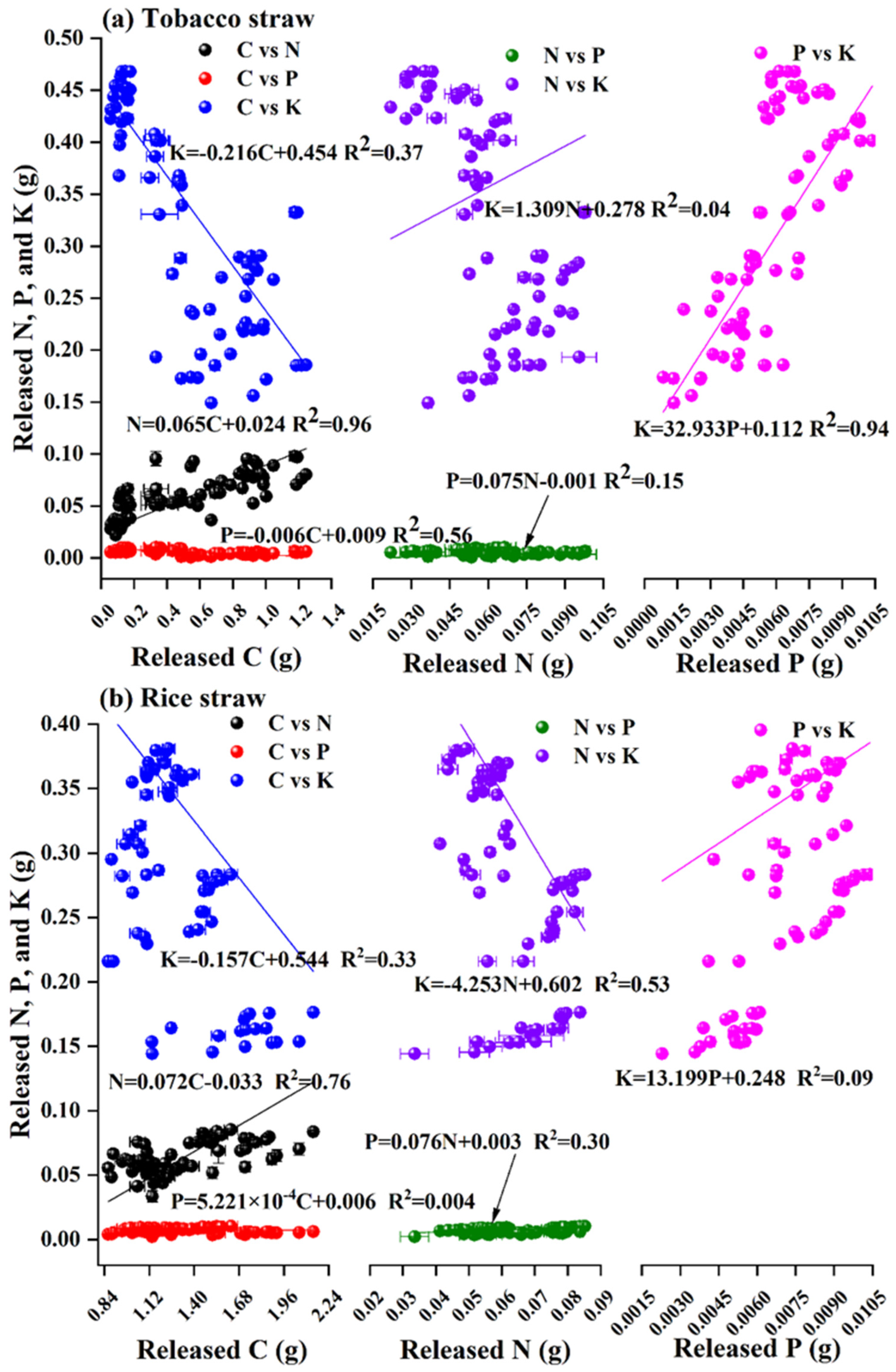
3.4. Interaction between Released C, N, P, and K from Straw Biochar
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, X.L.; Yang, Z.L.; Liu, X.; Huang, G.Q.; Xiao, W.H.; Han, L.J. The composition characteristics of different crop straw types and their multivariate analysis and comparison. Waste Manag. 2020, 110, 87–97. [Google Scholar] [CrossRef]
- Li, H.; Dai, M.W.; Dai, S.L.; Dong, X.J. Current status and environment impact of direct straw return in China’s cropland—A review. Ecotox. Environ. Safe. 2018, 159, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Yan, B.B.; Tao, J.Y.; Li, J.; Kumari, L.; Oba, B.T.; Aborisade, M.A.; Jamro, I.A.; Chen, G.Y. Co-pyrolysis of de-oiled microalgal biomass residue and waste tires: Deeper insights from thermal kinetics, behaviors, drivers, bio-oils, bio-chars, and in-situ evolved gases analyses. Chem. Eng. J. 2022, 446, 137160. [Google Scholar] [CrossRef]
- Kumar, A.; Jamro, I.A.; Rong, H.W.; Kumari, L.; Laghari, A.A.; Cui, B.H.; Aborisade, M.A.; Oba, B.T.; Nkinahamira, F.; Ndagijimana, P.; et al. Assessing bioenergy prospects of algal biomass and yard waste using an integrated hydrothermal carbonization and pyrolysis (HTC–PY): A detailed emission–to–ash characterization via diverse hyphenated analytical techniques and modelling strategies. Chem. Eng. J. 2024, 492, 152335. [Google Scholar] [CrossRef]
- Wang, B.; Shen, X.; Chen, S.; Bai, Y.C.; Yang, G.; Zhu, J.P.; Shu, J.C.; Xue, Z.Y. Distribution characteristics, resource utilization and popularizing demonstration of crop straw in southwest China: A comprehensive evaluation. Ecol. Indic. 2018, 93, 998–1004. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Wang, Z.F.; Can, M.Y.; Bai, H.H.; Ta, N. Analysis of yield and current comprehensive utilization of crop straws in China. J. China Agric. Univ. 2021, 26, 30–41. [Google Scholar]
- Gao, F.; Li, B.; Ren, B.Z.; Zhao, B.; Liu, P.; Zhang, J.W. Achieve simultaneous increase in straw resources efficiency and nitrogen efficiency under crop yield stabilization—A case study of NCP in China for up to 8 years. Field Crop. Res. 2022, 278, 108431. [Google Scholar] [CrossRef]
- Song, K.L.; Zhou, C.H.; Li, H.P.; Zhou, Z.C.; Ni, G.R.; Yin, X. Effects of rumen microorganisms on straw returning to soil at different depths. Eur. J. Soil Biol. 2023, 114, 103454. [Google Scholar] [CrossRef]
- Lu, J.W.; Li, Y.F.; Cai, Y.J.; Jiang, P.K.; Yu, B. Co-incorporation of hydrotalcite and starch into biochar-based fertilizers for the synthesis of slow-release fertilizers with improved water retention. Biochar 2023, 5, 44. [Google Scholar] [CrossRef]
- Xin, H.J.; Yang, J.; Lu, Y.Y.; Xiao, H.K.; Wang, H.T.; Eltohamy, K.M.; Zhu, X.Q.; Liu, C.L.; Fang, Y.Y.; Ye, Y.; et al. Potentials of emergent plant residue derived biochar to be alternative carbon-based phosphorus fertilizer by Fe (II)/Fe(III) magnetic modification. Biochar 2024, 6, 15. [Google Scholar] [CrossRef]
- Dinh, V.M.; Nguyen, H.T.; Nguyen, A.M.; Nguyen, T.T.; Nguyen, T.L.; Uteau, D.; Nguyen, N.H.; Tran, T.M.; Dultz, S.; Nguyen, M.N. Pelletized rice-straw biochar as a slow-release delivery medium: Potential routes for storing and serving of phosphorus and potassium. J. Environ. Chem. Eng. 2022, 10, 107237. [Google Scholar] [CrossRef]
- Chen, K.; Li, N.; Zhang, S.Y.; Liu, N.; Yang, J.F.; Zhan, X.M.; Han, X.R. Biochar-induced changes in the soil diazotroph community abundance and structure in a peanut field trial. Biochar 2022, 4, 26. [Google Scholar] [CrossRef]
- Huang, M.; Fan, L.; Jiang, L.G.; Yang, S.Y.; Zou, Y.B.; Norman, U. Continuous applications of biochar to rice: Effects on grain yield and yield attributes. J. Integr. Agr. 2019, 18, 563–570. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, B.S.; Shen, J.L.; Zhu, X.; Yi, W.Y.; Li, Y.; Wu, J.S. Contrasting effects of straw and straw-derived biochar applications on soil carbon accumulation and nitrogen use efficiency in double-rice cropping systems. Agr. Ecosyst. Environ. 2021, 311, 107286. [Google Scholar] [CrossRef]
- Lin, S.Y.; Wang, W.Q.; Sardans, J.; Lan, X.F.; Fang, Y.Y.; Singh, B.P.; Xu, X.P.; Wiesmeier, M.; Tariq, A.; Zeng, F.J.; et al. Effects of slag and biochar amendments on microorganisms and fractions of soil organic carbon during flooding in a paddy field after two years in southeastern China. Sci. Total Environ. 2022, 824, 153783. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.X.; Dong, C.; Chen, Z.Y.; Liu, Y.J.; Zhang, Y.Y.; Gou, P.X.; Zhao, X.; Ma, D.Y.; Kang, G.Z.; Wang, C.Y.; et al. Successive biochar amendment affected crop yield by regulating soil nitrogen functional microbes in wheat-maize rotation farmland. Environ. Res. 2021, 194, 110671. [Google Scholar] [CrossRef]
- Hu, Y.J.; Sun, B.H.; Wu, S.F.; Feng, H.; Gao, M.X.; Zhang, B.B.; Liu, Y.Y. After-effects of straw and straw-derived biochar application on crop growth, yield, and soil properties in wheat (Triticum aestivum L.)-maize (Zea mays L.) rotations: A four-year field experiment. Sci. Total Environ. 2021, 780, 146560. [Google Scholar] [CrossRef]
- Jin, Z.W.; Chen, C.; Chen, X.M.; Jiang, F.; Hopkins, I.; Zhang, X.L.; Han, Z.Q.; Billy, G.; Benavides, J. Soil acidity, available phosphorus content, and optimal biochar and nitrogen fertilizer application rates: A five-year field trial in upland red soil, China. Field Crop. Res. 2019, 232, 77–87. [Google Scholar] [CrossRef]
- Zhao, Y.Z.; Jiang, H.F.; Gao, J.P.; Feng, Y.Y.; Yan, B.C.; Li, K.; Lan, Y.; Zhang, W.Z. Effects of nitrogen co-application by different biochar materials on rice production potential and greenhouse gas emissions in paddy fields in northern China. Environ. Technol. Inno. 2023, 32, 103242. [Google Scholar] [CrossRef]
- Sun, T.; Sun, Y.B.; Huang, Q.Q.; Xu, Y.M.; Jia, H.T. Sustainable exploitation and safe utilization of biochar: Multiphase characterization and potential hazard analysis. Bioresour. Technol. 2023, 383, 129241. [Google Scholar] [CrossRef]
- Jia, X.X.; Yin, T.; Wang, Y.; Zhou, S.X.; Zhao, X.; Chen, W.T.; Hu, G.Z. Porous honeycomb cork biochar for efficient and highly selective removal of phosphorus from wastewater. Biochar 2023, 5, 84. [Google Scholar] [CrossRef]
- Chen, L.M.; Sun, S.L.; Yao, B.; Peng, Y.T.; Gao, C.F.; Qin, t.; Zhou, Y.Y.; Sun, C.R.; Quan, W. Effects of straw return and straw biochar on soil properties and crop growth: A review. Front. Plant Sci. 2022, 13, 986763. [Google Scholar] [CrossRef] [PubMed]
- Ippolito, J.A.; Cui, L.Q.; Kammann, C.; Wrage-Mönnig, N.; Estavillo, J.M.; Fuertes-Mendizabal, T.; Cayuela, M.L.; Sigua, G.; Novak, J.; Spokas, K.; et al. Feedstock choice, pyrolysis temperature and type influence biochar characteristics: A comprehensive meta-data analysis review. Biochar 2020, 2, 421–438. [Google Scholar] [CrossRef]
- He, X.Y.; Liu, Z.X.; Niu, W.J.; Yang, L.; Zhou, T.; Qin, D.; Niu, Z.Y. Effects of pyrolysis temperature on the physicochemical properties of gas and biochar obtained from pyrolysis of crop residues. Energy 2018, 143, 746–756. [Google Scholar] [CrossRef]
- Waqas, M.; Aburiazaiza, A.S.; Minadad, R.; Rehan, M.; Barakat, M.A.; Nizami, A.S. Development of biochar as fuel and catalyst in energy recovery technologies. J. Clean. Prod. 2018, 188, 477–488. [Google Scholar] [CrossRef]
- Batista, G.; Souza, R.B.A.; Pratto, B.; Santos-Rocha, M.S.R.; Cruz, A.J.G. Effect of severity factor on the hydrothermal pretreatment of sugarcane straw. Bioresour. Technol. 2019, 275, 321–327. [Google Scholar] [CrossRef]
- Bai, N.L.; Zhang, H.L.; Li, S.X.; Zheng, X.Q.; Zhang, J.Q.; Zhang, H.Y.; Zhou, S.; Sun, H.F.; Lv, W.G. Long-term effects of straw and straw-derived biochar on soil aggregation and fungal community in a rice-wheat rotation system. PeerJ 2019, 6, e6171. [Google Scholar] [CrossRef]
- Cai, H.L.; Zhu, L.; Ma, Z.Q. Research progress of the effect of torrefaction pretreatment on the properties of biomass and its pyrolysis product. Energy Environ. Prot. 2024, 1–11. [Google Scholar] [CrossRef]
- Singh, A.; Tsai, M.L.; Chen, C.W.; Singhania, R.R.; Patel, A.K.; Tambat, V.; Dong, C.D. Role of hydrothermal pretreatment towards sustainable biorefinery. Bioresour. Technol. 2023, 367, 128271. [Google Scholar] [CrossRef]
- Liu, J.Z.; Zhong, F.; Niu, W.J.; Zhao, Y.; Su, J.; Feng, Y.X.; Meng, H.B. Effects of temperature and catalytic methods on the physicochemical properties of microwave-assisted hydrothermal products of crop residues. J. Clean. Prod. 2021, 279, 123512. [Google Scholar] [CrossRef]
- Chi, W.C.; Nan, Q.; Liu, Y.X.; Dong, D.; Qin, Y.; Li, S.J.; Wu, W.X. Stress resistance enhancing with biochar application and promotion on crop growth. Biochar 2024, 6, 43. [Google Scholar] [CrossRef]
- Nan, Q.; Wang, C.; Yi, Q.Q.; Zhang, L.; Ping, F.; Thies, J.E.; Wu, W.X. Biochar amendment pyrolysed with rice straw increases rice production and mitigates methane emission over successive three years. Waste. Manag. 2020, 118, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.W.; Zou, W.X.; Chen, J.J.; Chen, H.; Yu, Z.B.; Huang, J.; Tang, H.R.; Wei, X.Y.; Gao, B. Biochar amendment improves crop production in problem soils: A review. J. Environ. Manag. 2019, 232, 8–21. [Google Scholar] [CrossRef] [PubMed]
- Weber, K.; Quicker, P. Properties of biochar. Fuel 2018, 217, 240–261. [Google Scholar] [CrossRef]
- Yan, C.; Yan, S.S.; Jia, T.Y.; Dong, S.K.; Ma, C.M.; Gong, Z.P. Decomposition characteristics of rice straw returned to the soil in northeast China. Nutr. Cycl. Agroecosys. 2019, 114, 211–224. [Google Scholar] [CrossRef]
- Gao, X.Y.; Liu, W.Z.; Li, X.Q.; Zhang, W.Z.; Bu, S.L.; Wang, A.J. A novel fungal agent for straw returning to enhance straw decomposition and nutrients release. Environ. Technol. Inno. 2023, 30, 103064. [Google Scholar] [CrossRef]
- Zhang, X.X.; Zhang, P.Z.; Yuan, X.R.; Li, Y.F.; Han, L.J. Effect of pyrolysis temperature and correlation analysis on the yield and physicochemical properties of crop residue biochar. Bioresour. Technol. 2020, 296, 122318. [Google Scholar] [CrossRef]
- Tan, G.C.; Wang, H.Y.; Xu, N.; Junaid, M.; Liu, H.; Zhai, L.M. Effects of biochar application with fertilizer on soil microbial biomass and greenhouse gas emissions in a peanut cropping system. Environ. Technol. 2021, 42, 9–19. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, J.R.; Gao, W.H.; Guo, Z.H.; Xue, C.; Pang, J.Y.; Shu, L.Z. Effects of biochar amendment on bacterial and fungal communities in the reclaimed soil from a mining subsidence area. Environ. Sci. Pollut. Res. Int. 2019, 26, 34368–34376. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Zhuo, Y.; Zhou, Y.; Chen, Q.; Peng, Y.; Liu, H.; Deng, J.; Xiao, J.; Ai, W.; Sun, S.; et al. Oxidative Degradation Characteristics of Low-Temperature Pyrolysis Biochar and the Synergistic Effect on Released Nutrients. Agronomy 2024, 14, 1898. https://doi.org/10.3390/agronomy14091898
Chen L, Zhuo Y, Zhou Y, Chen Q, Peng Y, Liu H, Deng J, Xiao J, Ai W, Sun S, et al. Oxidative Degradation Characteristics of Low-Temperature Pyrolysis Biochar and the Synergistic Effect on Released Nutrients. Agronomy. 2024; 14(9):1898. https://doi.org/10.3390/agronomy14091898
Chicago/Turabian StyleChen, Limei, Yuchen Zhuo, Yaoyu Zhou, Qing Chen, Yutao Peng, Haoyuan Liu, Jia Deng, Jiahong Xiao, Wenke Ai, Songlin Sun, and et al. 2024. "Oxidative Degradation Characteristics of Low-Temperature Pyrolysis Biochar and the Synergistic Effect on Released Nutrients" Agronomy 14, no. 9: 1898. https://doi.org/10.3390/agronomy14091898




