Effect of Bacillus velezensis GHt-q6 on Cucumber Root Soil Microecology and Root-Knot Nematodes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Greenhouse Conditions
2.2. Greenhouse Experiment Design
2.3. Determination of Soil Microorganisms and Rhizosphere Colonization Capacity of GHt-q6-Rif
2.4. Effects of Soil Enzyme Activity and the Physicochemical Properties of the Strain GHt-q6
2.5. High-Throughput Sequencing and Data Analysis
2.6. Effect of the Strain GHt-q6 on the Soil Root-Knot Nematodes Population Density and Control Efficacy
2.7. Data Analysis
3. Results
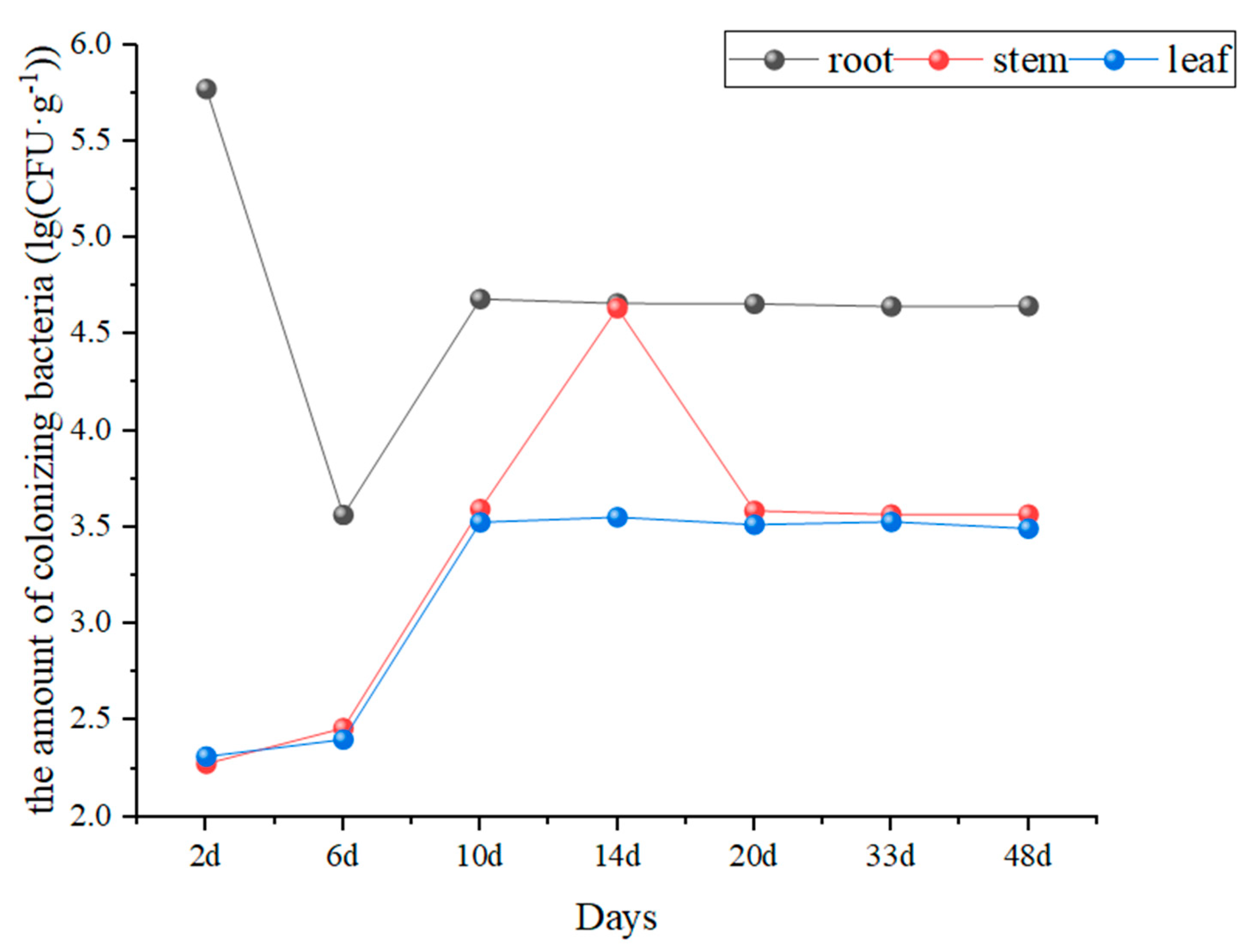
3.1. Colonization of Cucumber Plants by the Strain GHt-q6-Rif
3.2. Effects of the Strain GHt-q6 on the Soil Microbial Population
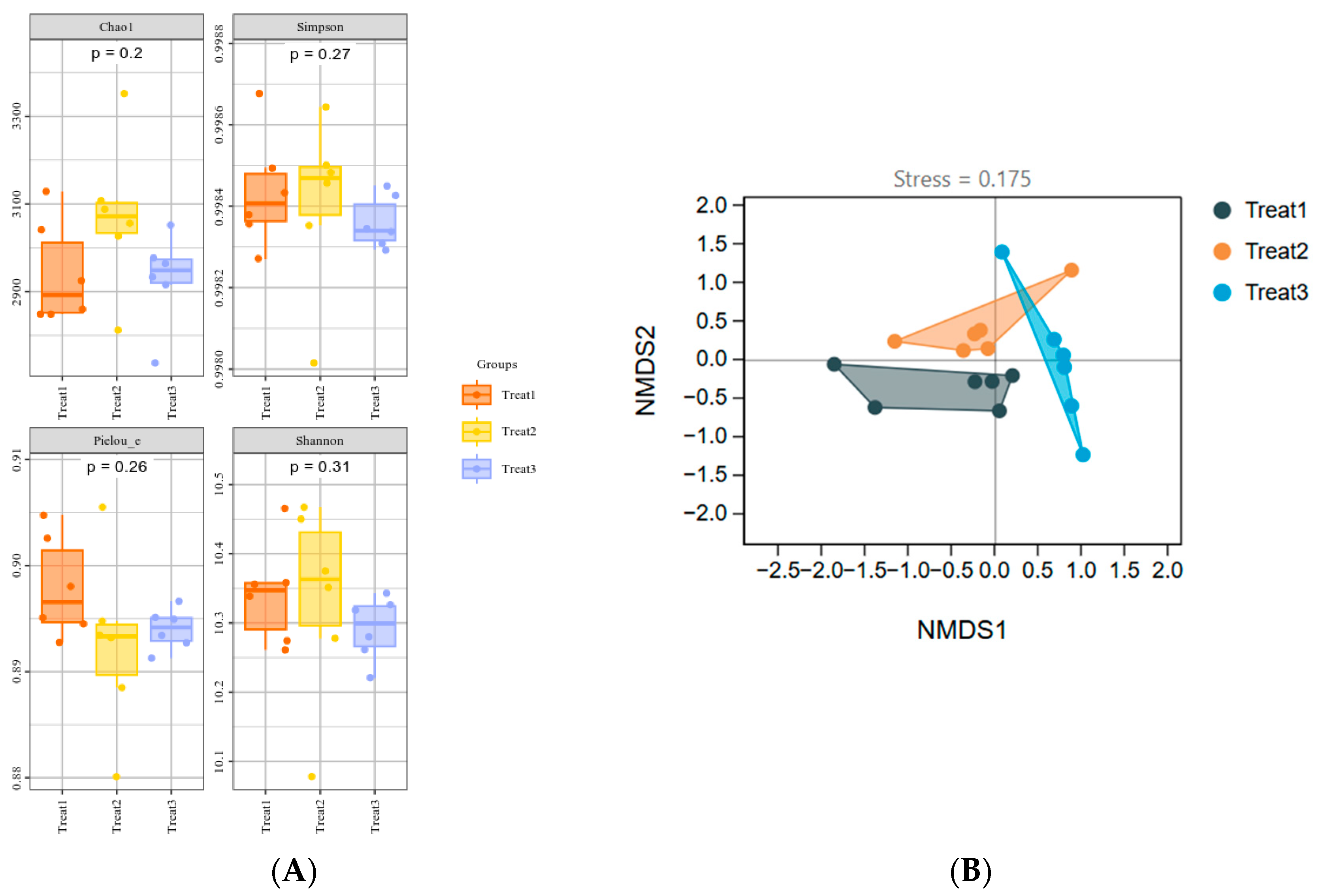
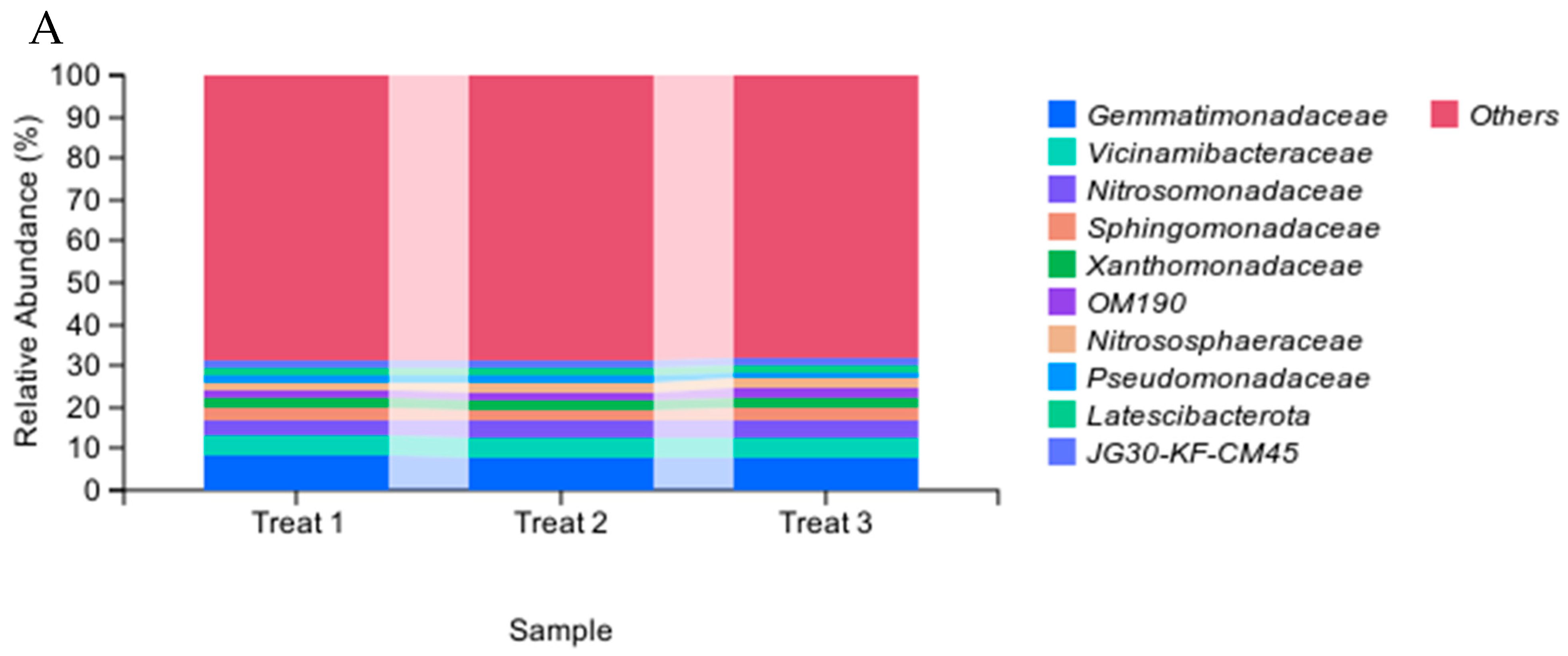
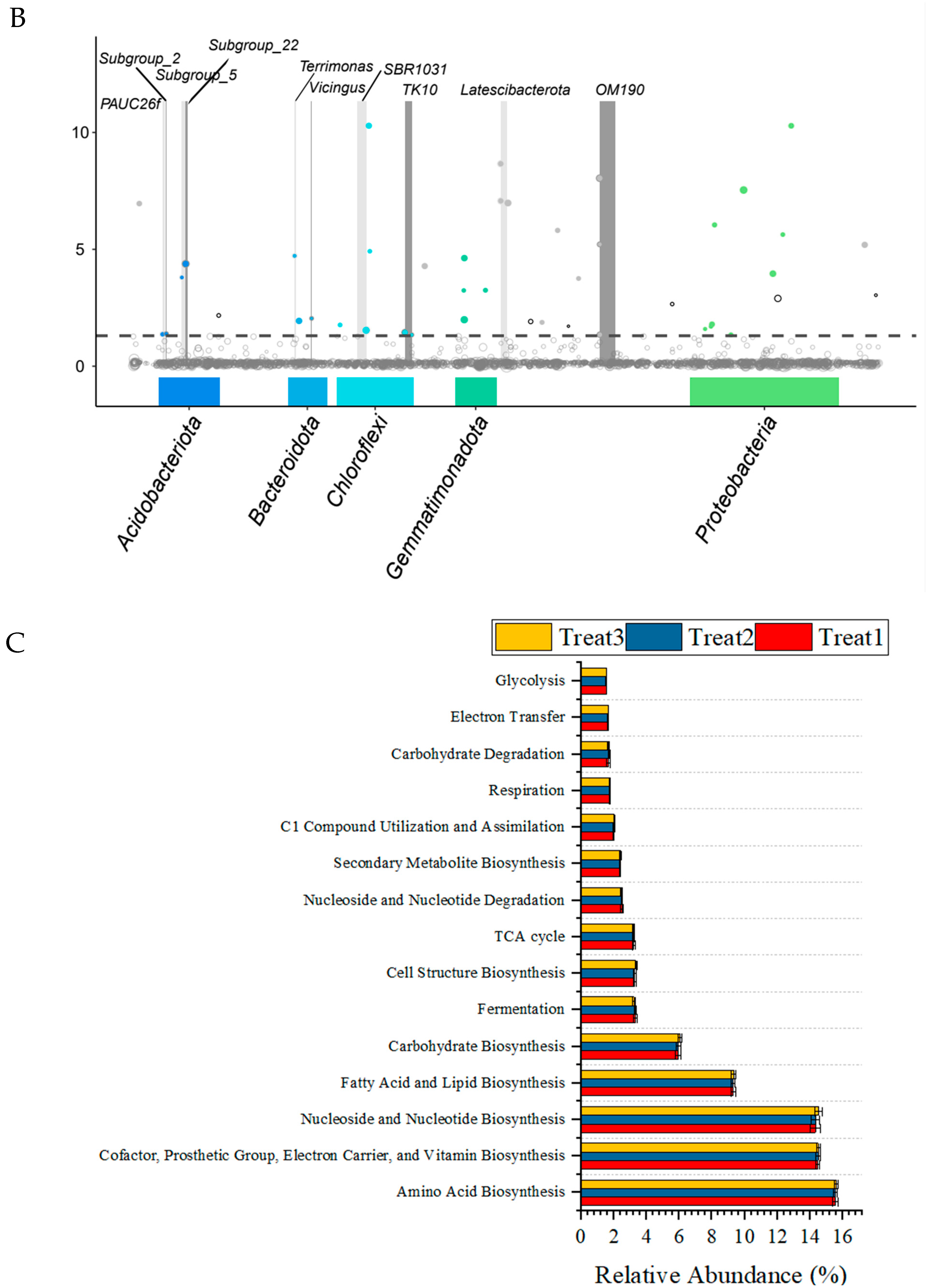
3.3. Effects of the Strain GHt-q6 on Soil Bacterial Community Diversity
3.4. Effect of the Strain GHt-q6 on Soil Enzyme Activity
3.5. Effects of Strain GHt-q6 on Soil Physicochemical Properties
3.6. Efficacy of the Strain GHt-q6 Against Cucumber Root-Knot Nematodes
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- John, T.J.; Annelies, H.; Etienne, G.; Hari, S.G.; Johannes, H.; Michael, G.; Taisei, K.; Rosa, M.; Juan, E.P.; Wim, M.; et al. Top 10 plant-parasitic nematodes in molecular plant pathology. Mol. Plant Pathol. 2013, 14, 946–961. [Google Scholar] [CrossRef]
- Aydinli, G.; Mennan, S. Identification of root-knot nematodes (Meloidogyne spp.) from greenhouses in the Middle Black. Sea Region of Turkey. Turk. J. Zool. 2016, 40, 675–685. [Google Scholar] [CrossRef]
- Yaseen, I.; Mukhtar, T. Impact of sequential and concurrent inoculations of Meloidogyne incognita and Fusarium oxysporum f. sp. vasinfectum on the growth performance of diverse okra cultivars. Plant Prot. 2024, 8, 303–313. [Google Scholar] [CrossRef]
- Tagiev, M.M. Gall nematodes (Meloidogyne) on the Apsheron Peninsula and their control. Succes. Mod. Sci. Educ. 2015, 5, 22–24. [Google Scholar]
- Aziz, S.; Jamshed, S.A.; Mukhtar, T.; Irshad, G.; Ijaz, S.S.; Raja, M.U. Evaluation of Bacillus spp. as biocontrol agents against chili leaf spot caused by Xanthomonas vesicatoria. J. Plant Dis. Prot. 2024, 131, 987–997. [Google Scholar] [CrossRef]
- Asghar, A.; Mukhtar, T.; Raja, M.U.; Gulzar, A. Interaction between Meloidogyne javanica and Ralstonia solanacearum in chili. Pak. J. Zool. 2020, 52, 1525–1530. [Google Scholar] [CrossRef]
- Muhammad, S.; Simon, R.G.; Muhammad, B.; Muhammad, Z.N.; Muhammad, A. Differential responses of Meloidogyne spp. to pasteuria isolates over crop cycles. Plant Prot. 2024, 8, 257–267. [Google Scholar] [CrossRef]
- Yang, L.L.; Huang, Y.; Liu, J.; Li, M.; Mo, M.H.; Li, W.J.; Yang, F.X. Lysinibacillus mangiferahumi sp. nov., a new bacterium producing nematicidal volatiles. Antonie Van Leeuwenhoek 2012, 102, 53–59. [Google Scholar] [CrossRef]
- Park, E.J.; Jang, H.J.; Park, J.Y.; Yang, Y.K.; Kim, M.J.; Park, C.S.; Lee, S.; Yun, B.S.; Lee, S.J.; Lee, S.W.; et al. Efficacy evaluation of Streptomyces nigrescens KA-1 against the root-knot nematode Meloidogyne incognita. Biol. Control 2023, 179, 105–150. [Google Scholar] [CrossRef]
- Gowda, M.T.; Meena, B.R.; Krishnan, N.; Manjunath, M.; Sellaperumal, C.; Rai, A.B.; Singh, A.; Manimurugan, C.; Patil, J.; Pandey, K.K.; et al. Antimicrobial peptides producing native Bacillus spp. for the management of root-knot nematode Meloidogyne incognita infecting okra (Abelmoschus esculentus L. Moench). Biol. Control 2022, 171, 104951. [Google Scholar] [CrossRef]
- Glick, B.R.; Gamalero, E. Recent developments in the study of plant microbiomes. Microorganisms 2021, 9, 1533. [Google Scholar] [CrossRef] [PubMed]
- Bhat, A.A.; Shakeel, A.; Waqar, S.; Handoo, Z.A.; Khan, A.A. Microbes vs. nematodes: Insights into biocontrol through antagonistic organisms to control root-knot nematodes. Plants 2023, 12, 451. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.L.; Zhao, X.M.; Zhao, S.Y.; Zhao, J.L.; Mao, Z.C. The biocontrol functions of Bacillus velezensis strain Bv-25 against Meloidogyne incognita. Front. Microbiol. 2022, 13, 843041. [Google Scholar] [CrossRef]
- Ye, S.; Zhou, S.; Ma, Y. Biocontrol activity and potential mechanism of Bacillus cereus G5 against Meloidogyne graminicola. Pestic. Biochem. Physiol. 2024, 204, 106079. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, L.; Zhai, H.; Dong, H.; Wang, J.; Zhao, Z.; Xu, Y. Bacillus velezensis A-27 as a potential biocontrol agent against Meloidogyne incognita and effects on rhizosphere communities of celery in field. Sci. Rep. 2025, 15, 1057. [Google Scholar] [CrossRef]
- Devindrappa, M.; Kamra, A.; Singh, D.; Gawade, B.; Sirohi, A. Plant growth promoting Bacillus species elicit defense against Meloidogyne incognita infecting tomato in polyhouse. J. Basic Microbiol. 2023, 63, 1233–1241. [Google Scholar] [CrossRef]
- Kulkova, I.; Dobrzyński, J.; Kowalczyk, P.; Bełżecki, G.; Kramkowski, K. Plant growth promotion using Bacillus cereus. Int. J. Mol. Sci. 2023, 24, 9759. [Google Scholar] [CrossRef]
- Asaturova, A.M.; Bugaeva, L.N.; Homyak, A.I.; Slobodyanyuk, G.A.; Kashutina, E.V.; Yasyuk, L.V.; Sidorov, N.M.; Nadykta, V.D.; Garkovenko, A.V. Bacillus velezensis Strains for Protecting Cucumber Plants from Root-Knot Nematode Meloidogyne incognita in a Greenhouse. Plants 2022, 11, 275. [Google Scholar] [CrossRef] [PubMed]
- Vinothini, K.; Nakkeeran, S.; Saranya, N.; Jothi, P.; Richard, J.I.; Perveen, K.; Bukhari, N.A.; Glick, B.R.; Sayyed, R.Z.; Mastinu, A. Rhizosphere Engineering of Biocontrol Agents Enriches Soil Microbial Diversity and Effectively Controls Root-Knot Nematodes. Microb. Ecol. 2024, 87, 120. [Google Scholar] [CrossRef]
- Pei, L.F.; Fan, G.P.; Xiao, M.L. Comparison of two methods for the determination of catalase in soil. Sci. Technol. Innov. Appl. 2019, 15, 145–149. [Google Scholar]
- Feng, X.; Duan, J.P.; Pu, X.P. Comparison of two methods for the determination of soil urease activity. Grassl. Turf. 2008, 2, 70–72. [Google Scholar]
- Li, C.Y.; Zheng, L. Selection of soil sucrase detection conditions based on DNS colorimetric method. China Agron. Bull. 2016, 32, 171–176. [Google Scholar]
- Ang, Q.; Fan, P.Z.; Zhang, Y. Determination of soil quick-acting potassium content by atomic absorption spectrophotometer. Agric. Eng. Technol. 2020, 40, 46–47. [Google Scholar]
- Han, Z.X.; Li, M.; Duang, A.L. Rapid determination of effective phosphorus in soil. Agric. Technol. 2021, 41, 33–35. [Google Scholar]
- Du, M.; Zhong, H.Q. Improvement of method for the determination of organic matter in soil by potassium dichromate oxidation-volumetric method. Chem. Manag. 2021, 25, 16–17. [Google Scholar]
- GB/T 17980 38-2000; Pesticide—Guidelines for the Field Efficacy Trials(I)—Nematocides Against Root-Knot Nematode Disease. Standards Press of China: Beijing, China, 2000.
- Zboralski, A.; Filion, M. Genetic factors involved in rhizosphere colonization by phytobeneficial Pseudomonas spp. Comput. Struct. Biotechnol. J. 2020, 18, 3539–3554. [Google Scholar] [CrossRef]
- Kloepper, J.W. A review of issues related to messunring colonization of plant roots by bacteria. Can. J. Microbiol. 1992, 38, 667–672. [Google Scholar] [CrossRef]
- Shu, J.; Ce, S.; Hai, D.; Yang, X.J.; Li, X.H.; Ji, M.S.; Chu, J. Evaluation of efficacy and mechanism of Bacillus velezensis CB13 for controlling peanut stem rot caused by Sclerotium rolfsii. Front. Microbiol. 2023, 16, 1111965. [Google Scholar]
- Sa, R.B.; Zhang, J.L.; Sun, J.Z.; Gao, Y.X. Colonization Characteristics of Poplar Fungal Disease Biocontrol Bacteria N6-34 and the Inhibitory Effect on Pathogenic Fungi by Real-Time Fluorescence Quantitative PCR Detection. Curr. Microbiol. 2021, 78, 2916–2925. [Google Scholar] [CrossRef]
- Garbeva, P.; Veen, J.A.V.; Elsas, J.D.V. Microbial diversity in soil: Selection of microbial populations by plant and soil type and implications for disease suppressiveness. Annu. Rev. Phytopathol. 2004, 42, 243–270. [Google Scholar] [CrossRef]
- Han, L.J.; Wang, Z.Y.; Li, N.; Wang, Y.H.; Feng, J.T.; Zhang, X. Bacillus amyloliquefaciens B1408 suppresses Fusarium wilt in cucumber by regulating the rhizosphere microbial community. Appl. Soil Ecol. 2019, 136, 55–66. [Google Scholar] [CrossRef]
- Wan, T.T.; Zhao, H.H.; Wang, W. Effect of biocontrol agent Bacillus amyloliquefaciens SN16-1 and plant pathogen Fusarium oxysporum on tomato rhizosphere bacterial community composition. Biol. Control 2017, 112, 1–9. [Google Scholar] [CrossRef]
- Khanna, K.; Kohli, S.K.; Sharma, P.; Kour, J.; Singh, A.D.; Sharma, N.; Ohri, P.; Bhardwaj, R. Antioxidant potential of plant growth-promoting rhizobacteria (PGPR) in agricultural crops infected with root-knot nematodes. In Antioxidants in Plant-Microbe Interaction; Springer: Singapore, 2021; pp. 339–379. [Google Scholar]
- Liu, H.; Wei, L.L.; Zhu, L.; Wei, H.F.; Bai, Y.X.; Liu, X.L.; Li, S.B. Research progress of Sphingomonas. Microbiol. China 2023, 50, 2738–2752. [Google Scholar]
- Kour, D.; Kour, H.; Khan, S.S.; Khan, R.T.; Bhardwaj, M.; Kailoo, S.; Kumari, C. Biodiversity and functional attributes of rhizospheric microbiomes: Potential tools for sustainable agriculture. Curr. Microbiol. 2023, 80, 192. [Google Scholar] [CrossRef] [PubMed]
- Adedayo, A.A.; Fadiji, A.E.; Babalola, O.O. Plant health status affects the functional diversity of the rhizosphere microbiome associated with Solanum lycopersicum. Front. Sustain. Food Syst. 2022, 6, 894312. [Google Scholar] [CrossRef]
- Moreno, L.F.; Mayer, V.; Voglmayr, H.; Rumsaïs, B.; Benjamin, S.J.; Marcus, M.T.; Vania, A.V.; Sybren, H. Genomic analysis of ant domatia-associated melanized fungi (Chaetothyriales, Ascomycota). Mycol. Prog. 2019, 18, 541–552. [Google Scholar] [CrossRef]
- Tang, H.; Xiao, C.H.; Ma, J.Z.; Yu, M.; Li, Y.M.; Wang, G.L. Prokaryotic diversity in continuous cropping and rotational cropping soybean soil. FEMS Microbiol. Lett. 2009, 298, 267–273. [Google Scholar] [CrossRef]
- Alori, E.T.; Glick, B.R.; Babalola, O.O. Microbial phosphorus solubilization and its potential for use in sustainable agricultre. Front. Microbiol. 2017, 8, 971. [Google Scholar] [CrossRef]
- Tian, L.; Shi, S.H.; Ji, L.; Nasir, F.; Ma, L.; Tian, C.J. Effect of the biocontrol bacterium Bacillus amyloliquefaciens on the rhizosphere in ginseng plantings. Int. Microbiol. 2018, 21, 153–162. [Google Scholar] [CrossRef]
- Zhao, J.; Ni, T.; Li, J.; Lu, Q.; Fang, Z.Y.; Huang, Q.W.; Zhang, R.F.; Li, R.; Shen, B.; Shen, Q.R. Effects of organic–inorganic compound fertilizer with reduced chemical fertilizer application on crop yields, soil biological activity and bacterial community structure in a rice–wheat cropping system. Appl. Soil Ecol. 2016, 99, 1–12. [Google Scholar] [CrossRef]
- Rashid, M.I.; Mujawar, L.H.; Shahzad, T.; Almeelbi, T.; Iqbal, M.I.; Ismail, O.M. Bacteria and fungi can contribute to nutrients bioavailability and aggregate formation in degraded soils. Microbiol. Res. 2016, 183, 26–41. [Google Scholar] [CrossRef]
- Fira, D.; Dimkić, I.; Berić, T.; Lozo, J.; Stanković, S. Biological control of plant pathogens by Bacillus species. J. Biotechnol. 2018, 285, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Ghahremani, Z.; Escudero, N.; Beltrán-Anadón, D.; Saus, E.; Cunquero, M.; Andilla, J.; Loza-Alvarez, P.; Gabaldón, T.; Sorribas, F.J. Bacillus firmus strain I-1582, a nematode antagonist by itself and through the plant. Front. Plant Sci. 2020, 11, 796. [Google Scholar] [CrossRef]
- Ye, L.; Wang, J.Y.; Liu, X.F.; Guan, Q.; Dou, N.X.; Li, J.; Zhang, Q.; Gao, Y.M.; Wang, M.; Li, J.S.; et al. Nematicidal activity of volatile organic compounds produced by Bacillus altitudinis AMCC 1040 against Meloidogyne incognita. Arch. Microbiol. 2022, 204, 521. [Google Scholar] [CrossRef] [PubMed]
- Briar, S.S.; Wichman, D.; Reddy, G.V.P. Plant-parasitic nematode problems in organic agriculture. In Organic Farming for Sustainable Agriculture; Springer: Berlin/Heidelberg, Germany, 2016; Volume 9, pp. 107–122. [Google Scholar]
- Hu, Y.F.; You, J.; Wang, Y.; Long, Y.; Wang, S.R.; Pan, F.J.; Yu, Z.H. Biocontrol efficacy of Bacillus velezensis strain YS-AT-DS1 against the root-knot nematode Meloidogyne incognita in tomato plants. Front. Microbiol. 2022, 13, 1035748. [Google Scholar] [CrossRef]

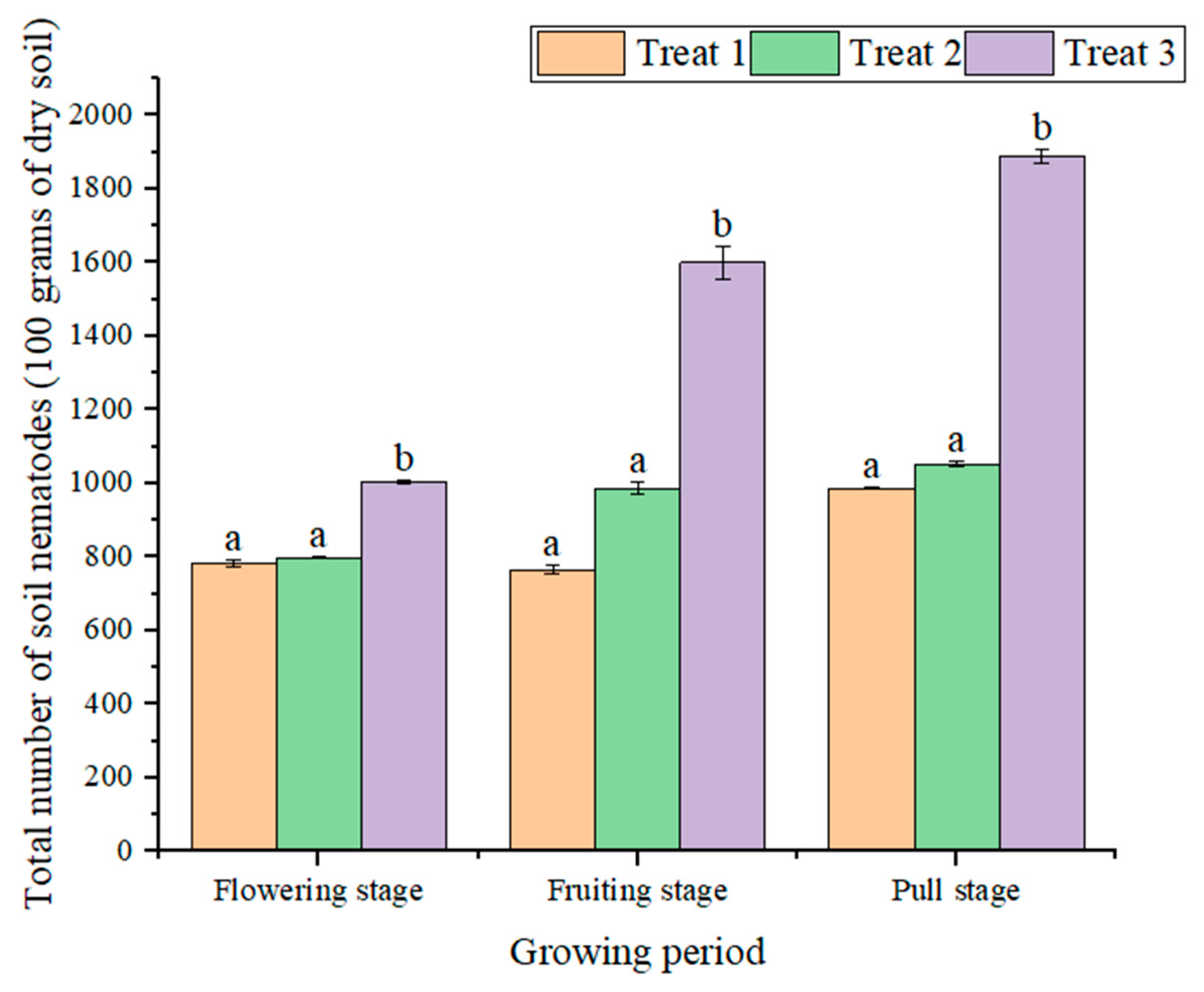
| Microbe | Disposal | 2 d | 7 d | 20 d | 34 d | 48 d |
|---|---|---|---|---|---|---|
| Bacteria (108 cfu·g−1) | GHt-q6 | 3.47 ± 0.19 a | 5.35 ± 0.15 a | 2.39 ± 0.16 a | 2.50 ± 0.15 a | 3.94 ± 0.12 a |
| B. subtilis | 2.00 ± 0.09 b | 3.30 ± 0.12 b | 2.00 ± 0.19 b | 1.77 ± 0.12 b | 3.20 ± 0.19 b | |
| CK | 1.24 ± 0.14 c | 2.01 ± 0.04 c | 0.84 ± 0.19 c | 2.05 ± 0.14 c | 3.50 ± 0.14 b | |
| Fungi (106 cfu·g−1) | GHt-q6 | 1.74 ± 0.07 a | 1.49 ± 0.24 a | 7.11 ± 0.86 a | 1.86 ± 0.33 a | 4.05 ± 0.31 a |
| B. subtilis | 3.39 ± 0.22 b | 2.48 ± 0.25 b | 7.77 ± 0.51 a | 3.07 ± 0.53 b | 3.96 ± 0.40 a | |
| CK | 3.98 ± 0.78 b | 4.21 ± 0.61 c | 8.38 ± 0.91 a | 1.22 ± 0.23 a | 3.32 ± 0.67 a | |
| Actinomycetes (107 cfu·g−1) | GHt-q6 | 1.30 ± 0.06 a | 1.05 ± 0.03 a | 1.01 ± 0.09 a | 0.63 ± 0.06 a | 2.66 ± 0.11 a |
| B. subtilis | 1.03 ± 0.09 b | 0.64 ± 0.08 b | 1.86 ± 0.16 b | 0.08 ± 0.01 b | 2.54 ± 0.17 b | |
| CK | 1.08 ± 0.09 b | 1.44 ± 0.18 c | 2.44 ± 0.13 c | 2.12 ± 0.07 c | 4.05 ± 0.06 c |
| Soil Enzymes | Disposal | 2 d | 7 d | 20 d | 34 d | 48 d |
|---|---|---|---|---|---|---|
| Soil urease activity (mg·g−1) | GHt-q6 | 6.75 ± 0.19 a | 5.38 ± 0.61 a | 4.33 ± 0.47 a | 3.75 ± 0.33 a | 5.50 ± 0.09 a |
| B. subtilis | 5.41 ± 0.09 ab | 5.08 ± 0.20 ab | 5.80 ± 0.08 b | 4.21 ± 0.03 b | 5.63 ± 0.02 b | |
| CK | 4.28 ± 0.01 b | 4.51 ± 0.03 b | 5.14 ± 0.14 c | 3.05 ± 0.07 c | 5.37 ± 0.01 c | |
| Soil sucrase activity (mg·g−1) | GHt-q6 | 1.74 ± 0.04 a | 1.94 ± 0.01 ab | 1.50 ± 0.07 b | 2.14 ± 0.04 a | 1.20 ± 0.01 a |
| B. subtilis | 1.22 ± 0.06 a | 1.88 ± 0.01 b | 1.42 ± 0.01 a | 1.97 ± 0.03 b | 1.42 ± 0.08 b | |
| CK | 1.12 ± 0.03 b | 1.07 ± 0.00 a | 1.05 ± 0.05 b | 1.73 ± 0.05 c | 1.24 ± 0.01 a | |
| Soil catalase activity mg H2O2·(g·20 min) −1 | GHt-q6 | 4.52 ± 0.08 a | 3.40 ± 0.12 ab | 2.65 ± 0.11 a | 3.78 ± 0.15 a | 3.93 ± 0.14 a |
| B. subtilis | 4.70 ± 0.11 a | 3.32 ± 0.09 a | 2.98 ± 0.16 b | 4.61 ± 0.17 b | 3.60 ± 0.15 b | |
| CK | 5.10 ± 0.11 b | 3.64 ± 0.17 b | 2.57 ± 0.18 a | 3.41 ± 0.09 c | 4.00 ± 0.14 a |
| Growing Period | Disposal | AP (mg·kg−1) | AK (mg·kg−1) | SOM (g·kg−1) |
|---|---|---|---|---|
| Seedling stage | GHt-q6 | 150.25 ± 7.62 b | 448.80 ± 12.75 a | 35.14 ± 0.40 a |
| B. subtilis | 129.45 ± 7.41 ab | 313.37 ± 10.81 b | 29.53 ± 0.53 a | |
| CK | 104.90 ± 8.63 a | 207.40 ± 10.14 c | 24.71 ± 0.47 b | |
| Flowering stage | GHt-q6 | 113.75 ± 11.13 a | 401.20 ± 5.91 a | 33.45 ± 1.73 a |
| B. subtilis | 100.45 ± 6.89 a | 309.97 ± 17.74 ab | 27.71 ± 0.04 ab | |
| CK | 106.27 ± 7.75 a | 272.00 ± 16.43 b | 23.45 ± 0.76 b | |
| Fruiting stage | GHt-q6 | 127.88 ± 8.29 a | 307.40 ± 3.05 a | 34.03 ± 0.81 a |
| B. subtilis | 101.46 ± 2.88 a | 296.93 ± 15.82 ab | 30.33 ± 0.76 a | |
| CK | 99.75 ± 7.62 a | 253.30 ± 4.14 b | 23.13 ± 0.87 b | |
| Pull stage | GHt-q6 | 192.69 ± 8.29 a | 233.60 ± 6.05 a | 17.47 ± 0.93 a |
| B. subtilis | 187.42 ± 8.91 a | 210.80 ± 10.89 a | 14.41 ± 0.96 a | |
| CK | 166.00 ± 11.12 a | 158.10 ± 18.25 b | 13.09 ± 0.72 a |
| Disposal | Disease Index | Control Effect (%) |
|---|---|---|
| Treat 1 | 13.13 ± 1.61 b | 43.35 ± 5.46 a |
| Treat 2 | 15.00 ± 1.02 b | 35.81 ± 3.31 a |
| Treat 3 | 23.13 ± 0.72 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Wang, L.; Peng, J.; Wang, C.; Wang, M. Effect of Bacillus velezensis GHt-q6 on Cucumber Root Soil Microecology and Root-Knot Nematodes. Agronomy 2025, 15, 1000. https://doi.org/10.3390/agronomy15041000
Liu Y, Wang L, Peng J, Wang C, Wang M. Effect of Bacillus velezensis GHt-q6 on Cucumber Root Soil Microecology and Root-Knot Nematodes. Agronomy. 2025; 15(4):1000. https://doi.org/10.3390/agronomy15041000
Chicago/Turabian StyleLiu, Yuanyuan, Luwei Wang, Jiale Peng, Chunwei Wang, and Meiqin Wang. 2025. "Effect of Bacillus velezensis GHt-q6 on Cucumber Root Soil Microecology and Root-Knot Nematodes" Agronomy 15, no. 4: 1000. https://doi.org/10.3390/agronomy15041000
APA StyleLiu, Y., Wang, L., Peng, J., Wang, C., & Wang, M. (2025). Effect of Bacillus velezensis GHt-q6 on Cucumber Root Soil Microecology and Root-Knot Nematodes. Agronomy, 15(4), 1000. https://doi.org/10.3390/agronomy15041000







