Abstract
Utilizing nanotechnology for weed management offers a sustainable alternative to synthetic herbicides. This study evaluated the effectiveness of sunn hemp extract (SH), Fe3O4 nanoparticles (NPs), and Fe3O4/sunn hemp NPs in inhibiting the germination of redroot pigweed (Amaranthus retroflexus L.), wild mustard (Sinapis arvensis L.), and lamb’s quarters (Chenopodium album L.) weeds. The structural characteristics of the NPs were analyzed using Scanning electron microscopy (SEM), Scanning X-ray diffraction (XRD), Thermogravimetric analysis (TGA), Vibrating sample magnetometer (VSM), Brunner–Emmet–Teller (BET), and Fourier-transform infrared spectroscopy (FTIR). The optimal Fe3O4 NP concentration for reducing seed germination ranged from 3000 to 3100 mg L−1. Higher concentrations of SH extract (100, 150, and 200 g L−1) effectively inhibited weed seed germination with A. retroflexus displaying the highest sensitivity. The maximal effective concentration (NOECmax) for Fe3O4/sunn hemp NPs was 10 g L−1 for S. arvensis, 150 g L−1 for A. retroflexus, and 200 g L−1 for C. album. Fe3O4/sunn hemp NPs led to a reduction in 1/D50 and an increase in EEC50, indicating a rise in sensitivity to Fe3O4 NPs, particularly in S. arvensis. Variations in species responses to SH, Fe3O4 NPs, and Fe3O4/sunn hemp NPs are likely influenced by genetic, physiological, and ecological factors. Overall, the findings suggest that utilizing Fe3O4/sunn hemp NPs offers an effective strategy for sustainable weed management.
1. Introduction
Competition with weeds poses a significant biological challenge to crop production, contributing up to a 34% reduction in crop yields [1,2]. While the use of relatively low-cost synthetic chemicals for weed control offers short-term effectiveness and ease of application, there is an increasing need for farming practices that offer more sustainable and environmentally friendly strategies [3,4,5,6,7].
Nanotechnology has emerged as an alternative weed control [8,9]. Nanoparticles (NPs), typically less than 100 nm in size, exhibit unique electrochemical, optical, and thermal properties. Nevertheless, the synthesis of metallic NPs may pose environmental hazards attributable to the application of highly reactive and toxic reducing agents, including sodium borohydride and hydrazine hydrate [10]. To mitigate these concerns, green chemistry methods have been proposed to produce more environmentally friendly NPs, using extracts from plants such as barley (Hordeum vulgare) and sorrel (Rumex acetosa) [11] and methanolic extracts from grape leaves [12] to stabilize Fe3O4 NPs. These processes highlight the role of biomolecules, such as phytols, terpenoids, and antioxidants in synthesizing Fe3O4 NPs.
Sunn hemp, a C3 tropical crop from Fabaceae family, is known for its resilience to harsh environmental conditions and traditionally is used as a cover crop for nitrogen biological fixation [13]. It offers various ecosystem benefits, including suppression of weeds [14], parasitic nematodes [15], in addition to the improvements in soil health [16]. Sunn hemp’s ability to produce substantial biomass contributes to its effectiveness in weed suppression [17], but also releases allelopathic compounds such as hydroxy norleucine, a phytotoxic non-protein amino acid, from its decomposing residues [18]. Sunn hemp seeds also contain 2-amino-5-hydroxyhexanoic acid, another amino acid with weed-suppressing properties [19]. Furthermore, sunn hemp’s root, stem, and seeds contain dehydropyrrolizidine alkaloids, which contribute to weed suppression abilities [20]. These alkaloids are found in varying concentrations in different plant parts, including 0.150% w/w in seeds, 0.115% w/w in stems, 0.053% w/w in roots, and 0.008% w/w in leaves [21]. Alkaloids such as riddelliine, senecionine, and seneciphylline are known for their allelopathic effects in weed suppression [19]. However, concerns have been raised about relying solely on the allelopathic effect of cover crops for weed management due to the negligible amounts of allelopathic compounds in the plants. Combining allelopathic compounds from plant extracts with NPs may present a novel approach to integrated weed management.
Redroot pigweed (Amaranthus retroflexus), lamb’s quarters (Chenopodium album), and wild mustard (Sinapis arvensis) are amongst most common weeds in many agricultural fields. These species hinder crop growth and reduce yield due to their rapid growth, prolific seed production, and ability to germinate under diverse environmental conditions. Several studies have explored the effects of aqueous and methanolic extracts, as well as essential oils derived from allelopathic plants, on the germination of these invasive weed species [22,23,24]. The application of sunn hemp leaf extract significantly reduced germination in various crops, including Poaceae (cereal rye, sweet corn, winter wheat 2–22%), Solanaceae (tomato, bell pepper 100%), Fabaceae (Austrian winter pea, cowpea, crimson clover 39–8%), Liliaceae (onion 95%), Brassicaceae (turnip 69%), Malvaceae (okra 49%), and Cucurbitaceae (cucumber 2%) [25].
In this study, we hypothesized that an aqueous extract derived from sunn hemp leaves can significantly prevent the germination of weed species. Previous research has shown that natural extracts that possess phytotoxic properties can make them viable alternatives to synthetic herbicides. We propose that green synthesis of Fe3O4 NPs using sunn hemp leaf extract may further enhance the inhibition of germination, surpassing the effects of the extract alone. This investigation aims to evaluate the effectiveness of the sunn hemp aqueous extract, Fe3O4 NPs, and the synthesized Fe3O4/sunn hemp NPs in controlling the germination of the three common weed species. We seek to determine whether the combination of NPs and plant extracts offers a more effective approach to weed management. This research therefore may contribute to the sustainability of farming by providing an eco-friendly alternative to synthetic herbicides and promoting an environmentally sustainable approach to managing weed growth.
2. Materials and Methods
2.1. Sunn Hemp Biomass and Extract Preparation
Sunn hemp (Crotalaria Juncea L.), (Global Sunn brand) was grown in the field at the Experimental Research Station of the Department of Plant Production and Genetics, University of Mohaghegh Ardabili, Iran, with geographical coordinates 48°20′ E, 38°19′ N in 2022. Sunn hemp was planted at the recommended seeding rate, 50 kg ha–1 [26]. The leaves of the plants at the onset of the blooming phase, approximately 65 days after planting, were harvested by hand [25]. The collected material was completely dried at room temperature (23 ± 2 °C) for 30 days because phenolics are not stable for drying in an oven at high temperatures [27]. The extract was prepared utilizing the maceration technique outlined by Trusheva et al. [28]. In brief, specified amounts of sunn hemp powder (0, 10, 50, 100, 150, and 200 g L−1) were added to one liter of distilled water, and placed on the shaker for 24 h. The solution was filtered through Whatman No. 42 filter paper and centrifuged for 15 min at 5000 rpm to remove the solids. The separated supernatant, referred to hereafter as the sunn hemp extract, was kept in the refrigerator at 4 °C until germination tests were conducted.
2.2. Seed Gathering and Storage
The experiment aimed to study the impact of the aqueous extract of sunn hemp, Fe3O4 NPs, and Fe3O4/sunn hemp NPs on the germination of the three weed species lamb’s quarters (Chenopodium album L.), redroot pigweed (Amaranthus retroflexus L.), and wild mustard (Sinapis arvensis L.). The weed seeds were collected from fields in Moghan Research and Natural Resources Center located at 48°20′ E, 38°19′ N during the summer of 2022. Weed-ripening inflorescences were gathered and air-dried at 23 ± 2 °C for three weeks, after which seeds were separated by rubbing. Germination tests on freshly harvested seed showed that C. album, A. retroflexus, and S. arvensis had high primary dormancy. We broke the primary seed dormancy by treating them with ultrasound technology and gibberellic acid at 15 min and 1500, and 1000 mg L−1, respectively [29,30,31]. The weed seeds were stored in a dry environment at room temperature (23 ± 2 °C) for four days before the experiment started.
2.3. Biosynthesis of Fe3O4 NPs
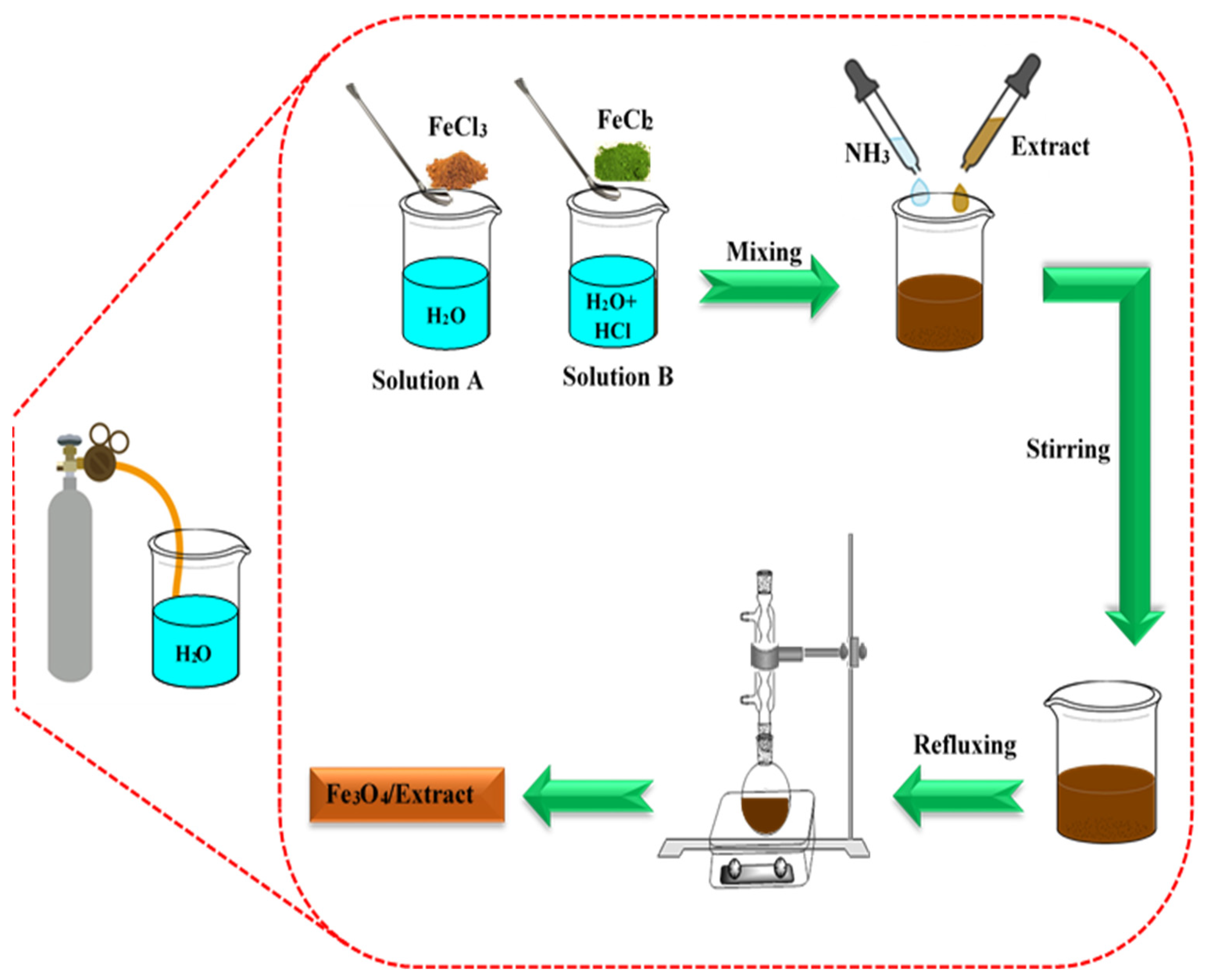
The Fe3O4 NPs were synthesized through the co-precipitation technique described by Massart, [32]. Initially, water was degassed by blowing N2 gas for 20 min. For the synthesis, 0.476 g of FeCl3·6H2O was dissolved in 20 mL of the degassed water (Solution A). In a separate preparation, 0.166 g of FeCl2·4H2O was added to 5 mL of degassed water and 0.8 mL of 2 M HCl (Solution B). Solutions A and B were combined with 13.1 mL of NH3 and 20 mL of sunn hemp extract, while N2 gas was continuously blown into the solutions. After stirring for 60 min, the brown precipitate was refluxed for an additional hour to ensure a complete reaction. The resulting precipitate was then washed with water and ethanol before drying. Additionally, pure Fe3O4 NPs was synthesized using the same method, omitting the sunn hemp extract (Scheme 1, Reaction Equation (1)).
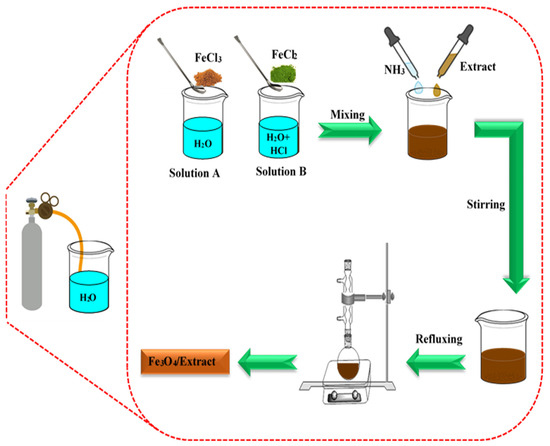
Scheme 1.
Schematic of Fe3O4/sunn hemp extract synthesis.
2.4. Characterization Techniques
Thermogravimetric analysis (TGA, LINSEIS STA PT-1000, LINSEIS Messgeräte GmbH, Selb, Germany) and vibrating sample magnetometer (VSM, MDKF, Magnetic Daneshpajoh Kashan (MDK) Co., Kashan, Iran), Fourier-transform infrared spectroscopy (FTIR, Perkin Elmer Spectrum RXI, PerkinElmer Co., Hopkinton, MA, USA), Scanning electron microscope (SEM, LEO 1430VP, LEO Electron Microscopy Ltd., Cambridge, UK and Carl Zeiss AG, Jena, Germany), X-ray diffraction (XRD, Philips Xpert diffractometer, Philips Co., Eindhoven, The Netherlands), and Brunner–Emmet–Teller (BET, Belsorp Mini II, MicrotracBEL Corp., Osaka, Japan) analysis were employed to assess the thermal and magnetic properties, analyze the chemical structure and functional groups, investigate the microstructures and crystal structure of the nanomaterials, and evaluate their textural characteristics.
2.5. Experimental Design
The study was executed in 2022 at the Weed Science Laboratory, Faculty of Agriculture and Natural Resources at Mohaghegh Ardabili University. The bioassay experiments were conducted in two parts. In the first part, the focus was on optimizing the impact of Fe3O4 NPs on the germination of seeds. A germination test was carried out using different concentrations of Fe3O4 NPs (0, 250, 500, 1000, 2000, and 3000 mg L−1) to determine the half-maximal effective concentration (NOEC50) and maximal effective concentration (NOECmax) within two defined ranges. Subsequently, Fe3O4 NPs containing sunn hemp extract were synthesized using green methods. In the second part, a germination test was performed using 0, 10, 50, 100, 150, and 200 g L−1 (or 0, 1, 5, 10, 15, and 20%) of pure sunn hemp extract and Fe3O4/sunn hemp NPs in three points (SH (Sunn hemp extract), SH + NOEC50, and SH + NOECmax). Both parts of the experiment were conducted using a completely randomized design with three replications for each weed seed. Each Petri dish contained 50 seeds disinfected with 1% sodium hypochlorite placed on filter paper within 9 mm diameter Petri dishes. Ten milliliters of the treatments (SH extract, Fe3O4 NPs, and Fe3O4/sunn hemp NPs) were added to each Petri dish, which was then covered with transparent plastic wrap and transferred to a seed germinator (BINDER KBW 240, Binder GmbH, Tuttlingen, Deutschland, Germany) set at a temperature of 25 °C without light. Germination experiments lasted 14 days. Seeds exhibiting healthy radicles that reached a length of two millimeters were classified as successfully germinated [33].
2.6. Gas Chromatography–Mass Spectrometry (GC-MS)
The analytical procedures utilized an Agilent 7890B Gas Chromatography system paired with an Agilent 5977A Mass Selective Detector (MSD), Agilent Technologies Inc, Palo Alto, CA, USA. A sample injection volume of 0.1 μL was used, with helium gas serving as the carrier. The multimode inlet (MMI) operated in split mode with a 15:1 ratio and a flow rate of 48 mL/min. The MMI’s initial temperature started at 100 °C and was raised to 380 °C at 8 °C/min, while the oven began at 40 °C and increased to 325 °C at 4 °C/min before rising to 400 °C at 10 °C/min, held for 12.5 min. The total analytical duration was 91.25 min.
2.7. Statistical Analysis
Cumulative germination percentage: The cumulative germination percentage represents the overall count of seeds that successfully sprouted within the experimental sample.
Final germination percentage (Gmax): Scott et al.’s [34]. and Burnett et al.’s [35] Equation (2) was used to calculate the Gmax.
where S indicates the number of germinated seeds, and T refers to the total number of seeds included in the sample.
For the determination of the regression trend of parameters, we used the non-linear regression function of Logistic (Equation (3)) and Comperze (Equation (4)) using R 2.4.1 software.
where Ymax represents the maximum value of y, and EC50 denotes the half-maximal effective concentration. The parameters’ best estimates were assessed using R² (coefficient of determination) and RMSE (root mean square error), which were computed using Equations (5) and (6), respectively.
where SSR denotes the sum of squares due to regression, and SST represents the total sum of squares. Yobs refers to the observed value, Ypred indicates the predicted value, and n signifies the number of samples.
3. Results
3.1. Characterization of Prepared NPs
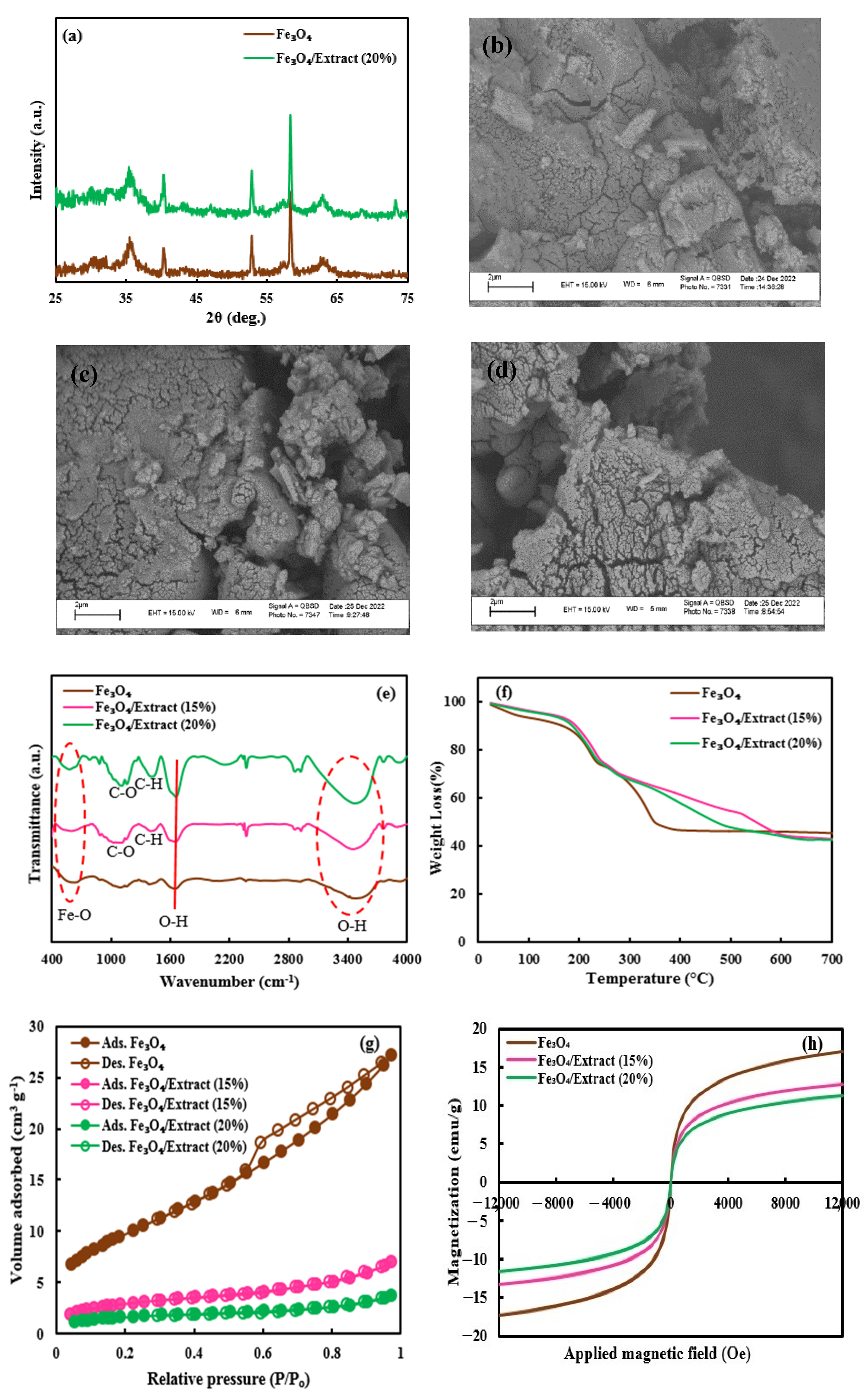
The XRD analysis was executed to assay the purity and crystallinity of the Fe3O4 NPs and Fe3O4/sunn hemp NPs (20%) samples, as demonstrated in Figure 1a. The diffraction patterns of both Fe3O4 NPs and Fe3O4/sunn hemp NPs (20%) revealed peaks corresponding to the (220), (311), (400), (422), (511), and (440) planes, positioned at 2θ values of 30.1°, 35.5°, 43.3°, 53.5°, 57.1°, and 62.8°, respectively [36]. No alterations in the crystal structure of Fe3O4 NPs were detected following the addition of the extract. The distinct diffraction peaks of samples were consistent with cubic inverse spinel structures (JCPDS file No. 01–075–0033) [37,38]. Furthermore, the absence of impurity peaks in the XRD patterns suggests that the synthesized NPs are of high purity.
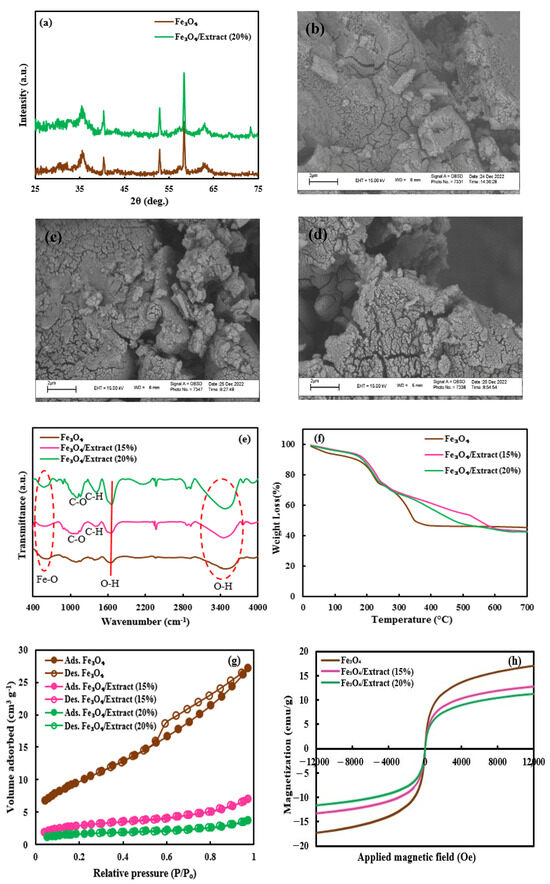
Figure 1.
XRD (a), SEM images (b–d), FTIR (e), TGA (f), BET (g), and VSM (h) analyses of the prepared nanomaterials. Fe3O4, Pure Fe3O4; Fe3O4/Extract (15 and 20%), Fe3O4/sunn hemp (150 and 200 g L−1) NPs.
The SEM images provided insights into the microstructures of the Fe3O4 NPs, Fe3O4/sunn hemp NPs (15%), and Fe3O4/sunn hemp NPs (20%) samples, showing that Fe3O4 NPs and Fe3O4/sunn hemp NPs samples possessed nearly spherical shapes (Figure 1b–d) [39].
FTIR analysis was performed to detect the functional groups present in the Fe3O4 NPs, Fe3O4/sunn hemp NPs (15%), and Fe3O4/sunn hemp NPs (20%) nanomaterials, as shown in Figure 1e. The absorbance bands for O-H stretching and bending vibrations were identified at 3200–3500 cm−1 and 1662 cm−1, respectively [40,41]. The bands ranging from 579 to 632 cm−1 corresponded to the stretching vibration of Fe-O [42]. The characteristic peaks of C-O stretching, and C-H stretching were found around 1120 and 1440 cm−1, respectively [43,44].
Thermal stability of the Fe3O4 NPs and Fe3O4/sunn hemp NPs (20%) was assessed through TGA, revealing minimal weight loss below 200 °C attributed to the evaporation of adsorbed water from the samples. The maximum weight loss of samples occurred between 250 and 700 °C, due to the decomposition of organic compounds surrounding the samples (Figure 1f).
BET analysis was used to assess the textural characteristics of the Fe3O4 NPs, Fe3O4/sunn hemp NPs (15%), and Fe3O4/sunn hemp NPs (20%) samples. The N2 adsorption–desorption isotherms of the samples are displayed in Figure 1g, revealing a type II nitrogen isotherm characteristic of mesoporous structures for all the samples. Pure Fe3O4 NPs had the largest surface area followed by Fe3O4/sunn hemp NPs samples, with values of 35.8, 10.7, and 6.2 m2g−1, for the Fe3O4 NPs, Fe3O4/sunn hemp NPs (15%), and Fe3O4/sunn hemp NPs (20%) samples, respectively. Table 1 demonstrates the details of porosity measurement information.

Table 1.
Textural properties of the prepared sunn hemp samples.
To explore the magnetic characteristics of the nanomaterials, VSM analysis was performed. Figure 1h reveals the magnetization curves of Fe3O4 NPs, Fe3O4/sunn hemp NPs (15%), and Fe3O4/sunn hemp NPs (20%) with a magnetic field of −12,000 to 12,000 Oe at 300 K. The saturation magnetization (Ms) of 17.05 emug−1 was obtained for Fe3O4 NPs, while the Ms value reduced to 12.71 and 11.27 emug−1 for Fe3O4/sunn hemp NPs (15%) and Fe3O4/sunn hemp NPs (20%) samples, respectively. With the incorporation of a non-magnetic extract to the surface of Fe3O4 NPs, the Ms value of the Fe3O4/sunn hemp NPs samples was reduced when compared with the Fe3O4 NPs. Nevertheless, the magnetic properties of the Fe3O4/sunn hemp NPs samples were sufficient to allow separation from the solution using an external magnetic field.
3.2. Optimizing the Fe3O4 NPs
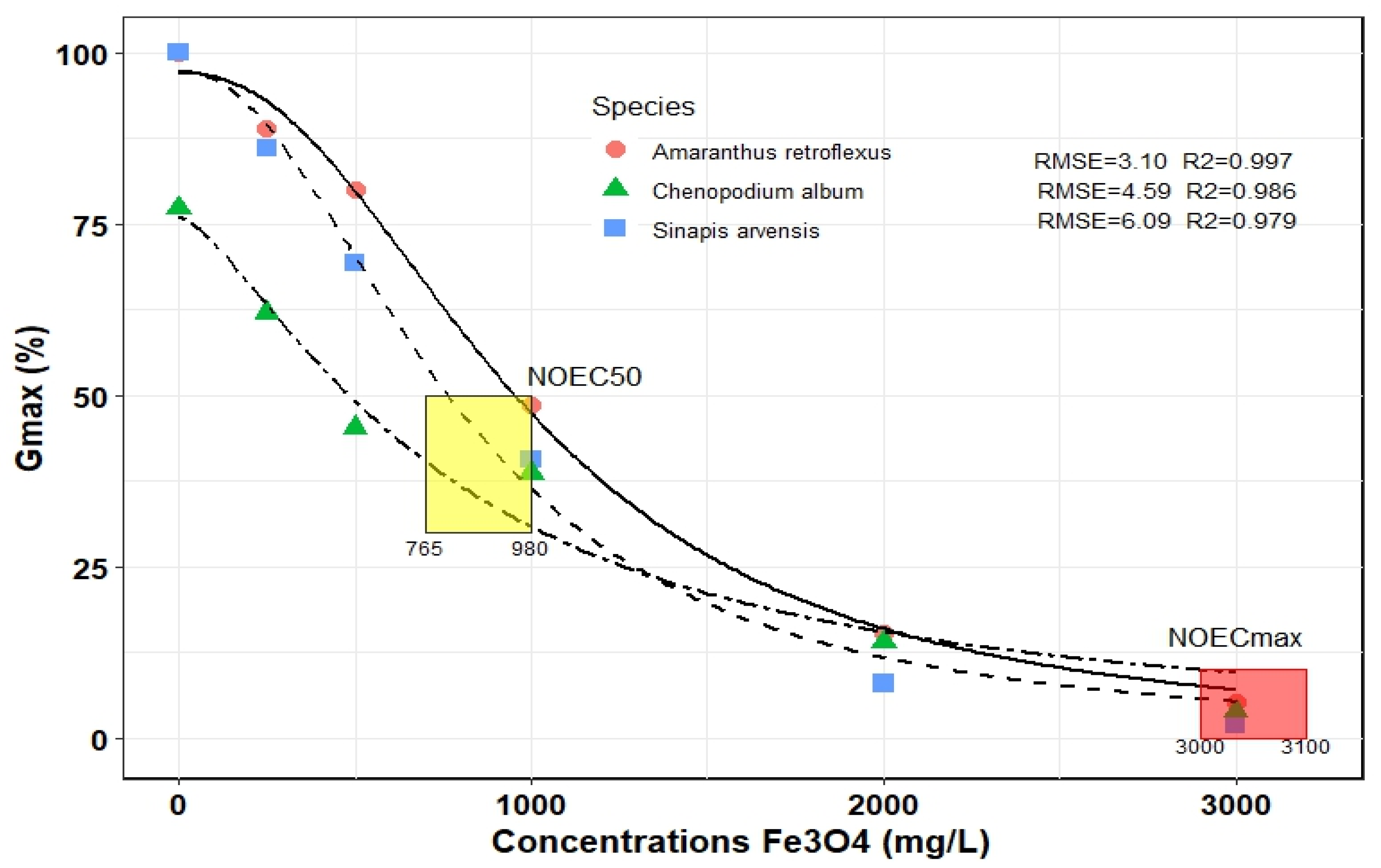
Figure 2 displays the logistic function’s optimized output for various concentrations of Fe3O4 NPs and indicates that the model fits the data well (RMSE = 3.10–6.09 and R2 = 0.846–0.999). The final germination percentage (Gmax) of three weed species was evaluated, and the use of Fe3O4 NPs up to 200 mg L−1 had no significant effect on weed seed germination. For example, the Gmax in A. retroflexus in 0 and 200 mg L−1 of Fe3O4 NPs was about 100% and 80%, respectively. The Gmax of S. arvensis and C. album in similar ranges from 100 to 80% and 77 to 68%, respectively. Furthermore, the NOEC50 of Fe3O4 NPs, was obtained in concentrations between 765 and 980 mg L−1, depending on the species. The A. retroflexus and C. album had the highest and lowest NOEC50, respectively. The NOECmax was recorded at about 3000–3100 mg L−1 and weed species were not statistically different (Figure 2).
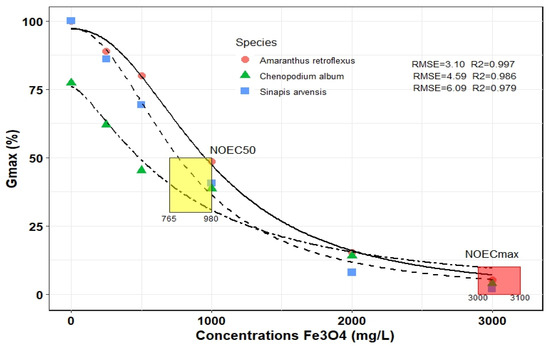
Figure 2.
Final germination (Gmax) of three weed species influenced by different concentrations of Fe3O4 NPs by fitted logistic model. The points correspond to the observed values, whereas the lines represent the predicted values. NOEC50, half maximal effective concentration of Fe3O4 NPs; NOECmax, maximal effective concentration of Fe3O4 NPs.
3.3. Effect of Fe3O4/Sunn Hemp NPs on Cumulative Germination
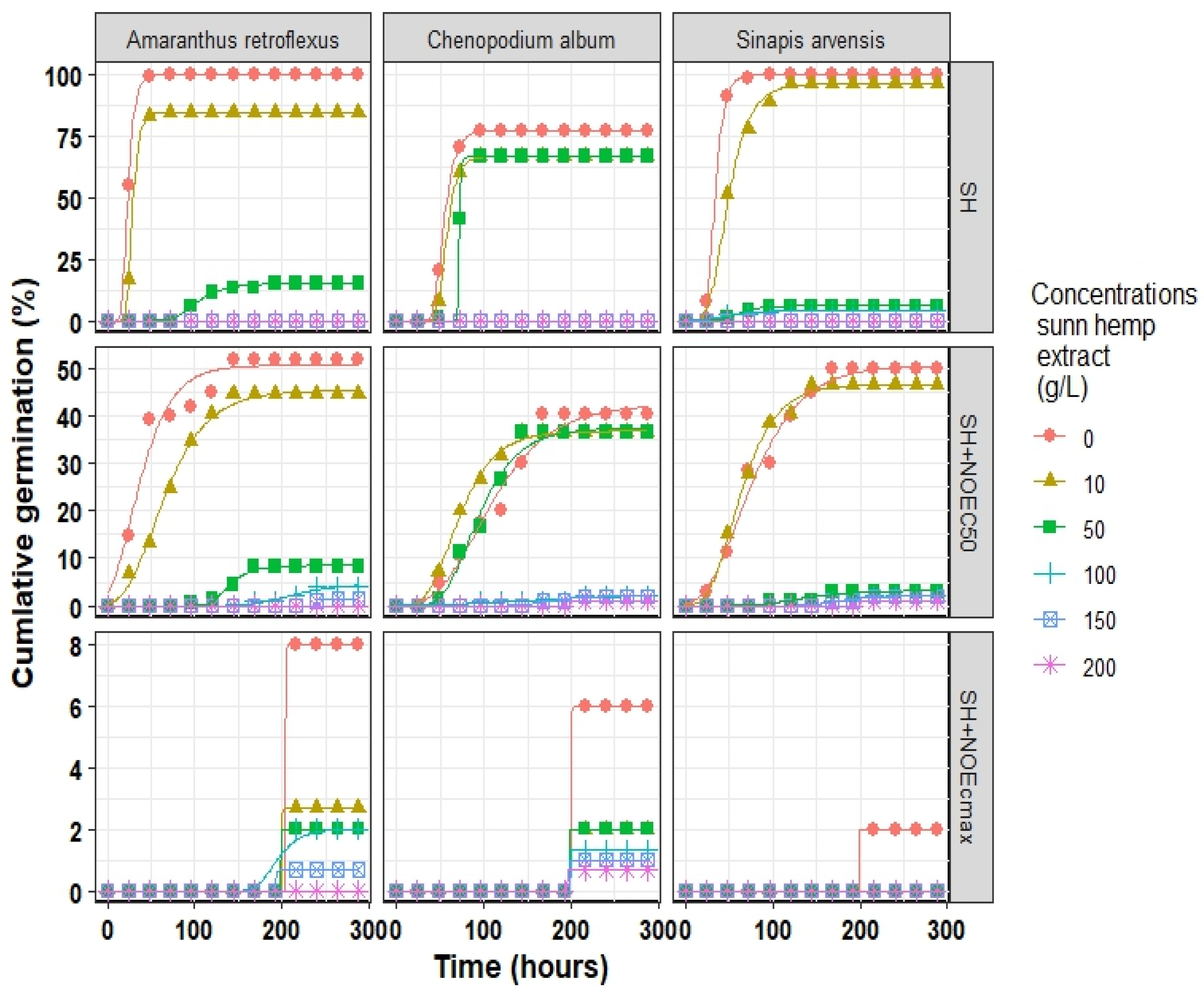
The Comperze model successfully fitted cumulative germination to investigate the germination initiation process of all three weed species, where R2 ranged from 0.859 to 0.999 for A. retroflexus, 0.934 to 0.999 for S. arvensis, and 0.893 to 0.999 for C. album (Table A1, Table A2 and Table A3 (Appendix A)). The estimated parameters indicated that A. retroflexus and S. arvensis reached their Gmax (estimated by the model) of approximately 100%, while C. album reached its peak germination rate of 77% under normal conditions (distilled water) (Figure 3).
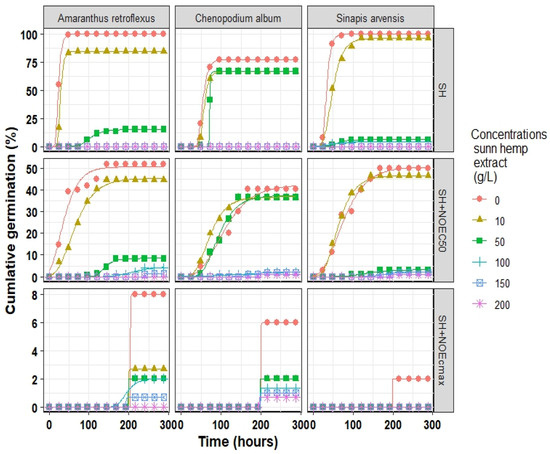
Figure 3.
Cumulative germination of three weeds species influenced by different concentrations of sunn hemp extract (SH) and two points of Fe3O4/sunn hemp NPs (NOEC50 and NOECmax) fitted Gompertz model. The points correspond to the observed values, whereas the lines represent the predicted values. NOEC50, half maximal effective concentration of Fe3O4/sunn hemp NPs; NOECmax, maximal effective concentration of Fe3O4/sunn hemp NPs.
The use of SH extract resulted in a reduction of Gmax and an increase in D50 (time to reach 50% germination); thus, the germination rate (1/D50) of all weed species slowed down. For instance, when 50 g L−1 of SH was applied in the absence of Fe3O4 NPs, the Gmax dropped by 85, 93, and 17 units in A. retroflexus, S. arvensis, and C. album, respectively. The results indicate that the use of Fe3O4 NPs at the optimized values for NOEC50 and NOECmax alleviated the toxic effects of SH extract. For example, in the absence of Fe3O4 NPs, the application of 100 g L−1 of SH extract in A. retroflexus and C. album and 150 g L−1 in S. arvensis inhibited their Gmax. However, in the presence of SH + NOEC50 and SH + NOECmax of Fe3O4 NPs, the germination inhibition occurred at the presence of 150 and 200 g L−1 of SH extract, respectively. The results indicated that the S. arvensis was relatively more tolerant to the extract of SH than C. album, while Gmax of S. arvensis was 4% and 1% under 100 and 200 g L−1 SH extract and SH + NOEC50 conditions, respectively, and the Gmax of the other two species was stopped. In the presence of Fe3O4 NPs, the concentration of SH extract for the complete inhibition of germination of C. album increased from 100 g L−1 to 200 g L−1.
3.4. Optimizing Sunn Hemp Extract Effect
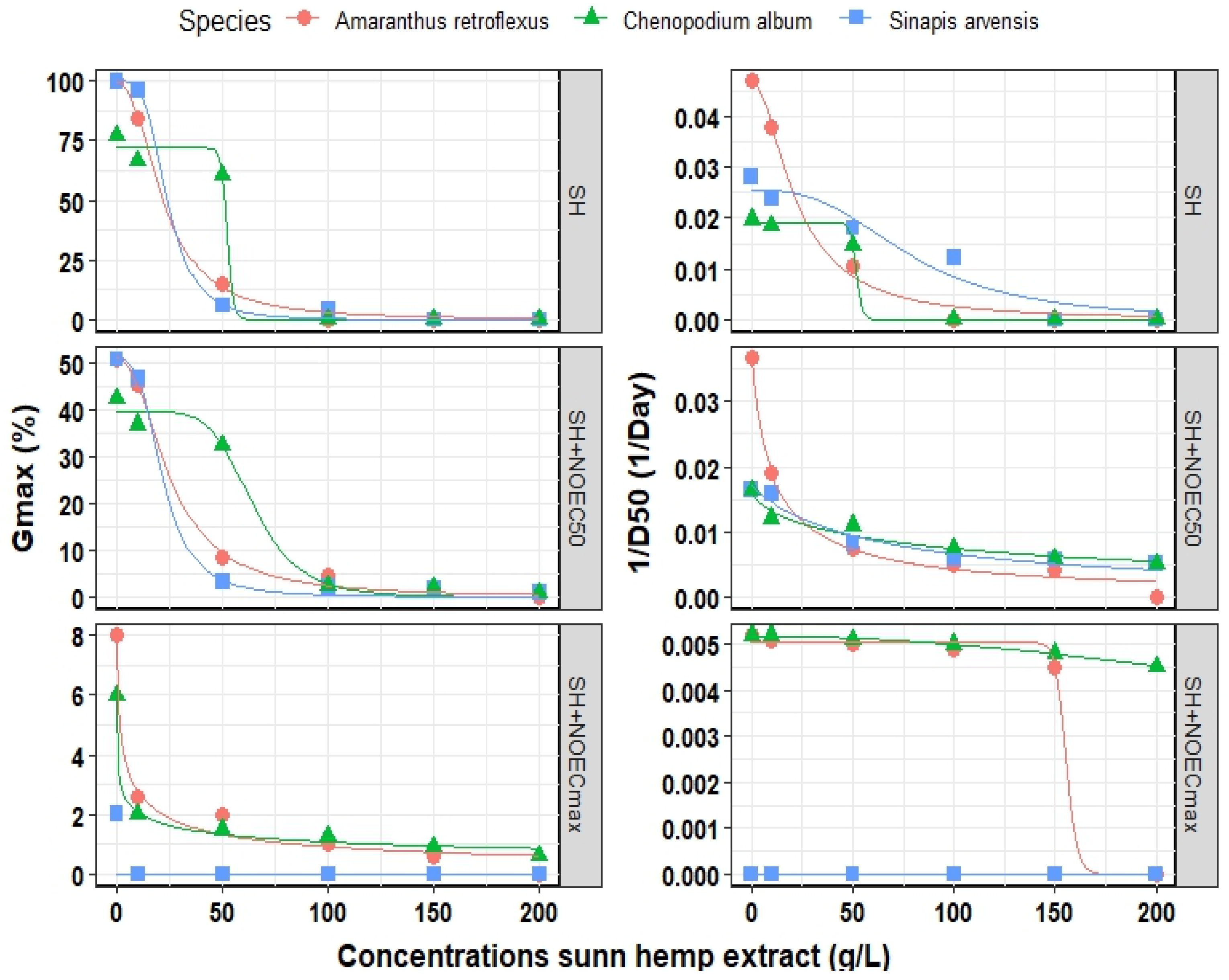
Figure 4 and Table 2 illustrate the fitted logistic model for optimizing the effect of SH extract on the Gmax of all three weed species. Our findings indicated that the logistic model successfully fitted the Gmax count and 1/D50 for all three weed species. The R2 values ranged from 0.940 to 0.999 and RMSE values varied from 0.001 to 4.32 when different concentrations of SH extract were used, depending on the estimated parameter. For example, the maximum EEC50 (half maximal effective concentration) of SH extract for Gmax was estimated at 23.9 g L−1 for S. arvensis but 52.3 g L−1 for C. album. The presence of Fe3O4 NPs resulted in an increase in EEC50 of A. retroflexus from 21.9 to 25.3 g L−1, from 52.3 to 64.5 g L−1 in C. album, and from 23.9 to 21.7 g L−1 in S. arvensis. On the contrary, EEC50 for 1/D50 in A. retroflexus and S. arvensis decreased whereas in C. album increased in the presence of Fe3O4 NPs at the optimized point (Table 2).
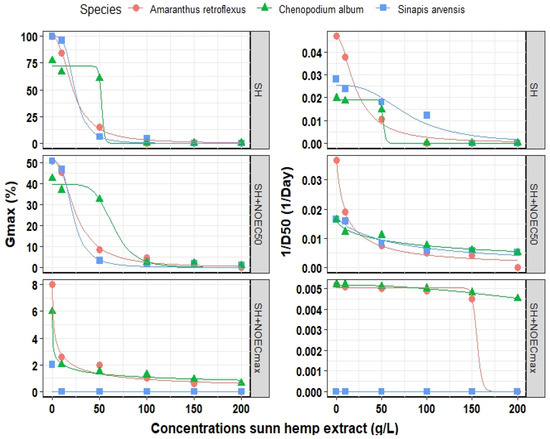
Figure 4.
Final germination (Gmax) and germination rate (1/D50) of three weeds species influenced by different concentrations of sunn hemp extract and three points of Fe3O4/sunn hemp NPs (SH, SH + NOEC50 and SH + NOECmax) when fitted by logistic model. The points correspond to the observed values, whereas the lines represent the predicted values. SH, Sunn hemp extract; NOEC50, half maximal effective concentration of Fe3O4/sunn hemp NPs; NOECmax, maximal effective concentration of Fe3O4/sunn hemp NPs.

Table 2.
Estimated parameters of final germination percentage (Gmax) and germination rate (1/D50) of three weed species influenced by different concentrations of sunn hemp extract and Fe3O4/sunn hemp NPs.
3.5. Extract Components
Table 3 displays the results of the sunn hemp GC-MS analysis utilized in this experiment. The analysis identified 37 dominant components in the extract, with Nonadecane (105.8 mg/g), Heneicosane (58.1 mg/g), Pentadecanone, 6, 10, 14-trimethyl (33.3 mg/g), Heptadecane (31.8 mg/g), Triallylsilane (33.7 mg/g), Phytol (39.5 mg/g), and Crotonic acid, Menthyl ester (35.4 mg/g) being the most prevalent compounds. Among these components, nonadecane was the dominant compound, with concentration (0.06–21.16 g L−1) across concentrations of sunn hemp, ranging from 10 to 200 g L−1. Interestingly, the concentrations of the other components of the extract remained relatively constant.

Table 3.
GC-MS results for sunn hemp at the minimum and maximum tested concentrations [45].
4. Discussion
Biosynthesis of NPs using plant extracts with allelopathic properties is cost-effective and environmentally friendly, and can be implemented as an alternative to synthetic herbicides for weed management [46,47]. This study evaluates the effectiveness of using green synthesis of Fe3O4 NPs with allelopathic sunn hemp (SH) extract in inhibiting the germination of three common weeds.
Our results demonstrated that SH aqueous extract, Fe3O4 NPs, and Fe3O4/sunn hemp NPs considerably inhibited the germination of A. retroflexus, S. arvensis, and C. album. The extent of inhibition, however, varied among species, reflecting differences in sensitivity. This variation in germination potential is among species’ inherent biological differences and ecological adaptations that influence their competitive abilities in agricultural settings [48,49,50]. As our results exhibited, A. retroflexus had the highest sensitivity to SH extract among other species, whereas C. album displayed relative tolerance. This variability in response may stem from differences in seed coat permeability and water absorption mechanisms, as thicker seed coats in C. album likely limited water and allelochemical uptake [51], and C. album had 77% germinating rate in control conditions.
Sunn hemp extract, notably lowered Gmax while raising the 1/D50 values for all three weed species, suggesting a delay in the time needed to achieve 50% germination. Sunn hemp extracts inhibited germination because allelochemicals can hinder endosperm weakening and embryo growth (processes required for endosperm rupture and root protrusion) [52,53]. Gas chromatography–mass spectrometry (GC-MS) analysis of SH extract identified phytol, terpenes, and other compounds; however, isolating individual compounds is necessary to elucidate their specific modes of action. Prior research has indicated that the SH aqueous extract contains a variety of phytochemicals, including alkaloids, and flavonoids [18,54]. Prior studies reported that alkaloids impact seeds by hindering water uptake, disrupting germination enzymes, and affecting hormonal balance [55]. The effect of phenolic compounds varies by their concentration and receptor sensitivity. Additionally, flavonoids inhibit germination by disrupting cell membrane permeability and causing leakage [56,57]. Our results are aligned with Skinner et al. [25], and Abdelmalik et al. [58], who found that dried ground sunn hemp residues and leaf extracts inhibited germination and seedling growth of various weeds and cover crops and also that there is a correlation between extract concentration and reduced germination.
The comprehensive characterization of Fe3O4 NPs synthesized using SH extract underscores the adaptability of these materials. Analytical techniques, including XRD, SEM, FTIR, TGA, BET, and VSM, demonstrated the structural integrity, presence of functional groups, microstructural features, thermal stability, surface characteristics, and magnetic properties of the NPs. The findings affirmed the viability of employing SH extracts for functionalization, highlighting the practical potential of NP synthesis with SH [59]. The results of this study indicated that in optimizing the Fe3O4 NPs effect in the control group, Gmax ranged from 100 to 77% for A. retroflexus, S. arvensis, and C. album. Germination significantly decreased by 80–68% at the highest Fe3O4 NPs concentration (3000 mg L−1), while concentrations up to 200 mg L−1 did not inhibit germination in these three weed species (Figure 2). These findings suggest that low concentrations of Fe3O4 NPs do not inhibit seed germination and therefore may not significantly threaten weed establishment. However, a high concentration of Fe3O4 NPs (3000 mg L−1) significantly reduced germination, possibly due to insufficient interaction of the functional groups of the NPs at this level. The functional groups of Fe3O4 NPs effective in stabilizing reactive oxygen species (ROS) during seed germination mainly include oxygen-containing groups (COOH-, OH-, C=O, and -O) that interact with ROS and regulate oxidative stress [60]. Fourier-transform infrared spectroscopy (FTIR) analysis of Fe3O4 NPs revealed C-O and O-H bonds (Figure 1e), yet there was probably insufficient interaction to neutralize radicals and control ROS at high Fe3O4 NPs concentrations, led to a severe reduction and inhibiting seeds germination. These findings align with previous research [61], which indicated that Parthenium-mediated green synthesis of Fe3O4 NPs greatly reduced corn seed germination at 400 ppm, while lower concentrations acted as micronutrients. Additionally, based on the NOEC50 results, A. retroflexus seeds demonstrated the highest tolerance to Fe3O4 NPs. Increased Fe3O4 NPs concentration reduces seed germination in A. retroflexus, S. arvensis, and C. album. Despite the high concentrations utilized in this study, it is noteworthy that the evaluated weed species did not show statistically significant differences in NOECmax values. This underscores the toxic effects of elevated concentrations of Fe3O4 NPs, necessitating further research into the mechanisms of action of seeds against Fe3O4 NPs. Another report indicated that high concentrations of Fe3O4 (≥100 mg L−1) exacerbated oxidative stress markers (malondialdehyde, H2O2) in rice seedlings [62]. Our findings are also aligned with Kornarzyński et al. [63], who investigated the effects of Fe3O4 NPs on seed germination and element concentration in Helianthus annuus L., reporting Fe3O4 NPs’ toxic effects on the reduction of germination parameters.
Green-synthesized Fe3O4 NPs with SH extract (SH + NOEC50) reduced or completely stopped the germination of S. arvensis, C. album, and A. retroflexus. In addition to the varying sensitivities of weed seeds to SH extract, Fe3O4 NPs, and Fe3O4/sunn hemp NPs, increasing the concentration of Fe3O4/sunn hemp NPs reduced or eliminated germination. This reduction in germination is likely due to the decreased NP surface area at higher extract concentrations. As shown by the BET results (Table 1), surface area decreases for Fe3O4/sunn hemp (15% and 20%) NPs with increasing extract concentration. The reduced BET surface area of green synthesized Fe3O4 NPs with SH extract may hinder seed germination by limiting water and NPs uptake, interaction with seeds, and possibly nutrient delivery [63,64]. However, increasing SH concentration in the Fe3O4 green synthesis enhanced the functional groups (C-O and O-H) (Figure 1e). In this study, the observed reduction in NPs surface area at 15% and 20% concentrations (150 and 200 g L−1) may limit the interaction of these functional groups, reducing their ability to alleviate oxidative stress during germination [60,65]. While Fe3O4 NPs can have both positive and negative effects on germination [66,67,68], the unique properties of Fe3O4 NPs, including their small size, high surface area, modifiable surface, functional groups, and magnetic properties, likely influence their mechanism of action [69]. For instance, one study found that root water uptake decreased to 57% of the control at 50 mg L−1 and further decreased to 26% at 100 mg L−1 [63].
5. Conclusions
The use of natural products, such as plant extracts, for weed control has gained popularity due to their short environmental half-lives, low toxicity, and eco-friendliness. This study examined the effectiveness of sunn hemp leaf extract, Fe3O4 NPs, and their green synthesis on the seed germination of three globally common weeds. Weed species exhibited variable responses to sunn hemp extracts, Fe3O4 NPs, and Fe3O4/sunn hemp NPs, underscoring the importance of tailoring weed management strategies to specific species. The combination of Fe3O4 NPs and sunn hemp extract enhanced their inhibitory effects on weed germination, suggesting a synergistic interaction between nanoparticles and allelochemicals. Understanding the mechanisms underlying these differences, such as seed coat permeability, functional groups of NPs, and water absorption, may improve the efficacy of natural herbicides. The results indicated that the concentration of NPs and sunn hemp extract plays a critical role in their effectiveness. While low concentrations had minimal impact, higher concentrations significantly inhibited germination, emphasizing the need for precise dosing in practical applications.
Author Contributions
Conceptualization, M.H. and F.A.; methodology, F.A. and M.T.A.; software, F.A., S.F. and G.P.; validation, M.H.; formal analysis, G.P. and F.A.; investigation, F.A.; resources, M.H., F.A. and S.F.; data curation, F.A. and G.P.; writing—original draft preparation, F.A. and M.H.; writing—review and editing, M.H., F.A., A.E. and G.P.; visualization, M.H.; supervision, M.H.; project administration, F.A.; funding acquisition, M.H. and A.E. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data that supports the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
The authors gratefully acknowledge the support from the Faculty of Agricultural Sciences and Natural Resources at the University of Mohaghegh Ardabili, Iran, and the Stockbridge School of Agriculture at the University of Massachusetts Amherst, USA.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| BET | Brunner–Emmet–Teller |
| EEC50 | Half-maximal effective concentration |
| FTIR | Fourier-transform infrared spectroscopy |
| GC-MS | Gas chromatography–mass spectrometry |
| Gmax | Final germination percentage or Maximum germination |
| NOEC50 | Half maximal effective concentration of Fe3O4 NPs and Fe3O4/sunn hemp NPs |
| NOECmax | The maximal effective concentration of Fe3O4 NPs and Fe3O4/sunn hemp NPs |
| NPs | Nanoparticles |
| SEM | Scanning electron microscopy |
| SH | Sunn hemp extract |
| TGA | Thermogravimetric analysis |
| VSM | Vibrating sample magnetometer |
| XRD | Scanning X-ray diffraction |
Appendix A

Table A1.
Estimated parameters of cumulative germination changes on A. retroflexus influenced by different concentrations of sunn hemp extract and Fe3O4/sunn hemp NPs.
Table A1.
Estimated parameters of cumulative germination changes on A. retroflexus influenced by different concentrations of sunn hemp extract and Fe3O4/sunn hemp NPs.
| Sunn Hemp Extract Concentrations (g L−1) | Sunn Hemp Aqueous Extract | Fe3O4/Sunn Hemp NPs | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SH | SH + NOEC50 | SH + NOECmax | |||||||||||||
| Estimate Parameter | R2 | RMSE | Estimate Parameter | R2 | RMSE | Estimate Parameter | R2 | RMSE | |||||||
| Gmax (%) | D50 (h) | Slope | Gmax (%) | D50 (h) | Slope | Gmax (%) | D50 (h) | Slope | |||||||
| 0 | 99.9 ± 0.05 | 21.3 ± 0.01 | 5.3 ± 0.01 | 0.999 | 0.002 | 50.8 ± 1.3 | 27.3 ± 3.6 | 25.8 ± 7.7 | 0.961 | 3.54 | 8.0 ± 0.00 | 192.6 ± 0.39 | 0.30 ± 0.02 | 0.999 | 0.001 |
| 10 | 84.6 ± 0.01 | 26.5 ± 0.02 | 5.2 ± 0.01 | 0.999 | 0.004 | 45.5 ± 0.5 | 52.7 ± 1.7 | 33.5 ± 2.3 | 0.995 | 1.25 | 2.6 ± 0.00 | 196.1 ± 0.30 | 0.36 ± 0.02 | 0.999 | 0.001 |
| 50 | 15.1 ± 0.24 | 95.5 ± 1.74 | 21.0 ± 2.0 | 0.995 | 0.530 | 8.5 ± 0.2 | 133.6 ± 2.1 | 16.7 ± 2.5 | 0.991 | 0.41 | 2.0 ± 0.00 | 200.1 ± 0.30 | 0.30 ± 0.01 | 0.999 | 0.002 |
| 100 | - | - | - | - | - | 4.7 ± 3.2 | 200.7 ± 8.1 | 38.0 ± 10.6 | 0.859 | 0.34 | 1.0 ± 0.00 | 204.8 ± 0.36 | 0.30 ± 0.01 | 0.995 | 0.067 |
| 150 | - | - | - | - | - | 1.4 ± 3.2 | 239.4 ± 2.7 | 38.2 ± 1.3 | 0.999 | 0.01 | 0.6 ± 0.00 | 222.1 ± 0.34 | 0.26 ± 0.01 | 0.999 | 0.002 |
| 200 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
Gmax, Final germination; D50, Time to reach 50% germination; SH, Sunn hemp extract; NOEC50, Half maximal effective concentration of Fe3O4/sunn hemp NPs; NOECmax, Maximal effective concentration of Fe3O4/sunn hemp NPs; RMSE, Root Mean Square Error; R2, Coefficient of determination.

Table A2.
Estimated parameters of cumulative germination changes on S. arvensis influenced by different concentrations of sunn hemp extract and Fe3O4/sunn hemp NPs.
Table A2.
Estimated parameters of cumulative germination changes on S. arvensis influenced by different concentrations of sunn hemp extract and Fe3O4/sunn hemp NPs.
| Sunn Hemp Extract Concentrations (g L−1) | Sunn Hemp Aqueous Extract | Fe3O4/Sunn Hemp NPs | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SH | SH + NOEC50 | SH + NOECmax | |||||||||||||
| Estimate Parameter | R2 | RMSE | Estimate Parameter | R2 | RMSE | Estimate Parameter | R2 | RMSE | |||||||
| Gmax (%) | D50 (h) | Slope | Gmax (%) | D50 (h) | Slope | Gmax (%) | D50 (h) | Slope | |||||||
| 0 | 99.8 ± 0.01 | 30.6 ± 0.11 | 7.2 ± 0.01 | 0.999 | 0.30 | 50.8 ± 1.10 | 60.9 ± 2.9 | 38.7 ± 4.1 | 0.989 | 2.17 | 2.0 ± 0.00 | 198.2 ± 0.29 | 0.05 ± 0.02 | 0.999 | 0.001 |
| 10 | 96.2 ± 0.87 | 42.1 ± 1.17 | 16.8 ± 1.4 | 0.996 | 2.49 | 46.8 ± 0.47 | 63.0 ± 1.4 | 27.0 ± 1.8 | 0.996 | 1.16 | - | - | - | - | - |
| 50 | 6.0 ± 0.06 | 55.3 ± 1.08 | 15.9 ± 1.3 | 0.996 | 0.16 | 3.2 ± 0.29 | 121.2 ± 9.0 | 39.8 ± 9.2 | 0.934 | 0.39 | - | - | - | - | - |
| 100 | 3.9 ± 0.12 | 69.9 ± 4.05 | 21.5 ± 5.0 | 0.958 | 0.33 | 2.0 ± 0.13 | 169.0 ± 4.8 | 26.3 ± 7.2 | 0.971 | 0.17 | - | - | - | - | - |
| 150 | - | - | - | - | - | 1.9 ± 0.03 | 172.0 ± 1.3 | 26.3 ± 7.2 | 0.921 | 0.14 | - | - | - | - | - |
| 200 | - | - | - | - | - | 1.0 ± 0.03 | 199.0 ± 0.8 | 0.26 ± 0.2 | 0.999 | 0.00 | - | - | - | - | - |
Gmax, Final germination; D50, Time to reach 50% germination; SH, Sunn hemp extract; NOEC50, Half maximal effective concentration of Fe3O4/sunn hemp NPs; NOECmax, Maximal effective concentration of Fe3O4/sunn hemp NPs; RMSE, Root Mean Square Error; R2, Coefficient of determination.

Table A3.
Estimated parameter of cumulative germination change on C. album influenced by different concentrations of sunn hemp extract and Fe3O4/sunn hemp NPs.
Table A3.
Estimated parameter of cumulative germination change on C. album influenced by different concentrations of sunn hemp extract and Fe3O4/sunn hemp NPs.
| Sunn Hemp Extract Concentrations (g L−1) | Sunn Hemp Aqueous Extract | Fe3O4/Sunn Hemp NPs | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SH | SH + NOEC50 | SH + NOECmax | |||||||||||||
| Estimate Parameter | R2 | RMSE | Estimate Parameter | R2 | RMSE | Estimate Parameter | R2 | RMSE | |||||||
| Gmax (%) | D50 (h) | Slope | Gmax (%) | D50 (h) | Slope | Gmax (%) | D50 (h) | Slope | |||||||
| 0 | 77.3 ± 0.05 | 50.7 ± 0.04 | 8.8 ± 0.07 | 0.999 | 0.14 | 42.6 ± 1.72 | 61.3 ± 1.32 | 45.9 ± 7.12 | 0.982 | 2.49 | 6.0 ± 0.001 | 192.6 ± 0.38 | 0.35 ± 0.02 | 0.999 | 0.001 |
| 10 | 66.7 ± 0.03 | 54.0 ± 0.05 | 7.9 ± 0.04 | 0.999 | 0.10 | 36.9 ± 0.36 | 82.9 ± 2.92 | 27.5 ± 1.73 | 0.997 | 0.87 | 2.0 ± 0.001 | 192.5 ± 0.38 | 0.05 ± 0.02 | 0.999 | 0.001 |
| 50 | 60.6 ± 0.15 | 68.7 ± 1.24 | 1.6 ± 0.085 | 0.999 | 0.42 | 32.5 ± 0.89 | 90.9 ± 4.68 | 30.2 ± 3.98 | 0.989 | 1.85 | 1.5 ± 0.001 | 196.0 ± 0.35 | 0.06 ± 0.01 | 0.999 | 0.001 |
| 100 | - | - | - | - | - | 2.5 ± 0.70 | 134.9 ± 32.7 | 87.8 ± 42.2 | 0.893 | 0.29 | 1.3 ± 0.002 | 200.1 ± 0.30 | 0.30 ± 0.01 | 0.999 | 0.001 |
| 150 | - | - | - | - | - | 2.0 ± 0.13 | 169.0 ± 4.8 | 26.3 ± 7.2 | 0.971 | 0.17 | 0.9 ± 0.002 | 208.3 ± 0.40 | 0.26 ± 0.01 | 0.999 | 0.001 |
| 200 | - | - | - | - | - | 1.0 ± 0.13 | 199.8 ± 2.4 | 0.26 ± 0.2 | 0.999 | 0.001 | 0.6 ± 0.001 | 221.2 ± 0.35 | 0.19 ± 0.01 | 0.999 | 0.001 |
Gmax, Final germination; D50, Time to reach 50% germination; SH, Sunn hemp extract; NOEC50, Half maximal effective concentration of Fe3O4/sunn hemp NPs; NOECmax, Maximal effective concentration of Fe3O4/sunn hemp NPs; RMSE, Root Mean Square Error; R2, Coefficient of determination.
References
- Kostina-Bednarz, M.; Plonka, J.; Barchanska, H. Allelopathy as a source of bioherbicides: Challenges and prospects for sustainable agriculture. Rev. Environ. Sci. Biotechnol. 2023, 22, 471–504. [Google Scholar] [CrossRef]
- Yadav, T.; Chopra, N.K.; Chopra, N.K.; Kumar, R.; Soni, P.G. Assessment of critical period of crop-weed competition in forage cowpea (Vigna unguiculata) and its effect on seed yield and quality. Indian J. Agron. 2018, 63, 124–127. [Google Scholar]
- Hunter, J.E.; Gannon, T.W.; Richardson, R.J.; Yelverton, F.H.; Leon, R.G. Integration of remote-weed mapping and an autonomous spraying unmanned aerial vehicle for site-specific weed management. Pest. Manag. Sci. 2020, 76, 1386–1392. [Google Scholar] [CrossRef]
- Esposito, M.; Crimaldi, M.; Cirillo, V.; Sarghini, F.; Maggio, A. Drone and sensor technology for sustainable weed management: A review. Chem. Biol. Technol. Agric. 2021, 8, 18. [Google Scholar] [CrossRef]
- Gawel, A.; Seiwert, B.; Sühnholz, S.; Schmitt-Jansen, M.; Mackenzie, K. In-situ treatment of herbicide-contaminated groundwater–Feasibility study for the cases atrazine and bromacil using two novel nanoremediation-type materials. J. Hazard. Mater. 2020, 393, 1–10. [Google Scholar] [CrossRef]
- Li, Z.F.; Dong, J.X.; Vasylieva, N.; Cui, Y.L.; Wan, D.B.; Hua, X.D.; Huo, J.Q.; Yang, D.C.; Gee, S.J.; Hammock, B.D. Highly specific nanobody against herbicide 2,4-dichlorophenoxyacetic acid for monitoring of its contamination in environmental water. Sci. Total Environ. 2021, 753, 141950. [Google Scholar] [CrossRef] [PubMed]
- Ghahremani, S.; Ebadi, A.; Tobeh, A.; Hashemi, M.; Sedghi, M.; Gholipoouri, A.; Barker, V. Short-term impact of monoculture and mixed cover crops on soil properties, weed suppression, and lettuce yield. Commun. Soil. Sci. Plant Anal. 2021, 52, 406–415. [Google Scholar] [CrossRef]
- Yadav, A.S.; Srivastava, D.S. Application of nano-technology in weed management: A Review. Res. Rev. J. Crop Sci. Technol. 2015, 4, 21–23. [Google Scholar]
- Choudhary, S.K.; Kumar, A.; Kumar, R. Novel nanotechnological tools for weed management—A review. Chem. Rev. Lett. 2020, 9, 886–894. [Google Scholar]
- Mondal, P.; Anweshan, A.; Purkait, M.K. Green synthesis and environmental application of iron-based nanomaterials and nanocomposite: A review. Chemosphere 2020, 259, 1–111. [Google Scholar] [CrossRef]
- Markova, Z.; Novak, P.; Kaslik, J.; Plachtova, P.; Brazdova, M.; Jancula, D.; Siskova, K.; Machala, L.; Marsalek, B.; Zboril, R.; et al. Iron (II, III)-Polyphenol complex nanoparticles derived from green tea with remarkable ecotoxicological impact. ACS Sustain. Chem. Eng. 2014, 2, 1674–1680. [Google Scholar] [CrossRef]
- Luo, F.; Chen, Z.; Megharaj, M.; Naidu, R. Biomolecules in grape leaf extract involved in one-step synthesis of iron-based nanoparticles. RSC Adv. 2014, 96, 53467–53474. [Google Scholar] [CrossRef]
- Besançon, T.E.; Wasacz, M.H.; Heckman, J.R. Weed suppression, nitrogen availability, and cabbage production following Sunn hemp or Sorghum-Sudan grass. HortTechnology 2021, 31, 439–447. [Google Scholar] [CrossRef]
- Cho, A.H.; Chase, C.A.; Treadwell, D.D.; Koenig, R.L.; Morris, J.B.; Morales-Payan, J.P. Apical dominance and planting density effects on weed suppression by Sunn Hemp (Crotalaria juncea L.). HortScience 2015, 50, 263–267. [Google Scholar] [CrossRef]
- Parenti, A.; Cappelli, G.; Zegada-Lizarazu, W.; Sastre, C.M.; Christou, M.; Monti, A.; Ginaldi, F. SunnGro: A new crop model for the simulation of Sunn hemp (Crotalaria juncea L.) grown under alternative management practices. Biomass Bioenergy 2021, 146, 1–16. [Google Scholar] [CrossRef]
- Meagher, R.L.; Rodney, J.R.; Nagoshi, N.; Brown, J.T. Flowering of the cover crop Sunn hemp, Crotalaria juncea L. HortScience 2017, 52, 986–990. [Google Scholar] [CrossRef]
- Bundit, A.; Ostlie, M.; Prom-U-Thai, C. Sunn hemp (Crotalaria juncea) weed suppression and allelopathy at different timings. Biocontrol Sci. Technol. 2021, 31, 694–704. [Google Scholar] [CrossRef]
- Javaid, M.M.; Bhan, M.; Johnson, J.V.; Rathinasabapathi, B.; Chase, C.A. Biological and chemical characterizations of allelopathic potential of diverse accessions of the cover crop Sunn hemp. J. Am. Soc. Hortic. Sci. 2015, 140, 532–541. [Google Scholar] [CrossRef]
- Morris, J.B.; Chase, C.; Treadwell, D.; Koenig, R.; Cho, A.; Morales-Payan, J.P.; Murphy, T.; Antonious, G.F. Effect of Sunn hemp (Crotalaria juncea L.) cutting date and planting density on weed suppression in Georgia, USA. J. Environ. Sci. Health [B] 2015, 50, 614–621. [Google Scholar] [CrossRef]
- Colegate, S.M.; Gardner, D.R.; Joy, R.J.; Betz, J.M.; Panter, K.E. Dehydropyrrolizidine alkaloids, including monoesters with an unusual esterifying acid, from cultivated Crotalaria juncea (Sunn hemp cv. ‘Tropic Sun’). J. Agric. Food Chem. 2012, 60, 3541–3550. [Google Scholar] [CrossRef]
- Adams, R.; Gianturco, M. The alkaloids of Crotalaria juncea. J. Am. Chem. Soc. 1956, 78, 1919–1921. [Google Scholar] [CrossRef]
- Li, A.; Zheng, R.; Tian, L.; Wei, Y.; Wu, J.; Hou, X. Allelopathic effects of switchgrass on redroot pigweed and crabgrass growth. J. Plant Ecol. 2021, 222, 1–12. [Google Scholar] [CrossRef]
- Mousavi, S.A.; Feizi, H.; Ahmadian, A.; Izadi Darbandi, E. Extracts obtained from organs of saffron (Crocus sativus) alter growth and seed germination of common lamb’s quarters (Chenopodium album) and barnyard grass (Echinochloa crus-galli). Plant Biosyst.-An. Int. J. Deal. All Asp. Plant Biol. 2023, 157, 1–8. [Google Scholar] [CrossRef]
- Boukhili, M.; Szilágyi, A.; Cheradil, A. Allelopathic effect of five invasive plants on seed germination and growth of wild mustard. Rev. Agric. Rural. Dev. 2022, 11, 1–5. [Google Scholar] [CrossRef]
- Skinner, E.M.; Díaz-Pérez, J.C.; Phatak, S.C.; Schomberg, H.H.; Vencill, W. Allelopathic effects of Sunn hemp (Crotalaria juncea L.) on germination of vegetables and weeds. HortScience 2012, 47, 138–142. [Google Scholar] [CrossRef]
- Rotar, P.P.; Joy, R.J. ‘Tropic Sun’ Sunn Hemp (Crotalaria juncea L.); Research Extension Series; University of Hawaii: Honolulu, HI, USA, 1983. [Google Scholar]
- Iftikhar Hussain, M.; El-Sheikh, M.A.; Reigosa, M.J. Allelopathic potential of aqueous extract from Acacia melanoxylon R. Br. on Lactuca sativa. Plants 2020, 9, 1228. [Google Scholar] [CrossRef] [PubMed]
- Trusheva, B.; Trunkova, D.; Bankova, V. Different extraction methods of biologically active components from propolis: A preliminary study. Chem. Cent. J. 2007, 1, 1–4. [Google Scholar] [CrossRef]
- Babaei-Ghaghelestany, A.; Alebrahim, M.T.; MacGregor, D.R.; Khatami, S.A.; Hasani Nasab Farzaneh, R. Evaluation of ultrasound technology to break seed dormancy of common lamb’s quarters (Chenopodium album). Food Sci. Nutr. 2020, 8, 2662–2669. [Google Scholar] [CrossRef]
- Ahmadnia, F.; Alebrahim, M.T.; Nabati Souha, L.; MacGregor, D.R. Evaluation of techniques to break seed dormancy in Redroot pigweed (Amaranthus retroflexus). Food Sci. Nutr. 2024, 12, 2334–2345. [Google Scholar] [CrossRef]
- Şin, B.; Kadıoğlu, I. A Study on germination biology of Wild Mustard (Sinapis arvensis L.). Turk. J. Agric.-Food Sci. Technol. 2021, 9, 728–732. [Google Scholar] [CrossRef]
- Massart, R. Preparation of aqueous magnetic liquids in alkaline and acidic media. IEEE Trans. Magn. 1981, 17, 1247–1248. [Google Scholar] [CrossRef]
- Perry, D. Methodology and application of vigour tests. In Handbook of Vigour Test Methods International Seed Testing Association; International Seed Testing Association: Wallisellen, Switzerland, 1981; pp. 3–7. [Google Scholar]
- Scott, S.; Jones, R.; Williams, W. Review of data analysis methods for seed germination 1. Crop Sci. 1984, 24, 1192–1199. [Google Scholar] [CrossRef]
- Burnett, S.E.; Pennisi, S.V.; Thomas, P.A.; Van Iersel, M.W. Controlled drought affects morphology and anatomy of Salvia splendens. J. Am. Soc. Hortic. Sci. 2005, 130, 775–781. [Google Scholar]
- Antarnusa, G.; Jayanti, P.D.; Denny, Y.R.; Suherman, A. Utilization of co-precipitation method on synthesis of Fe3O4/PEG with different concentrations of PEG for biosensor applications. Materialia 2022, 25, 101525. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, S.; Zhang, W.; Peng, K. Influence of processed parameters on the magnetic properties of Fe/Fe3O4 composite cores. J. Mater. Sci.: Mater. Electron. 2021, 32, 1233–1241. [Google Scholar] [CrossRef]
- Sahu, S.R.; Jagannatham, M.; Gautam, R.; Rikka, V.R.; Prakash, R.; Mallikarjunaiah, K.J.; Reddy, G.S. A facile synthesis of raspberry-shaped Fe3O4 nanoaggregate and its magnetic and lithium-ion storage properties. Mater. Sci. Eng. B 2022, 282, 115771. [Google Scholar] [CrossRef]
- Bhuiyan, M.S.H.; Miah, M.Y.; Paul, S.C.; Aka, T.D.; Saha, O.; Rahaman, M.M.; Sharif, M.J.I.; Habiba, O.; Ashaduzzaman, M. Green synthesis of iron oxide nanoparticle using Carica papaya leaf extract: Application for photocatalytic degradation of remazol yellow RR dye and antibacterial activity. Heliyon 2020, 6, e04603. [Google Scholar] [CrossRef] [PubMed]
- Elgorban, A.M.; Marraiki, N.; Akber Ansari, S.; Syed, A. Green synthesis of Cu/Fe3O4 nanocomposite using Calendula extract and evaluation of its catalytic activity for chemo selective oxidation of sulfides to sulfoxides with aqueous hydrogen peroxide. J. Org. Chem. 2021, 954–955, 122077. [Google Scholar] [CrossRef]
- Tiama, T.M.; Ismail, A.M.; Elhaes, H.; Ibrahim, M.A. Structural and spectroscopic studies for chitosan/Fe3O4 nanocomposites as glycine biosensors. Biointerface Res. Appl. Chem. 2023, 13, 547. [Google Scholar] [CrossRef]
- Sari, E.K.; Tumbelaka, R.M.; Ardiyanti, H.; Istiqomah, N.I.; Chotimah Suharyadi, E. Green synthesis of magnetically separable and reusable Fe3O4/Cdots nanocomposites photocatalyst utilizing Moringa oleifera extract and watermelon peel for rapid dye degradation. Carbon. Resour. Convers. 2023, 6, 274–286. [Google Scholar] [CrossRef]
- Inbaraj, B.S.; Chen, B.Y.; Liao, C.W.; Chen, B.H. Green synthesis, characterization and evaluation of catalytic and antibacterial activities of chitosan, glycol chitosan and poly (γ-glutamic acid) capped gold nanoparticles. Int. J. Biol. Macromol. 2020, 161, 1484–1495. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wei, D.; Liu, L.; Nai, J.; Liu, Y.; Xiong, Y.; Peng, J.; Mahmud, S.; Liu, H. Green synthesis of Konjac glucomannan templated palladium nanoparticles for catalytic reduction of azo compounds and hexavalent chromium. Mater. Chem. Phys. 2021, 267, 124651. [Google Scholar] [CrossRef]
- Ahmadnia, F.; Ebadi, A.; Hashemi, M.; Ghavidel, A.; Alebrahim, M.T. Investigating the effect of aqueous extracts of Sunn hemp (Crotalaria juncea) and Oats (Avena sativa L.) on the germination of wild mustard weed (Sinapis arvensis). Iran. J. Seed Sci. Res. 2023, 10, 1–19. [Google Scholar]
- Ojo, S.K.S.; Otugboyega, J.O.; Ayo, I.O.; Ojo, A.M.; Oluwole, B.R. Mitigating Action of Nanobioherbicides from Natural Products on Agricultural Produce. In Handbook of Agricultural Biotechnology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2024; Volume 2, pp. 19–44. [Google Scholar] [CrossRef]
- Mondéjar-López, M.; García-Simarro, M.P.; Navarro-Simarro, P.; Gómez-Gómez, L.; Ahrazem, O.; Niza, E. A review on the encapsulation of “eco-friendly” compounds in natural polymer-based nanoparticles as next generation nano-agrochemicals for sustainable agriculture and crop management. Int. J. Biol. Macromol. 2024, 280, 136030. [Google Scholar] [CrossRef]
- Hao, J.H.; Lv, S.S.; Bhattacharya, S.; Fu, J.G. Germination response of four alien congeneric Amaranthus species to environmental factors. PLoS ONE 2017, 12, e0170297. [Google Scholar] [CrossRef]
- Singh, A.; Mahajan, G.; Chauhan, B.S. Germination ecology of wild mustard (Sinapis arvensis) and its implications for weed management. Weed Sci. 2022, 70, 103–111. [Google Scholar] [CrossRef]
- Bana, R.S.; Kumar, V.; Sangwan, S.; Singh, T.; Kumari, A.; Dhanda, S.; Dawar, R.; Godara, S.; Singh, V. Seed germination ecology of Chenopodium album and Chenopodium murale. Biology 2022, 11, 1599. [Google Scholar] [CrossRef]
- Loades, E.; Pérez, M.; Turečková, V.; Tarkowská, D.; Strnad, M.; Seville, A.; Nakabayashi, K.; Leubner-Metzger, G. Distinct hormonal and morphological control of dormancy and germination in Chenopodium album dimorphic seeds. Front. Plant Sci. 2023, 14, 1156794. [Google Scholar] [CrossRef]
- Voegele, A.; Graeber, K.; Oracz, K.; Tarkowská, D.; Jacquemoud, D.; Tarkowská, V.; Urbanová, T.; Strnad, M.; Leubner-Metzger, G. Embryo growth, testa permeability, and endosperm weakening are major targets for the environmentally regulated inhibition of Lepidium sativum seed germination by myrigalone. J. Exp. Bot. 2012, 63, 5337–5350. [Google Scholar] [CrossRef]
- Bachheti, A.; Sharma, A.; Bachheti, R.; Husen, A.; Pandey, D. Plant allelochemicals and their various applications. In Co-Evolution of Secondary Metabolites; Reference Series in Phytochemistry; Springer: Cham, Switzerland, 2020; pp. 441–465. [Google Scholar]
- Pandharmise, P.; Tambe, A.N.; Kamble, S.; Tuwar, D.A. Preliminary phytochemical screening and HPTLC analysis of leaf extract of Crotalaria juncea from Vidarbha region, MS, India. J. Maharaja Sayajirao Univ. Baroda 2022, 56, 269–274. [Google Scholar]
- Samajdar, S.; Mukherjee, S.; Das, P.P. Seed germination inhibitors: Molecular and phytochemical aspects. Int. J. Appl. Pharm. Sci. Res. 2018, 3, 12–23. [Google Scholar] [CrossRef]
- Nikolova, M.; Yankova-Tsvetkova, E.; Stefanova, T.; Berkov, S. Exudate flavonoids of Primula veris leaves and their inhibitory activity on Lolium perrene seed germination. Pap. Present. Proc. Bulg. Acad. Sci. 2023, 76, 388–393. [Google Scholar] [CrossRef]
- Torawane, S.; Mokat, D. Allelopathic potential of weed Neanotis lancifolia (Hook. f.) W.H. Lewis on seed germination and metabolism of mungbean and rice. Allelopath. J. 2021, 52, 277–290. [Google Scholar] [CrossRef]
- Abdelmalik, A.M.; Alshahrani, T.S.; Alqarawi, A.A.; Ahmed, E.M. Allelopathic potential of Nicotiana glauca aqueous extract on seed germination and seedlings of Acacia gerrardii. Diversity 2024, 16, 26. [Google Scholar] [CrossRef]
- Mahur, B.K.; Ahuja, A.; Singh, S.; Maji, P.K.; Rastogi, V.K. Different nanocellulose morphologies (cellulose nanofibers, nanocrystals and nanospheres) extracted from Sunn hemp (Crotalaria Juncea). Int. J. Biol. Macromol. 2023, 253, 126657. [Google Scholar] [CrossRef]
- Lesiak, B.; Rangam, N.; Jiricek, P.; Gordeev, I.; Tóth, J.; Kövér, L.; Mohai, M.; Borowicz, P. Surface study of Fe3O4 nanoparticles functionalized with biocompatible adsorbed molecules. Front. Chem. 2019, 7, 642. [Google Scholar] [CrossRef]
- Periakaruppan, R.; Kumar, T.S.; Vanathi, P.; Al-Awsi, G.R.L.; Al-Dayan, N.; Dhanasekaran, S. Phyto-synthesis and characterization of parthenium-mediated iron oxide nanoparticles and an evaluation of their antifungal and antioxidant activities and effect on seed germination. Miner. Met. Mater. Soc. 2023, 75, 5235–5242. [Google Scholar] [CrossRef]
- Sainao, W.; Shi, Z.; Pang, H.; Feng, H. Alleviative effects of magnetic Fe3O4 nanoparticles on the physiological toxicity of 3-nitrophenol to rice (Oryza sativa L.) seedlings. Open Life Sci. 2022, 17, 626–640. [Google Scholar] [CrossRef]
- Kornarzyński, K.; Sujak, A.; Czernel, G.; Wiącek, D. Effect of Fe3O4 nanoparticles on germination of seeds and concentration of elements in Helianthus annuus L. under constant magnetic field. Sci. Rep. 2020, 10, 8068. [Google Scholar]
- Serpoush, M.; Kiyasatfar, M.; Ojaghi, J. Impact of Fe3O4 nanoparticles on wheat and barley seeds germination and early growth. Mater. Today 2022, 65, 2915–2919. [Google Scholar] [CrossRef]
- Das, C.K.; Srivastava, G.; Dubey, A.; Verma, S.; Saxena, M.; Roy, M.; Sethy, N.K.; Bhargava, K.; Singh, S.K.; Sarkar, S.; et al. The seed stimulant effect of nano iron pyrite is compromised by nano cerium oxide: Regulation by the trace ionic species generated in the aqueous suspension of iron pyrite. RSC Adv. 2016, 6, 67029–67038. [Google Scholar] [CrossRef]
- Gupta, N.; Singh, P.M.; Sagar, V.; Pandya, A.; Chinnappa, M.; Kumar, R.; Bahadur, A. Seed priming with ZnO and Fe3O4 nanoparticles alleviate the lead toxicity in Basella alba L. through reduced lead uptake and regulation of ROS. Plants 2022, 11, 2227. [Google Scholar] [CrossRef] [PubMed]
- Alkhatib, R.; Alkhatib, B.; Abdo, N. Effect of Fe3O4 nanoparticles on seed germination in tobacco. Environ. Sci. Pollut. 2021, 28, 53568–53577. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Akhtar, N.; Rehman, S.U.; Shujah, S.; Rha, E.S.; Jamil, M. Biosynthesized iron oxide nanoparticles (Fe3O4 NPs) mitigate arsenic toxicity in rice seedlings. Toxics 2021, 9, 2. [Google Scholar] [CrossRef]
- Xu, P.; Zeng, G.M.; Huang, D.L.; Feng, C.L.; Hu, S.; Zhao, M.H.; Lai, C.; Wei, Z.; Huang, C.; Xie, G.X.; et al. Use of iron oxide nanomaterials in wastewater treatment: A review. Sci. Total Environ. 2012, 424, 1–10. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).