The Impact of Fulvic Acids on Cotton Growth, Yield and Phosphorus Fertilizer Use Efficiency Under Different Phosphorus Fertilization Rates in Xinjiang, China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Experimental Design
2.3. Measurement and Calculation
2.4. Statistical Analysis
3. Results
3.1. Soil pH, Olsen P and Alkaline Phosphatase Activity
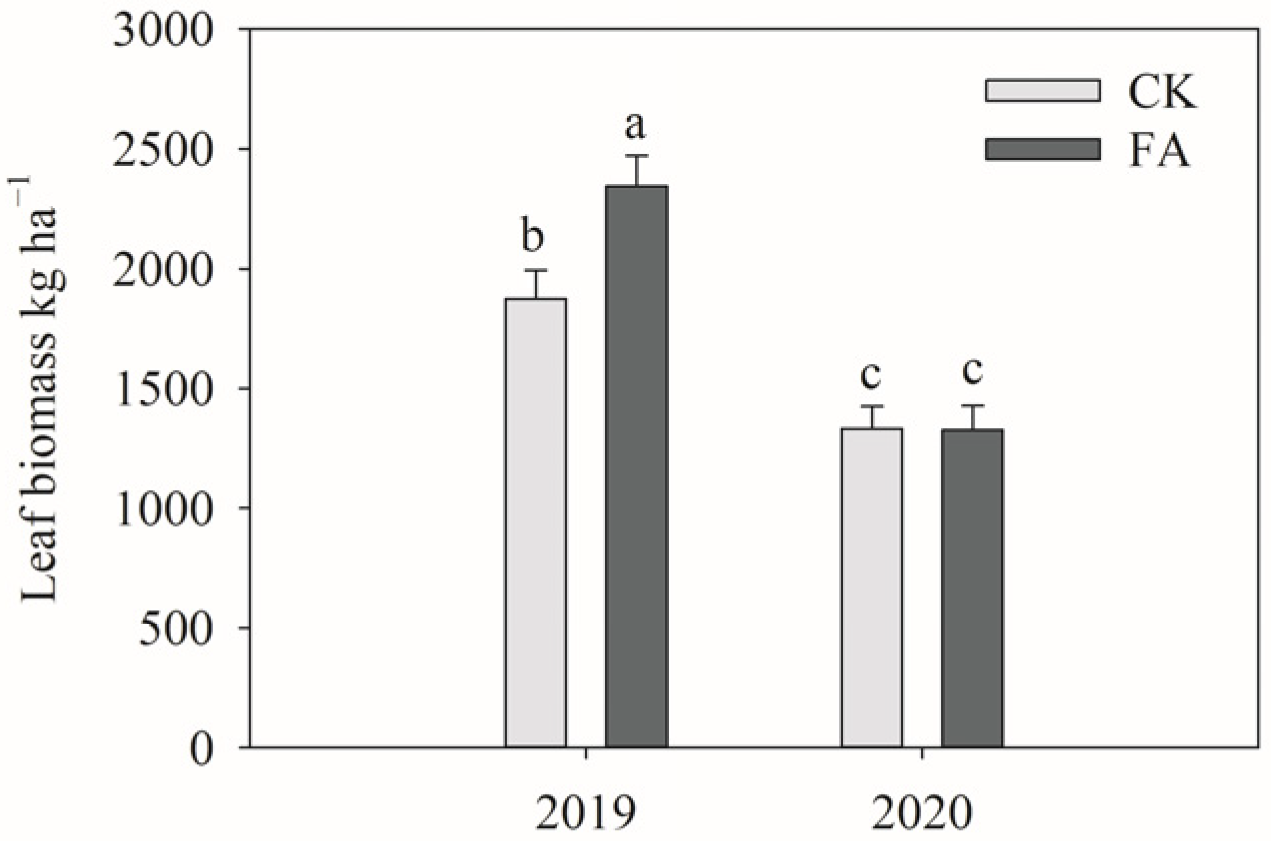
3.2. Cotton Biomass
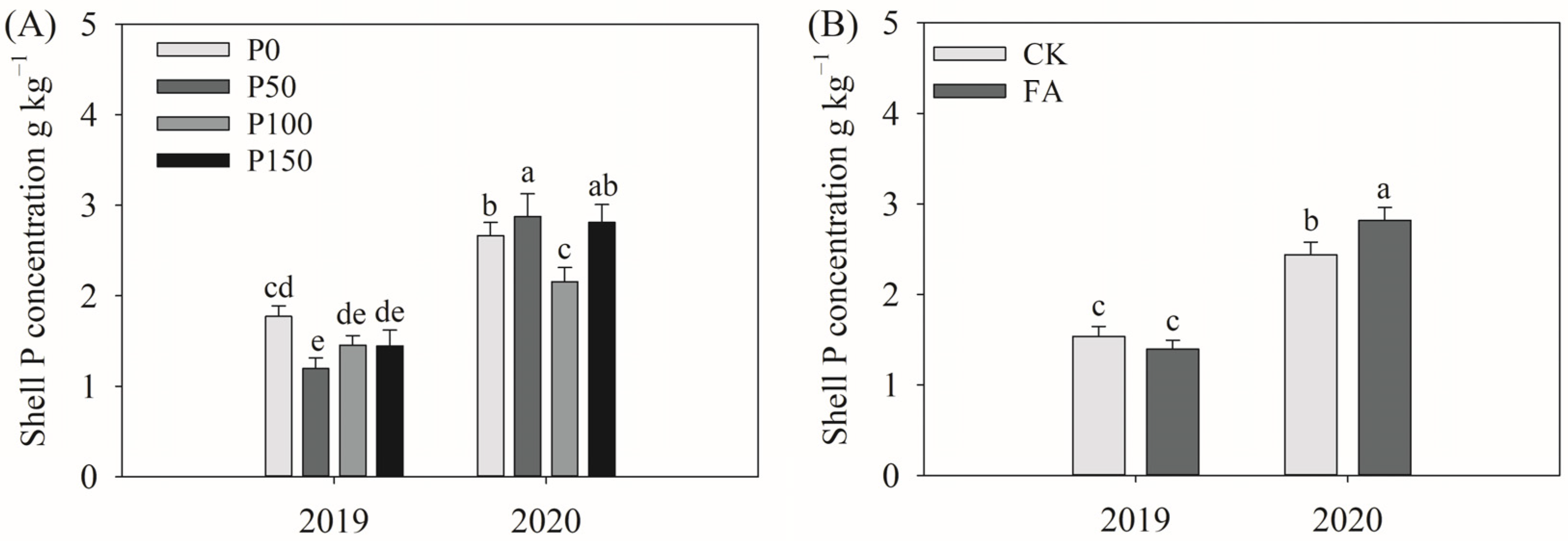
3.3. Cotton P Concentration
3.4. Cotton P Uptake
3.5. Yield and Components
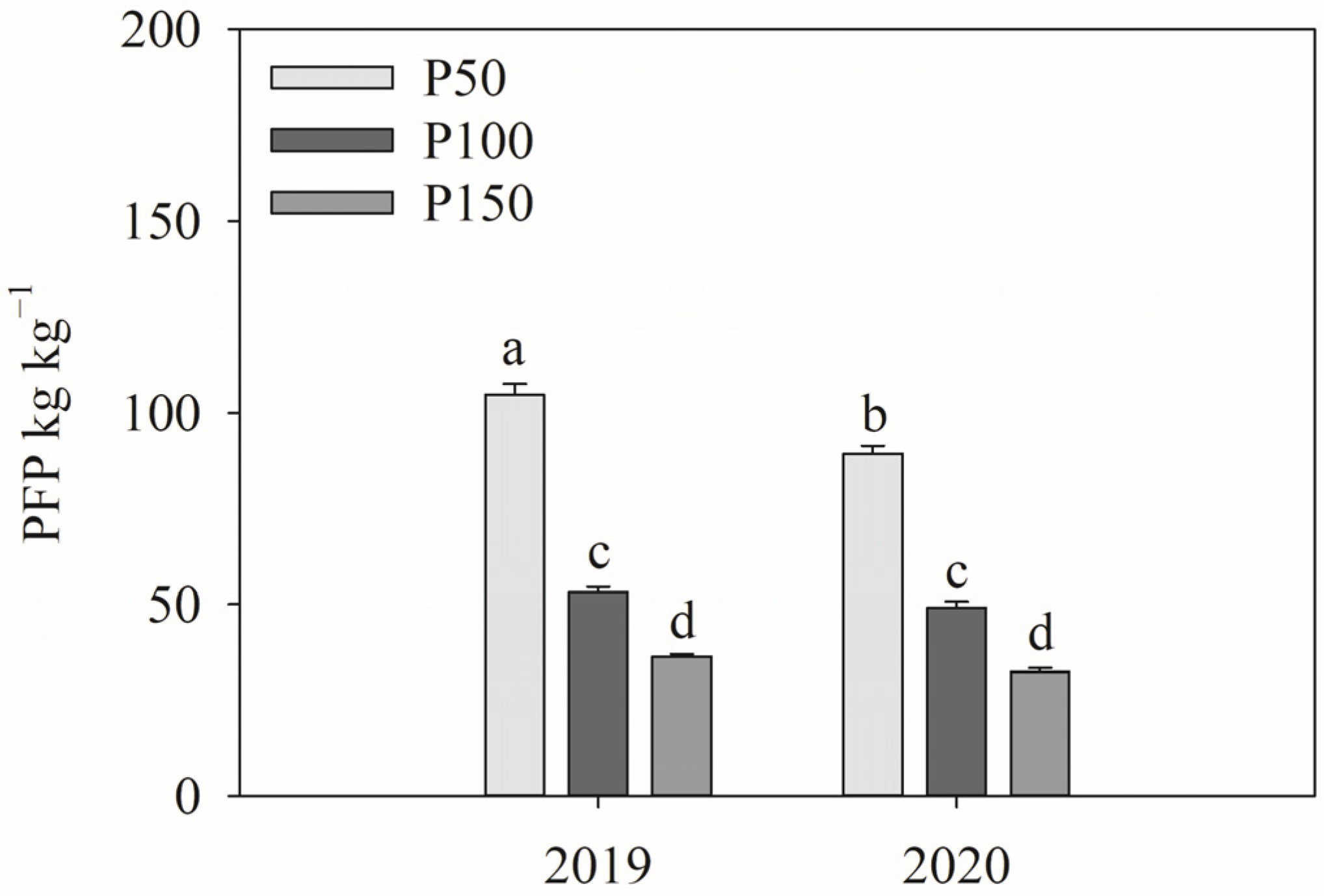
3.6. Phosphorus Use Efficiency
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Khan, M.A.; Wahid, A.; Ahmad, M.; Tahir, M.T.; Ahmed, M.; Ahmad, S.; Hasanuzzaman, M. World cotton production and consumption: An overview. In Cotton Production and Uses: Agronomy, Crop Protection, and Postharvest Technologies; Shakeel, A., Mirza, H., Eds.; Springer Nature Singapore Pte Ltd.: Singapore, 2020; pp. 1–7. [Google Scholar]
- Bai, Y.; Mao, S.; Tian, L.; Li, L.; Dong, H. Advances and prospects of high-yielding and simplified cotton cultivation technology in Xinjiang cotton-growing area. Sci. Agric. Sin. 2017, 50, 38–50. [Google Scholar] [CrossRef]
- Feng, L.; Wan, S.; Zhang, Y.; Dong, H. Xinjiang cotton: Achieving super-high yield through efficient utilization of light, heat, water, and fertilizer by three generations of cultivation technology systems. Field Crops Res. 2024, 312, 109401. [Google Scholar] [CrossRef]
- Chen, J.; Guan, Y.; Wang, J.; Chu, G. The long-term evolution of phosphate fertilizer application amount, efficiency and types on main crops in Xinjiang. Xinjiang Agric. Sci. 2016, 53, 1858–1866. [Google Scholar]
- Tang, M.; Shen, C.; Zhang, Y.; Yan, C.; Fu, G.; Xu, Y.; Tang, G.; Geng, Q. Investigation of fertilizer use efficiency for cotton in Xinjiang. Soil Fertil. Sci. China 2022, 4, 161–168. [Google Scholar] [CrossRef]
- Muindi, E.D.M. Understanding soil phosphorus. Int. J. Plant Soil Sci. 2019, 31, 1–18. [Google Scholar] [CrossRef]
- Penn, C.J.; Camberato, J.J. A critical review on soil chemical processes that control how soil pH affects phosphorus availability to plants. Agriculture 2019, 9, 120. [Google Scholar] [CrossRef]
- Merwad, A.R.M.A. Effect of humic and fulvic substances and Moringa leaf extract on Sudan grass plants grown under saline conditions. Can. J. Soil Sci. 2017, 97, 703–716. [Google Scholar] [CrossRef]
- Shi, X.; Gu, D.; Yang, H.; Li, Y.; Jiang, Y.; Zhan, N.; Cui, X. Effect of exogenous organic matter on phosphorus forms in middle-high fertility cinnamon soil. Plants 2024, 13, 1313. [Google Scholar] [CrossRef]
- Liu, X.; Yang, J.; Tao, J.; Yao, R. Integrated application of inorganic fertilizer with fulvic acid for improving soil nutrient supply and nutrient use efficiency of winter wheat in a salt-affected soil. Appl. Soil Ecol. 2022, 170, 104255. [Google Scholar] [CrossRef]
- Alsudays, I.M.; Alshammary, F.H.; Alabdallah, N.M.; Alatawi, A.; Alotaibi, M.M.; Alwutayd, K.M.; Alharbi, M.M.; Alghanem, S.M.S.; Alzuaibr, F.M.; Gharib, H.S.; et al. Applications of humic and fulvic acid under saline soil conditions to improve growth and yield in barley. BMC Plant Biol. 2024, 24, 191. [Google Scholar] [CrossRef]
- Bustos, I.; Schoebitz, M.; Zagal, E.; Muñoz, C. Evaluation of liquid phosphorus fertilizers and fulvic acids in a potato crop in an Andisol type soil. Int. J. Agric. Nat. Resour. 2022, 49, 123–129. [Google Scholar] [CrossRef]
- Hareem, M.; Danish, S.; Al Obaid, S.; Ansari, M.J.; Datta, R. Mitigation of drought stress in chili plants (Capsicum annuum L.) using mango fruit waste biochar, fulvic acid and cobalt. Sci. Rep. 2024, 14, 14270. [Google Scholar] [CrossRef] [PubMed]
- Ampong, K.; Thilakaranthna, M.S.; Gorim, L.Y. Understanding the role of humic acids on crop performance and soil health. Front. Agron. 2022, 4, 848621. [Google Scholar] [CrossRef]
- Bezuglova, O.; Klimenko, A. Application of humic substances in agricultural industry. Agronomy 2022, 12, 584. [Google Scholar] [CrossRef]
- Canellas, L.P.; Olivares, F.L.; Aguiar, N.O.; Jones, D.L.; Nebbioso, A.; Mazzei, P.; Piccolo, A. Humic and fulvic acids as biostimulants in horticulture. Sci. Hortic. 2015, 196, 15–27. [Google Scholar] [CrossRef]
- Rathor, P.; Gorim, L.Y.; Thilakarathna, M.S. Plant physiological and molecular responses triggered by humic based biostimulants-A way forward to sustainable agriculture. Plant Soil 2023, 492, 31–60. [Google Scholar] [CrossRef]
- Olk, D.C.; Dinnes, D.L.; Scoresby, J.R.; Callaway, C.R.; Darlington, J.W. Humic products in agriculture: Potential benefits and research challenges-a review. J. Soils Sed. 2018, 18, 2881–2891. [Google Scholar] [CrossRef]
- Jordan, D.L.; Hare, A.T.; Wells, R. Peanut response to a commercial blend of nitrogen, phosphorus, potassium, and fulvic acid. Crop Forage Turf. Man. 2022, 8, e20180. [Google Scholar] [CrossRef]
- Kumar Sootahar, M.; Zeng, X.; Su, S.; Wang, Y.; Bai, L.; Zhang, Y.; Li, T.; Zhang, X. The effect of fulvic acids derived from different materials on changing properties of albic black soil in the Northeast Plain of China. Molecules 2019, 24, 1535. [Google Scholar] [CrossRef]
- Santos, M.D.; Cavalcanti, M.T.; Pessoa, L.N.; Silva, Z.G.D.; da Silva, A.M.; Souza, T.; Henschel, J.M.; Pereira, E.M.; Neto, M.A.D.; Diniz, B.L.M.T. Exploring the impact of humic biostimulants on cassava yield and nutrition in Northeast Brazil. Sustainability 2024, 16, 4088. [Google Scholar] [CrossRef]
- Rose, M.T.; Patti, A.F.; Little, K.R.; Brown, A.L.; Jackson, W.R.; Cavagnaro, T.R. A meta-analysis and review of plant-growth response to humic substances: Practical implications for agriculture. Adv. Agron. 2014, 124, 37–89. [Google Scholar] [CrossRef]
- Yuan, F.; Zhang, K.; Ma, C.; Zhang, N.; Sheng, J.; Zhang, W. Effect of organic fertilizer addition on field phosphorus status and yield under different amounts of phosphorus fertilization in cotton field in Xinjiang, China. J. Agric. Resour. Environ. 2022, 39, 118–128. [Google Scholar] [CrossRef]
- Bao, S. Soil Agro-Chemistrical Analysis, 3rd ed.; China Agriculture Press: Beijing, China, 2000. [Google Scholar]
- Tabatabai, M. Soil enzymes. In Methods of Soil Analysis: Part 2 Microbiological and Biochemical Properties; Weaver, R.W., Angle, S., Bottomley, P., Bezdicek, D., Smith, S., Tabatabai, A., Wollum, A., Eds.; Soil Science Society of America, Inc.: Madison, WI, USA, 1994; Volume 5, pp. 775–833. [Google Scholar]
- Chen, B.; Sheng, J.; Jiang, P.; Liu, Y. Effect of applying different forms and rates of phosphoric fertilizer on phosphorus efficiency and cotton yield. Cotton Sci. 2010, 22, 49–56. [Google Scholar]
- Liu, T.; Dong, X.; Guo, K.; Wang, J.; Liu, X.; Sun, H. Effects of fertilizer types and application levels on phosphorus availability of saline soils and crops: A meta-analysis. Land Degrad. Dev. 2024, 35, 4068–4080. [Google Scholar] [CrossRef]
- Delgado, A.; Madrid, A.; Kassem, S.; Andreu, L.; del Campillo, M.D. Phosphorus fertilizer recovery from calcareous soils amended with humic and fulvic acids. Plant Soil 2002, 245, 277–286. [Google Scholar] [CrossRef]
- Shen, Y.; Ma, Z.; Chen, H.; Lin, H.; Li, G.; Li, M.; Tan, D.; Gao, W.; Jiao, S.; Liu, P.; et al. Effects of macromolecular organic acids on reducing inorganic phosphorus fixation in soil. Heliyon 2023, 9, e14892. [Google Scholar] [CrossRef]
- Yuan, Y.; Tang, C.; Jin, Y.; Cheng, K.; Yang, F. Contribution of exogenous humic substances to phosphorus availability in soil-plant ecosystem: A review. Crit. Rev. Environ. Sci. Technol. 2023, 53, 1085–1102. [Google Scholar] [CrossRef]
- Rauthan, B.S.; Schnitzer, M. Effects of a soil fulvic-acid on the growth and nutrient content of cucumber (Cucumis sativus) plants. Plant Soil 1981, 63, 491–495. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, H.; Wu, G.; Chen, X.; Gruda, N.; Li, X.; Dong, J.; Duan, Z. Dose-dependent application of straw-derived fulvic acid on yield and quality of tomato plants grown in a greenhouse. Front. Plant Sci. 2021, 12, 736613. [Google Scholar] [CrossRef]
- Dorahy, C.G.; Rochester, I.J.; Blair, G.J. Response of field-grown cotton (Gossypium hirsutum L.) to phosphorus fertilisation on alkaline soils in eastern Australia. Aust. J. Soil Res. 2004, 42, 737–746. [Google Scholar] [CrossRef]
- Iqbal, B.; Kong, F.; Ullah, I.; Ali, S.; Li, H.; Wang, J.; Khattak, W.A.; Zhou, Z. Phosphorus application improves the cotton yield by enhancing reproductive organ biomass and nutrient accumulation in two cotton cultivars with different phosphorus sensitivity. Agronomy 2020, 10, 153. [Google Scholar] [CrossRef]
- Malhotra, H.; Vandana; Sharma, S.; Pandey, R. Phosphorus nutrition: Plant growth in response to deficiency and excess. In Plant Nutrients and Abiotic Stress Tolerance; Mirza, H., Masayuki, F., Hirosuke, O., Kamrun, N., Barbara, H.-N., Eds.; Springer Nature Singapore Pte Ltd.: Singapore, 2018; pp. 171–190. [Google Scholar]
- Khan, F.; Siddique, A.B.; Shabala, S.; Zhou, M.; Zhao, C. Phosphorus plays key roles in regulating plants’ physiological responses to abiotic stresses. Plants 2023, 12, 2861. [Google Scholar] [CrossRef] [PubMed]
- Rehman, A.; Farooq, M. Morphology, physiology and ecology of cotton. In Cotton Production; Khawar, J., Bhagirath, S.C., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2019; pp. 23–46. [Google Scholar] [CrossRef]
- Anjum, S.A.; Wang, L.; Farooq, M.; Xue, L.; Ali, S. Fulvic acid application improves the maize performance under well-watered and drought conditions. J. Agron. Crop Sci. 2011, 197, 409–417. [Google Scholar] [CrossRef]
- Mai, W.; Xue, X.; Feng, G.; Yang, R.; Tian, C. Can optimization of phosphorus input lead to high productivity and high phosphorus use efficiency of cotton through maximization of root/mycorrhizal efficiency in phosphorus acquisition? Field Crops Res. 2018, 216, 100–108. [Google Scholar] [CrossRef]
- Wang, H.; Wu, L.; Wang, X.; Zhang, S.; Cheng, M.; Feng, H.; Fan, J.; Zhang, F.; Xiang, Y. Optimization of water and fertilizer management improves yield, water, nitrogen, phosphorus and potassium uptake and use efficiency of cotton under drip fertigation. Agric. Water Manage. 2021, 245, 106662. [Google Scholar] [CrossRef]
- Yuan, F.; Ma, D.; Zhao, K.; Zhang, K. Effect of phosphorus fertilization rate on cotton field phosphorus condition, yield and phosphorus balance in Xinjiang. Southwest China J. Agric. Sci. 2021, 34, 1033–1041. [Google Scholar] [CrossRef]
- Zhang, Y.; Yao, Y.; Hu, W.; Gao, Y.; Tang, M. Effects of phosphate fertilizer application on P accumulation, distribution, utilization and yield of cotton. Xinjiang Agric. Sci. 2020, 57, 2004–2011. [Google Scholar]
- de Wit, C.d. Resource use efficiency in agriculture. Agric. Syst. 1992, 40, 125–151. [Google Scholar] [CrossRef]

| Time (Day Month Year) | Irrigation Amount m3 ha−1 | Time (Day Month Year) | Irrigation Amount m3 ha−1 |
|---|---|---|---|
| 10 May 2019 | 656 | 28 April 2020 | 623 |
| 5 July 2019 | 578 | 14 June 2020 | 600 |
| 16 July 2019 | 237 | 26 June 2020 | 293 |
| 25 July 2019 | 320 | 15 July 2020 | 387 |
| 5 August 2019 | 554 | 2 August 2020 | 453 |
| 15 August 2019 | 492 | 17 August 2020 | 476 |
| 24 August 2019 | 359 | 26 August 2020 | 452 |
| Total irrigation amount in 2019 | 3196 | Total irrigation amount in 2020 | 3284 |
| Treatment | Urea kg ha−1 | Monoammonium Phosphate kg ha−1 | Potassium Sulfate kg ha−1 | Fulvic Acid kg ha−1 |
|---|---|---|---|---|
| P0 + CK | 543 | 0 | 60 | 0 |
| P0 + FA | 543 | 0 | 60 | 45 |
| P50 + CK | 522 | 82 | 60 | 0 |
| P50 + FA | 522 | 82 | 60 | 45 |
| P100 + CK | 501 | 164 | 60 | 0 |
| P100 + FA | 501 | 164 | 60 | 45 |
| P150 + CK | 479 | 246 | 60 | 0 |
| P150 + FA | 479 | 246 | 60 | 45 |
| pH | Available P mg kg−1 | Alkaline Phosphatase Activity mg kg−1 h−1 | |
|---|---|---|---|
| P fertilization rates | |||
| P0 | 8.63 ± 0.03 a | 19.20 ± 0.91 c | 200.73 ± 14.14 |
| P50 | 8.61 ± 0.03 a | 22.94 ± 0.93 a | 175.65 ± 13.40 |
| P100 | 8.59 ± 0.06 ab | 21.43 ± 0.76 b | 167.57 ± 13.82 |
| P150 | 8.52 ± 0.05 b | 22.07 ± 0.64 ab | 172.46 ± 12.19 |
| FA addition | |||
| CK | 8.61 ± 0.03 | 21.06 ± 0.57 b | 179.28 ± 9.46 |
| FA | 8.57 ± 0.03 | 21.89 ± 0.67 a | 178.09 ± 9.81 |
| Year | |||
| 2019 | 8.71 ± 0.02 a | 19.40 ± 0.36 b | 140.78 ± 4.54 |
| 2020 | 8.46 ± 0.02 b | 24.30 ± 0.49 a | 227.78 ± 5.04 |
| ANOVA (p values) | |||
| P fertilization rates | 0.038 | <0.001 | 0.022 |
| FA addition | 0.107 | 0.0444 | 0.582 |
| Year | <0.001 | <0.001 | <0.001 |
| P × FA | 0.362 | 0.906 | 0.693 |
| P × Year | 0.082 | 0.833 | 0.976 |
| FA × Year | 0.945 | 0.878 | 0.966 |
| P × FA × Year | 0.499 | 0.946 | 0.926 |
| Shoot kg ha−1 | Leaf kg ha−1 | Shell kg ha−1 | Fiber kg ha−1 | Seed kg ha−1 | Total kg ha−1 | |
|---|---|---|---|---|---|---|
| P fertilization rates | ||||||
| P0 | 1968 ± 104 c | 1408 ± 142 c | 1925 ± 158 b | 1667 ± 74 b | 2501 ± 107 c | 9469 ± 484 c |
| P50 | 2443 ± 147 b | 1613 ± 150 bc | 2320 ± 155 ab | 1901 ± 91 ab | 2802 ± 129 bc | 11,079 ± 541 b |
| P100 | 2470 ± 142 b | 1784 ± 159 b | 2530 ± 141 a | 2049 ± 106 a | 3074 ± 159 ab | 11,907 ± 583 ab |
| P150 | 2951 ± 112 a | 2074 ± 114 a | 2606 ± 134 a | 2164 ± 77 a | 3191 ± 120 a | 12,985 ± 381 a |
| FA addition | ||||||
| CK | 2391 ± 118 | 1604 ± 88 b | 2184 ± 112 b | 1927 ± 77 | 2890 ± 114 | 10,996 ± 432 |
| FA | 2525 ± 97 | 1836 ± 122 a | 2507 ± 107 a | 1963 ± 61 | 2893 ± 89 | 11,724 ± 394 |
| Year | ||||||
| 2019 | 2444 ± 110 | 2110 ± 96 a | 2229 ± 126 | 1942 ± 74 | 2885 ± 109 | 11,608 ± 469 |
| 2020 | 2473 ± 107 | 1330 ± 67 b | 2462 ± 93 | 1945 ± 65 | 2898 ± 95 | 11,111 ± 356 |
| ANOVA (p values) | ||||||
| P fertilization rates | <0.001 | <0.001 | 0.009 | 0.003 | 0.003 | <0.001 |
| FA addition | 0.295 | 0.023 | 0.032 | 0.695 | 0.985 | 0.173 |
| Year | 0.822 | <0.001 | 0.118 | 0.939 | 0.920 | 0.349 |
| P × FA | 0.338 | 0.758 | 0.949 | 0.495 | 0.462 | 0.808 |
| P × Year | 0.905 | 0.703 | 0.821 | 0.746 | 0.717 | 0.619 |
| FA × Year | 0.689 | 0.020 | 0.071 | 0.946 | 0.927 | 0.311 |
| P × FA × Year | 0.846 | 0.749 | 0.834 | 0.572 | 0.307 | 0.985 |
| Shoot g kg−1 | Leaf g kg−1 | Shell g kg−1 | Fiber g kg−1 | Seed g kg−1 | |
|---|---|---|---|---|---|
| P fertilization rates | |||||
| P0 | 1.34 ± 0.11 | 1.94 ± 0.12 | 2.22 ± 0.15 | 1.28 ± 0.06 | 9.80 ± 0.40 |
| P50 | 1.27 ± 0.11 | 1.71 ± 0.11 | 2.04 ± 0.25 | 1.15 ± 0.05 | 10.11 ± 0.29 |
| P100 | 1.24 ± 0.09 | 1.84 ± 0.13 | 1.80 ± 0.13 | 1.10 ± 0.05 | 10.72 ± 0.36 |
| P150 | 1.22 ± 0.10 | 1.82 ± 0.15 | 2.13 ± 0.22 | 1.08 ± 0.05 | 10.10 ± 0.40 |
| FA addition | |||||
| CK | 1.31 ± 0.08 | 1.87 ± 0.09 | 1.99 ± 0.12 | 1.15 ± 0.04 | 10.16 ± 0.24 |
| FA | 1.23 ± 0.07 | 1.78 ± 0.09 | 2.10 ± 0.15 | 1.16 ± 0.04 | 10.21 ± 0.28 |
| Year | |||||
| 2019 | 0.95 ± 0.03 b | 1.45 ± 0.05 b | 1.46 ± 0.07 b | 1.12 ± 0.04 | 9.46 ± 0.20 b |
| 2020 | 1.59 ± 0.06 a | 2.20 ± 0.07 a | 2.63 ± 0.10 a | 1.19 ± 0.04 | 10.91 ± 0.24 a |
| ANOVA (p values) | |||||
| P fertilization rates | 0.508 | 0.342 | 0.063 | 0.058 | 0.224 |
| FA addition | 0.197 | 0.314 | 0.290 | 0.839 | 0.854 |
| Year | <0.001 | <0.001 | <0.001 | 0.244 | <0.001 |
| P × FA | 0.145 | 0.287 | 0.116 | 0.685 | 0.199 |
| P × Year | 0.579 | 0.510 | 0.012 | 0.264 | 0.370 |
| FA × Year | 0.197 | 0.369 | 0.022 | 0.710 | 0.916 |
| P × FA × Year | 0.380 | 0.539 | 0.744 | 0.662 | 0.514 |
| Shoot kg ha−1 | Leaf kg ha−1 | Shell kg ha−1 | Fiber kg ha−1 | Seed kg ha−1 | Total kg ha−1 | |
|---|---|---|---|---|---|---|
| P fertilization rates | ||||||
| P0 | 2.62 ± 0.25 b | 2.60 ± 0.23 b | 4.40 ± 0.55 b | 2.15 ± 0.16 | 24.35 ± 1.26 c | 36.12 ± 1.99 c |
| P50 | 3.14 ± 0.32 ab | 2.65 ± 0.20 b | 4.75 ± 0.70 ab | 2.16 ± 0.11 | 28.18 ± 1.26 bc | 40.88 ± 2.12 bc |
| P100 | 2.98 ± 0.21 b | 3.10 ± 0.23 ab | 4.53 ± 0.39 ab | 2.24 ± 0.15 | 33.09 ± 2.11 a | 45.94 ± 12.68 ab |
| P150 | 3.68 ± 0.39 a | 3.64 ± 0.27 a | 5.55 ± 0.68 a | 2.34 ± 0.13 | 31.94 ± 1.42 ab | 47.28 ± 2.20 a |
| FA addition | ||||||
| CK | 3.11 ± 0.24 | 2.91 ± 0.17 | 4.46 ± 0.42 | 2.20 ± 0.11 | 29.28 ± 1.30 | 41.95 ± 1.80 |
| FA | 3.10 ± 0.20 | 3.08 ± 0.19 | 5.16 ± 0.41 | 2.25 ± 0.09 | 29.50 ± 1.19 | 43.16 ± 1.71 |
| Year | ||||||
| 2019 | 2.33 ± 0.14 b | 3.04 ± 0.16 | 3.14 ± 0.18 b | 2.15 ± 0.10 | 27.23 ± 1.16 b | 37.8 ± 1.49 b |
| 2020 | 3.88 ± 0.20 a | 2.95 ± 0.20 | 6.48 ± 0.37 a | 2.30 ± 0.09 | 31.56 ± 1.20 a | 47.23 ± 1.61 a |
| ANOVA (p values) | ||||||
| P fertilization rates | 0.010 | 0.014 | 0.162 | 0.765 | 0.001 | 0.002 |
| FA addition | 0.984 | 0.475 | 0.078 | 0.687 | 0.890 | 0.569 |
| Year | <0.001 | 0.727 | <0.001 | 0.318 | 0.009 | <0.001 |
| P × FA | 0.065 | 0.446 | 0.171 | 0.328 | 0.791 | 0.993 |
| P × Year | 0.284 | 0.365 | 0.057 | 0.623 | 0.971 | 0.782 |
| FA × Year | 0.232 | 0.410 | 0.487 | 0.816 | 0.970 | 0.940 |
| P × FA × Year | 0.240 | 0.992 | 0.735 | 0.397 | 0.892 | 0.952 |
| Density ×103 Plant ha−1 | Bolls per Plant | Single Boll Weight g | Seed Cotton Yield kg ha−1 | |
|---|---|---|---|---|
| P fertilization rates | ||||
| P0 | 122.90 ± 1.38 c | 6.20 ± 0.27 c | 5.29 ± 0.08 b | 4019 ± 176 c |
| P50 | 130.66 ± 2.07 b | 6.65 ± 0.21 b | 5.61 ± 0.05 a | 4851 ± 130 b |
| P100 | 130.65 ± 1.92 b | 6.99 ± 0.24 a | 5.65 ± 0.09 a | 5117 ± 115 a |
| P150 | 135.68 ± 5.07 a | 6.63 ± 0.24 b | 5.79 ± 0.09 a | 5161 ± 123 a |
| FA addition | ||||
| CK | 129.10 ± 1.81 | 6.39 ± 0.18 b | 5.59 ± 0.07 | 4592 ± 120 b |
| FA | 130.84 ± 1.87 | 6.84 ± 0.16 a | 5.58 ± 0.05 | 4982 ± 123 a |
| Year | ||||
| 2019 | 127.13 ± 1.80 b | 7.44 ± 0.09 a | 5.43 ± 0.05 b | 5143 ± 94 a |
| 2020 | 132.82 ± 1.64 a | 5.79 ± 0.10 b | 5.74 ± 0.07 a | 4431 ± 123 b |
| ANOVA (p values) | ||||
| P fertilization rates | <0.001 | <0.001 | <0.001 | <0.001 |
| FA addition | 0.264 | <0.001 | 0.829 | <0.001 |
| Year | 0.001 | <0.001 | <0.001 | <0.001 |
| P × FA | 0.376 | 0.323 | 0.582 | 0.990 |
| P × Year | 0.896 | 0.484 | 0.002 | 0.068 |
| FA × Year | 0.371 | 0.390 | 0.498 | 0.332 |
| P × FA × Year | 0.199 | 0.059 | 0.350 | 0.208 |
| RE % | PNB % | AE kg kg−1 | PFP kg kg−1 | |
|---|---|---|---|---|
| P fertilization rates | ||||
| P0 | - | - | - | - |
| P50 | 26.73 ± 6.97 | 186 ± 10 a | 20.36 ± 1.77 a | 97.02 ± 2.61 a |
| P100 | 24.86 ± 5.72 | 104 ± 6 b | 12.84 ± 1.23 b | 51.17 ± 1.15 b |
| P150 | 18.42 ± 2.10 | 71 ± 3 c | 8.85 ± 0.71 c | 34.40 ± 0.82 c |
| FA addition | ||||
| CK | 22.25 ± 4.27 | 119 ± 11 | 11.56 ± 1.10 b | 58.41 ± 5.42 b |
| FA | 23.43 ± 4.47 | 122 ± 12 | 16.48 ± 1.57 a | 63.32 ± 5.91 a |
| Year | ||||
| 2019 | 21.60 ± 4.49 | 106 ± 10 b | 12.14 ± 1.43 b | 64.76 ± 6.14 a |
| 2020 | 25.08 ± 3.77 | 135 ± 12 a | 15.89 ± 1.37 a | 56.97 ± 5.06 b |
| ANOVA (p values) | ||||
| P fertilization rates | 0.581 | <0.001 | <0.001 | <0.001 |
| FA addition | 0.749 | 0.749 | <0.001 | <0.001 |
| Year | 0.611 | <0.001 | 0.004 | <0.001 |
| P × FA | 0.984 | 0.984 | 0.162 | 0.162 |
| P × Year | 0.642 | 0.052 | 0.593 | <0.001 |
| FA × Year | 0.895 | 0.895 | 0.386 | 0.386 |
| P × FA × Year | 0.999 | 0.999 | 0.310 | 0.310 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, K.; Gao, X.; Ma, C.; Chen, B.; Yuan, F.; Sheng, J. The Impact of Fulvic Acids on Cotton Growth, Yield and Phosphorus Fertilizer Use Efficiency Under Different Phosphorus Fertilization Rates in Xinjiang, China. Agronomy 2025, 15, 992. https://doi.org/10.3390/agronomy15040992
Zhang K, Gao X, Ma C, Chen B, Yuan F, Sheng J. The Impact of Fulvic Acids on Cotton Growth, Yield and Phosphorus Fertilizer Use Efficiency Under Different Phosphorus Fertilization Rates in Xinjiang, China. Agronomy. 2025; 15(4):992. https://doi.org/10.3390/agronomy15040992
Chicago/Turabian StyleZhang, Kai, Xiaopeng Gao, Chao Ma, Bing Chen, Fang Yuan, and Jiandong Sheng. 2025. "The Impact of Fulvic Acids on Cotton Growth, Yield and Phosphorus Fertilizer Use Efficiency Under Different Phosphorus Fertilization Rates in Xinjiang, China" Agronomy 15, no. 4: 992. https://doi.org/10.3390/agronomy15040992
APA StyleZhang, K., Gao, X., Ma, C., Chen, B., Yuan, F., & Sheng, J. (2025). The Impact of Fulvic Acids on Cotton Growth, Yield and Phosphorus Fertilizer Use Efficiency Under Different Phosphorus Fertilization Rates in Xinjiang, China. Agronomy, 15(4), 992. https://doi.org/10.3390/agronomy15040992






