Molecular Cloning of the scd1 Gene and Its Expression in Response to Feeding Artificial Diets to Mandarin Fish (Siniperca chuatsi)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Ethics
2.2. Fish Sampling
2.3. Total RNA Isolation and cDNA Synthesis
2.4. cDNAs Cloning of scd1 Gene
2.5. Sequence Analyses and Data Processing
2.6. Tissue Distribution Levels of Sc-scd1
2.7. Statistical Analysis
3. Results
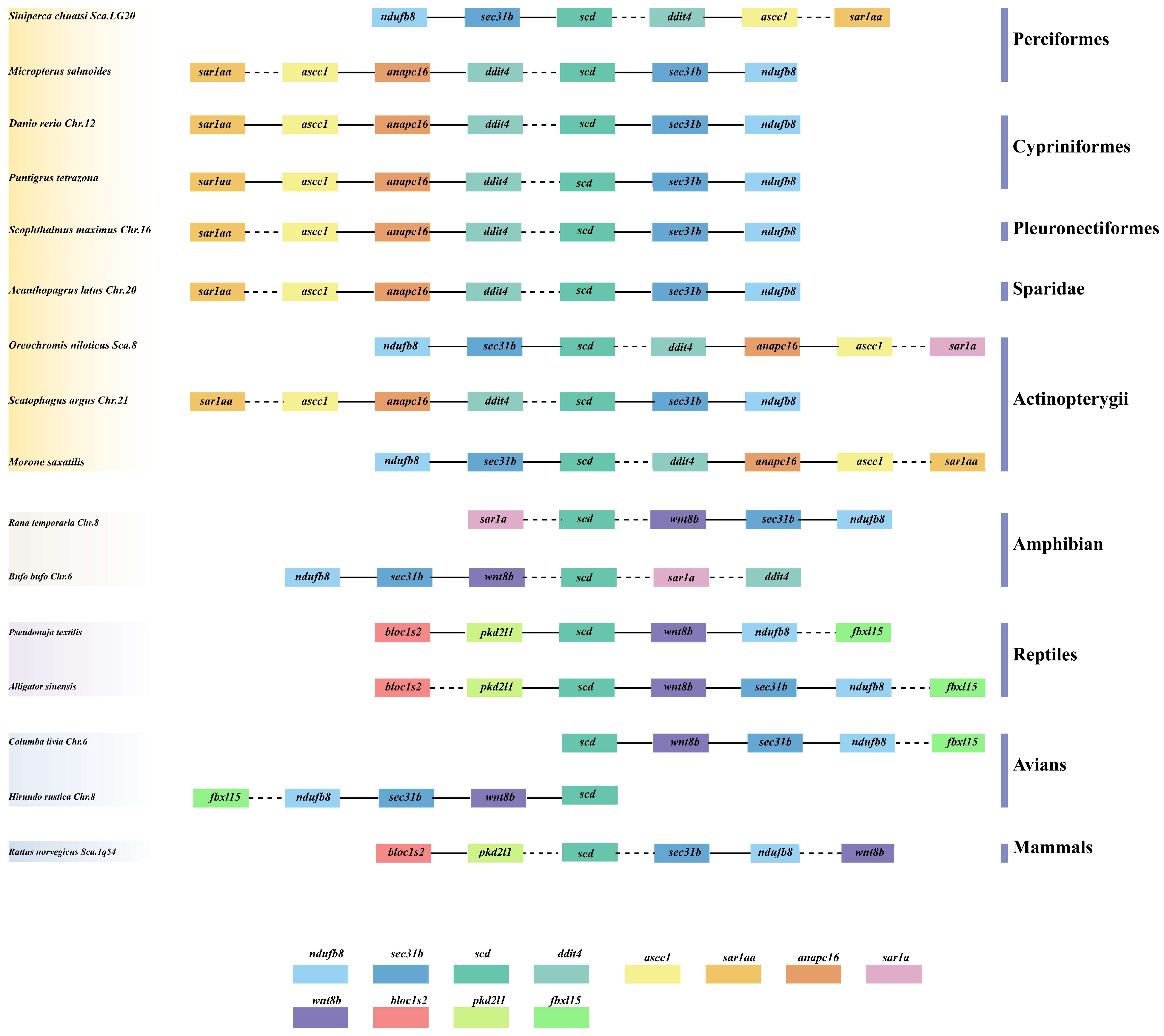
3.1. Comparative Syntenic and Structural Analyses of scd1 Genes in Vertebrates
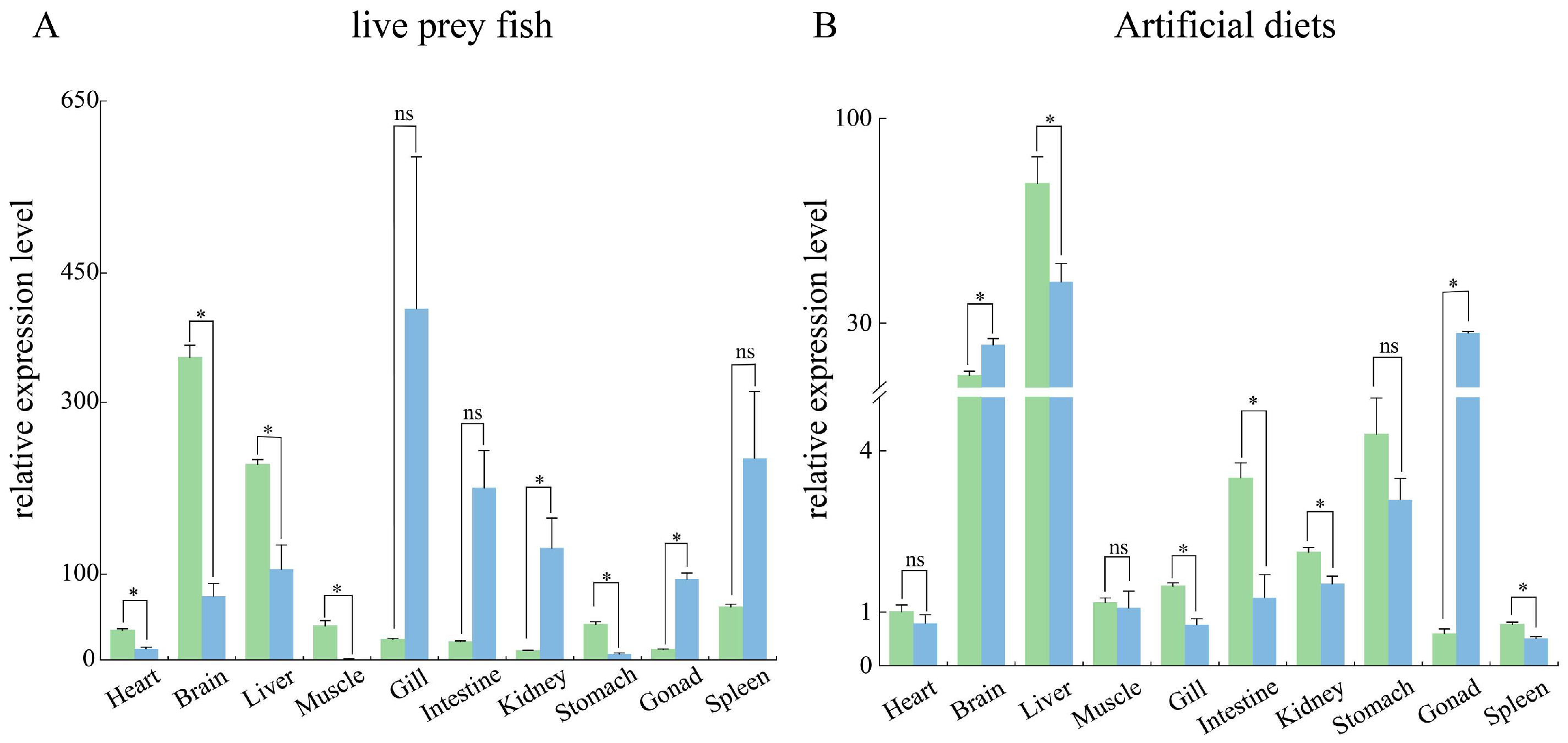
3.2. Tissue Distribution Pattern of Sc-scd1
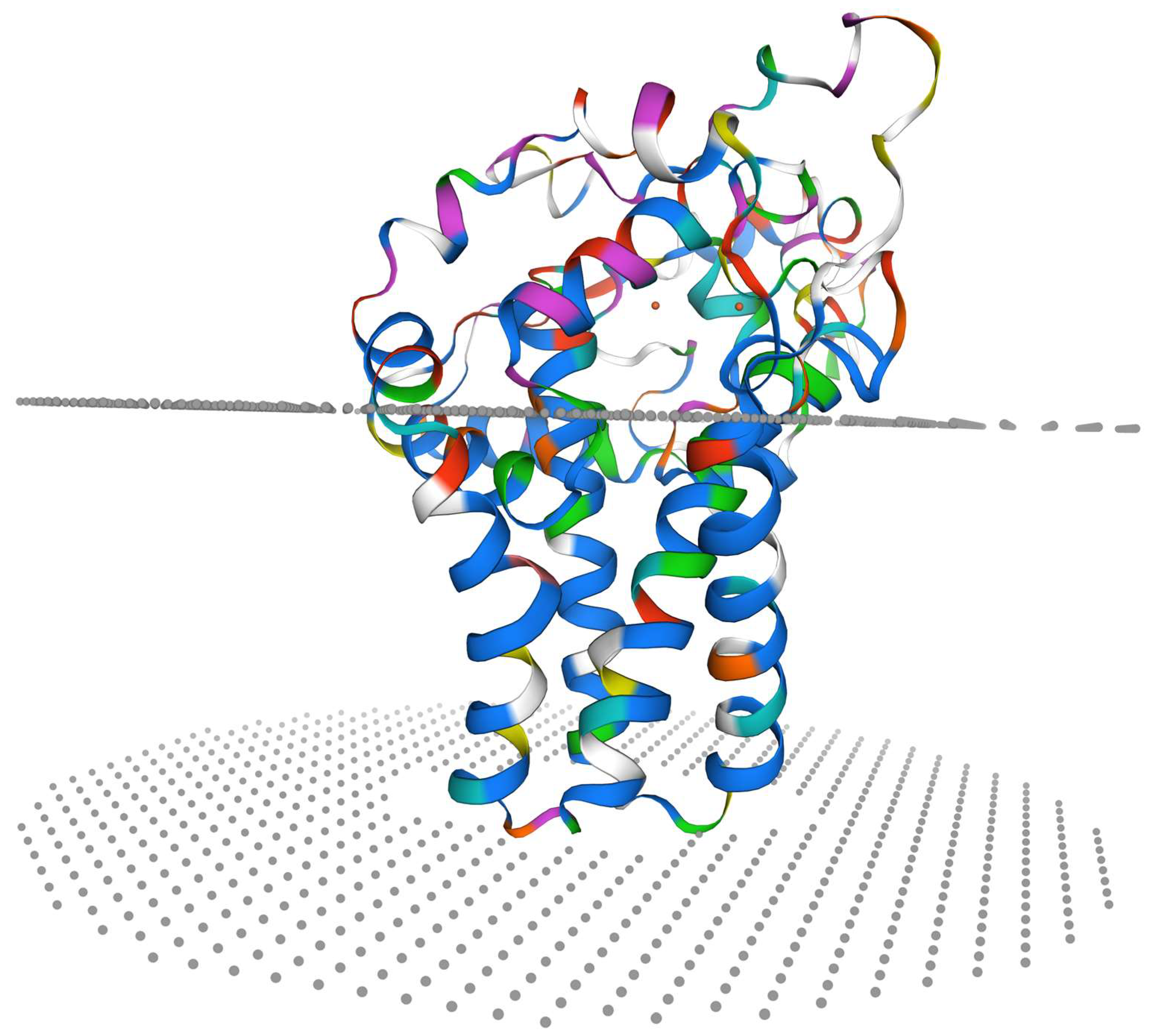
3.3. Molecular Cloning and Characterization of Sc-scd1
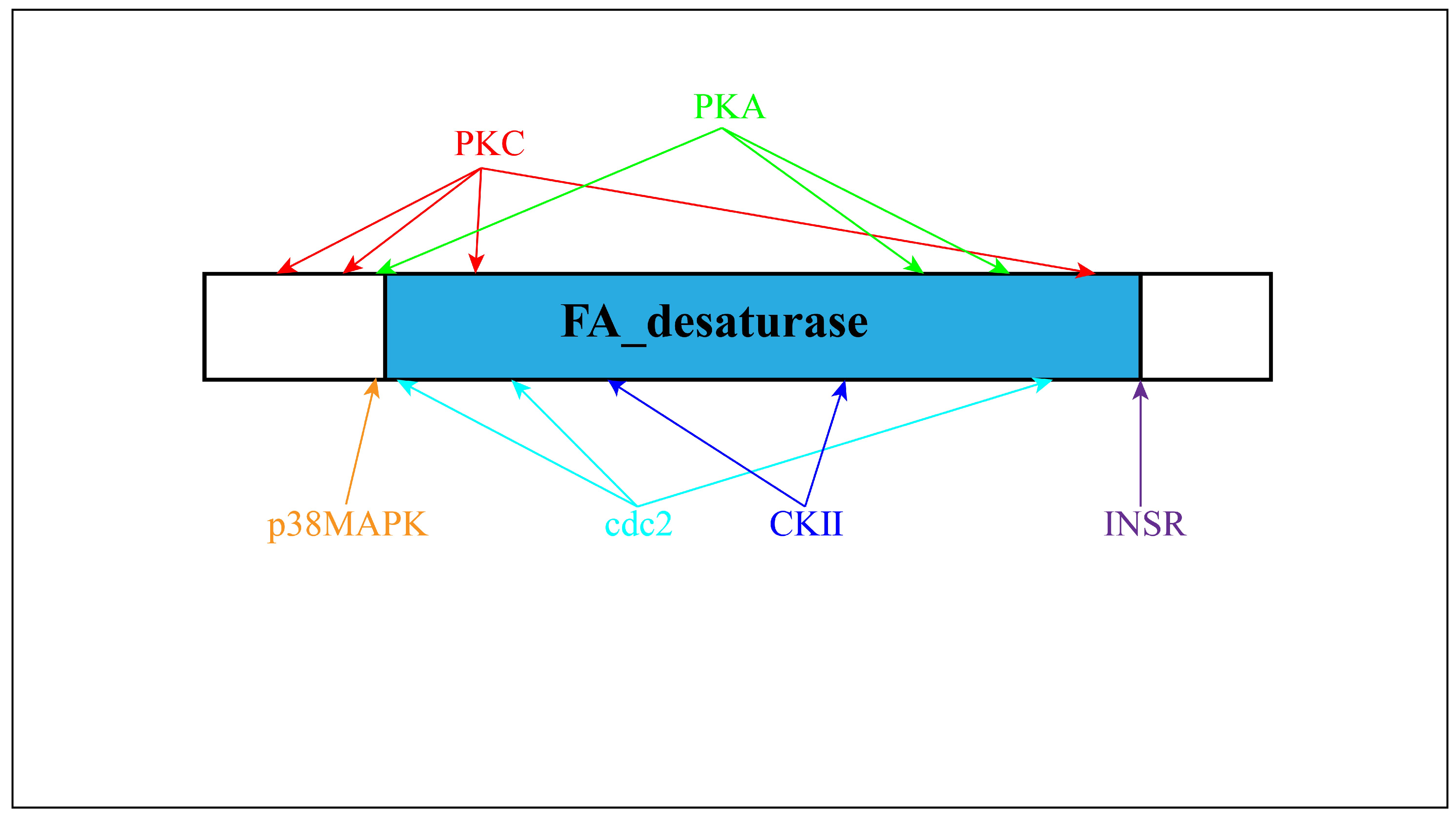
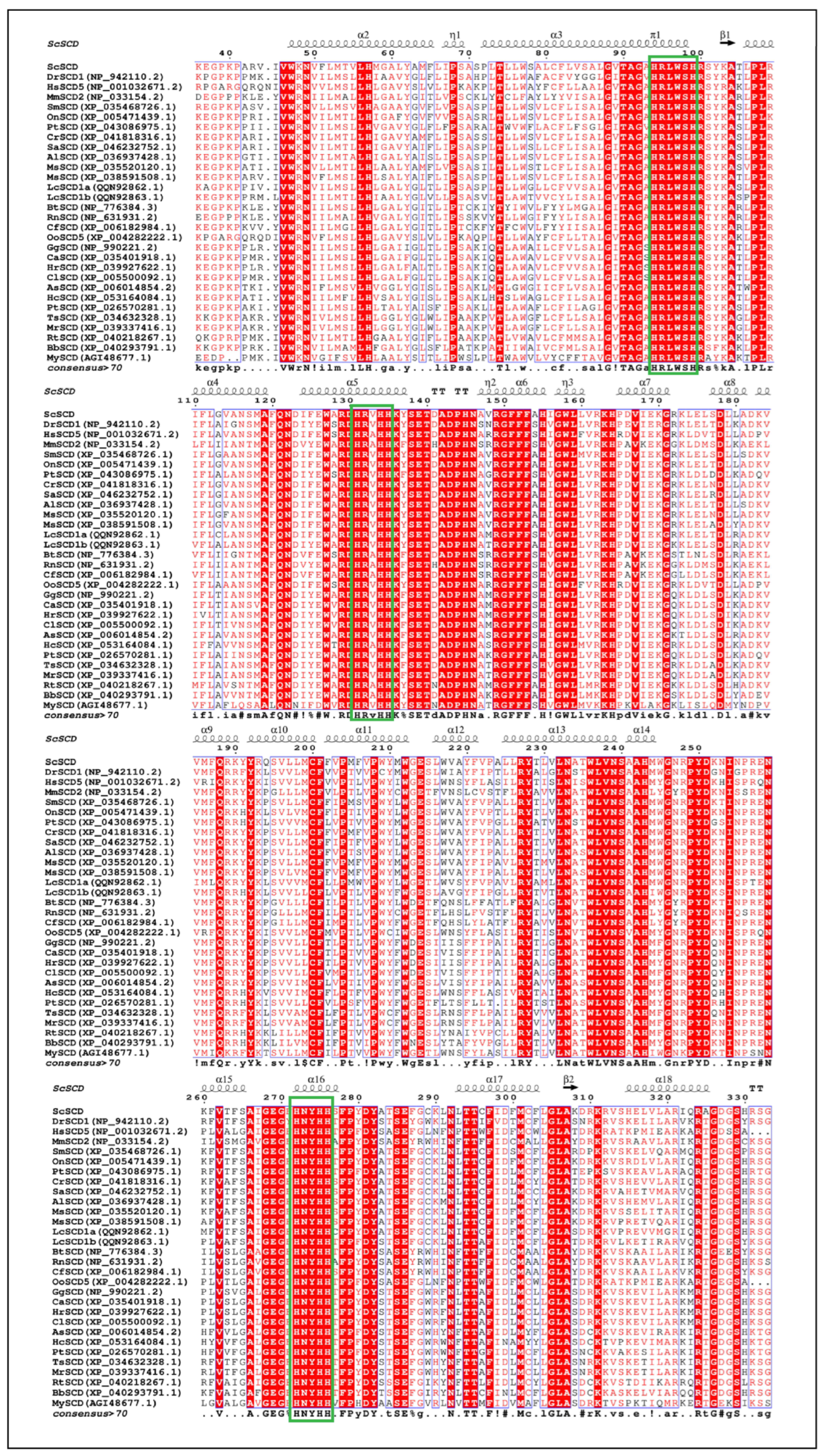
3.4. Alignment of SCD Protein Sequence
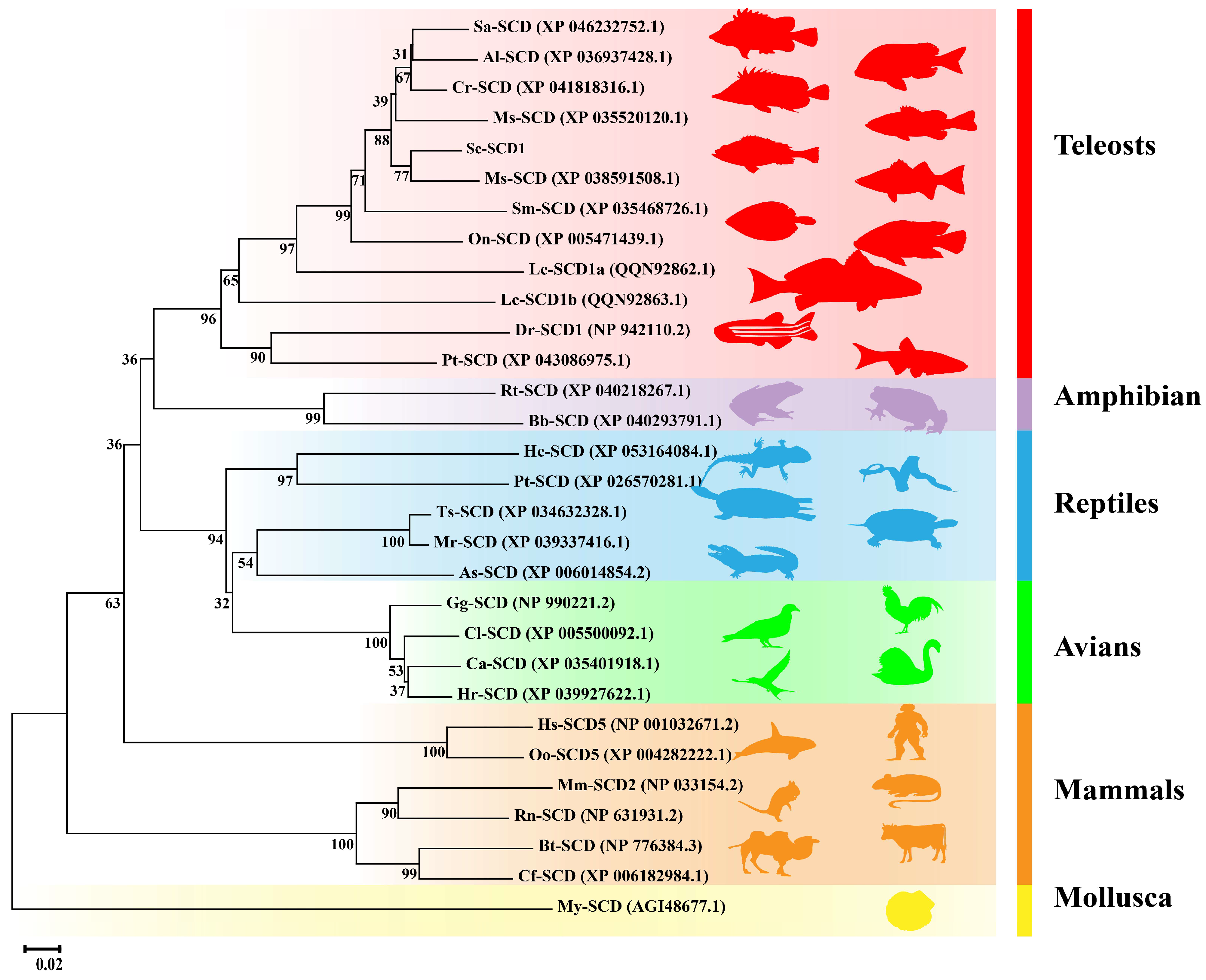
3.5. SCD Phylogenetic Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, C.F.; Dong, J.J.; Li, W.H.; Tian, Y.Y.; Hu, J.; Ye, X. Response to four generations of selection for growth performance traits in mandarin fish (Siniperca chuatsi). Aquaculture 2022, 548, 737590. [Google Scholar] [CrossRef]
- Administration of Fisheries and Fishery Administration; Ministry of Agriculture and Rural Affairs; National Aquatic Technology Extension General Station; China Fisheries Society. China Fishery Statistical Yearbook; China Agricultural Publishing House: Beijing, China, 2024. [Google Scholar]
- Liu, L.; Liang, X.F.; Fang, J. The optimal stocking density for hybrid of Siniperca chuatsi (♀)× Siniperca scherzeri (♂) mandarin fish fed minced prey fish. Aquac. Res. 2017, 48, 1342–1345. [Google Scholar] [CrossRef]
- Chen, X.H.; Hong, C.Q.; Tan, Y.J.; Mo, W.L.; Zhao, S.Y.; Hong, Y.C.; Sun, K.H.; Wang, X.S.; Li, J.Y. Effects of Siniperca chuatsi compound feeds on the growth of warble-billed mandarin fish and the domestication culture technology of Siniperca chuatsi. Contemp. Aquac. 2022, 47, 76–78+81. (In Chinese) [Google Scholar]
- Shen, Y.W.; Li, H.Y.; Zhao, J.L.; Tang, S.J.; Zhao, Y.; Bi, Y.H.; Chen, X.W. The digestive system of mandarin fish (Siniperca chuatsi) can adapt to domestication by feeding with artificial diet. Aquaculture 2021, 538, 736546. [Google Scholar] [CrossRef]
- Zhang, Y.; He, Z.Y.; Huabf, J.Q.; Liang, X.; Huang, F.Y.; Yao, G.H.; Xu, X.J.; Guang, W.Z.; Lou, B. Effects of compound feed and live bait feeding on muscle metabolism gene expression of Siniperca chuatsi (Siniperca chuatsi). J. Zhejiang Agric Sci. 2023, 64, 1380–1384. (In Chinese) [Google Scholar]
- Huang, X.P.; Yao, X.L.; Zheng, J.; Zhao, J.L. Effects of compound feed on the intestinal structure and immune function of Siniperca chuatsi. Freshw. Fish. 2023, 53, 77–85. (In Chinese) [Google Scholar]
- Zhang, Z.; Yuan, X.P.; Wu, H.; Gao, J.W.; Wu, J.Y.; Xiong, Z.Z.; Feng, Z.F.; Xie, M.; Li, S.M.; Xie, Z.G.; et al. The Effect of Short-Term Artificial Feed Domestication on the Expression of Oxidative-Stress-Related Genes and Antioxidant Capacity in the Liver and Gill Tissues of Mandarin Fish (Siniperca chuatsi). Genes 2024, 15, 487. [Google Scholar] [CrossRef]
- Fang, W.; Leng, X.J.; Yun, B.; Wang, L.; Qian, X.Q. Artificial diets affect glucose and lipid metabolism, antioxidant capacity, and inflammatory response in the muscle of mandarin fish (Siniperca chuatsi). Front. Mar. Sci. 2024, 11, 1445902. [Google Scholar] [CrossRef]
- Peng, D.; Liang, X.F.; Chai, F.; Feng, H.X.; Li, J.; Tang, S.L.; Lu, K.; Zhang, Q.W. Effects of dietary carbohydrate to lipid ratios on growth, biochemical indicators, lipid metabolism, and appetite in Chinese perch (Siniperca chuatsi). Fish Physiol. Biochem. 2022, 48, 101–116. [Google Scholar] [CrossRef]
- Floers, M.T.; Ntambi, J.M. Stearoyl-CoA desaturase and its relation to high-carbohydrate diets and obesity. Biochim. Biophys. Acta 2009, 1791, 85–91. [Google Scholar] [CrossRef]
- Da Silva-Santi, L.G.; Antunes, M.M.; Caparroz-Assef, S.M.; Carbonera, F.; Masi, L.N.; Curi, R.; Visentainer, J.V.; Bazotte, R.B. Liver Fatty Acid Composition and Inflammation in Mice Fed with High-Carbohydrate Diet or High-Fat Diet. Nutrients 2016, 8, 682. [Google Scholar] [CrossRef]
- Polley, S.D.; Tiku, P.E.; Trueman, R.T.; Caddick, M.X.; Morozov, I.Y.; Cossins, A.R. Differential expression of cold- and diet-specific genes encoding two carp liver delta 9-acyl-CoA desaturase isoforms. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 284, 41–50. [Google Scholar] [CrossRef]
- Guo, Z.H.; Yang, Z.G.; Cheng, Y.X.; Ji, L.Y.; Que, Y.Q.; Liu, Z.W.; Zeng, Q.T. Molecular characterization, tissue expression of acyl-CoA Δ9-desaturase-like gene, and effects of dietary lipid levels on its expression in the hepatopancreas of the Chinese mitten crab (Eriocheir sinensis). Aquaculture 2013, 402–403, 58–65. [Google Scholar] [CrossRef]
- Ma, X.Y.; Qiang, J.; Xu, P.; He, J. Stearoyl Coenzyme A Desaturase Gene Structure, Function, Regulatory Factors, and Progress in Fishes. Jiangsu Agric Sci. 2015, 43, 9–13. (In Chinese) [Google Scholar]
- Qiang, J.; Tao, Y.F.; He, J.; Sun, Y.L.; Xu, P. miR-29a modulates SCD expression and is regulated in response to a saturated fatty acid diet in juvenile genetically improved farmed tilapia (Oreochromis niloticus). J. Exp. Biol. 2017, 220, 1481–1489. [Google Scholar]
- Ntambi, J.M.; Miyazaki, M.; Stoehr, J.P.; Lan, H.; Kendziorski, C.M.; Yandell, B.S.; Song, Y.; Cohen, P.; Friedman, J.M.; Attie, A.D. Loss of stearoyl–CoA desaturase-1 function protects mice against adiposity. Proc. Natl. Acad. Sci. USA 2002, 99, 11482–11486. [Google Scholar] [CrossRef]
- He, S.; You, J.J.; Liang, X.F.; Zhang, Z.L.; Zhang, Y.P. Transcriptome sequencing and metabolome analysis of food habits domestication from live prey fish to artificial diets in mandarin fish (Siniperca chuatsi). BMC Genom. 2021, 22, 129. [Google Scholar] [CrossRef]
- Sampath, H.; Ntambi, J.M. Role of stearoyl-CoA desaturase-1 in skin integrity and whole body energy balance. J. Biol. Chem. 2014, 289, 2482–2488. [Google Scholar] [CrossRef]
- Igal, R.A. Stearoyl CoA desaturase-1: New insights into a central regulator of cancer metabolism. Biochim. Biophys. Acta 2016, 1861, 1865–1880. [Google Scholar] [CrossRef]
- Sen, U.; Coleman, C.; Sen, T. Stearoyl coenzyme A desaturase-1: Multitasker in cancer, metabolism, and ferroptosis. Trends Cancer 2023, 9, 480–489. [Google Scholar] [CrossRef]
- Luo, J.X.; Gao, X.W.; Zhang, L.H.; Sun, Y.F.; Wu, C.B. Transcriptomic analysis of the impact of artificial compound feed cultivation on gene expression in the intestinal tissue of mandarin fish. Hebei Fish. 2024, 365, 1–5. (In Chinese) [Google Scholar]
- GB/T 35892-2018; Laboratory Animal-Guideline for Ethical Review of Animal Welfare. General Administration of Quality Supervision, Inspection and Quarantine of the People's Republic of China. Standardization Administration of the People's Republic of China: Beijing, China, 2017.
- Liang, X.F. Research on Artificial Feeding of Siniperca chuatsi. Fish. Sci. Technol. Inf. 2002, 29, 64–67. (In Chinese) [Google Scholar]
- Zhang, L.H.; Luo, Z.; Song, Y.F.; Shi, X.; Pan, Y.X.; Fan, Y.F.; Xu, Y.H. Effects and mechanisms of waterborne copper exposure influencing ovary development and related hormones secretion in yellow catfish Pelteobagrus fulvidraco. Aquat. Toxicol. 2016, 178, 88–98. [Google Scholar] [CrossRef]
- Yang, C.; Chen, L.M.; Huang, R.; Gui, B.; Li, Y.Y.; Li, Y.Y.; Li, Y.M.; Liao, L.J.; Zhu, Z.Y.; Wang, Y.P. Screening of Genes Related to Sex Determination and Differentiation in Mandarin Fish (Siniperca chuatsi). Int. J. Mol. Sci. 2022, 23, 7692. [Google Scholar] [CrossRef]
- Jones, D.T.; Taylor, W.R.; Thormtom, J.M. The rapid generation of mutation data matrices from protein sequences. Comput. Appl. Biosci. 1992, 8, 275–282. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Liang, X.F.; He, S.; Wang, J.; Li, L.; Zhang, Z.; Li, J.; Chen, X.; Alam, M.S. Metabolic responses of Chinese perch (Siniperca chuatsi) to different levels of dietary carbohydrate. Fish Physiol Biochem. 2021, 47, 1449–1465. [Google Scholar] [CrossRef]
- Castro, L.F.C.; Wilson, J.M.; Goncalves, O.; Galante-Oliveira, S.; Rocha, E.; Cunha, I. The evolutionary history of the stearoyl-CoA desaturase gene family in vertebrates. BMC Evol. Biol. 2011, 11, 132. [Google Scholar] [CrossRef]
- Ren, H.T.; Yu, J.H.; Xu, P.; Tang, Y.K.; Li, H.X.; Li, J.L. Cloning and expression of two Δ6 fatty acid desaturase genes from Jian carp (Cyprinus carpio). Chin. J. Biochem. Mol. Biol. 2012, 28, 677–684. (In Chinese) [Google Scholar]
- Ai, Q.H.; Yan, J.; Mai, K.S. Research progresses of lipids and fatty acids transport in fish. Acta Hydrobiol. Sin. 2016, 40, 859–868. (In Chinese) [Google Scholar]
- Kjær, M.A.; Vegusdal, A.; Berge, G.M.; Galloway, T.F.; Hillestad, M.; Krogdahl, A.; Holm, H.; Ruyter, B. Characterisation of lipid transport in Atlantic cod (Gadus morhua) when fasted and fed high or low fat diets. Aquaculture 2009, 288, 325–336. [Google Scholar] [CrossRef]
- Chen, X.; Sun, C.F.; Dong, J.J.; Li, W.H.; Tian, Y.Y.; Hu, J.; Ye, X. Comparative Analysis of the Gut Microbiota of Mandarin Fish (Siniperca chuatsi) Feeding on Compound Diets and Live Baits. Front. Genet. 2022, 13, 797420. [Google Scholar] [CrossRef]
- Feng, T.T.; Tao, Y.F.; Ma, X.Y.; Lu, S.Q.; Zhang, X.; Liu, W.T.; Pan, Y.F.; Qiang, J. Cloning and expression analysis of the stearoyl-CoA desaturse gene in the genetic improvement of farmed tilapia (Oreochromis niloticus) and transference analysis in zebrafish. J. Fish. Sci. China 2023, 30, 11–24. (In Chinese) [Google Scholar]
- Hsieh, S.L.; Chang, H.T.; Wu, C.H.; Kuo, C.M. Cloning, tissue distribution and hormonal regulation of stearoyl-CoA desaturase in tilapia, Oreochromis mossambicus. Aquaculture 2004, 230, 527–546. [Google Scholar] [CrossRef]
- Chen, Z.Q.; Zhang, Z.Y.; Zhang, Z.W.; Wei, M.L.; Lin, Z.J.; Zhu, F.; Jia, C.F.; Chen, S.Y.; Meng, Q. Cloning of Acanthopagrus schlegelii scd1a and scd1b genes associated with response to acute low temperature. J. Fish. Sci. China 2022, 29, 200–210. (In Chinese) [Google Scholar]
- Jeffcoat, R.; James, A.T. The control of stearoyl-CoA desaturase by dietary linoleic acid. FEBS Lett. 1978, 85, 114–118. [Google Scholar] [CrossRef]
- Wang, Y.X.; Zheng, Q.; Xu, S.F.; Wang, Y.W.; Huang, Y.H.; Huang, X.H.; Wei, J.G.; Qin, Q.W.; Wei, S.N. Fish SCD1 promotes SGIV infection via modulating the formation of lipid droplets and TBK1/MDA5-activated IFN signal pathway. Aquaculture 2023, 575, 739766. [Google Scholar] [CrossRef]
- Chang, B.E.; Hsieh, S.L.; Kuo, C.M. Molecular cloning of full-length cDNA encoding delta-9-desaturase through PCR strategies and its genomic organization and expression in grass carp (Ctenopharyngodon idella). Mol. Reprod. Dev. 2001, 58, 245–254. [Google Scholar] [CrossRef]
- Hsieh, S.L.; Liao, W.L.; Kuo, C.M. Molecular cloning and sequence analysis of stearoyl-CoA desaturase in milkfish, Chanos chanos. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2001, 130, 467–477. [Google Scholar] [CrossRef]
- Zhang, Q.; Sun, S.Y.; Zhang, Y.L.; Wang, X.; Li, Q. Identification of Scd5 as a functional regulator of visceral fat deposition and distribution. Iscience 2022, 25, 103916. [Google Scholar] [CrossRef]
- Bai, Y.; McCoy, J.G.; Levin, E.L.; Sobrado, P.; Rajashankar, K.R.; Fox, B.G.; Zhou, M. X-ray structure of a mammalian stearoyl-CoA desaturase. Nature 2015, 524, 252–256. [Google Scholar] [CrossRef]
- Wang, H.; Klein, M.G.; Zou, H.; Lane, W.; Snell, G.; Levin, I.; Li, K.; Sang, B.C. Crystal structure of human stearoyl-coenzymeA in complex with substrate. Nat. Struct. Mol. Biol. 2015, 22, 581–585. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.L.; Zhang, R.; Wang, X.W.; Zhu, H. Analysis of sequence, function and expression of PtScd1 gene in tropical stenothermal fish tiger skin barb Puntius tetrazona. Fish. Sci. 2020, 39, 820–828. (In Chinese) [Google Scholar]
- Shen, J.M.; Wu, G.; Pierce, B.S.; Tsai, A.L.; Zhou, M. Free ferrous ions sustain activity of mammalian stearoyl-CoA desaturase-1. J. Biol. Chem. 2023, 299, 104897. [Google Scholar] [CrossRef] [PubMed]



| Primers | Sequences (5′–3′) |
|---|---|
| scd1-01-F | F: GGTTAGGCAGACCATCTTCATC |
| scd1-01-R | R: TCTGGGTTCCCTCACTCTTCC |
| scd1-02-F | F: TACTCTTCTGCCCGTCTTTG |
| scd1-02-R | R: TTCAACAATCTTAGCCACTCC |
| scd1-qF | F: TTGAGAAAGGACGCAAGCTG |
| scd1-qR | R: AAGAAGCACATGAGCAGCAC |
| β-actin-qF | F: AATCGTGCGTGACATCAAGG |
| β-actin-qF | R: TTGCCAATGGTGATGACCTG |
| Applications | Softwares | Websites |
|---|---|---|
| sequences download | NCBI | https://www.ncbi.nlm.nih.gov/ (accessed on 11 September 2024) |
| open reading frame (ORF) | ORF Finder | https://www.ncbi.nlm.nih.gov/orffinder (accessed on 11 September 2024) |
| physicochemical properties of proteins | Expasy | https://web.expasy.org (accessed on 11 September 2024) |
| protein domain features | Conserved Domain Database (CDD) | https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi (accessed on 11 September 2024) |
| N-linked glycosylation sites | NetNGlyc-1.0 | https://services.healthtech.dtu.dk/services/NetNGlyc-1.0/ (accessed on 11 September 2024) |
| phosphorylation sites | NetPhos-3.1 | https://services.healthtech.dtu.dk/services/NetPhos-3.1/ (accessed on 11 September 2024) |
| Putative signal peptide predictions | SignalP-6.0 Server) | https://services.healthtech.dtu.dk/services/SignalP-6.0/ (accessed on 11 September 2024) |
| Putative transmembrane regions | TMHMM | https://services.healthtech.dtu.dk/services/TMHMM-2.0/ (accessed on 11 September 2024) |
| protein secondary structures | SOPMA | https://npsa.lyon.inserm.fr/ (accessed on 11 September 2024) |
| protein tertiary structures | Swiss-Model | https://swissmodel.expasy.org/ (accessed on 14 September 2024) |
| Amino Acids | Number | Percentage | Amino Acids | Number | Percentage |
|---|---|---|---|---|---|
| Ala (A) | 29 | 8.7 | Leu (L) | 35 | 10.5 |
| Arg (R) | 20 | 6.0 | Lys (K) | 19 | 5.7 |
| Asn (N) | 14 | 4.2 | Met (M) | 11 | 3.3 |
| Asp (D) | 14 | 4.2 | Phe (F) | 23 | 6.9 |
| Cys (C) | 5 | 1.5 | Pro (P) | 14 | 4.2 |
| Gln (Q) | 5 | 1.5 | Ser (S) | 20 | 6.0 |
| Glu (E) | 16 | 4.8 | Thr (T) | 14 | 4.2 |
| Gly (G) | 18 | 5.4 | Trp (W) | 10 | 3.0 |
| His (H) | 17 | 5.1 | Tyr (Y) | 13 | 3.9 |
| Ile (I) | 10 | 3.0 | Val (V) | 26 | 7.8 |
| Subcellular Localization | Score | Probability |
|---|---|---|
| Cytoplasmic | 21.5 | 48.31 |
| Cytoplasmic/nuclear | 12.5 | 28.09 |
| Plasma membrane | 3 | 6.74 |
| Nuclear | 2.5 | 5.62 |
| Peroxisome | 2 | 4.49 |
| Mitochondrial | 1 | 2.25 |
| Endoplasmic reticulum | 1 | 2.25 |
| Golgi apparatus | 1 | 2.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Zhang, L.; Gao, X.; Sun, Y.; Zhao, C.; Gao, X.; Wu, C. Molecular Cloning of the scd1 Gene and Its Expression in Response to Feeding Artificial Diets to Mandarin Fish (Siniperca chuatsi). Genes 2024, 15, 1211. https://doi.org/10.3390/genes15091211
Wang J, Zhang L, Gao X, Sun Y, Zhao C, Gao X, Wu C. Molecular Cloning of the scd1 Gene and Its Expression in Response to Feeding Artificial Diets to Mandarin Fish (Siniperca chuatsi). Genes. 2024; 15(9):1211. https://doi.org/10.3390/genes15091211
Chicago/Turabian StyleWang, Jiangjiang, Lihan Zhang, Xiaowei Gao, Yanfeng Sun, Chunlong Zhao, Xiaotian Gao, and Chengbin Wu. 2024. "Molecular Cloning of the scd1 Gene and Its Expression in Response to Feeding Artificial Diets to Mandarin Fish (Siniperca chuatsi)" Genes 15, no. 9: 1211. https://doi.org/10.3390/genes15091211






