Concentrations and Sources of Atmospheric PM, Polycyclic Aromatic Hydrocarbons and Nitropolycyclic Aromatic Hydrocarbons in Kanazawa, Japan
Abstract
:1. Introduction
2. Experimental
2.1. Sampling of PM2.5 and PM>2.5
2.2. Determination of the PAHs and NPAHs
2.3. Calculation of Source Contributions
2.4. Lidar Observation
2.5. Backward Trajectory and Weather Map
2.6. Health Risk Assessment
3. Results and Discussion
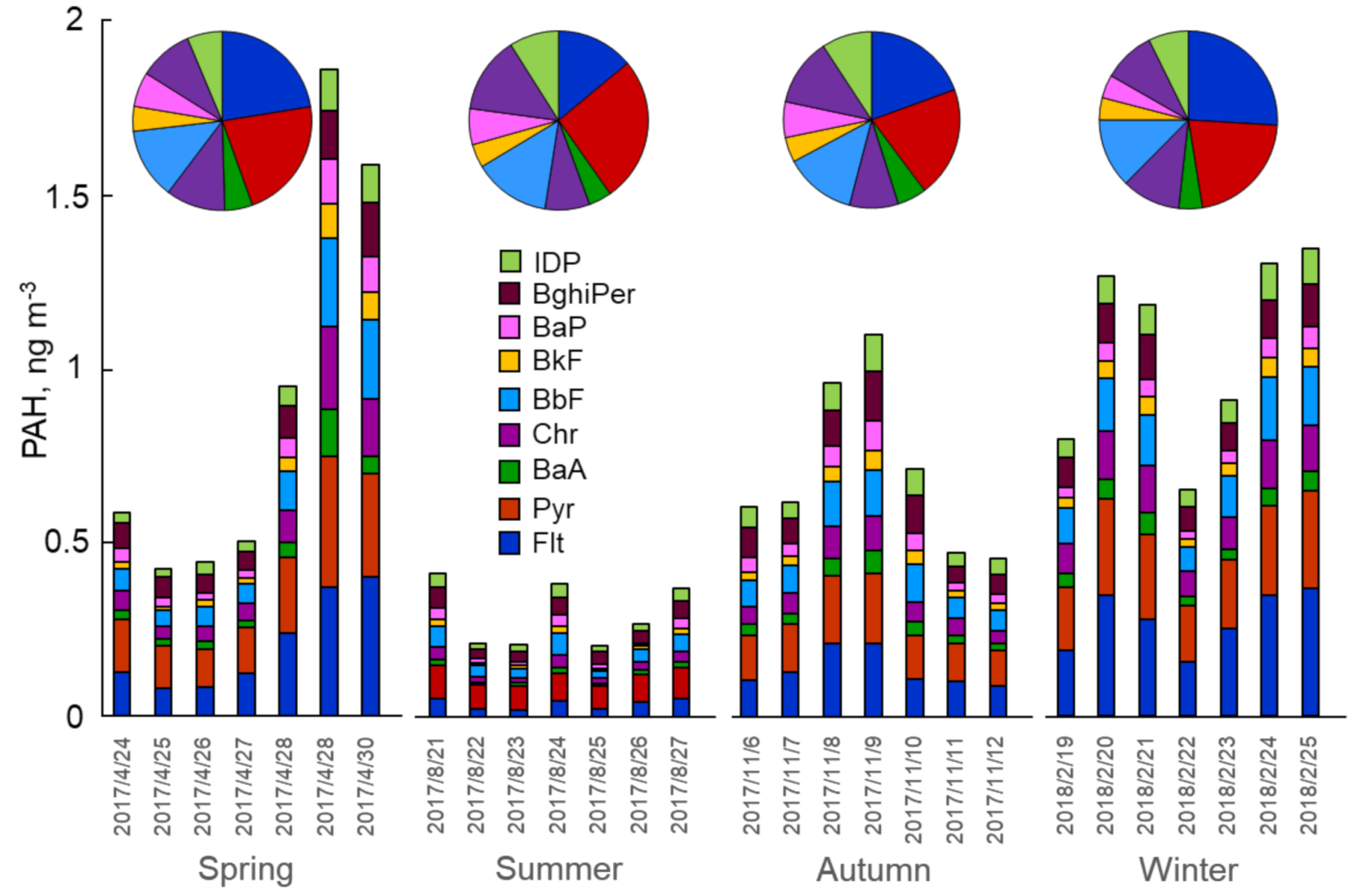
3.1. Seasonal Variations of PM, PAH and NPAH Concentrations
3.2. Source Analysis of PM2.5, PAHs and NPAHs
3.3. Health Risk Assessment
4. Conclusions
- The atmospheric concentrations of PAHs and NPAHs showed similar seasonal variations: they were highest in the winter and lowest in the summer.
- The percentage of Pc in the PM2.5 was much smaller (2.1%). However, the atmospheric concentration of Pc showed seasonal variation. The annual average contributions of coal heating facilities/industries and vehicles to Pc were 69% and 31%, respectively.
- The high concentrations of Pc and PAHs in the winter and during the Asian dust event in the spring were largely attributed to the long-range transport of emissions from coal heating facilities and industries in China. The NPAHs were mainly emitted from vehicles in Kanazawa.
- The Pc concentrations in the summer of 2017 and the winter of 2018 were respectively 1/13 and 1/16 of those detected in 1999. This significant improvement was mostly attributed to the decrease in the Pc quantities emitted from vehicles in Kanazawa. However, the decrease in the Pc amount transported from China was much lower.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Ambient Air Pollution: A Global Assessment of Exposure and Burden of Disease; WHO: Geneva, Switzerland, 2016; pp. 1–131. ISBN 9789241511353. [Google Scholar]
- WHO. Air Pollution and Child Health: Prescribing Clean Air; WHO/CED/PHE/18.01; WHO News Releases: Geneva, Switzerland, 2018. [Google Scholar]
- Anyenda, E.O.; Higashi, T.; Kambayashi, Y.; Nguyen, T.T.T.; Michigami, Y.; Fujimura, M.; Hara, A.J.; Tsujiguchi, H.; Kitaika, M.; Asakura, H.; et al. Associations of Cough Prevalence with Ambient Polycyclic Aromatic Hydrocarbons, Nitrogen and Sulphur Dioxide: A Longitudinal Study. Int. J. Environ. Res. Public Health 2016, 13, 800. [Google Scholar] [CrossRef] [Green Version]
- Hayakawa, K.; Tang, N.; Nagato, E.G.; Toriba, A.; Sakai, S.; Kano, F.; Goto, S.; Endo, O.; Arashidani, K.; Kakimoto, H. Long Term Trends in Atmospheric Concentrations of Polycyclic Aromatic Hydrocarbons and Nitropolycyclic Aromatic Hydrocarbons: A Study of Japanese Cities from 1997 to 2014. Environ. Pollut. 2018, 233, 474–482. [Google Scholar] [CrossRef]
- Hayakawa, K.; Nagao, S.; Inomata, Y.; Inoue, M.; Matsuki, A. Trans-Boundary Pollution in North-East Asia; Nova Science Publishers: New York, NY, USA, 2018; ISBN 978-1-53613-742-2. [Google Scholar]
- Hayakawa, K.; Suzuki, N. Special Issue “Recent Advances in Polycyclic Aromatic Hydrocarbons Research: Occurrence, Fate, Analysis and Risk Assessment”. Available online: https://www.mdpi.com/journal/ijerph/special_issues/PAHs (accessed on 31 December 2019).
- Tang, N.; Hakamata, M.; Sato, K.; Okada, Y.; Yang, X.-Y.; Tatematsu, M.; Toriba, A.; Kameda, T.; Hayakawa, K. Atmospheric behaviors of polycyclic aromatic hydrocarbons at a Japanese remote background site, Noto peninsula, from 2004. Atmos. Environ. 2015, 120, 144–151. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Zhang, L.; Yang, L.; Zhou, Q.; Zhang, X.; Xing, W.; Hayakawa, K.; Toriba, A.; Tang, N. Impact of COVID-19 Outbreak on the Long-range Transport of Common Air Pollutants in KUWAMS. Biol. Pharm. Bull. 2021, 20, 2035–2046. [Google Scholar]
- Yang, L.; Zhang, L.; Zhang, H.; Zhou, Q.; Zhang, X.; Xing, W.; Takami, A.; Sato, K.; Shimizu, A.; Yoshino, A.; et al. Comparative analysis of PM2.5-bound polycyclic aromatic hydrocarbons (PAHs), nitro-PAHs (NPAHs) and water-soluble inorganic ions (WSIIs) at two background sites in Japan. Int. J. Environ. Res. Public Health 2020, 17, 8224. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, K.; Tang, N.; Toriba, A.; Nagato, E.G. Calculating sources of combustion-derived particulates using 1-nitropyrene and pyrene as markers. Environ. Pollut. 2020, 265, 114730. [Google Scholar] [CrossRef] [PubMed]
- Xing, W.; Zhang, L.; Yang, L.; Zhou, Q.; Zhang, X.; Toriba, A.; Hayakawa, K.; Tang, N. Characteristics of PM2.5-bound polycyclic aromatic hydrocarbons and nitro-polycyclic aromatic hydrocarbons at a roadside air pollution monitoring station in Kanazawa, Japan. Int. J. Environ. Res. Public Health 2020, 17, 805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wise, S.A.; Sander, L.C.; Schantz, M.M. Analytical methods for determination of polycyclic aromatic hydrocarbons (PAHs)—A historical perspective on the U.S. EPA priority pollutant PAHs. Polycycl. Aromat. Compd. 2015, 35, 187–247. [Google Scholar] [CrossRef]
- Hayakawa, K.; Kitamura, R.; Butoh, M.; Imaizumi, N.; Miyazaki, M. Determination of diamino- and aminopyrenes by high-performance liquid chromatography with chemiluminescence detection. Anal. Sci. 1991, 7, 573–577. [Google Scholar] [CrossRef] [Green Version]
- Hayakawa, K.; Murahashi, T.; Butoh, M.; Miyazaki, M. Determination of 1,3-, 1,6-, and 1,8-dinitropyrenes and 1-nitropyrene in urban air by high-performance liquid chromatography using chemiluminescence detection. Environ. Sci. Technol. 1995, 29, 928–932. [Google Scholar] [CrossRef]
- Tang, N.; Taga, R.; Hattori, T.; Toriba, A.; Kizu, R.; Hayakawa, K. Simultaneous determination of twenty-one mutagenic nitropolycyclic aromatic hydrocarbons by high-performance liquid chromatography with chemiluminescence detection. In Proceedings of the 13th International Symposium, Bioluminescence and Chemiluminescence Progress and Perspective, Yokihama, Japan, 2–6 August 2005; Tsuji, A., Maeda, M., Matsumoto, M., Kricka, L., Stanley, P.E., Eds.; World Science: London, UK, 2005; pp. 441–9812561183. [Google Scholar]
- Shimizu, A.; Sugimoto, N.; Matsui, I.; Arao, K.; Uno, I.; Murayama, T.; Kagawa, N.; Aoki, K.; Uchiyama, A.; Yamazaki, A. Continuous observations of Asian dust and other aerosols by polarization lidar in China and Japan during ACE-Asia. J. Geophys. Res. 2004, 109, D19S17. [Google Scholar] [CrossRef]
- National Oceanic and Atmospheric Administration. Lagrangian Integrated Trajectory Model. Available online: https://ready.arl.noaa.gov/hypub-bin/trajtype.pl (accessed on 12 February 2020).
- Japan Meteorological Agency. Daily Weather Map. Available online: http://www.data.jma.go.jp/fcd/yoho/hibiten/index.html (accessed on 12 February 2020).
- USEPA. Provisional Guidance for Quantitative Risk Assessment of Polycyclic Aromatic Hydrocarbons EAP/600/R-93/089. 1993. Available online: https://www.epa.gov/risk/relative-potency-factors-carcinogenic-polycyclic-aromatic-hydrocarbons-pahs (accessed on 12 February 2021).
- Hayakawa, K.; Nakamura, A.; Terai, N.; Kizu, R.; Ando, K. Nitroarene concentrations and direct-acting mutagenicity of diesel exhaust particulates fractionated by silica-gel column chromatograph. Chem. Pharm. Bull. 1997, 45, 1820–1822. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Suzuki, G.; Zhang, L.; Zhou, Q.; Zhang, X.; Xing, W.; Shima, M.; Yoda, Y.; Nakatsubo, R.; Hiraki, T.; et al. The characteristics of polycyclic aromatic hydrocarbons in different emission source areas in Shenyang, China. Int. J. Environ. Public Heath 2019, 16, 2817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Morisaki, H.; Wei, Y.; Li, Z.; Yang, L.; Zhou, Q.; Zhang, X.; Xing, W.; Hu, M.; Shima, M.; et al. PM2.5-bound polycyclic aromatic hydrocarbons and nitro-polycyclic aromatic hydrocarbons inside and outside a primary school classroom in Beijing: Concentration, composition, and inhalation cancer risk. Sci. Total Environ. 2020, 705. [Google Scholar] [CrossRef]
- Ansmann, A.; Bösenberg, J.; Chaikovsky, A.; Comerón, A.; Eckhardt, S.; Eixmann, R.; Freudenthaler, V.; Ginoux, P.; Komguem, L.; Linné, H.; et al. Long-range transport of Sahara dust to northern Europe: The 11–16 October 2001 outbreak observed with EARLINET. J. Geogr. Res. 2003, 108, D24. [Google Scholar] [CrossRef]
- Nguyen, H.; Riley, M.; Leys, J.; Salter, D. Dust storm event of February 2019 in central and east coast of Australia and evidence of long-range transport to New Zealand and Antarctica. Atmosphere 2019, 10, 653. [Google Scholar] [CrossRef] [Green Version]
- Hayakawa, K. Polycyclic Aromatic Hydrocarbons: Environmental Behavior and Toxicity in East Asia; Springer: Berlin/Heidelberg, Germany, 2018; ISBN 978-981-10-6774-7. [Google Scholar]
- Cetin, B.; Ozturk, F.; Keles, M.; Yurdakul, S. PAHs and PCBs in an eastern Mediterranean megacity, Istanbul: Their spatial and temporal distributions, air–soil exchange and toxicological effects. Environ. Pollut. 2017, 220, 1322–1332. [Google Scholar] [CrossRef] [PubMed]
- Franco, J.; de Resende, F.; de Almeida Furtado, L.; Brasil, F.; Eberlin, N.; Netto, P. Polycyclic aromatic hydrocarbons (PAHs) in street dust of Rio de Janeiro and Niterói, Brazil: Particle size distribution, sources and cancer risk assessment. Sci. Total Environ. 2017, 599–600, 305–313. [Google Scholar] [CrossRef]
- Hamid, N.; Syed, H.; Junaid, M.; Mahmood, A.; Li, J.; Zhang, G.; NaseemMalik, R. Elucidating the urban levels, sources and health risks of polycyclic aromatic hydrocarbons (PAHs) in Pakistan: Implications for changing energy demand. Sci. Total Environ. 2018, 619–620, 165–175. [Google Scholar] [CrossRef]
- Yang, J.; Xu, W.; Cheng, H. Seasonal variations and sources of airborne polycyclic aromatic hydrocarbons (PAHs) in Chengdu, China. Atmosphere 2018, 9, 63. [Google Scholar] [CrossRef] [Green Version]
- Byambaa, B.; Yang, L.; Matsuki, A.; Nagato, E.G.; Gankhuyang, K.; Chuluunpurev, B.; Banzragch, L.; Chonokhuu, S.; Tang, N.; Hayakawa, K. Sources and characteristics of polycyclic aromatic hydrocarbons in ambient total suspended particles in Ulaanbaatar city, Mongolia. Int. J. Environ. Res. Public Health 2019, 16, 442. [Google Scholar] [CrossRef] [Green Version]
- Hu, H.; Tian, M.; Zhang, L.; Yang, F.; Peng, C.; Chen, Y.; Shi, G.; Yao, X.; Jiang, C.; Wang, J. Sources and gas-particle partitioning of atmospheric parent, oxygenated, and nitrated polycyclic aromatic hydrocarbons in a humidcity in southwest China. Atmos. Environ. 2019, 206, 1–10. [Google Scholar] [CrossRef]
- Emine, A.; Fatma, E. Atmospheric polycyclic aromatic hydrocarbons (PAHs) at two sites, in Bursa, Turkey: Determination of concentrations, gas–particle partitioning, sources, and health risk. Arch. Environ. Contam. Toxicol. 2020, 78, 350–366. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, X.; Xing, W.; Zhou, Q.; Zhang, L.; Wu, Q.; Zhou, Z.; Chen, R.; Toriba, A.; Hayakawa, K.; et al. Yearly variation in characteristics and health risk of polycyclic aromatic hydrocarbons and nitro-PAHs in urban Shanghai from 2010–2018. J. Environ. Sci. 2021, 99, 72–79. [Google Scholar] [CrossRef] [PubMed]
- WHO. Review of Evidence on Health Aspects of Air Pollution—REVIHAAP Project: Final Technical Report, WHO Regional Office for Europe (2013). Available online: https://www.euro.who.int/en/health-topics/environment-and-health/air-quality/publications/2013/review-of-evidence-on-health-aspects-of-air-pollution-revihaap-project-final-technical-report (accessed on 12 February 2021).
- European Environment Agency. Annual Mean Bap Concentrations in 2018. 2020. Available online: https://www.eea.europa.eu/data-and-maps/figures/annual-mean-bap-concentrations-in-4 (accessed on 12 February 2021).




| Spring, 2017 | Summer, 2017 | Autumn, 2017 | Winter, 2018 | Annual a | |
|---|---|---|---|---|---|
| PM>2.5, μg m−3 | 9.5 ± 9.9 | 7.6 ± 1.7 | 7.7 ± 4.2 | 7.4 ± 2.7 | 8.0 ± 4.2 |
| PM2.5, μg m−3 | 21.7 ± 12.1 | 10.6 ± 4.3 | 12.3 ± 5.1 | 14.2 ± 2.6 | 14.7 ± 7.9 |
| PM2.5/TSP b, % | 69.6 | 58.2 | 61.5 | 65.7 | 64.6 |
| Spring, 2017 | Summer, 2017 | Autumn, 2017 | Winter, 2018 | Annual a | |
|---|---|---|---|---|---|
| ΣPAH b, ng m−3 | 0.86 ± 0.56 | 0.30 ± 0.09 | 0.66 ± 0.23 | 1.00 ± 0.26 | 0.71 ± 0.41 |
| ΣNPAH c, pg m−3 | 7.90 ± 3.02 | 3.32 ± 1.18 | 6.53 ± 2.47 | 9.56 ± 4.06 | 6.83± 3.68 |
| Spring, 2017 | Summer, 2017 | Autumn, 2017 | Winter, 2018 | Annual a | |
|---|---|---|---|---|---|
| Pc, μg m−3 | 0.36 ± 0.14 | 0.18 ± 0.10 | 0.26 ± 0.08 | 0.47 ± 0.08 | 0.33 ± 0.15 |
| Po, μg m−3 | 21.4 ± 12.1 | 10.2 ± 6.2 | 12.0 ± 5.0 | 13.6 ± 2.6 | 14.6 ± 8.1 |
| Pc/PM2.5, % | 1.7 | 1.7 | 2.1 | 3.3 | 2.1 |
| Pl, μg m−3 | 0.26 ± 0.14 | 0.10 ± 0.04 | 0.20 ± 0.05 | 0.31 ± 0.07 | 0.23 ± 0.11 |
| Ph, μg m−3 | 0.10 ± 0.03 | 0.07 ± 0.07 | 0.06 ± 0.03 | 0.16 ± 0.06 | 0.10 ± 0.06 |
| Ph/Pc, % | 27.8 | 41.2 | 23.1 | 34.0 | 31.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hayakawa, K.; Tang, N.; Xing, W.; Oanh, P.K.; Hara, A.; Nakamura, H. Concentrations and Sources of Atmospheric PM, Polycyclic Aromatic Hydrocarbons and Nitropolycyclic Aromatic Hydrocarbons in Kanazawa, Japan. Atmosphere 2021, 12, 256. https://doi.org/10.3390/atmos12020256
Hayakawa K, Tang N, Xing W, Oanh PK, Hara A, Nakamura H. Concentrations and Sources of Atmospheric PM, Polycyclic Aromatic Hydrocarbons and Nitropolycyclic Aromatic Hydrocarbons in Kanazawa, Japan. Atmosphere. 2021; 12(2):256. https://doi.org/10.3390/atmos12020256
Chicago/Turabian StyleHayakawa, Kazuichi, Ning Tang, Wanli Xing, Pham Kim Oanh, Akinori Hara, and Hiroyuki Nakamura. 2021. "Concentrations and Sources of Atmospheric PM, Polycyclic Aromatic Hydrocarbons and Nitropolycyclic Aromatic Hydrocarbons in Kanazawa, Japan" Atmosphere 12, no. 2: 256. https://doi.org/10.3390/atmos12020256






