Experimental Research on Regulated and Unregulated Emissions from E20-Fuelled Vehicles and Hybrid Electric Vehicles
Abstract
1. Introduction
2. Experimental Setup
2.1. Fuel Properties
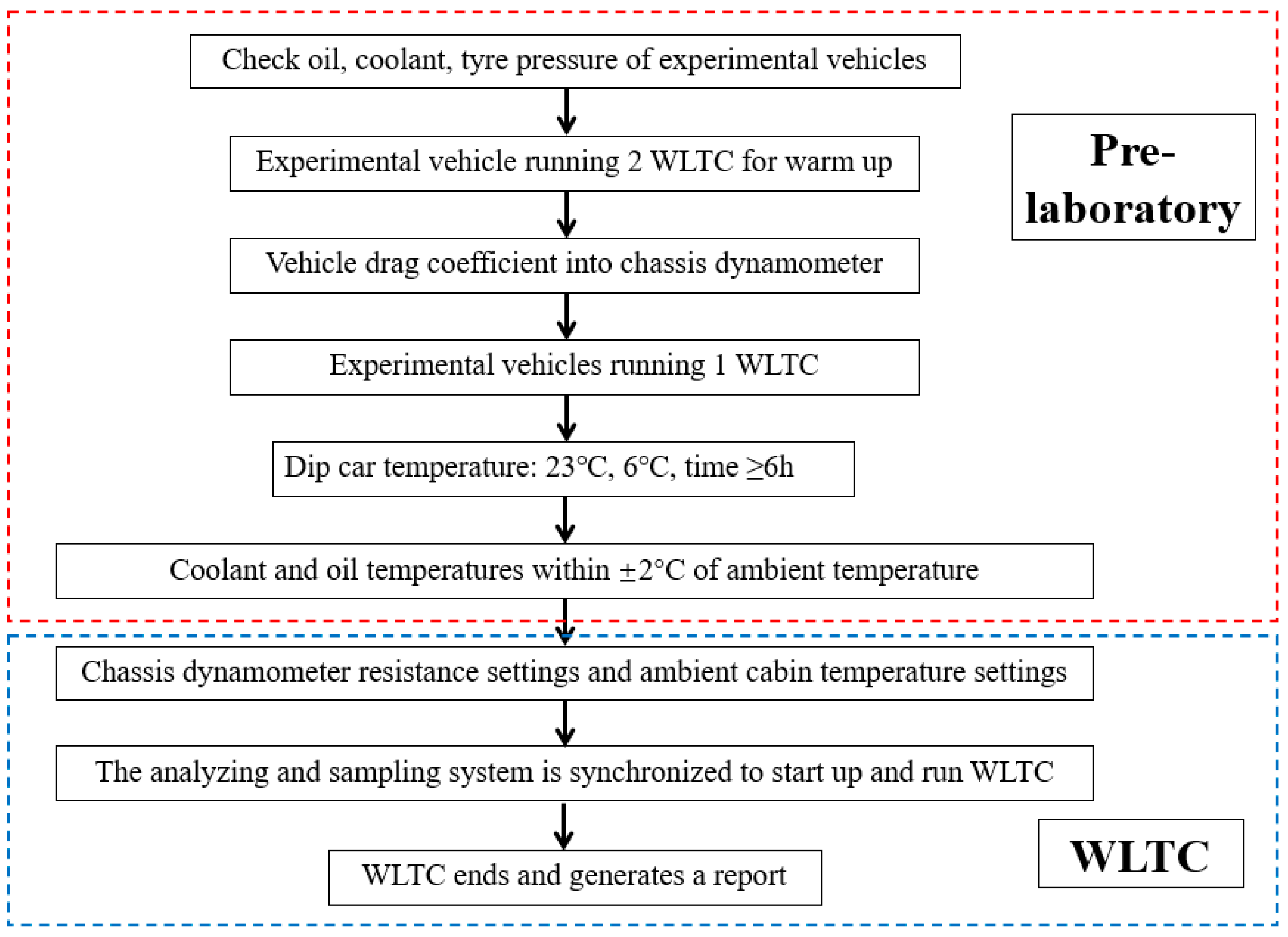
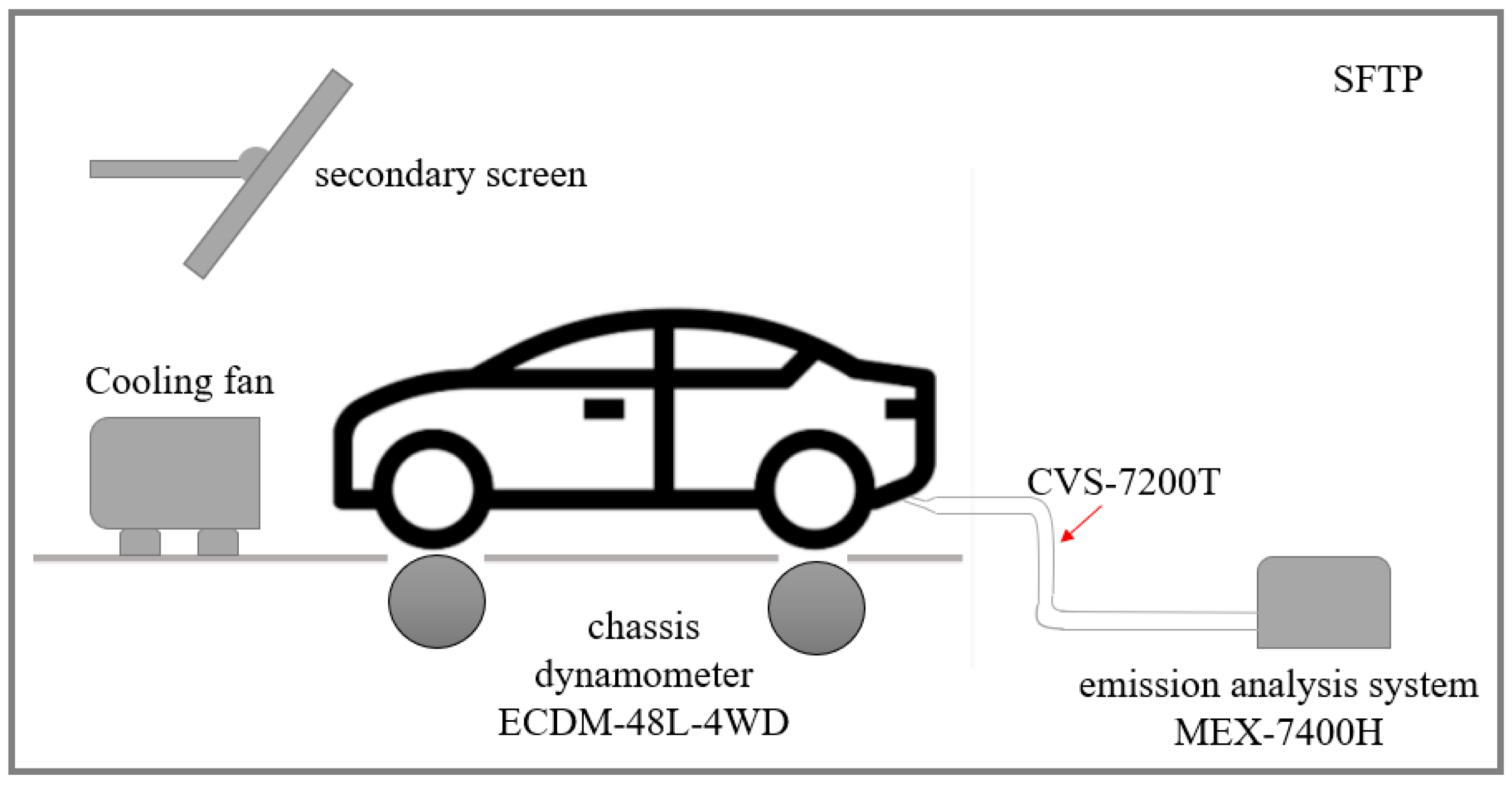
2.2. Chassis Dynamometer Experiments
- (1)
- Empty the fuel tank of the experimental vehicle and fill the tank with experimental fuel.
- (2)
- Place the vehicle on the chassis dynamometer, run a WLTC test cycle, and then empty the fuel tank.
- (3)
- Fill the fuel tank with experimental fuel again and repeat step (2).
- (4)
- Fill up the experimental vehicle with experimental fuel and run the WLTC test cycle.
- (5)
- Switch to a different experimental fuel and repeat steps (1) to (4).
2.3. Sampling and Analytical Instrumentation
2.4. Data Processing Methods
2.5. Ambient Temperature Error Analysis
3. Results and Discussion
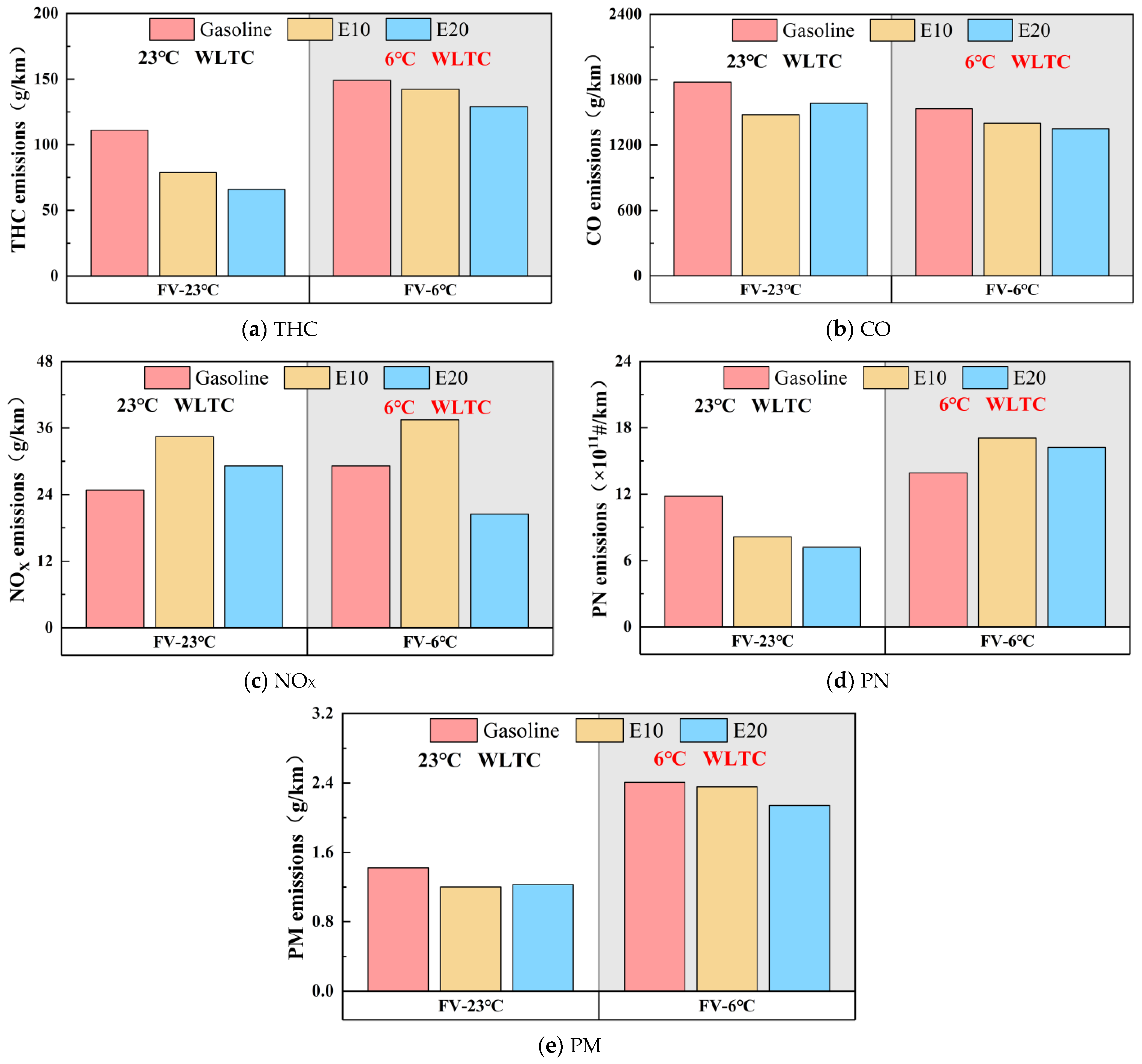
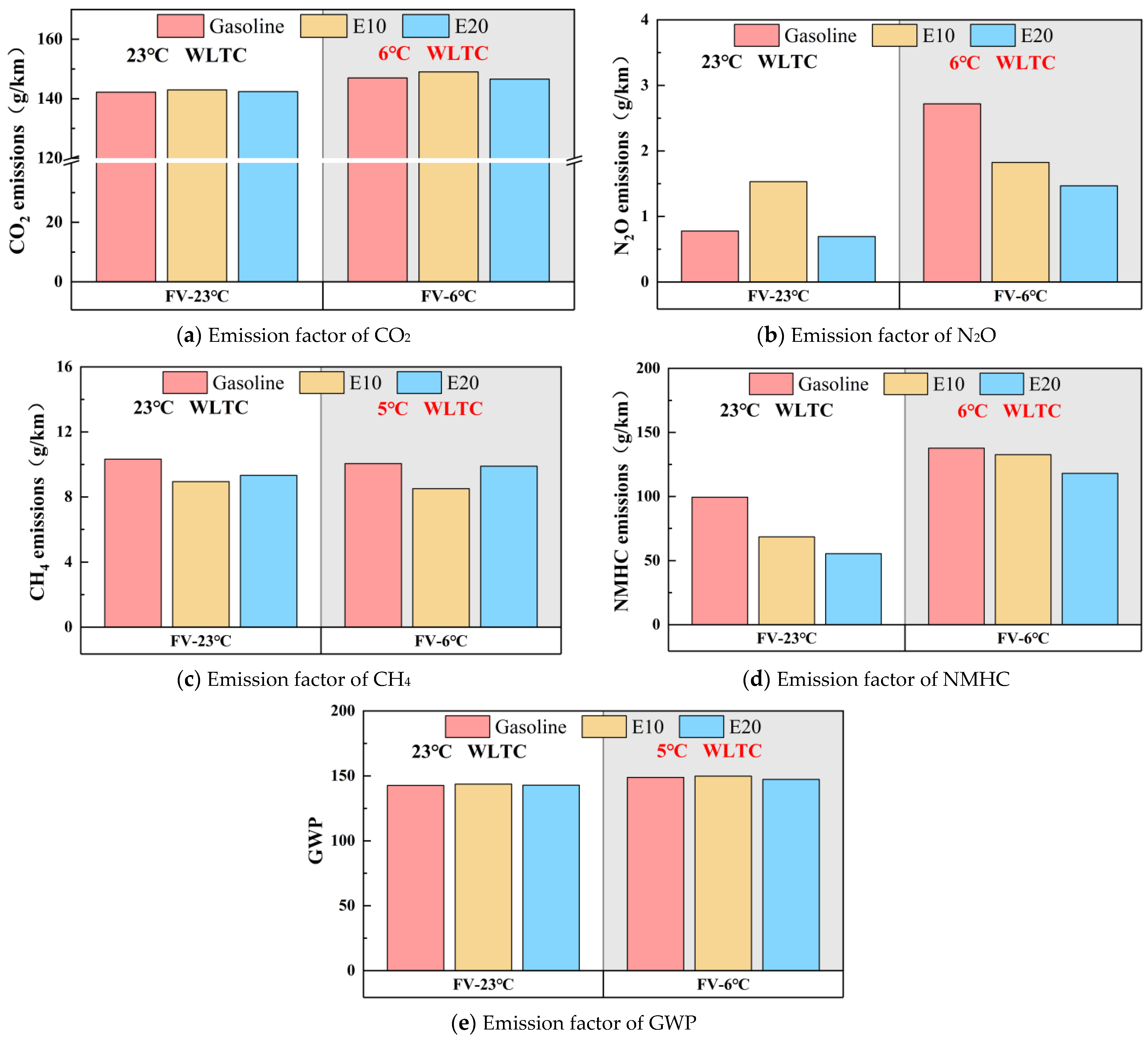
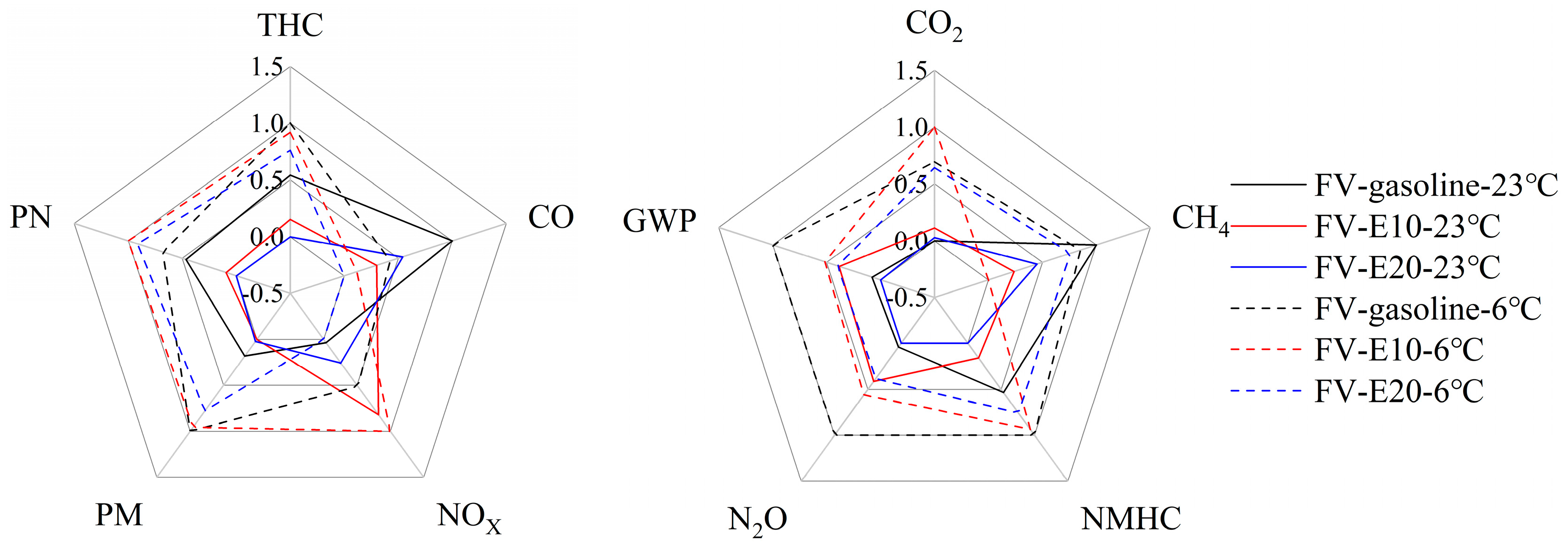
3.1. Regulated and Unregulated Emissions
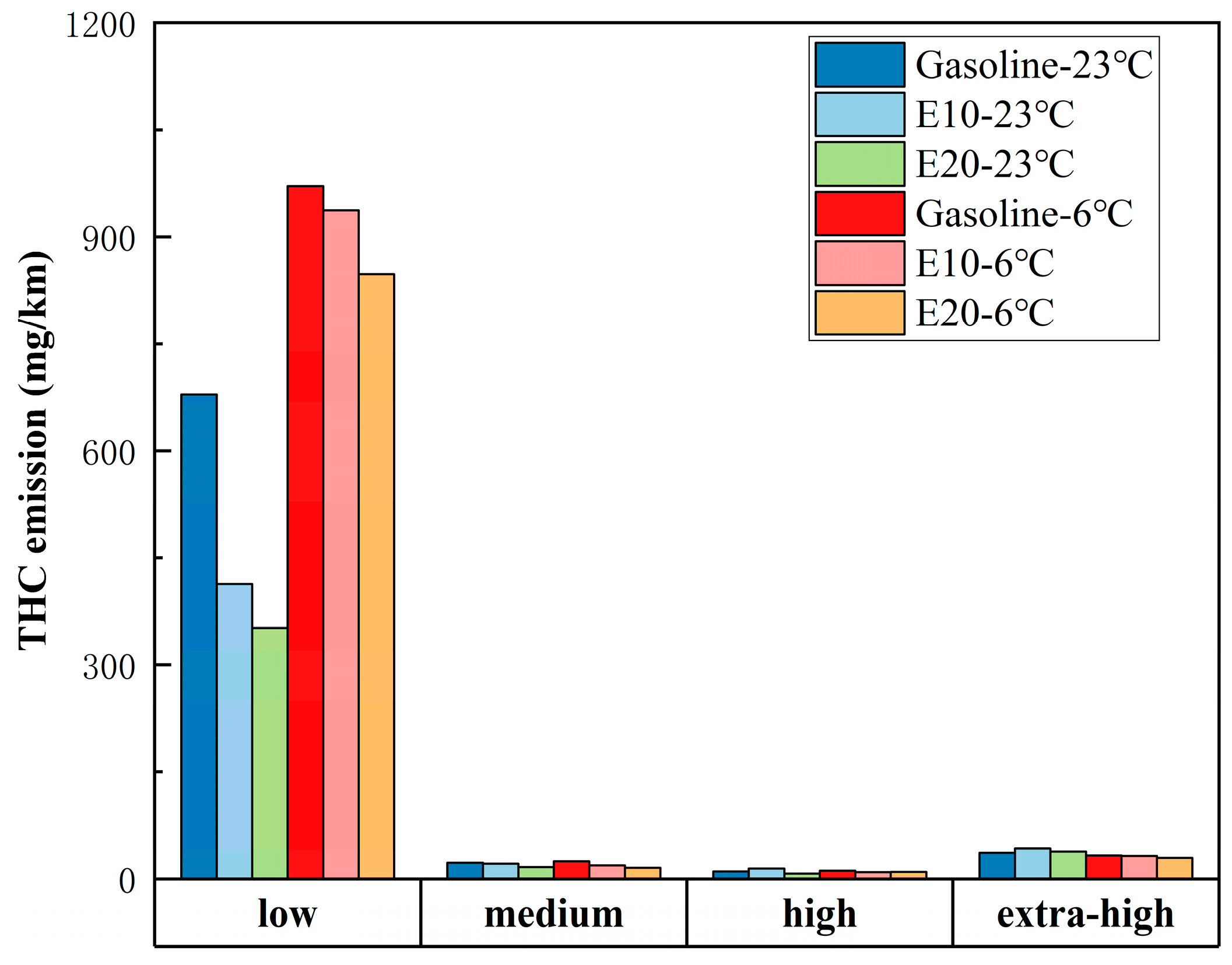
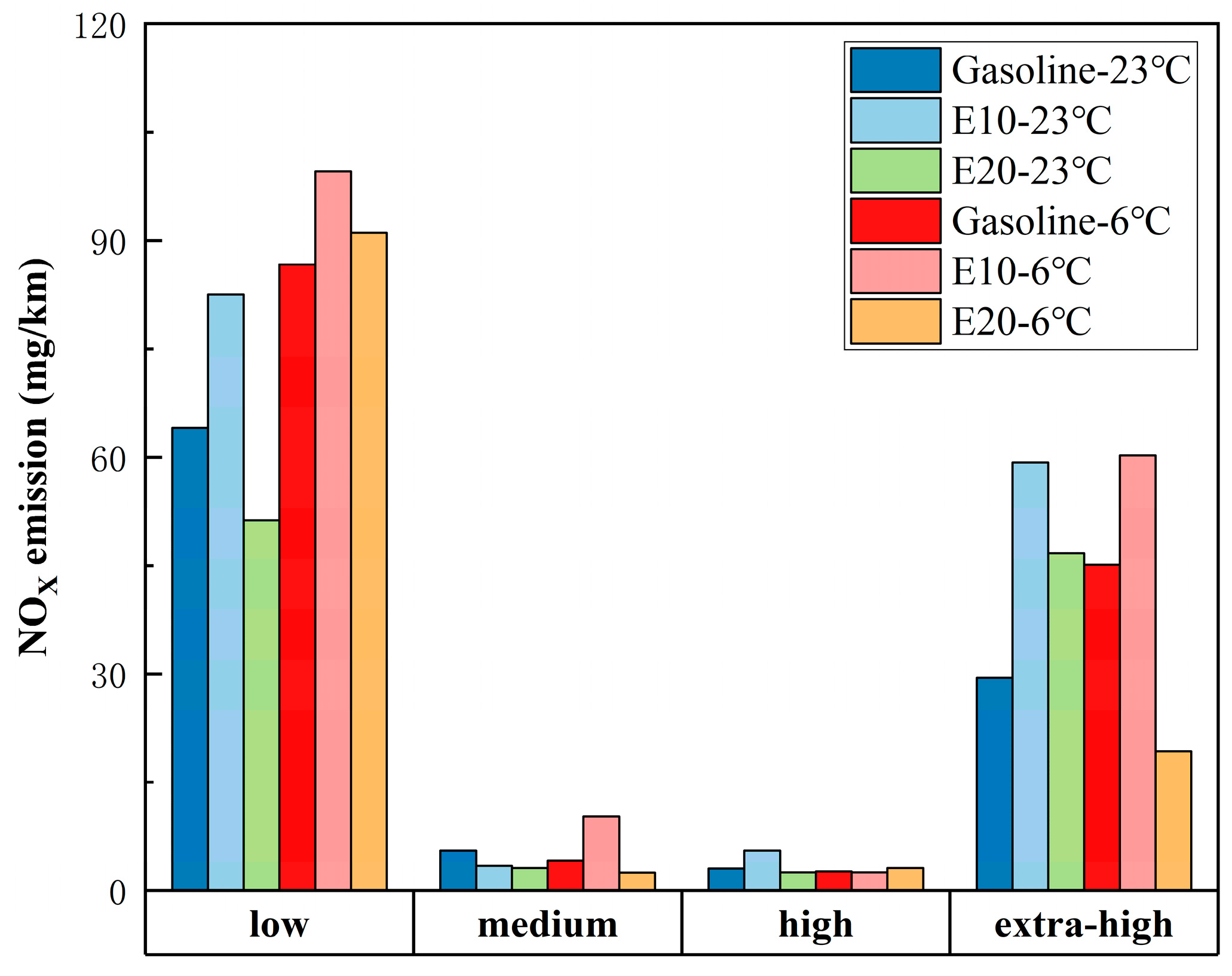
3.2. WLTC Emissions in Different Speed Bands
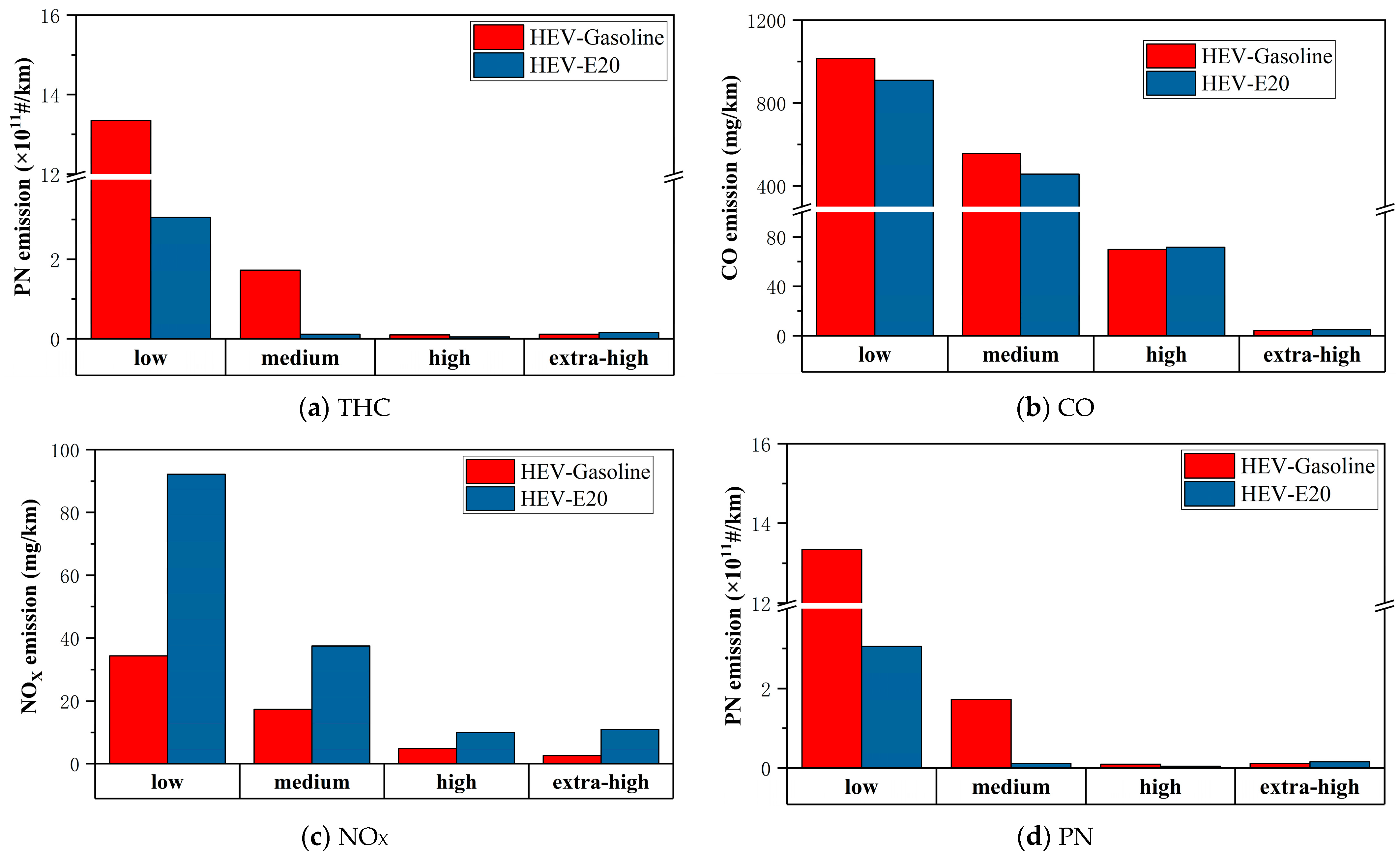
3.3. Emissions from the Combustion of E20 Ethanol Gasoline in Hybrid Vehicles
4. Conclusions
- The use of E10 and E20 in FVs effectively reduces the conventional emissions of the vehicles: at 23 °C, E10 and E20 reduce the conventional emissions (THC, CO, NMHC, PM, PN) by 15.40–31.11% and 11.00–40.51%, but increase NOX emissions by 63.42% and 17.86%; at 6 °C, E10 and E20 reduce the conventional emissions (THC, CO, PM) by 2.15–8.61% and 11.02–13.34%, but increase PN emissions by 44.75% and 37.56%. At 6 °C, E10 and E20 reduced the conventional emissions (THC, CO, PM) by 2.15–8.61% and 11.02–13.34%, but increased the PN emissions by 44.75% and 37.56%. Overall, the emission reduction effect of E20 is better than that of E10, but the emission reduction effect of ethanol gasoline for conventional emissions is reduced at low temperatures.
- Gasoline vehicles burning E10 and E20 do not have a significant trend in reducing the non-conventional emissions (CH4, N2O, CO2) of the vehicles. At 23 °C, E10 and E20 reduce CH4 emissions by 3.44% and 9.47%. E10 and E20 do not have the same trend in N2O emissions, in which E10 increases N2O emissions by 96.87%, and E20 reduces them by 10.70%. The CO2 emissions were close, and the CO2 emissions of E10 and E20 were slightly higher than that of gasoline; at 6 °C, E10, and E20 made the CH4 emission decrease by 15.30% and 1.53%, the N2O emission decrease by 32.87% and 46.00%. The CO2 emissions were close, and the CO2 emissions of E20 were slightly lower than that of gasoline, and the emission reduction of E20 was overall better than that of E10. The overall emission reduction effect of E20 is better than that of E10.
- A lower ambient temperature increases vehicle emissions. Except for CO, vehicle emissions at 6 °C increase significantly compared to 23 °C. Compared to gasoline, E10 and E20 are more susceptible to lower ambient temperatures making the emissions increase. As the ambient temperature decreases, ethanol gasoline’s advantage for vehicle emissions reduction is reduced; analyzed from the speed band, lower ambient temperatures increase the emissions of the vehicle in the low-speed band, but it will lower the vehicle emissions in the ultra-high-speed range.
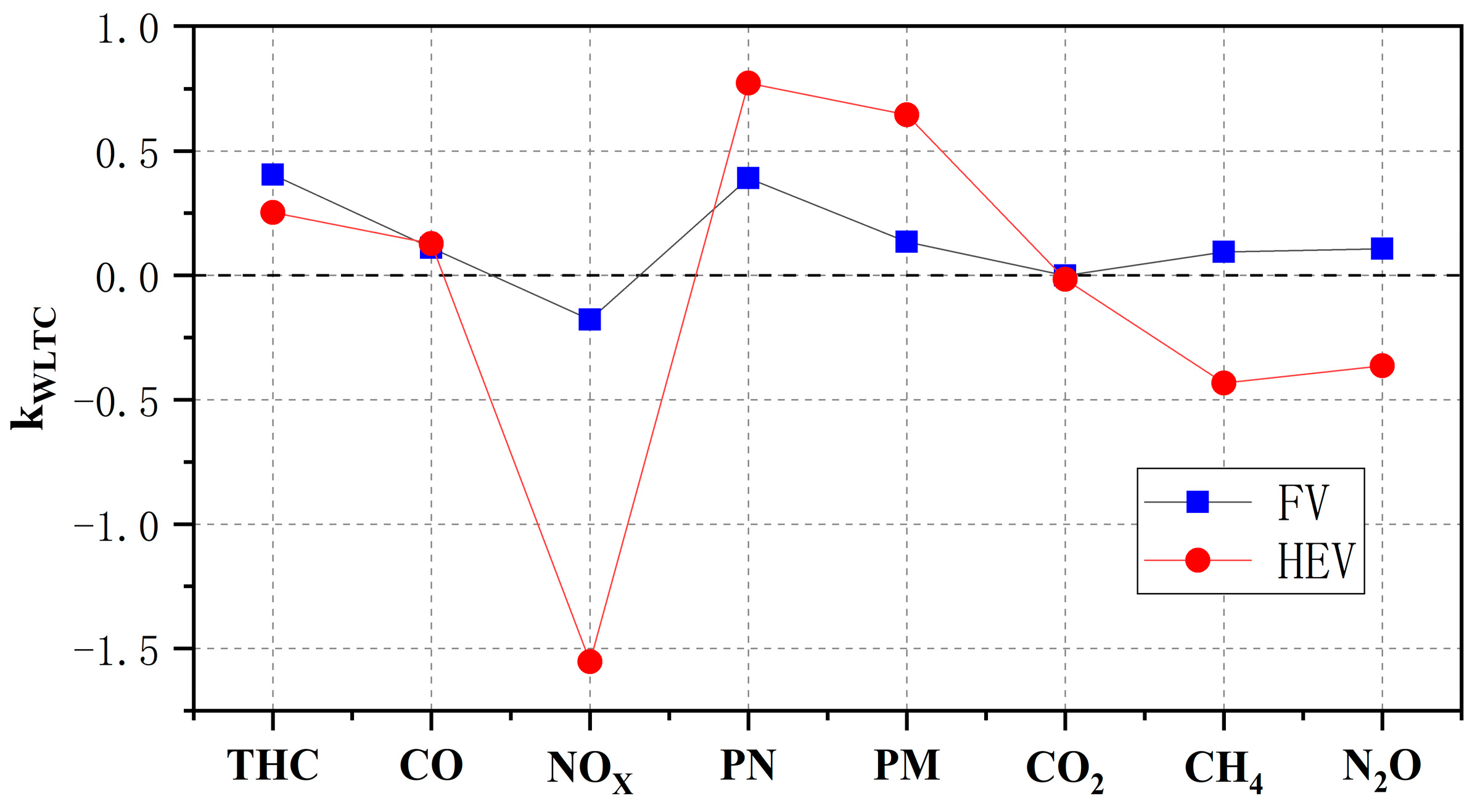
- The emissions of THC, CO, PN, and PM from hybrid vehicles burning E20 are reduced by 25.28%, 12.72%, 77.34%, and 64.59% compared with those of gasoline, but the emissions of NOX, CH4, and N2O appear to increase, and hybrid vehicles burning E20 can further reduce the emissions efficiently; the comparison of the emission reduction effects of hybrid vehicles burning E20 and gasoline vehicles burning E20 shows that the emission reduction effects of other emissions are not as good as those of gasoline vehicles. A comparison of the emission reduction effect of hybrid vehicles using E20 and gasoline vehicles using E20 shows that the reduction effect of particulate matter of hybrid vehicles is better, and the reduction effect of other emissions is not as good as that of gasoline vehicles.
- In summary, compared with gasoline, vehicle emissions can be effectively reduced by burning E10 and E20. At room temperature, the promotion of E10 and E20 ethanol gasoline can effectively reduce vehicle emissions. However, in low-temperature areas, the emission reduction effect of ethanol gasoline decreases, and the promotion of ethanol gasoline should be carefully considered.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ma, T.; Li, C.; Luo, J.; Frederickson, C.; Tang, T.; Durbin, T.D.; Johnson, K.C.; Karavalakis, G. In-use NOX and black carbon emissions from heavy-duty freight diesel vehicles and near-zero emissions natural gas vehicles in California’s San Joaquin Air Basin. Sci. Total Environ. 2024, 907, 168188. [Google Scholar] [CrossRef]
- Luo, Z.; Wang, Y.; Lv, Z.; He, T.; Zhao, J.; Wang, Y.; Gao, F.; Zhang, Z.; Liu, H. Impacts of vehicle emission on air quality and human health in China. Sci. Total Environ. 2022, 813, 152655. [Google Scholar] [CrossRef] [PubMed]
- Jhang, S.; Lin, Y.; Chen, K.; Lin, S.; Batterman, S. Evaluation of fuel consumption, pollutant emissions and well-to-wheel GHGs assessment from a vehicle operation fueled with bioethanol, gasoline and hydrogen. Energy 2020, 209, 118436. [Google Scholar] [CrossRef]
- Hoque, N.; Biswas, W.; Mazhar, I.; Howard, I. Environmental Life Cycle Assessment of Alternative Fuels for Western Australia’s Transport Sector. Atmosphere 2019, 10, 398. [Google Scholar] [CrossRef]
- Storch, M.; Hinrichsen, F.; Wensing, M.; Will, S.; Zigan, L. The effect of ethanol blending on mixture formation, combustion and soot emission studied in an optical DISI engine. Appl. Energy 2015, 156, 783–792. [Google Scholar] [CrossRef]
- Huang, J.; Yuan, Z.; Duan, Y.; Liu, D.; Fu, Q.; Liang, G.; Li, F.; Huang, X. Quantification of temperature dependence of vehicle evaporative volatile organic compound emissions from different fuel types in China. Sci. Total Environ. 2022, 813, 152661. [Google Scholar] [CrossRef] [PubMed]
- Sakthivel, P.; Subramanian, K.A.; Mathai, R. Experimental study on unregulated emission characteristics of a two-wheeler with ethanol-gasoline blends (E0 to E50). Fuel 2020, 262, 116504. [Google Scholar] [CrossRef]
- Zhang, M.; Ge, Y.; Wang, X.; Xu, H.; Tan, J.; Hao, L. Effects of ethanol and aromatic compositions on regulated and unregulated emissions of E10-fuelled China-6 compliant gasoline direct injection vehicles. Renew. Energy 2021, 176, 12. [Google Scholar] [CrossRef]
- Karavalakis, G.; Durbin, T.D.; Shrivastava, M.; Zheng, Z.; Villela, M.; Jung, H. Impacts of ethanol fuel level on emissions of regulated and unregulated pollutants from a fleet of gasoline light-duty vehicles. Fuel 2012, 93, 549–558. [Google Scholar] [CrossRef]
- Lv, Z.; Yang, L.; Wu, L.; Peng, J.; Zhang, Q.; Sun, M.; Mao, H.; Min, J. Comprehensive Analysis of the Pollutant Characteristics of Gasoline Vehicle Emissions under Different Engine, Fuel, and Test Cycles. Energies 2022, 15, 622. [Google Scholar] [CrossRef]
- Hilton, B.; Duddy, B. The effect of E20 ethanol fuel on vehicle emissions. Proc. Inst. Mech. Eng. Part D-J. Automob. Eng. 2009, 22, 1577–1586. [Google Scholar] [CrossRef]
- Zhu, R.; Hu, J.; He, L.; Zu, L.; Bao, X.; Lai, Y.; Su, S. Effects of ambient temperature on regulated gaseous and particulate emissions from gasoline-, E10-and M15-fueled vehicles. Front. Environ. Sci. Eng. 2021, 15, 14. [Google Scholar] [CrossRef]
- Suarez-Bertoa, R.; Astorga, C. Impact of cold temperature on Euro 6 passenger car emissions. Environ. Pollut. 2018, 234, 12. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, R.; Weilenmann, M. Effect of low ambient temperature on fuel consumption and pollutant and CO2 emissions of hybrid electric vehicles in real-world conditions. Fuel 2012, 97, 6. [Google Scholar] [CrossRef]
- Clairotte, M.; Adam, T.W.; Zardini, A.A.; Manfredi, U.; Martini, G.; Krasenbrink, A.; Vicet, A.; Tournie, E.; Astorga, C. Effects of low temperature on the cold start gaseous emissions from light duty vehicles fuelled by ethanol-blended gasoline. Appl. Energy 2013, 102, 11. [Google Scholar] [CrossRef]
- Amanda, A.M.; Rodrigo AD, R.; José, R.S. Cold start and full cycle emissions from a flexible fuel vehicle operating with natural gas, ethanol and gasoline. J. Nat. Gas Sci. Eng. 2014, 17, 94–98. [Google Scholar]
- Challa, R.; Kamath, D.; Anctil, A. Well-to-wheel greenhouse gas emissions of electric versus combustion vehicles from 2018 to 2030 in the US. J. Environ. Manag. 2022, 308, 114592. [Google Scholar] [CrossRef] [PubMed]
- Derwent, R.G. Global Warming Potential (GWP) for Methane: Monte Carlo Analysis of the Uncertainties in Global Tropospheric Model Predictions. Atmosphere 2020, 11, 486. [Google Scholar] [CrossRef]
- Yu, A.; Wei, Y.; Chen, W.; Peng, N.; Peng, L. Life cycle environmental impacts and carbon emissions: A case study of electric and gasoline vehicles in China. Transp. Res. 2018, 65, 409–420. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, F.; Gong, X.; Wang, Z.; Liu, Y. Comparison of Climate Change Impact Between Power System of Electric Vehicles and Internal Combustion Engine Vehicles. In Proceedings of the Chinese Materials Conference, Yinchuan, China, 6–12 July 2017; Springer: Singapore, 2017. [Google Scholar]
- Suarez-Bertoa, R.; Astorga, C. Unregulated emissions from light-duty hybrid electric vehicles. Atmos. Environ. 2016, 136, 10. [Google Scholar] [CrossRef]
- Mandev, A.; Ploetz, P.; Sprei, F. The effect of plug-in hybrid electric vehicle charging on fuel consumption and tail-pipe emissions. Environ. Res. Commun. 2021, 3, 081001. [Google Scholar] [CrossRef]
- Selleri, T.; Melas, A.; Ferrarese, C.; Franzetti, J.; Giechaskiel, B.; Suarez-Bertoa, R. Emissions from a Modern Euro 6d Diesel Plug-In Hybrid. Atmosphere 2022, 13, 1175. [Google Scholar] [CrossRef]
- Ehrenberger, S.; Konrad, M.; Philipps, F. Pollutant emissions analysis of three plug-in hybrid electric vehicles using different modes of operation and driving conditions. Atmos. Environ. 2020, 234, 117612. [Google Scholar] [CrossRef]
- He, L.; You, Y.; Zheng, X.; Zhang, S.; Li, Z.; Zhang, Z.; Wu, Y.; Hao, J. The impacts from cold start and road grade on real-world emissions and fuel consumption of gasoline, diesel and hybrid-electric light-duty passenger vehicles. Sci. Total Environ. 2022, 851, 158045. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Surawski, N.C.; Organ, B.; Zhou, J.L.; Tang, O.H.H.; Chan, E.F.C. Fuel consumption and emissions performance under real driving: Comparison between hybrid and conventional vehicles. Sci. Total Environ. 2019, 659, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Prati, M.V.; Costagliola, M.A. Real driving emissions of Euro 6 electric/gasoline hybrid and natural gas vehicles. Transp. Res. Part D Transp. Environ. 2022, 113, 103509. [Google Scholar] [CrossRef]
- Garcia, A.; Monsalve-Serrano, J.; Martinez-Boggio, S.; Roso, V.R.; Santos, N.D.S.A. Potential of bio-ethanol in different advanced combustion modes for hybrid passenger vehicles. Renew. Energy 2020, 150, 20. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, S.; Lei, N.; Fan, Q.; Wang, Z. Leveraging the benefits of ethanol-fueled advanced combustion and supervisory control optimization in hybrid biofuel-electric vehicles. Appl. Energy 2022, 326, 15. [Google Scholar] [CrossRef]
- Stettler, M.E.J.; Woo, M.; Ainalis, D.; Achurra-Gonzalez, P.; Speirs, J.; Cooper, J.; Lim, D.; Brandon, N.; Hawkes, A. Review of Well-to-Wheel lifecycle emissions of liquefied natural gas heavy goods vehicles. Appl. Energy 2023, 333, 15. [Google Scholar] [CrossRef]
- Zhang, M.; Ge, Y.; Li, J.; Wang, X.; Tan, J.; Hao, L.; Xu, H.; Hao, C.; Wang, J.; Qian, L. Effects of ethanol and aromatic contents of fuel on the non-regulated exhaust emissions and their ozone forming potential of E10-fueled China-6 compliant vehicles. Atmos. Environ. 2021, 264, 118688. [Google Scholar] [CrossRef]
- Dardiotis, C.; Fontaras, G.; Marotta, A.; Martini, G.; Manfredi, U. Emissions of modern light duty ethanol flex-fuel vehicles over different operating and environmental conditions. Fuel 2015, 140, 531–540. [Google Scholar] [CrossRef]
- Myung, C.; Choi, K.; Cho, J.; Kim, K.; Baek, S.; Lim, Y.; Park, S. Evaluation of regulated, particulate, and BTEX emissions inventories from a gasoline direct injection passenger car with various ethanol blended fuels under urban and rural driving cycles in Korea. Fuel 2020, 262, 116406. [Google Scholar] [CrossRef]
- Song, C.; Zhou, Y.; Huang, R.; Wang, Y.; Huang, Q.; Lue, G.; Liu, K. Influence of ethanol–diesel blended fuels on diesel exhaust emissions and mutagenic and genotoxic activities of particulate extracts. J. Hazard. Mater. 2007, 149, 355–363. [Google Scholar] [CrossRef]
- Hsieh, W.D.; Chen, R.H.; Wu, T.L.; Lin, T.H. Engine performance and pollutant emission of an SI engine using ethanol-gasoline blended fuels. Atmos. Environ. 2002, 36, 403–410. [Google Scholar] [CrossRef]
- Lin, Y.; Jhang, S.; Lin, S.; Chen, K. Comparative effect of fuel ethanol content on regulated and unregulated emissions from old model vehicles: An assessment and policy implications. Atmos. Pollut. Res. 2021, 12, 66–75. [Google Scholar] [CrossRef]
- Zhu, R.; Hu, J.; Bao, X.; He, L.; Lai, Y.; Zu, L.; Li, Y.; Su, S. Investigation of tailpipe and evaporative emissions from China IV and Tier 2 passenger vehicles with different gasolines. Transp. Res. Part D Transp. Environ. 2017, 50, 305–315. [Google Scholar] [CrossRef]
- McCaffery, C.; Durbin, T.D.; Johnson, K.C.; Karavalakis, G. The effect of ethanol and iso-butanol blends on polycyclic aromatic hydrocarbon (PAH) emissions from PFI and GDI vehicles. Atmos. Pollut. Res. 2020, 11, 2056–2067. [Google Scholar] [CrossRef]
- Catapano, F.; Di Iorio, S.; Magno, A.; Sementa, P.; Vaglieco, B.M. Effect of ethanol blends, E10, E25 and E85 on sub-23 nm particle emissions and their volatile fraction at exhaust of a high-performance GDI engine over the WLTC. Fuel 2022, 327, 125184. [Google Scholar] [CrossRef]
- Mendoza-Villafuerte, P.; Suarez-Bertoa, R.; Giechaskiel, B.; Riccobono, F.; Bulgheroni, C.; Astorga, C.; Perujo, A. NOX, NH3, N2O and PN real driving emissions from a Euro Ⅵ heavy-duty vehicle. Impact of regulatory on-road test conditions on emissions. Sci. Total Environ. 2017, 609, 546–555. [Google Scholar] [CrossRef]
- Mejia-Centeno, I.; Fuentes, G.A. Nitrous oxide formation during light-off over a commercial pd-containing three-way catalytic converter: The effect of low-sulfur gasoline. Chem. Eng. Commun. 2009, 196, 1140–1151. [Google Scholar] [CrossRef]
- Brinklow, G.; Herreros, J.M.; Rezaei, S.Z.; Doustdar, O.; Tsolakis, A.; Kolpin, A.; Millington, P. Non-carbon greenhouse gas emissions for hybrid electric vehicles: Three-way catalyst nitrous oxide and ammonia trade-off. Int. J. Environ. Sci. Technol. 2023, 20, 12521–12532. [Google Scholar] [CrossRef]
- Suarez-Bertoa, R.; Zardini, A.A.; Keuken, H.; Astorga, C. Impact of ethanol containing gasoline blends on emissions from a flex-fuel vehicle tested over the Worldwide Harmonized Light duty Test Cycle (WLTC). Fuel 2015, 143, 10. [Google Scholar] [CrossRef]




| Units | Gasoline | E10 | E20 | |
|---|---|---|---|---|
| Ethanol content | % | 0 | 10.47 | 19.63 |
| Density (20 °C) | kg/m3 | 741.7 | 744 | 743.2 |
| Reid vapor pressure (RVP) | kPa | 57.5 | 58.1 | 58.1 |
| 10% volume evaporated temperature | °C | 53.2 | 57.3 | 56 |
| 50% volume evaporated temperature | °C | 93.4 | 104.4 | 75 |
| 90% volume evaporated temperature | °C | 168.9 | 157.4 | 152 |
| Final boiling point | °C | 196.1 | 192.6 | 179.5 |
| Olefins | %v/v | 10.2 | 7.1 | 9.5 |
| Aromatics | %v/v | 30.2 | 31 | 25.1 |
| Benzene | %v/v | 0.58 | 0.69 | 0.51 |
| Oxygen | %m/m | 2.36 | 4.31 | 6.9 |
| Sulfur | mg/kg | 3.4 | 5.5 | 4.3 |
| Parameter | Fuel Vehicle (FV) * | Hybrid Electric Vehicle (HEV) * |
|---|---|---|
| Vehicle type | Gasoline | Plug-in hybrid |
| Length × width × height (mm) | 4663 × 1815 × 1462 | 4765 × 1837 × 1495 |
| Preparation/total weight (kg) | 1270/1730 | 1500/1875 |
| Intake mode | Natural inhalation | Natural inhalation |
| Displacement (L) | 1.5 | 1.5 |
| Maximum power (kW) | 85 | 81 |
| Maximum torque (N·m) | 150 | 135 |
| Post-processing | TWC | TWC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, T.; Zhao, Y.; Lei, Y.; Chen, Z.; Guo, D.; Shi, F.; Wang, T. Experimental Research on Regulated and Unregulated Emissions from E20-Fuelled Vehicles and Hybrid Electric Vehicles. Atmosphere 2024, 15, 669. https://doi.org/10.3390/atmos15060669
Qiu T, Zhao Y, Lei Y, Chen Z, Guo D, Shi F, Wang T. Experimental Research on Regulated and Unregulated Emissions from E20-Fuelled Vehicles and Hybrid Electric Vehicles. Atmosphere. 2024; 15(6):669. https://doi.org/10.3390/atmos15060669
Chicago/Turabian StyleQiu, Tao, Yakun Zhao, Yan Lei, Zexun Chen, Dongdong Guo, Fulu Shi, and Tao Wang. 2024. "Experimental Research on Regulated and Unregulated Emissions from E20-Fuelled Vehicles and Hybrid Electric Vehicles" Atmosphere 15, no. 6: 669. https://doi.org/10.3390/atmos15060669
APA StyleQiu, T., Zhao, Y., Lei, Y., Chen, Z., Guo, D., Shi, F., & Wang, T. (2024). Experimental Research on Regulated and Unregulated Emissions from E20-Fuelled Vehicles and Hybrid Electric Vehicles. Atmosphere, 15(6), 669. https://doi.org/10.3390/atmos15060669








