Occurrence and Distribution of UV Filters in Beach Sediments of the Southern Baltic Sea Coast
Abstract
:1. Introduction
2. Materials and Methods
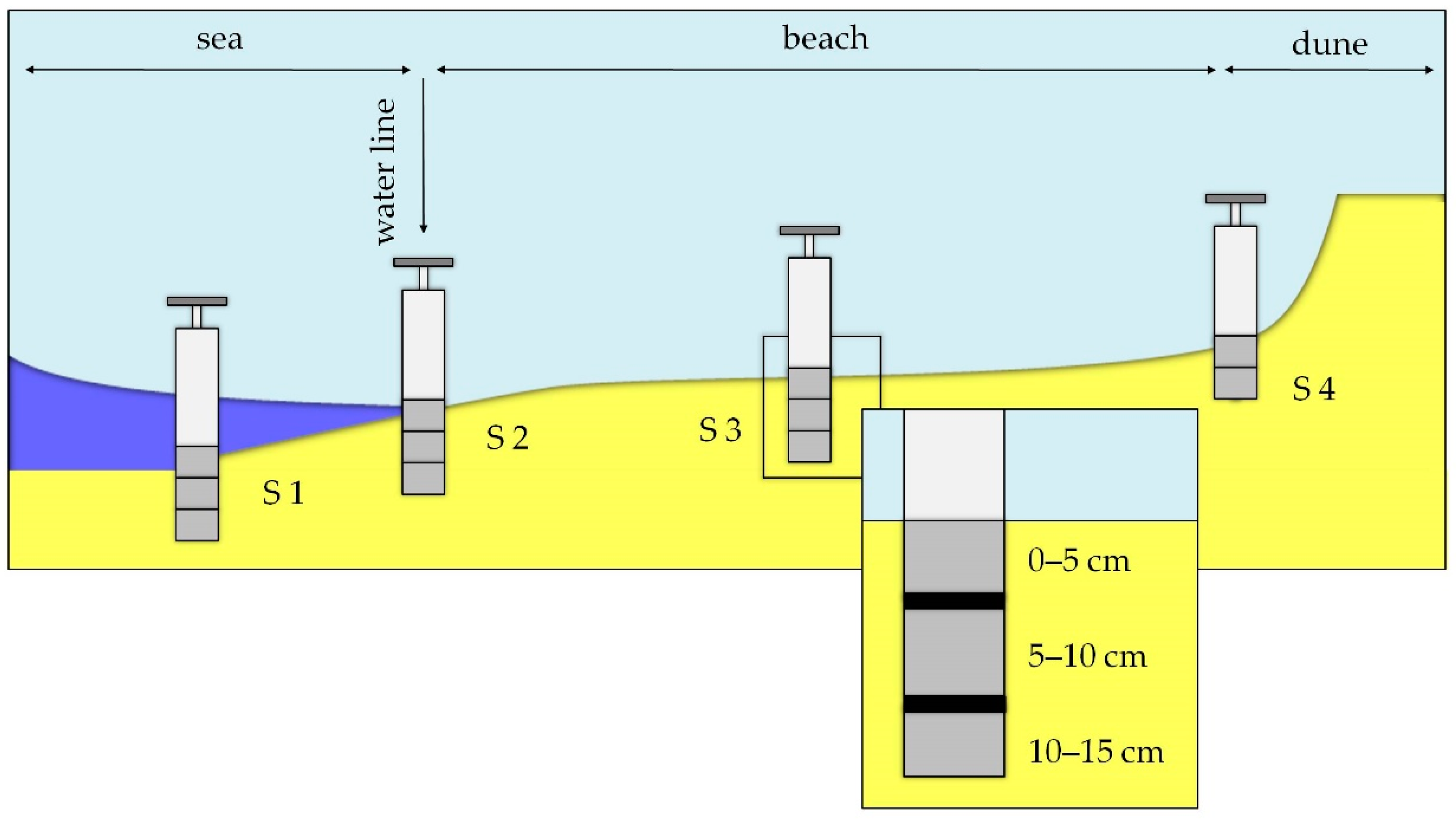
2.1. Beach Description
2.2. Sand Core Sampling
2.3. Sample Pretreatment and Analytical Methods
2.4. Quality Assurance and Quality Control
2.5. Statistical Procedures
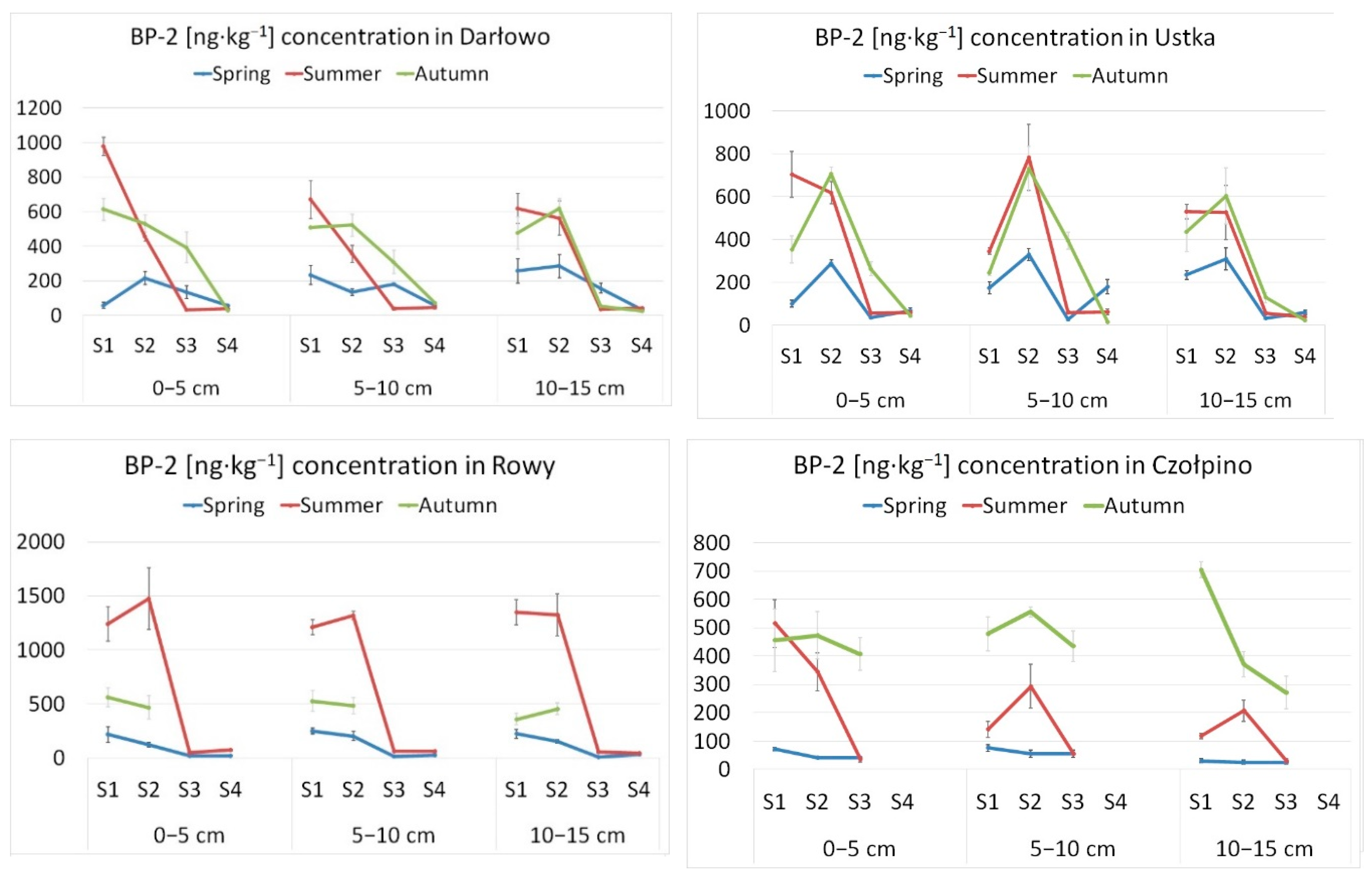
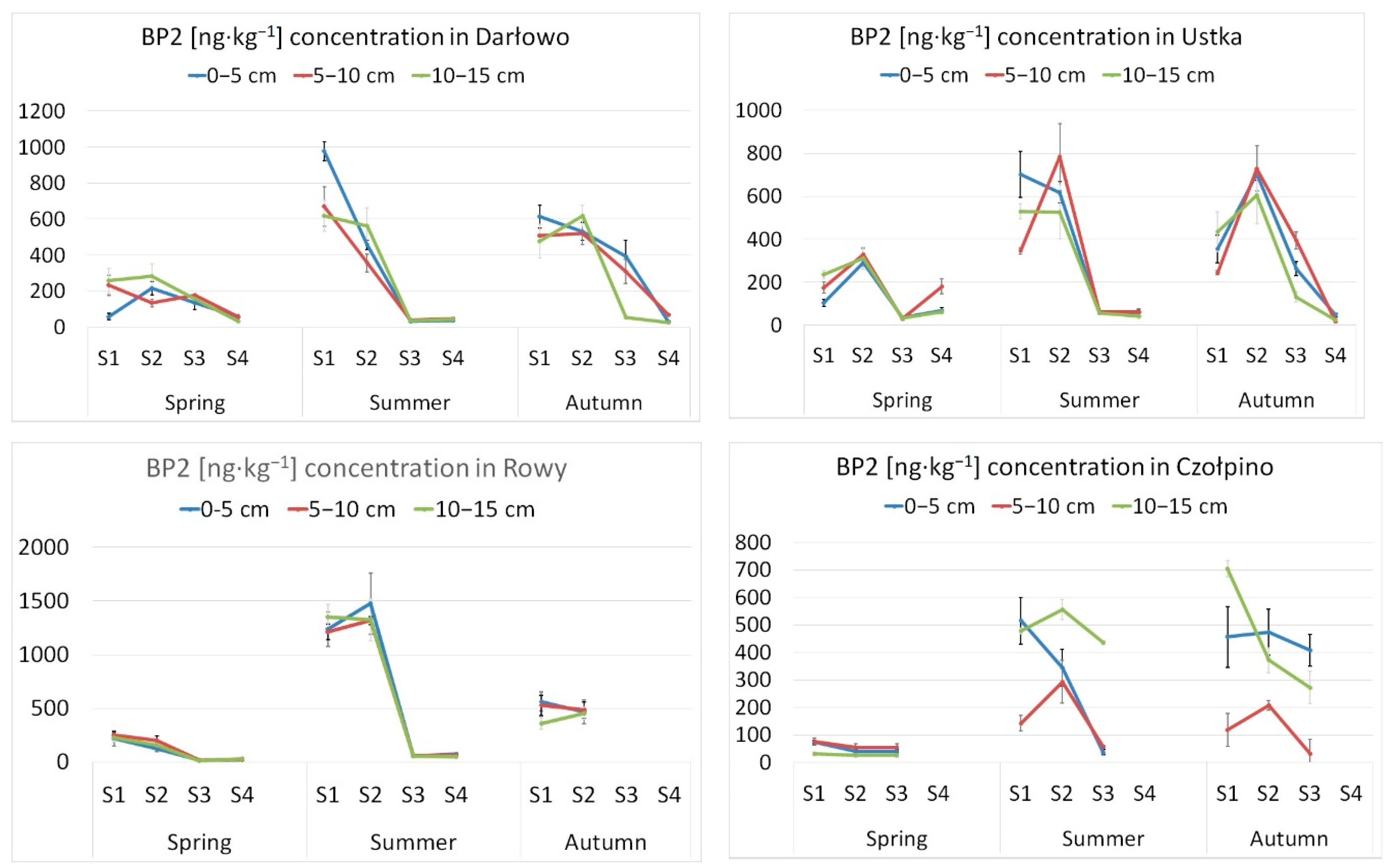
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Giokas, D.L.; Salvador, A.; Chisvert, A. UV filters: From sunscreens to human body and the environment. TRAC 2007, 26, 360–374. [Google Scholar] [CrossRef]
- Langford, K.H.; Reid, M.J.; Fjeld, E.; Øxnevad, S.; Thomas, K.V. Environmental occurrence and risk of organic UV filters and stabilizers in multiple matrices in Norway. Environ. Int. 2007, 80, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.; Margarida, S.; Miranda, M.; da Silva, J.C.E. The degradation products of UV filters in aqueous and chlorinated aqueous solutions. Water Res. 2012, 46, 3167–3176. [Google Scholar] [CrossRef] [PubMed]
- EC. European Commission Recommendation of 22 September 2006 on the efficacy of sunscreen products and the claims made relating thereto. Off. J. Eur. Union 2006, 265, 39–43. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2006:265:0039:0043:en:PDF (accessed on 29 May 2020).
- Tarras-Wahlberg, N.; Rosén, A.; Stenhagen, G.; Larkö, O.; Wennberg, A.M.; Wennerström, O. Changes in ultraviolet absorption of sunscreens after ultraviolet irradiation. J. Invest. Dermatol. 1999, 113, 547–553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- International Agency for Research on Cancer World Health Organization (IARC). Sunscreens. In IARC Handbook of Cancer Prevention; IARC: Lyon, France, 2001; Volume 5, pp. 23–24. [Google Scholar]
- Danovaro, R.; Bongiorni, L.; Corinaldesi, C.; Giovannelli, D.; Damiani, E.; Astolfi, P.; Greci, L.; Pusceddu, A. Sunscreens cause coral bleaching by promoting viral infections. Environ. Health Perspect. 2008, 116, 441–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poiger, T.; Buser, H.R.; Balmer, M.E.; Bergqvist, P.A.; Müller, M.D. Occurrence of UV filter compounds from sunscreens in surface waters: Regional mass balance in two Swiss lakes. Chemosphere 2004, 55, 951–963. [Google Scholar] [CrossRef] [PubMed]
- Sharifan, H.; Klein, D.; Morse, A.N. UV filters are an environmental threat in the Gulf of Mexico: A case study of Texas coastal zones. Oceanography 2016, 58, 327–335. [Google Scholar] [CrossRef] [Green Version]
- Norval, M.; Lucas, R.M.; Cullen, A.P.; de Grujil, F.R.; Longstreth, J.; Takizaw, Y.; van der Leun, J.C. The human health effects of ozone depletion and interactions with climate change. Photoch. Photobiol. Sci. 2011, 10, 199–225. [Google Scholar] [CrossRef]
- Mitchelmore, C.L.; He, K.; Gonsior, M.; Hain, E.; Heyes, A.; Clark, C.; Younger, R.; Schmitt-Kopplin, P.; Feerick, A.; Conway, A.; et al. Occurrence and distribution of UV-filters and other anthropogenic contaminants in coastal surface water, sediment, and coral tissue from Hawaii. Sci. Total Environ. 2019, 670, 398–410. [Google Scholar] [CrossRef]
- Gulson, B.; McCall, M.; Korsch, M.; Gomez, L.; Casey, P.; Oytma, Y.; Taylor, A.; McCulloch, M.; Trotter, J.; Kinsley, L. Small amounts of zinc from zinc oxide particles in sunscreens applied outdoors are absorbed through human skin. Toxicol. Sci. 2010, 118, 140–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gulson, B.; Wong, H.; Korsch, M.; Gomez, L.; Casey, P.; McCall, M.; McCulloch, M.; Trotter, J.; Stauber, J.; Greenoak, G. Comparison of dermal absorption of zinc from different sunscreen formulations and differing UV exposure based on stable isotope tracing. Sci. Total Environ. 2012, 420, 313–318. [Google Scholar] [CrossRef]
- Matta, M.K.; Zusterzeel, R.; Pilli, N.R.; Patel, V.; Volpe, D.A.; Florian, J.; Oh, L.; Bashaw, E.; Zineh, I.; Sanabria, C.; et al. Effect of Sunscreen Application Under Maximal Use Conditions on Plasma Concentration of Sunscreen Active Ingredients: A Randomized Clinical Trial. JAMA 2019, 321, 2082–2091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schlumpf, M.; Schmid, P.; Durrer, S.; Conscience, M.; Maerkel, K.; Henseler, M.; Gruetter, M.; Herzog, I.; Reolon, S.; Ceccatelli, R.; et al. Endocrine activity and developmental toxicity of cosmetic UV filters—An update. Toxicology 2004, 205, 113–122. [Google Scholar] [CrossRef]
- Blüthgen, N.; Zucchi, S.; Fent, K. Effects of the UV filter benzophenone-3 (oxybenzone) at low concentrations in zebrafish (Danio rerio). Toxicol. Appl. Pharm. 2012, 263, 184–194. [Google Scholar] [CrossRef]
- Calafat, A.M.; Wong, L.Y.; Ye, X.; Reidy, J.A.; Needham, L.L. Concentrations of the sunscreen agent benzophenone-3 in residents of the United States: National Health and Nutrition Examination Survey 2003–2004. Environ. Health Perspect. 2008, 116, 893–897. [Google Scholar] [CrossRef] [Green Version]
- Kunz, P.Y.; Fent, K. Estrogenic activity of UV filter mixtures. Toxicol. Appl. Pharm. 2006, 217, 86–99. [Google Scholar] [CrossRef]
- Fent, K.; Kunz, K.P.Y.; Gomez, E. UV filters in the aquatic environment induce hormonal effects and affect fertility and reproduction in fish. Chimia 2008, 62, 368–375. [Google Scholar] [CrossRef] [Green Version]
- Kunz, P.Y.; Galicia, H.F.; Fent, K. Comparison of in vitro and in vivo estrogenic activity of UV filters in fish. Toxicol. Sci. 2006, 90, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, D.; Sieratowicz, A.; Zielke, H.; Oetken, M.; Hollert, H.; Oeglmann, J. Ecotoxicological effect characterization of widely used organic UV filters. Environ. Pollut. 2012, 163, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Rodil, R.; Moeder, M.; Altenburger, R.; Schmitt-Jansen, M. Photostability and phytotoxicity of selected sunscreen agents and their degradation mixtures in water. Anal. Bional. Chem. 2009, 395, 1513–1524. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, Y.; Kameda, Y. Concentration of organic sun-blocking agents in seawater of beaches and coral reefs of Okinawa Island. Mar. Pollut. Bull. 2013, 77, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Downs, C.A.; Kramarsky-Winter, E.; Segal, R.; Fauth, J.; Kuntson, S.; Bronstein, F.; Jeger, R.; Lichtenfeld, Y.; Woodley, C.M.; Pennington, P.; et al. Toxicopathological Effects of the Sunscreen UV Filter, Oxybenzone (Benzophenone-3), on Coral Planulae and Cultured Primary Cells and Its Environmental Contamination in Hawaii and the U.S. Virgin Islands. Arch. Environ. Contam. Toxicol. 2016, 70, 265–288. [Google Scholar] [CrossRef]
- Fel, J.P.; Lacherez, C.; Bensetra, A.; Mezzache, S.; Béraud, E.; Léonard, M.; Allemand, D.; Ferrier-Pagès, C. Photochemical response of the scleractinian coral Stylophora pistillata to some sunscreen ingredients. Coral Reefs 2019, 38, 109–122. [Google Scholar] [CrossRef] [Green Version]
- Kroon, F.J.; Berry, K.L.E.; Brinkman, D.L.; Kookana, R.; Leusch, F.D.L.; Melvin, S.D.; Neale, P.A.; Negri, A.P.; Puotinen, M.; Tsang, J.J.; et al. Sources, presence and potential effects of contaminants of emerging concern in the marine environments of the Great Barrier Reef and Torres Strait, Australia. Sci. Total Environ. 2020, 719, 135140. [Google Scholar] [CrossRef]
- Rodríguez-Romero, A.; Ruiz-Gutiérrez, G.; Viguri, J.R.; Tovar-Sánchez, A. Sunscreens as a New Source of Metals and Nutrients to Coastal Waters. Environ. Sci. Technol. 2019, 53. [Google Scholar] [CrossRef]
- Sánchez-Quiles, D.; Tovar-Sánchez, A. Are sunscreens a new environmental risk associated with coastal tourism? Environ. Int. 2015, 83, 158–170. [Google Scholar] [CrossRef] [Green Version]
- Sendra, M.; Sánchez-Quiles, D.; Blasco, J.; Moreno-Garrido, I.; Lubián, L.M.; Pérez-García, S.; Tovar-Sánchez, A. Effects of TiO2 nanoparticles and sunscreens on coastal marine microalgae: Ultraviolet radiation is key variable for toxicity assessment. Environ. Int. 2017, 98, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Tovar-Sánchez, A.; Sánchez-Quiles, D.; Rodríguez-Romero, A. Massive coastal tourism influx to the Mediterranean Sea: The environmental risk of sunscreens. Sci. Total Environ. 2019, 656, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Labille, J.; Slomberg, D.; Catalano, R.; Robert, S.; Apres–Termelo, M.L.; Boudenne, J.L.; Manasfi, T.; Radakovitch, O. Assessing UV filter inputs into beach waters during recreational activity: A field study of three French Mediterranean beaches from consumer survey to water analysis. Sci. Total Environ. 2020, 706, 136010. [Google Scholar] [CrossRef]
- Montesdeoca-Esponda, S.; Álvarez-Raya, C.; Torres-Padrón, M.E.; Sosa-Ferrera, Z.; Santana-Rodríguez, J.J. Monitoring and environmental risk assessment of benzotriazole UV stabilizers in the sewage and coastal environment of Gran Canaria (Canary Islands, Spain). J. Environ. Manag. 2019, 233, 567–575. [Google Scholar] [CrossRef]
- Tsui, M.M.P.; Chen, L.; He, T.; Wan, Q.; Hu, C.; Lam, J.C.W.; Lam, P.K.S. Organic ultraviolet (UV) filters in the South China sea coastal region: Environmental occurrence, toxicological effects and risk assessment. Ecotoxicol. Environ. Saf. 2019, 181, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Fisch, K.; Waniek, J.J.; Schulz-Bull, D.E. Occurrence of pharmaceuticals and UV-filters in riverine run-offs and waters of the German Baltic Sea. Mar. Pollut. Bull. 2017, 124, 388–399. [Google Scholar] [CrossRef]
- Apel, C.; Joerss, H.; Ebinghaus, R. Environmental occurrence and hazard of organic UV stabilizers and UV filters in the sediment of European North and Baltic Seas. Chemosphere 2018, 212, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Dąbrowska, A.; Binkiewicz, K.; Nawrocki, J. Testing the content of benzophenones in surface waters using the GC-ECD technique. Prot. Environ. 2009, 31, 57–60. [Google Scholar]
- Mudryk, Z.; Skórczewski, P.; Perliński, P.; Wielgat, M. Studies concerning heterotrophic bacteria decomposing macromolecular compounds at two marine beaches. Oceanol. Hydrobiol. St. 2011, 40, 74–83. [Google Scholar] [CrossRef]
- Bigus, K.; Astel, A.; Niedzielski, P. Seasonal distribution of metals in vertical and horizontal profiles of sheltered and exposed beaches on Polish coast. Mar. Pollut. Bull. 2016, 106, 347–359. [Google Scholar] [CrossRef]
- Astel, A.; Bigus, K.; Stec, M. Microbial enzymatic activity and its relation to organic matter abundance on sheltered and exposed beaches on the Polish coast. Oceanologia 2018, 60, 312–330. [Google Scholar] [CrossRef]
- Jeon, H.K.; Chung, Y.; Ryu, J.C. Simultaneous determination of benzophenone-type UV filters in water and soil by gas chromatography-mass spectrometry. J. Chromatogr. A 2006, 113, 192–202. [Google Scholar] [CrossRef]
- EU. Commission Regulation (EU) 2017/238 of 10 February 2017 amending Annex VI to Regulation (EC) No 1223/2009 of the European Parliament and of the Council on cosmetic products. Off. J. Eur. Union 2017, 36, 37. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32017R0238&rid=7 (accessed on 2 October 2020).
- EC. Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on Cosmetic Products (Recast). 2009. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:02009R1223-20200501&from=EN (accessed on 2 October 2020).
- EC. European Commission Decision 2002/7657/EC of 12 August 2002 Implementing Council Directive 96/23/EC Concerning the Performance of Analytical Methods and the Interpretation of Results. Off. J. Eur. Comm. 2002, 221, 8–36. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2002:221:0008:0036:EN:PDF (accessed on 10 August 2020).
- Sánchez Rodríguez, A.; Sanz, R.M.; Betancort Rodríguez, J.R. Occurrence of eight UV filters in beaches of Gran Canaria (Canary Islands). An approach to environmental risk assessment. Chemosphere 2015, 131, 85–90. [Google Scholar] [CrossRef]
- Tarazona, I.; Chisvert, A.; Salwador, A. Development of a gas chromatography-mass spectrometry method for the determination of ultraviolet filters in beach sand samples. Anal. Methods 2014, 6, 7772–7780. [Google Scholar] [CrossRef]
- Benedé, J.L.; Chisvert, A.; Moyano, C.; Giokas, D.L. Expanding the application of stir bar sorptive-dispersive microextraction approach to solid matrices: Determination of ultraviolet filters in coastal sand Samales. J. Chromatogr. A 2018, 1564, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Vila, M.; Llompart, M.; Gracia-Jares, C.; Homem, V.; Dagnac, T. Development and optimization of a solid-phase microextraction gas chromatography–tandem mass spectrometry methodology to analyse ultraviolet filters in beach sand. J. Chromatogr. A 2018, 1564, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Vila, M.; Llopart, M.; Garcia-Jares, C.; Dagnac, T. Different miniaturized extraction methodologies followed by GC–MS/MS analysis for the determination of UV filters in beach sand. J. Sep. Sci. 2018, 41, 3347–3502. [Google Scholar] [CrossRef]
- EC. Annex VI, List of UV Filters Allowed in Cosmetic Products. 2020. Available online: https://ec.europa.eu/growth/tools-databases/cosing/pdf/COSING_Annex%20VI_v2.pdf (accessed on 6 August 2020).
- Kerr, A.C. A survey of the availability of sunscreen filters in the UK. Clin. Exp. Dermatol. 2011, 36, 541–543. [Google Scholar] [CrossRef]
- Manová, E.; von Goetz, N.; Hauri, U.; Bogdal, C.; Hungerbühler, K. Organic UV filters in personal care products in Switzerland: A survey of occurrence and concentrations. Int. J. Hyg. Environ. Health 2013, 216, 508–514. [Google Scholar] [CrossRef]
- Zhang, W.; Harff, J.; Schneider, B. Analysis of 50-year wind data of the southern Baltic Sea for modelling coastal morphological evolution—A case study from the Darss-Zingst Peninsula. Oceanologia 2011, 53, 489–518. [Google Scholar] [CrossRef] [Green Version]
- Balmer, M.; Buser, H.R.; Müller, M.D.; Poiger, T. Occurrence of some organic UV filters in wastewater, in surface waters, and in fish from Swiss Lakes. Environ. Sci. Technol. 2005, 39, 953–962. [Google Scholar] [CrossRef]
- Kameda, Y.; Kimura, K.; Miyazaki, M. Occurrence and profiles of organic sunblocking agents in surface waters and sediments in Japanese rivers and lakes. Environ. Pollut. 2011, 159, 1570–1576. [Google Scholar] [CrossRef] [PubMed]
- Bargar, T.A.; Alvarez, D.; Garrison, V.H. Synthetic ultraviolet light filtering chemical contamination of coastal waters of Virgin Islands national park, St. John, U.S. Virgin Islands. Mar. Poll. Bull. 2015, 101, 193–199. [Google Scholar] [CrossRef]
- Ekpeghere, K.I.; Un-Jung, K.; Sung-Hee, O.; Hee-Young, K.; Jeong-Eun, O. Distribution and seasonal occurrence of UV filters in rivers and wastewater treatment plants in Korea. Sci. Total Environ. 2016, 542, 121–128. [Google Scholar] [CrossRef]
- Kasprzyk-Hordern, B.; Dinsdale, R.M.; Guwy, A.J. The occurrence of pharmaceuticals, personal care products, endocrine disruptors and illicit drugs in surface water in South Wales, UK. Water Res. 2008, 42, 3498–3518. [Google Scholar] [CrossRef]
- Kasprzyk-Hordern, B.; Dinsdale, R.M.; Guwy, A.J. The removal of pharmaceuticals, personal care products, endocrine disruptors and illicit drugs during wastewater treatment and its impact on the quality of receiving waters. Water Res. 2009, 43, 363–380. [Google Scholar] [CrossRef]
- Ramos, S.; Homem, V.; Santos, L.; Alves, A. A review of organic UV-filters in wastewater treatment plants. Environ. Int. 2016, 86, 24–44. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ok, Y.S.; Kim, K.H.; Kwon, E.E.; Tsang, Y.F. Occurrences and removal of pharmaceuticals and personal care products (PPCPs) in drinking water and water/sewage treatment plants: A review. Sci. Total Environ. 2017, 596, 303–320. [Google Scholar] [CrossRef]
- Bachelot, M.; Li, Z.; Munaron, D.; Le Gall, P.; Casellas, C.; Fenet, H.; Gomez, E. Organic UV filter concentrations in marine mussels from French coastal regions. Sci. Total Environ. 2012, 420, 273–279. [Google Scholar] [CrossRef]
- Picot Groz, M.; Martinez Bueno, M.J.; Rosain, D.; Fenet, H.; Casellas, C.; Pereira, C.; Maria, V.; Bebianno, M.J.; Gomez, E. Detection of emerging contaminants (UV filters, UV stabilizers and musks) in marine mussels from Portuguese coast by QuEChERS extraction and GC-MS/MS. Sci. Total Environ. 2014, 493, 162–169. [Google Scholar] [CrossRef]
- Fent, K.; Zenker, A.; Rapp, M. Widespread occurrence of estrogenic UV-filters in aquatic ecosystems in Switzerland. Environ. Pollut. 2010, 158, 1817–1824. [Google Scholar] [CrossRef] [PubMed]
- Gago-Ferrero, P.; Díaz-Cruz, M.S.; Barceló, D. UV filters bioaccumulation in fish from Iberian river basins. Sci. Total Environ. 2015, 518, 518–525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zenker, A.; Schmutz, H.; Fent, K. Simultaneous trace determination of nine organic UV-absorbing compounds (UV filters) in environmental samples. J. Chromatogr. A 2008, 1202, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Gago-Ferrero, P.; Alonso, M.B.; Bertozzi, C.P.; Marigo, J.; Barbosa, L.; Cremer, M.; Secchi, E.R.; Domit, C.; Azevedo, A.; Lailson-Brito, J., Jr.; et al. First determination of UV filters in marine mammals. Octocrylene levels in Franciscana dolphins. Environ. Sci. Technol. 2013, 47, 5619–5625. [Google Scholar] [CrossRef]
- Tsui, M.M.P.; Leung, H.W.; Wai, T.C.; Yamashita, N.; Taniyasu, S.; Liu, W.; Lam, P.K.S.; Murphy, M.B. Occurrence, distribution and ecological risk assessment of multiple classes of UV filters in surface waters from different countries. Water Res. 2014, 67, 55–65. [Google Scholar] [CrossRef]
- Cuderman, P.; Heath, E. Determination of UV filters and antimicrobial agents in environmental water samples. Anal. Bioanal. Chem. 2007, 387, 1343–1350. [Google Scholar] [CrossRef]
- Román, I.P.; Chisvert, A.; Canals, A. Dispersive solid-phase extraction based on oleic acid-coated magnetic nanoparticles followed by gas chromatography-mass spectrometry for UV-filter determination in water samples. J. Chromatogr. A 2011, 1218, 2467–2475. [Google Scholar] [CrossRef] [PubMed]
- Grabicova, K.; Fedorova, G.; Burkina, V.; Steinbach, C.; Schmidt-Posthaus, H.; Zlabek, V.; Kocour Kroupova, K.H.; Grabic, R.; Randak, T. Presence of UV filters in surface water and the effects of phenylbenzimidazole sulfonic acid on rainbow trout (Oncorhynchus mykiss) following a chronic toxicity test. Ecotoxicol. Environ. Saf. 2013, 96, 41–47. [Google Scholar] [CrossRef]
- Wu, J.W.; Chen, H.C.; Ding, W.H. Ultrasound-assisted dispersive liquid–liquid microextraction plus simultaneous silylation for rapid determination of salicylate and benzophenone-type ultraviolet filters in aqueous samples. J. Chromatogr. A 2013, 1302, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Wick, A.; Fink, G.; Ternes, T.A. Comparison of electrospray ionization and atmospheric pressure chemical ionization for multi-residue analysis of biocides, UV-filters and benzothiazoles in aqueous matrices and activated sludge by liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2010, 1217, 2088–2103. [Google Scholar] [CrossRef]
- Benedé, J.L.; Chisvert, A.; Salvador, A.; Sánchez-Quiles, D.; Tovar-Sánchez, A. Determination of UV filters in both soluble and particulate fractions of seawaters by dispersive liquid-liquid microextraction followed by gas chromatography-mass spectrometry. Anal. Chim. Acta 2014, 812, 50–58. [Google Scholar] [CrossRef]
- Tarazona, I.; Chisvert, A.; León, Z.; Salvador, A. Determination of hydroxylated benzophenone UV filters in sea water samples by dispersive liquid-liquid microextraction followed by gas chromatography-mass spectrometry. J. Chromatogr. A 2010, 1217, 4771–4778. [Google Scholar] [CrossRef] [PubMed]
- Bratkovics, S.; Sapozhnikova, Y. Determination of seven commonly used organic UV filters in fresh and saline waters by liquid chromatography-tandem mass spectrometry. Anal. Methods 2011, 3, 2943–2950. [Google Scholar] [CrossRef]
- Giokas, D.L.; Sakkas, V.A.; Albanis, T.A. Determination of residues of UV filters in natural waters by solid-phase extraction coupled to liquid chromatography–photodiode array detection and gas chromatography–mass spectrometry. J. Chromatogr. A 2004, 1026, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.T.N.; Scapolla, C.; Di Carro, M.; Magi, E. Rapid and selective determination of UV filters in seawater by liquid chromatography-tandem mass spectrometry combined with stir bar sorptive extraction. Talanta 2011, 85, 2375–2384. [Google Scholar] [CrossRef] [PubMed]

| Compound (Abbreviation) /Systematic Name /CAS No | Chemical Formula | Chemical Structure | UV Protection | Average Content in Cosmetics 1 | Log Kow | λmax (nm) |
|---|---|---|---|---|---|---|
| Benzophenone-1 (BP-1) /2,4-dihydroxybenzophenone /131-56-6 | C13H10O3 |  | UV-A UV-B | up to 10% according to country | 3.15 | 290 |
| Benzophenone-2 (BP-2) /2,2’,4,4’-tetrahydroxybenzophenone /131-55-5 | C13H10O5 | 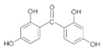 | UV-A UV-B | up to 10% according to country | 2.78 | 285 |
| Benzophenone-3 (BP-3) /2-hydroxy-4-methoxybenzophenone /131-57-7 | C14H12O3 | 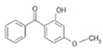 | UV-A UV-B | 6% [41] | 3.79 | 287 |
| Enzacamene (4-MBC) /(3E)-1,7,7-trimethyl-3-[(4- methylphenyl)methylene] -2-norbornanone /36861-47-9 | C18H22O | 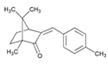 | UV-B | 4% | 4.95 | 300 |
| 3-benzylidene camphor (3BC) /1,7,7-trimethyl-3-(phenylmethylene) bicyclo[2.2.1] heptan-2-one /15087-24-8 | C17H20O | 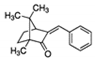 | UV-A UV-B | use prohibited in UE [42] | 5.37 | 289 |
| Compound | Correlation Coefficient (r2) | LOD (S/N = 3) | LOQ (S/N = 10) |
|---|---|---|---|
| Benzophenone-1 (BP-1) | 0.9999 | 0.010 µg·L−1 | 0.033 µg·L−1 |
| Benzophenone-2 (BP-2) | 0.9991 | 0.025 µg·L−1 | 0.083 µg·L−1 |
| Benzophenone-3 (BP-3) | 0.9999 | 0.035 µg·L−1 | 0.116 µg·L−1 |
| 3-Benzylidene camphor (3-BC) | 0.9999 | 0.030 µg·L−1 | 0.099 µg·L−1 |
| 3-(4-Methylbenzylidene)-camphor (4-MBC) | 0.9997 | 0.030 µg·L−1 | 0.099 µg·L−1 |
| Location | UV Filter | Range | Mean | S.D. | Detection Frequency (%) |
|---|---|---|---|---|---|
| Darłowo | BP-1 | <LOD-52.5 | 21.0 | 16.1 | 19.4 |
| BP-2 | 27.3-977.0 | 273.6 | 249.0 | 100 | |
| BP-3 | <LOD-74.2 | 63.3 | 10.3 | 16.7 | |
| 3-BC | n.d. | 0 | |||
| 4-MBC | <LOD-132.1 | 73.9 | 32.5 | 25.0 | |
| Ustka | BP-1 | <LOD-5.4 | 5.4 | 2.8 | |
| BP-2 | 17.0-782.7 | 267.7 | 241.9 | 100 | |
| BP-3 | <LOD-25.6 | 25.6 | 2.8 | ||
| 3-BC | n.d. | 0 | |||
| 4-MBC | <LOD-67.3 | 67.3 | 2.8 | ||
| Rowy | BP-1 | <LOD-14.1 | 11.0 | 2.5 | 22.2 |
| BP-2 | <LOD-1474.3 | 414.0 | 490.7 | 83.3 | |
| BP-3 | <LOD-65.7 | 40.4 | 24.9 | 8.3 | |
| 3-BC | n.d. | 0 | |||
| 4-MBC | <LOD-133.0 | 85.5 | 30.4 | 22.2 | |
| Czołpino | BP-1 | n.d. | 0 | ||
| BP-2 | <LOD-704.5 | 233.7 | 208.6 | 75.0 | |
| BP-3 | n.d. | 0 | |||
| 3-BC | n.d. | 0 | |||
| 4-MBC | <LOD-71.3 | 71.3 | 71.3 | 2.8 |
| Season | UV Filter | Range | Mean | S.D. | Detection Frequency (%) |
|---|---|---|---|---|---|
| spring | BP-1 | n.d. | 0 | ||
| BP-2 | <LOD-329.8 | 119.4 | 96.4 | 93.7 | |
| BP-3 | n.d. | 0 | |||
| 3-BC | n.d. | 0 | |||
| 4-MBC | n.d. | 0 | |||
| summer | BP-1 | <LOD-52.5 | 15.0 | 11.7 | 33.3 |
| BP-2 | <LOD-1474.3 | 393.9 | 444.6 | 93.7 | |
| BP-3 | <LOD-74.2 | 55.7 | 18.7 | 18.7 | |
| 3-BC | n.d. | 0 | |||
| 4-MBC | <LOD-132.1 | 72.7 | 33.1 | 20.8 | |
| autumn | BP-1 | n.d. | 0 | ||
| BP-2 | <LOD-729.6 | 387.5 | 207.3 | 81.2 | |
| BP-3 | <LOD-25.6 | 25.6 | 2.1 | ||
| 3-BC | n.d. | 0 | |||
| 4-MBC | <LOD-133.0 | 84.5 | 25.4 | 18.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Astel, A.; Stec, M.; Rykowska, I. Occurrence and Distribution of UV Filters in Beach Sediments of the Southern Baltic Sea Coast. Water 2020, 12, 3024. https://doi.org/10.3390/w12113024
Astel A, Stec M, Rykowska I. Occurrence and Distribution of UV Filters in Beach Sediments of the Southern Baltic Sea Coast. Water. 2020; 12(11):3024. https://doi.org/10.3390/w12113024
Chicago/Turabian StyleAstel, Aleksander, Marcin Stec, and Iwona Rykowska. 2020. "Occurrence and Distribution of UV Filters in Beach Sediments of the Southern Baltic Sea Coast" Water 12, no. 11: 3024. https://doi.org/10.3390/w12113024





